Over the past several decades, research on substances of misuse has vastly improved understanding of human behavior and physiology and the nature of substance use disorders (SUDs). Basic neurobiologic research has enhanced understanding of the biologic and genetic causes of SUDs. These discoveries have helped establish SUD as a biologic brain disease that is chronic and relapsing in nature (National Institute on Drug Abuse [NIDA], 2018c; Volkow et al., 2019). By mapping the neural pathways of pleasure and pain through the human brain, investigators are beginning to understand how psychoactive substances, including stimulants, interact with various cells and neurochemicals in the brain.
This new information has also improved understanding of appropriate treatment approaches for different SUDs. This chapter describes the effects that acute and chronic cocaine and methamphetamine (MA) use, and prescription stimulant misuse, have on a person's brain and behavior. The chapter also discusses how to assess for and diagnose stimulant use disorders. Knowledge of the neurobiologic effects of stimulants will give clinicians greater insight into people who use stimulants, how to detect a stimulant use disorder in an individual, and why the treatment approaches described in Chapter 4 are effective.
Substance Use Disorders
SUDs are complex phenomena with numerous psychological, social, familial, emotional, and systemic contributors. SUDs often co-occur, and people with stimulant use disorders often also use or misuse other substances (Timko et al., 2018). However, at the core, SUDs involve a biologic process: the effects of repeated exposure to an agent (a substance) on a biologic substrate (the brain) over time (MacNicol, 2017; Volkow et al., 2019). Ultimately, adaptations that substance exposure elicits in individual neurons alter the functioning of those neurons, which in turn alters the functioning of the neural circuits and networks in which those neurons operate. This eventually leads to the complex phenomena that characterize SUDs (MacNicol, 2017).
Chronic substance use results in a complex set of physiological and neurologic adaptations. These adaptations are the body's attempt to adjust to or compensate for the intermittent or chronic presence of substances. Repeated exposure to a substance can also lead to adaptations in the reward circuitry that oppose and/or neutralize the substance's effects (i.e., counteradaptation). See Exhibit 2.1 for the parts of the brain that make up the reward circuitry. SUD-related brain activity can be characterized in three stages:
Box
EXHIBIT 2.1. Brain Structures Involved in the Reward System.
- 1.
Acute intoxication/binge
- 2.
Withdrawal/negative affect stage
- 3.
Anticipation/craving
Each stage has its own complicated and intricate neurocircuitry that continues to reinforce the seeking and use of a specific substance (Koob & Volkow, 2016). There are more than 18 systems of neuromodulation involved in the perpetuation of the three stages of SUDs in the brain; three important structures and regions are the basal ganglia, the amygdala, and the prefrontal cortex (Koob & Volkow, 2016).
Box
A SIMPLE WAY OF THINKING ABOUT SUDS.
With increasing use and development of tolerance to the effects of a substance, people will need to increase the amount taken to produce the desired effects. As substance use increases, so does disruption of executive function and of the reward and stress pathways. (The stress pathway comprises the various neurobiologic mechanisms invoked in response to stressful stimuli, such as the “fight or flight” response triggered by the hypothalamic–pituitary–adrenal axis.) These disruptions result in patients continuing to use and seek substances despite adverse consequences—the very definition of an SUD (Koob & Volkow, 2016).
Box
HOW TO UNDERSTAND A PERSON'S STRUGGLE WITH SUDS.
Neurobiology
The human nervous system is an elegant communication system, and the brain is the control center. The brain processes sensory information from throughout the body, guides muscle movement and locomotion, regulates a multitude of bodily functions, forms thoughts and feelings, modulates perception and moods, and essentially controls all behavior. Neurotransmitters (chemicals that transfer information between neurons and help neurons communicate with one another) also play a key role in the neurobiology of SUDs.
The Reward System or Positive Reinforcement
The brain circuit that is considered essential to neurologic reinforcement is called the limbic reward system (also called the dopamine reward system or the brain reward system). This neural circuit extends across the ventral tegmental area, the nucleus accumbens, and the prefrontal cortex.
Substances of misuse—including stimulants—affect the reward system (Volkow et al., 2019). Normal functioning of the brain's circuitry results in inhibition and stimulation of neurotransmitters at multiple sites in the brain's reward systems. However, neuroadaptation and neuroplasticity that occur when substances are present can result in multiple neurotransmitters disrupting this normal circuitry, resulting in prolonged phases of withdrawal/negative affect and anticipation/craving (Koob & Volkow, 2016).
The neurotransmitter dopamine, which helps to regulate the feelings of pleasure (euphoria and satisfaction), is both directly and indirectly affected by stimulants (Volkow et al., 2019). Dopamine also plays an important role in the control of movement, cognition, motivation, and reward (Bromberg-Martin et al., 2010; Volkow et al., 2019). In addition, stimulant use causes the brain to release norepinephrine, which helps regulate mood, attention, learning, memory, and arousal and may play a role in substance withdrawal (Office of the Surgeon General, 2016). The neurotransmitter serotonin affects reinforcement, motivation, learning, and memory, and may play a role in SUDs by making people more susceptible to compulsive (rather than controlled) substance use, especially people with genetic vulnerabilities to SUDs (Müller & Homberg, 2015).
Activities such as eating, drinking, and sex activate the reward system, inducing considerable communication among this structure's neurons. This internal communication leads to the release of dopamine. But substance use causes a surge of dopamine release that is far beyond that of natural activities, like eating and sex. The released dopamine produces immediate, but short-lived, feelings of pleasure and elation.
As dopamine levels subside, so do the feelings of pleasure. But if the activity is repeated, then dopamine is again released, and more feelings of pleasure and euphoria are produced. The release of dopamine and the resulting pleasurable feelings positively reinforce such activities and motivate the repetition of these activities. Moreover, with substance use, the person needs more and more of the substance to achieve the same level of pleasure.
Dopamine is believed to play an important role in the reinforcement of and motivation for repetitive actions (Daw & Tobler, 2013; Nutt et al., 2015; Volkow et al., 2019). An increasing amount of scientific evidence suggests that neuroadaptations to the reward and stress systems play a considerable role in the development of compulsive use behaviors (Koob & Volkow, 2016).
When the nucleus accumbens is functioning normally, communication among its neurons occurs in a consistent and predictable manner (Koob & Volkow, 2016). First, an electrical signal within a stimulated neuron reaches its point of connection (i.e., the synapse) with the target (postsynaptic) neuron. The electrical signal in the transmitting (presynaptic) neuron triggers the release of dopamine into the synaptic gap (Koob & Volkow, 2016). Dopamine travels across the synaptic gap until it reaches the postsynaptic neuron. It then binds to the postsynaptic neuron's dopamine-specific receptors. The binding of dopamine to the receptor has an excitatory effect that generates an internal electrical signal within this neuron. However, not all of the released dopamine binds to the target neuron's receptors. Extra dopamine may be chemically deactivated, or it may be quickly reabsorbed by the presynaptic neuron through a system called the dopamine reuptake transporter (see Exhibit 2.2).
Box
EXHIBIT 2.2. Normal Dopamine Transmission.
The postsynaptic neuron receives messages in the form of neurotransmitters released from the presynaptic neuron, resulting in depolarization or hyperpolarization of the postsynaptic neuron membrane. If the membrane is depolarized to a certain degree, an action potential occurs that causes the neuron to release a neurotransmitter (i.e., to “send a message”).
To learn more about the reward circuit in stimulant use disorders, see the NIDA video “The Reward Circuit: How the Brain Responds to Cocaine” (https://www.drugabuse.gov/videos/reward-circuit-how-brain-responds-to-cocaine).
The Stress System or Negative Reinforcement
In addition to positive reinforcement through the brain's reward system, negative reinforcement can play a key role in the development and maintenance of chronic, compulsive substance use (Wise & Koob, 2014). The motivation to use a substance to avoid discomfort is an example of negative reinforcement. This motivation to continue using a substance occurs in the withdrawal/negative affect stage of substance use and also in the anticipation/craving stage.
As people experience negative withdrawal symptoms from not using a substance, their brain circuitry causes further dysregulation of executive function and other cognitive processes. This dysregulation creates negative effects in the absence of the substance, further driving the precontemplation/craving phase to reinforce the compulsive seeking and taking of the substance (Koob & Volkow, 2016).
Experimental evidence supports the theory that stimulants and other commonly misused substances imitate, facilitate, or block the neurotransmitters (especially dopamine) involved in brain reinforcement systems (Ashok et al., 2017; dela Peña et al., 2015; Nutt et al., 2015; Volkow et al., 2019). Negative reinforcement through overactivation of the stress system or the anti-reward system could also play a role in the perpetuation of chronic recurrent use to alleviate negative effects (Koob & Volkow, 2016).
Drug Craving and Memory
The degree to which learning and memory sustain the addictive process has also been addressed. Researchers believe that each time a neurotransmitter like dopamine floods across a synapse, circuits that trigger thoughts and memories and that motivate action become more strongly activated in the brain. Moreover, activation of the reward system creates a very powerful association between the euphoric and other rewarding effects of the substance and whatever people, objects, or places the individual is exposed to at the time; these people, objects, or places then can become cues for substance use (Office of the Surgeon General, 2016).
Craving, a central aspect of SUDs, is a very strong learned response with powerful motivational properties often associated with specific memories (i.e., conditioned cues and triggers; Carmack et al., 2017). Cues—any stimuli (e.g., drug paraphernalia, moods, friends who use substances, locations associated with substance use) repeatedly paired with substance use over the course of a patient's SUD—can become so strongly associated with the substance's effects that the associated (conditioned) stimuli can later trigger arousal and an intense desire for the substance and lead to recurrent use (Carmack et al., 2017). High recurrence rates are common in people with stimulant use disorders even after treatment (Brecht & Herbeck, 2014).
Brain imaging studies have shown that cue-induced drug craving may be linked to distinct brain systems involved in memory (Moreno-Rius & Miquel, 2017; Perry et al., 2014). Brain structures involved in memory and learning, including the dorsolateral prefrontal cortex, amygdala, and cerebellum, have been linked to cue-induced craving (Moreno-Rius & Miquel, 2017; Sinha, 2013). A network of these brain regions integrates emotional and cognitive aspects of memory and triggers craving when it reacts to cues and memories. These cues and memories also play an important role in reinforcing substance use (Carmack et al., 2017). In contrast, negative experiences (e.g., violence, trauma, paranoia) that occur during acute intoxication do not seem to reinforce avoidance of intoxication.
Most SUD treatment approaches recognize the power of these factors in triggering recurrent use and warn patients to avoid everything previously associated with their substance use. Treatment approaches that address these learning and memory issues of SUDs may prove effective. For example, cue exposure therapy uses extinction (i.e., breaking the individual's association between the trigger or cue—like seeing drug paraphernalia—and the conditioned response—such as experiencing feelings of craving) to help decrease physiologic reactions to triggers and decrease cravings (Carmack et al., 2017; Torregrossa & Taylor, 2013).
Researchers have examined other methods of similarly reducing physiologic responses to triggers and thereby reducing craving, such as inhibiting memory reconsolidation (i.e., the process of stabilizing newly formed memories so they can be stored long term) and using pharmacologic agents (e.g., propranolol) to enhance the extinction process used in cue exposure therapy (Torregrossa & Taylor, 2013). People with SUDs may further benefit from cue exposure therapy that is combined with other psychosocial interventions, like cognitive therapy and motivational enhancement (Kaplan et al., 2011).
Role of Technologies
The development of noninvasive brain imaging (e.g., positron emission tomography [PET] scans) has created a powerful new tool for demonstrating not only the short-term effects of substance use but also the longer term consequences of chronic substance use and SUDs. These tools have allowed researchers to go where they previously could not—literally into a living human brain. However, this research is still maturing, and many questions remain about whether and how these technologies might inform clinicians' care of people with stimulant use disorders.
Such noninvasive techniques can depict higher or lower activity levels of different brain areas by measuring metabolic activity (e.g., glucose use; Fakhoury, 2014). They can identify substance-induced structural changes and physiologic adaptations (Fakhoury, 2014). Through a combination of techniques, researchers and clinicians can observe the altered processing of information in various circuits as the brain responds to substance use.
Using noninvasive imaging techniques, investigators have been able to identify brain structures involved in craving, map the emotions of people who use substances, and plot the neurobiologic basis of substance-induced euphoria. For example, researchers have used functional magnetic resonance imaging to predict substance use relapse and maintenance of abstinence (S. J. Moeller & Paulus, 2018). Additional neuroimaging studies have demonstrated significant changes in gray matter and in neurochemistry and have also predicted long-term effects of substance use and potential for recurrent use (S. J. Moeller & Paulus, 2018).
PET has revealed subtle alterations in the dopamine receptors in the brains of people who use stimulants (Solingapuram Sai et al., 2019). A review of some PET studies has demonstrated not just reductions in dopamine receptor availability and sensitivity associated with cocaine and MA use, but also increased dopamine release (Wiers et al., 2016). Combining PET with the radiotracer [18F]-fluorodeoxyglucose—which is used to visualize brain glucose metabolism—has helped researchers understand changes in the brain's metabolic activity associated with craving, alterations in cognition, self-regulation, and intoxication (Wiers et al., 2016).
PET imaging has also provided insight into stimulant-related effects on neurofunction such as identifying inflammation in the brain, understanding the influence of cocaine on mu-opioid receptor binding, and pinpointing increases in norepinephrine in the synapses due to blockage of its reuptake (Wiers et al., 2016). Single photon emission computerized tomography—a form of PET that uses a different type of radiotracer—may prove to be a useful diagnostic and classification tool (e.g., differentiating people at high risk of recurrent use from those who are not).
Structural magnetic resonance imaging studies of people with MA use have shown clear gray matter deficits in cortical areas (i.e., frontal, insular, cingulate, temporal, and occipital cortices) and in the hippocampus, along with an increase in volume in the parietal lobe and the striatum (Hall et al., 2015; Jan et al., 2012). White matter enhancement appears to occur in the temporal and occipital lobes, accompanied by widespread white matter hyperintensities and aberrations in the corpus callosum (Jan et al., 2012). Structural changes documented in people with cocaine and amphetamine use include reduced gray matter in the insula, ventromedial prefrontal cortex, inferior frontal gyrus, pregenual anterior cingulate gyrus, and anterior thalamus (Ersche et al., 2013; Hall et al., 2015). Research is ongoing to better understand what these patterns mean and how structural deficits and changes affect substance use behaviors and outcomes.
Imaging research is also providing important evidence about changes that can take place in the brain with abstinence and SUD treatment. For instance, exercise training for people in treatment for MA use disorder (and who were abstinent) was associated with recovery of certain striatal dopamine receptors that are known to become deficient with MA exposure—although it should be noted that abstinence plus education did not produce these benefits (C. L. Robertson et al., 2016). Nonetheless, such research suggests that healing in the brain can occur, providing additional evidence for the importance of promoting abstinence and recovery.
Although mapping brain activity during stimulant use and withdrawal may allow researchers to further document substance-induced neuropsychological impairments, not much of this research has been conducted in humans. Animal models suggest stimulant withdrawal is accompanied by a reduction in modularity—the ability of independent and functionally separate networks within the brain to interact with one another, somewhat like an electric circuit board with independent circuits that can connect to one another (Kalvar & Medaglia, 2018). Reduced modularity appears to occur in thalamic regions for MA and in a combination of midbrain-cortico-thalamic-hypothalamic-amygdalar brain regions for cocaine (Kimbrough et al., 2019).
The continuing development and application of new technologies such as noninvasive brain imaging will allow researchers to improve their understanding of how stimulants affect the human brain. Greater understanding of the underlying neuronal impairments of stimulant use will aid in the development of new and more effective treatment approaches.
Stimulant Use and the Brain
To better understand underlying drivers for substance use, it is important to learn the effects of any particular substance on a given person. For instance, someone with long-term stimulant use who is taking a stimulant and an opioid at the same time will be affected differently by that stimulant than someone taking a stimulant alone and for the first time. How stimulants affect individuals (both universal effects as well as person-specific effects) can provide helpful information to providers to assess, treat, and prevent recurrent use of the substance (Volkow et al., 2017).
Once a substance enters the bloodstream, it is transported throughout the body to various organs and organ systems, including the brain. To enter the brain, a substance's molecules must first get through its chemical protection system, which consists mainly of the blood–brain barrier. Tight cell-wall junctions and a layer of cells around the blood vessels keep large or electrically charged molecules from entering the brain. However, small neutral molecules like those of cocaine and MA easily pass through the blood–brain barrier and enter the brain (Kousik et al., 2012; Turowski & Kenny, 2015). Once inside the brain, substances begin to exert psychoactive effects.
Stimulants' Mechanisms of Action
On a short-term basis, stimulants exert their effects by disrupting or modifying the normal communication that occurs among brain neurons and brain circuits. Cocaine and MA have both been shown to disrupt the dopamine neurotransmitter system—cocaine indirectly and MA both directly and indirectly (Ashok et al., 2017). Both cocaine and MA can inhibit the reuptake and release of dopamine by the presynaptic neuron, resulting in excess dopamine in the synaptic gap.
The two common forms of prescription stimulants—methylphenidate and amphetamine—affect the dopamine system differently, but, like cocaine and MA, both result in increased extracellular dopamine. Methylphenidate inhibits the reuptake of dopamine, as does amphetamine; but amphetamine also increases the amount of dopamine initially released into the synaptic gap (Yanofski, 2011).
Whereas the mechanism of action of prescription stimulants is not drastically different from that of cocaine and MA, differences in effects can occur based on who is taking the substance (e.g., someone with attention deficit hyperactivity disorder [ADHD] versus someone without it), the dose taken, and how it is administered. These differences influence whether the prescription stimulant is a helpful therapy or a drug that can change the brain at the cellular and structural levels. Specifically, if a person has ADHD and takes a prescription stimulant, the medication is provided at a dose that increases dopamine to a level that provides relief from ADHD. When taken as directed (i.e., orally at the prescribed dosage and according to schedule), it provides a constant blood level of the medication. People with ADHD will feel more focused and productive as a result. But when MA, cocaine, or prescription stimulants are injected or smoked (or, in the case of medications, taken in higher-than-prescribed amounts or taken by people without ADHD), they can lead to brain changes and stimulant use disorder.
The use of stimulants increases the amount of available dopamine in the brain (Paulus & Stewart, 2020). High levels of available dopamine in the brain generally enhance mood and increase body movement (i.e., motor activity) and motivation, but too much dopamine may produce symptoms that approximate positive symptoms of schizophrenia (e.g., delusions, hallucinations, paranoia; Kesby et al., 2018; Klein et al., 2019). With cocaine, the effects are generally short-lived, whereas with MA, the duration of effect is much longer.
As the stimulant level in the brain decreases, the dopamine levels subside to normal, and the pleasurable feelings dwindle. With repeated stimulant use, dopamine stores in the brain become temporarily depleted (Ashok et al., 2017), resulting in the depressive and exhaustive symptoms associated with stimulant withdrawal.
Although the neurochemical pathways of chronic stimulant use disorders are not definitively established, a few researchers have found evidence of changes in the structure and function of brain neurons after chronic stimulant use in humans. Some researchers propose that the changes may come from dopamine depletion, changes in neurotransmitter receptors or other structures, or changes in cellular components or other brain messenger pathways that could cause the changes in mood, behavior (e.g., compulsivity, decision making), and cognitive function associated with chronic stimulant misuse (Ashok et al., 2017; Jan et al., 2012). (The medical aspects of stimulant use disorders are discussed in Chapter 3.)
General Effects of Stimulants
Stimulants affect the normal functioning of the dopamine neurotransmitter system (Volkow et al., 2019). Stimulants appear to increase the brain's levels of free dopamine (Ashok et al., 2017; dela Peña et al., 2015; MacNicol, 2017; Volkow et al., 2019). The higher the substance dose, the greater the individual's feelings of wakefulness, mania, and euphoria. As the dopamine levels and pleasurable feelings subside, the individual experiences an intense desire to replicate the feelings of pleasure by administering another dose of the substance. As with substance use generally, this tendency toward repeated administration is characteristic of stimulant use disorders and underlies most of the other effects of stimulants, as well as most other addictive substances.
Half-lives of stimulants vary by drug. Cocaine, being naturally derived, has a much shorter half-life (around 60 minutes), whereas MA, being synthetic, has a half-life of around 10 hours (Coe et al., 2018; Cruickshank & Dyer, 2009). The half-lives of prescription stimulants also vary by drug and by formulation (e.g., short-acting versus long-acting). For example, short-acting prescription amphetamine has a half-life of approximately 9 hours, whereas the long-acting formulation has a half-life in the range of 10 to 13 hours (Pradeep & Standeven, 2019).
Continued use often leads to adverse consequences, which may include neuropsychologic impairment, mental health issues, and diminished physical health. Work performance and social and family relations can be adversely affected, and the risk of arrest and criminal/legal involvement increases (McKetin et al., 2020).
It is important to note that in small and measured doses, stimulants may serve a clinical purpose to heighten wakefulness, help focus attention, and enhance cognition (see Exhibit 2.3). This could explain why some people who misuse prescription stimulants—especially adolescents in high school and young adults in college—often do so to improve their concentration and alertness, which they perceive as helping with their studying and academic performance (Clemow & Walker, 2014; Weyandt et al., 2018). Increasing doses, higher potency, and more frequent use increase psychostimulation and may eventually result in the cognitive impairments often correlated with stimulant use disorder (Wood et al., 2014). In very high doses, stimulant use can lead to serious medical complications, including coma and circulatory collapse, or even death (Wood et al., 2014).
Box
EXHIBIT 2.3. Continuum of Psychostimulant Activation.
For patients with a stimulant use disorder, impairments in the brain's reward systems that lead to problems with cognition and neuropsychiatric functioning may persist even after cessation of stimulant use (Taylor et al., 2013). Cravings for the stimulant's effects tend to linger, even after abstinence has been achieved, and the potential for recurrent use is high.
Effects of Combining Psychostimulants With Opioids
Psychostimulant-related overdose deaths involving opioids have been increasing over the past 20 years, with heroin and synthetic opioids (like fentanyl) largely accounting for psychostimulant-associated fatalities since 2010 (McCall Jones et al., 2017). Polysubstance use, specifically the co-consumption of synthetic opioids and psychostimulants, like cocaine and MA, was largely responsible for the increases in cocaine- and MA-related overdose deaths observed from 2012 to 2017 (Kariisa et al., 2019).
Opioids can lead to potentially lethal respiratory depression. This is especially true of fentanyl and fentanyl analogs, which are rapid acting and are increasingly being taken in combination with cocaine or MA, by accident or on purpose (LaRue et al., 2019). Fentanyl can induce fatal respiratory depression in as little as 2 minutes (Kuczyńska et al., 2018). Even if nonfatal, respiratory depression is dangerous and can lead to hypoxic brain injury (Kiyatkin, 2019). Because people naïve to opioids lack opioid tolerance, they may be at an increased risk of unintentional overdose when combining stimulants, like cocaine, with fentanyl or other synthetic and nonsynthetic opioids (LaRue et al., 2019).
Effects of Route of Administration
The five most common routes of administering psychoactive (mood-changing) substances are:
- •
Oral consumption (i.e., swallowing, gumming [rubbing the substance on the gums]).
- •
Intranasal consumption (i.e., insufflation).
- •
Inhalation into the lungs (i.e., smoking).
- •
Intravenously (i.e., injection).
- •
Vaginal or anal insertion.
Cocaine and MA can be smoked, snorted, injected, ingested orally, or absorbed intrarectally or intravaginally. Prescription stimulants can be taken orally or crushed and snorted. The route of administration affects the amount (i.e., the dosage) of stimulant delivered to the brain, the speed at which it is delivered, and the resulting intensity of the stimulant's effects—which in turn may affect the course of an SUD. Because a person's preferred route of administration affects the extent and depth of chronic effects, it has implications for treatment decisions (see Chapter 5). (For specific information on drug use and peak effects, see Chapter 3, Exhibit 3.2.)
The long plateau effect and the much longer half-life of MA versus cocaine suggest considerable dangers in repeated use of MA (Cruickshank & Dyer, 2009). Because stimulants exert their effects in a dose-dependent manner, the route of administration has serious neurologic, physical, psychiatric, and neurocognitive implications for the person using the stimulant. Prolonged high doses of stimulants (e.g., during binges or chronic use) may cause greater and longer lasting neurologic damage, which in turn may lead to greater and longer lasting cognitive deficits.
The onset of stimulants' chronic effects varies across individuals, and although there are few data to predict how long it will take for any person to begin experiencing the chronic effects of stimulant use, onset is probably related to:
- •
The amount of stimulant used.
- •
The frequency of use.
- •
The route of administration.
- •
Significant medical comorbidities.
- •
Co-occurring mental disorders.
- •
Co-administration of other substances.
- •
The environment in which the substance is taken.
- •
Genetics and metabolic factors.
However, in general, higher, more frequent doses of stimulants used in combination with other substances result in more rapid transition to the effects of chronic stimulant exposure. (For a discussion of route-of-administration effects on toxicity and adverse reactions, see Chapter 3.)
Psychological and Neurocognitive Effects
The immediate psychological effects of stimulant administration include a heightened sense of well-being, euphoria, excitement, and alertness, and increases in motor activity, similar to what would be seen in a manic state. Stimulants also reduce appetite and may result in insomnia. Stimulants may also enhance focus and libido (Volkow et al., 2007).
High doses, particularly in the setting of sleep deprivation, may result in restlessness, agitation, and more profound psychiatric presentation, including altered perceptions of reality and hallucinations. Chronic psychological effects of stimulant use and withdrawal may include paranoia, psychosis, depression, and/or suicidal ideation.
Cocaine
Routes of Administration
Cocaine is most commonly taken by nasal insufflation (snorting), intravenous injection, or inhalation of smoke vapors (smoking/inhalation). Less often, it is taken orally, vaginally, rectally, or sublingually. The half-life of cocaine is about 60 minutes (Coe et al., 2018) but can range from 40 to 90 minutes (ARUP Laboratories, 2019).
Pharmacology
Cocaine has two main pharmacologic actions. It is both a local anesthetic and a central nervous system (CNS) stimulant (NIDA, 2016a). Cocaine exerts its local anesthetic actions by blocking the conduction of sensory impulses within nerve cells. This effect is most pronounced when cocaine is applied to the skin or to mucous membranes. Cocaine has approved medical use as a local anesthetic in some surgery of the eye, ear, and throat (NIDA, 2016a).
As a CNS stimulant, cocaine affects a number of neurotransmitter systems, but it is through its interaction with the dopamine and the limbic reward system that cocaine produces some of its most important effects, including positive reinforcing effects (NIDA, 2016a). The major influence of cocaine on the dopamine system is its ability to block the synaptic reuptake of dopamine.
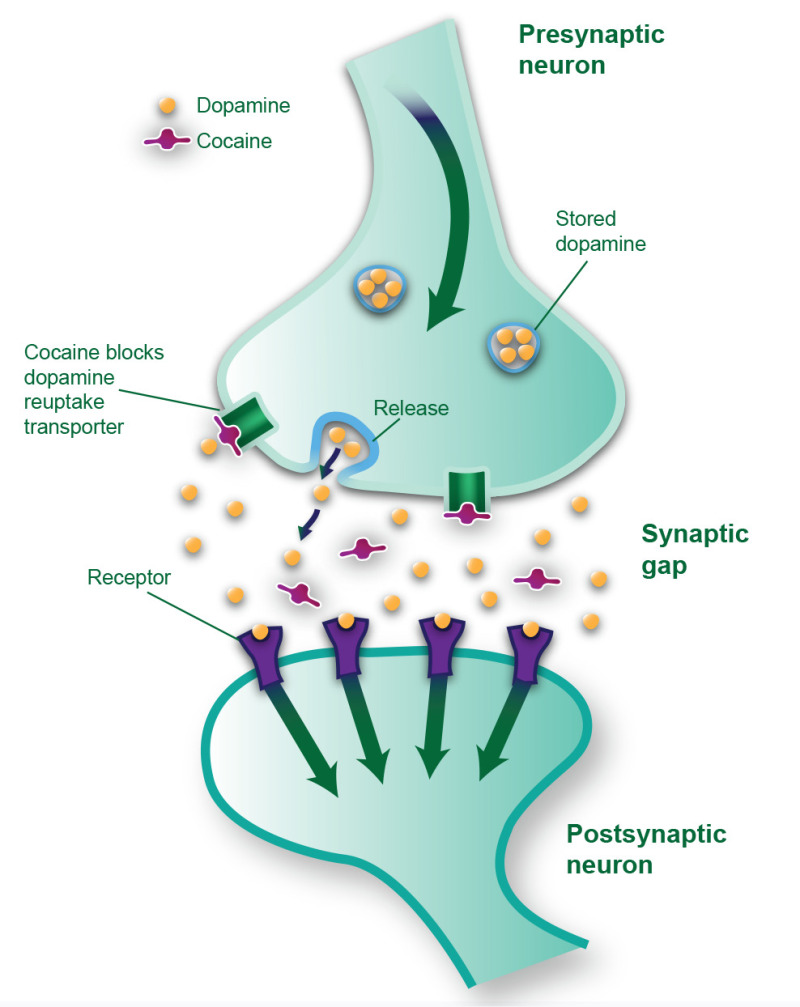
Cocaine does not directly stimulate the dopamine system; rather, it causes the system to be stimulated by preventing dopamine from being removed from the synaptic gap. Cocaine's blockade of the dopamine reuptake transporter extends the availability of dopamine in the synaptic space, where it continues to occupy the dopamine receptors and causes the postsynaptic neurons to fire for a longer-than-normal period (NIDA, 2016a). (See Exhibit 2.4.)
Box
EXHIBIT 2.4. Acute Effects of Cocaine on Dopamine Transmission.
Acute Physiologic Effects
Acute cocaine use can lead to narrowing of the blood vessels and an increase in body temperature, pulse, and blood pressure (NIDA, 2016a) as well as fatigue (Ciccarone, 2011). In some cases, tremors, dizziness, and muscle twitching can occur (NIDA, 2016a).
Acute Psychological Effects
The extended firing of the postsynaptic neurons resulting from prolonged dopamine receptor activity is initially experienced subjectively by people using cocaine as a positive sensation involving increased energy, arousal, and stimulation (NIDA, 2016a). The effects experienced during the initial period of cocaine use are generally mood altering in a positive manner. For most individuals, the subjective experience of the acute effects includes a generalized state of euphoria in combination with feelings of increased energy, talkativeness, mental alertness, and hypersensitivity to sight, sound, and touch (NIDA, 2016a).
Many people feel more intensely involved in their interactions with others and more playful and spontaneous when using cocaine. As patients use more, they may experience unpleasant adverse effects including increased anxiety, irritability, paranoia, and restlessness (NIDA, 2016a). As cocaine use subsides, particularly among patients with a stimulant use disorder, withdrawal symptoms will be present, including depressive symptoms and mood lability (Ciccarone, 2011).
With continued escalating use of cocaine, the individual becomes progressively tolerant to the positive effects and sensitized to the negative effects, which can increase the risk of unintentional overdose (NIDA, 2016a). People report that the positive effects of cocaine use are not as profound and that the rebound negative and adverse effects may increase over time, leading to a dysphoric, depressed state. This constant cycle of seeking additional positive effects and eliminating negative effects may perpetuate the cocaine use disorder. (For details on the medical aspects of acute cocaine use, see Chapter 3.)
Chronic Physiologic Effects
Initial experimental cocaine use often progresses to more steady use, requiring larger and larger doses to achieve the desired effects (NIDA, 2016a). Someone with regular cocaine use may become obsessed with the rituals of cocaine use and find that many common items or situations trigger cravings for the drug. Cocaine use disorder can develop, with overwhelming urges and cravings for cocaine, and an inability to self-limit or abstain from use.
The person addicted to cocaine will continue use despite the negative consequences. At this stage, the adverse consequences of cocaine use disorder have probably affected all aspects of the person's life.
There are no data that indicate how long it will take for any individual to begin to experience the chronic effects of cocaine use. Some individuals report an ability to use for extended periods with few signs of negative consequences. Others report a very dramatic onset of severe detrimental effects as soon as a few weeks or months after initiation of cocaine use. In general, however, similar to the effects of MA, the higher the doses and the more frequently the doses are administered, the more quickly the chronic effects of cocaine use will appear. In addition, intranasal administration (snorting) is associated with slower onset of chronic effects than is smoking cocaine (freebasing or smoking crack) or injecting it intravenously (Ciccarone, 2011).
Physically, the person with cocaine use disorder may appear thin or even emaciated. Personal hygiene and self-care may be neglected, and medical and dental needs may go unmet. Because cocaine suppresses appetite, the person fails to eat properly and may suffer from weight loss and nutritional deficiencies (Ciccarone, 2011). People with severe cocaine use disorder may ignore food, clothing, shelter, and sexual needs. Continued cocaine use can lead to erectile dysfunction and menstrual irregularities (Ciccarone, 2011), as well as anorexia, chest pain, and extreme fatigue.
Chronic Psychological Effects
Psychologically, cocaine's chronic effects oppose the often-desired initial effects. Chronic cocaine use increases paranoia and confusion (Ciccarone, 2011). The same substance that produced a mild sensation of arousal and decreased fatigue now causes insomnia and episodic depression.
Chronic use of cocaine may cause neuropsychological impairments (Quednow & Vonmoos, 2017; Spronk et al., 2013). Cocaine-induced cognitive deficits may affect multiple domains, but they appear to be reversible in patients with a mild or moderate cocaine use disorder within 1 year of cessation of use (Vonmoos et al., 2014).
The physical, psychological, and cognitive effects of chronic cocaine use reflect the underlying neurobiologic changes from cocaine's impact on the neurotransmitter dopamine. Spronk and colleagues (2013) found a strong association between the long-term use of cocaine and deficiencies in the following cognitive domains: attention, response inhibition (i.e., the ability to inhibit one's impulse to respond to a stimulus), working memory, cognitive flexibility, and psychomotor performance.
Although clinicians may easily pick up on the extensive health-compromising effects of cocaine use when examining the behavioral and psychological profile of patients entering SUD treatment, patients may need additional education to understand the correlation between their substance use and its negative health effects.
Methamphetamine
Routes of Administration
MA is typically taken orally, nasally (snorting/insufflation), intravenously, or by inhaling smoke vapors (smoking/inhalation). Less often, MA is taken vaginally, rectally, or sublingually. The half-life of a single dose of MA is about 10 hours across routes of administration (Cruickshank & Dyer, 2009).
Pharmacology
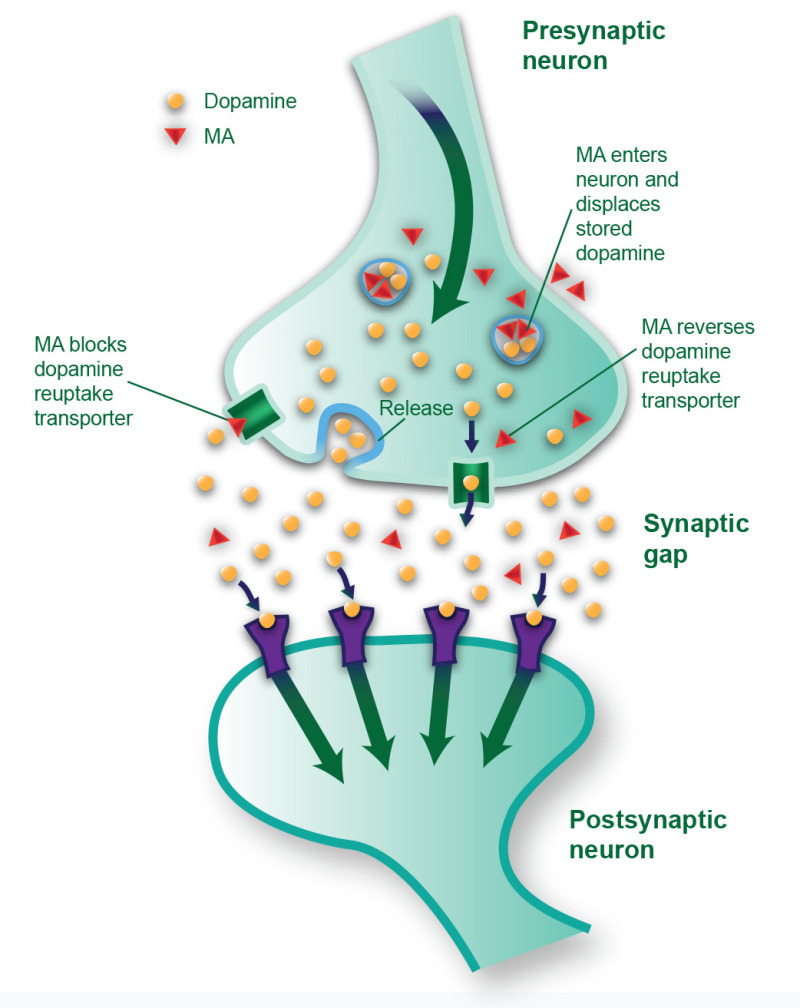
The course of MA use disorder development is similar to that of cocaine use disorder. The underlying neurologic effects of MA are similar to the effects produced by cocaine: essentially, it increases levels of free dopamine in the brain's limbic reward system. (Exhibit 2.5 illustrates some of the acute effects of MA on dopamine transmission.)
Box
EXHIBIT 2.5. Acute Effects of Methamphetamine on Dopamine Transmission.
Research has demonstrated that MA has neurotoxic effects, but the mechanism of action of neurotoxicity is still being studied, although it seems to be multifactorial. Oxidative stress, excitotoxicity, and neuroinflammation have all produced signals related to the neurotoxic effects of MA (Paulus & Stewart, 2020; Yang et al., 2018). Because MA crosses neuronal cell membranes and enters the microscopic sacs (called vesicles) where neurons store dopamine, it is believed that damage to the storage sacs and the neurons' axonal endings causes dopamine to leak uncontrollably into the synapse. MA can also cause neurotoxicity indirectly by moving dopamine out of the storage sacs and into the neuron's cytoplasm (i.e., the cell's internal material), where it is converted to toxic and reactive chemicals. (Exhibit 2.5 shows some of these processes.)
Exhibit 2.6 includes key terms discussed in this chapter.
Box
EXHIBIT 2.6. Key Terms.
Additionally, glutamate accumulation and microglial activity changes associated with MA use may be related to MA's neurotoxic effects. Medications that block the inflammatory effects of microglial activation may help prevent MA's neurotoxicity (Yang et al., 2018).
Numerous animal studies have demonstrated that MA can damage both dopamine and serotonin systems (Chiu & Schenk, 2012; Shin et al., 2017; Yang et al., 2018). MA toxicity occurs after repeated high-dose administration, and it is selective for certain neuronal systems, particularly those in the limbic reward system (e.g., striatum, substantia nigra, nucleus accumbens). Within these brain circuits, MA has been shown to reduce the number of nerve fibers, impair normal physiologic functioning, and destroy both axons and axon terminals (i.e., synaptic junctions). These studies have also shown that MA toxicity is highly dependent on dose, route of administration, and the frequency with which the drug is taken.
Long-term use of MA may deplete dopamine levels, decrease dopamine receptors, and lower dopamine transporter levels (Yang et al., 2018). Some postmortem studies have shown that even recreational doses of MA significantly expend dopamine levels (Boileau et al., 2016). A review on the neurotoxicity of MA (Yang et al., 2018) implicates dopamine in numerous harmful effects of MA exposure, including increased oxidative stress (i.e., an imbalance of free radicals and antioxidants in the body), impairments in mitochondrial metabolism, and inflammatory processes within the brain.
Prolonged or heavy use of MA decreases the brain's ability to manufacture dopamine. This impairment may persist for months or even years after one stops taking MA (Yang et al., 2018). Researchers believe that those changes in dopamine levels and the damage done to dopamine and serotonin neurons are responsible for the chronic effects of MA use (Shin et al., 2017).
Compared with cocaine, which is rapidly metabolized by plasma and tissue enzymes, MA is metabolized at a much slower rate, which results in a longer duration of action (NIDA, 2019a). Although the half-life of cocaine is about 1 hour, a single dose of MA may produce an effect for about 10 hours (Coe et al., 2018; Cruickshank & Dyer, 2009). MA's slower rate of metabolism extends the duration of its neurotoxic effects.
Acute Physiologic Effects
The acute physiologic effects of MA are generally similar to those of cocaine: increased heart and respiratory rates, elevated blood pressure and body temperature, and pupillary dilation (Matsumoto et al., 2014). Other acute effects include increased vigor, irregular heart rate, and damage to small blood vessels in the brain (Ciccarone, 2011; Kevil et al., 2019). Dangerously elevated body temperature and severe damage to the liver occur with high-dose MA (Matsumoto et al., 2014). If not treated immediately, these effects can result in death (Matsumoto et al., 2014).
Acute Psychological Effects
MA's psychological effects, like those of cocaine, include a heightened sense of well-being or euphoria, and increased alertness (Ciccarone, 2011). High doses may produce repetitive and compulsive acts and may cause irritability; excitement; visual, auditory, or tactile hallucinations; and altered perceptions of reality, characterized by delusions and psychosis (Bramness et al., 2012; Glasner-Edwards & Mooney, 2014; Wearne & Cornish, 2018). People using MA may engage in protective behaviors in response to irrational fears brought on by altered perceptions of reality. Mood lability secondary to elevated dopamine levels is common. With continued use, tolerance develops to the behavioral effects, and repeated exposure may produce sensitization.
MA withdrawal is like that of cocaine, but because of the longer effects of MA, withdrawal may be more intense and protracted (Courtney & Ray, 2014).
Over the course of 1 to 14 days after last use, the person using MA experiences a drastic drop in mood and energy levels. Sleep—which may be promoted by the use of secondary substances such as alcohol, barbiturates, and benzodiazepines—finally begins and may last more or less uninterrupted for several days. Upon awakening, the individual may experience mild to severe depression (Zorick et al., 2010), perhaps lasting for several weeks. While in this depressed state, the person has an increased risk of suicide (Lerner & Klein, 2019).
Chronic Physiologic Effects
Understanding the chronic physiologic effects of MA use is essential for treatment providers who serve this population. Chronic use of MA may result in multiple dysfunctions of the heart (e.g., hypertension, aortic dissection, acute coronary syndromes, pulmonary hypertension, cardiomyopathy [Kevil et al., 2019; Paratz et al., 2016; Paulus & Stewart, 2020; Petit et al., 2012]) and, among people who inject the drug, skin abscesses (Yasaei & Saadabadi, 2021) and damaged blood vessels at the injection site. Chronic use may also lead to episodes of protective behaviors, paranoia, anxiety, confusion, and insomnia (Glasner-Edwards & Mooney, 2014). Heavy use is linked to progressive social and occupational deterioration. Psychotic symptoms may sometimes persist for months or years after use has ceased (Wearne & Cornish, 2018).
Some of the most concerning research findings about MA suggest that its prolonged use not only modifies behaviors, but changes the brain in fundamental and long-lasting ways. MA impairs the functioning of both the dopamine system and the serotonin system (serotonin is another important CNS neurotransmitter; Thomas et al., 2010) and possibly other neurotransmitter systems (Ferrucci et al., 2019). MA-induced neuronal toxicity is specific to certain brain regions (primarily the limbic reward system), and this toxicity is reflected both biochemically and anatomically. Finally, these impairments in brain functioning may underlie the cognitive and emotional deficits seen in many people who use MA.
Chronic Psychological Effects
One of the potential negative effects of chronic MA use is psychosis. Patients with persistent psychosis are often treated with medications to return their brain functions to normal, and many antipsychotic medications work by affecting the activity of the dopamine and serotonin neurons. Current protocol in treating persistent MA psychosis is to manage the patient's symptoms—potentially through second-generation antipsychotics like olanzapine and risperidone—to try and improve overall quality of life and reduce the risk for recurrent use (Wearne & Cornish, 2018).
Characteristics of neurocognitive decline in people with MA use disorder are similar to those seen in patients with a cocaine use disorder. The changes to cognition are usually across multiple domains, including attention, psychomotor activity, memory, and decision making (Hart et al., 2012). Unlike with cocaine, the duration of the neurocognitive deficiencies has not been well described, but neuroimaging would suggest more long-lasting neurocognitive deficiencies likely related to the longer duration of action of MA itself and its more profound effect on the neuroplasticity of the brain.
Depletion of dopamine in the brains of people who use MA is similar to the loss of dopamine seen in patients with Parkinson's disease. Research has yet to define a clear correlation between MA use disorder and the Parkinson-like symptoms described by clinicians and patients (Christine et al., 2010; Granado et al., 2013; Kish et al., 2017). Determining the lasting effects of prolonged exposure to MA on the dopamine reward system may help clinicians better support patients entering recovery from MA use. Additionally, understanding how these Parkinsonian symptoms develop may reveal additional pathways for treatment of the condition and MA use disorder.
Prescription Stimulant Medications
Routes of Administration
Prescription stimulants are typically taken orally but, when misused, can also be taken intranasally (snorted; Yanofski, 2011). Half-lives vary by drug and by formulation (e.g., short acting versus long acting). For example, short-acting amphetamine has a half-life of approximately 9 hours, whereas the long-acting formulation has a half-life in the range of 10 to 13 hours (Pradeep & Standeven, 2019).
Pharmacology
Stimulant medications (e.g., d-amphetamine, mixed enantiomers/mixed salts amphetamine, lisdexamfetamine) exert their effect in much the same way that cocaine and MA do—by increasing levels of dopamine in the brain (NIDA, 2014). The prescription stimulants methylphenidate and d-amphetamine increase dopamine signaling—methylphenidate by blocking dopamine transporters and d-amphetamine by enhancing dopamine release from nerve terminals (Lakhan & Kirchgessner, 2012). Prescription stimulants are prescribed in such a way that, when taken appropriately, they produce slow and steady increases in dopamine (NIDA, 2014).
Acute Physiologic Effects
Acute adverse physiologic effects of stimulant medications include loss of appetite, insomnia, weight loss, headache, nausea, vomiting, abdominal cramps, increased blood pressure and heart rate, and, potentially, worsening of motor tics (Craig et al., 2015; Heal et al., 2013).
Chronic Physiologic Effects
Ongoing exposure to stimulants—such as repeatedly taking even the same doses of stimulant medication—can lead to tolerance to the stimulant, as well as tolerance to the brain's endogenous dopamine (Yanofski, 2011). This tolerance to dopamine means the brain becomes less sensitive to it; thus, it could become less sensitive to the medication's effects over time (Yanofski, 2011).
Other long-term effects of stimulant medication in children and adults are unclear, in part because of the lack of longitudinal treatment studies and poor long-term adherence to treatment (Molina & Swanson, 2020). For instance, it is unknown whether brain changes that occur with acute stimulant medication exposure (e.g., increased activation of areas of the prefrontal cortex that are normally underactive in ADHD) persist with chronic exposure (Molina & Swanson, 2020; Weyandt et al., 2013). There seems to be no link between prescription stimulants taken in adolescence and later development of SUDs (Quinn et al., 2017; Wilens et al., 2011). Appetite loss, headache, and digestive distress appear to continue with chronic use, but, again, few studies of long-term effects exist (Craig et al., 2015).
Acute Psychological Effects
Prescription stimulants are known to improve alertness, attention, and energy (NIDA, 2018b). Thus, much of the research on the short-term effects of these medications concerns cognitive functioning, including enhancement of several cognitive processes, such as attention, vigilance, response inhibition, memory, and working memory (D. M. Dougherty et al., 2016; Molina & Swanson, 2020; Swanson et al., 2011). People misusing prescription stimulants often do so because of the perceived neurocognitive benefit—largely that of improved concentration and alertness, such as for studying and academic performance (Clemow & Walker, 2014; Marraccini et al., 2016; Weyandt et al., 2013)—and not necessarily solely as a result of craving.
Chronic Psychological Effects
Research suggests that long-term use results in continued alleviation of ADHD symptoms, including inattention, hyperactivity, and impulsivity, but only while the medication is being taken (Craig et al., 2015).
Assessment and Diagnosis
Diagnosis can be based on criteria established in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association [APA], 2013) for a stimulant use disorder involving amphetamine or cocaine. For treatment reimbursement, the diagnosis may also need to reflect criteria according to the most recent version of the International Classification of Diseases, Clinical Modification (ICD–CM) found under “Coding” at https://www.cms.gov/Medicare/Medicare (the current version at the time of this Treatment Improvement Protocol [TIP] update's publication was ICD-10-CM). Arriving at a diagnosis is simplified by having information available from a relevant and accurate patient history, a urine toxicology screen or similar laboratory tests, and clinical observations of physical signs and mental status.
Box
WHAT TO DO IF A PATIENT SCREENS POSITIVE FOR STIMULANT USE.
History
An appropriate substance use history should include the substance(s) and medications used during the past 30 days; the specific substance(s) or combinations typically used with the usual dose, frequency, and route of administration; the duration of use; and the time and amount of last use (Substance Abuse and Mental Health Services Administration [SAMHSA], 2020l). If the patient has been bingeing, a brief description of this and previous episodes is helpful. In addition, the history should include information about any previous seizures, delirium tremens, heart and pulmonary problems, paranoid reactions (with or without altered perceptions of reality and hallucinations), and other serious medical and psychological conditions and psychiatric diagnoses and if they occurred pre- or post-stimulant use, as well as all medications the patient is taking. Additionally, check to see if there are substance use or psychiatric problems within the person's family (SAMHSA, 2020l).
For most patients presenting in an emergency department, the substance use and medical history will, of necessity, be brief and focus on the potential causes for the observed symptoms and complaints and any potential medical or psychological problems that are likely to complicate treatment and the patient's response. Emergency department personnel should stabilize the patient medically and assess potential danger to self and others before trying to take a history. Patients in a heightened state of arousal and experiencing persecutory perceptions may not give an accurate accounting of their current and past substance use. Information from significant others or from a reliable source can help clarify the patient's history. In situations where the patient is delirious, psychotic, or unable to respond, information from accompanying friends or significant others about the antecedents of the problem is particularly important. Sometimes, the substance use history must await symptomatic management.
The history may be supplemented by a variety of SUD screening instruments, although these are not notably reliable if used with individuals who are intoxicated or acutely psychotic.
A number of these screening instruments are described in detail in Appendix B of SAMHSA's TIP 42, Substance Use Disorder Treatment for People With Co-Occurring Disorders (https://store.samhsa.gov/product/tip-42-substance-use-treatment-persons-co-occurring-disorders/PEP20-02-01-004).
Urine Toxicology
A urine screen or toxicology test can be used to identify which substances the patient has used recently (Jaffe et al., 2016). This testing is vital to confirm clinicians' clinical assessments and observations. Some emergency departments have bedside or patient-side urine immunoassay testing kits (dipstick tests) that can be used for a quick turnaround without waiting on more formal assays. The kit's results can be validated by additional laboratory studies.
The results of either dipstick or Enzyme Multiplied Immunoassay Technique (EMIT) tests are appropriate to use for medical purposes. Alternative techniques for determining substance use are analyses of hair, blood, sweat, or tissue samples (Jaffe et al., 2016). In general, however, urine has become the standard method of determining substance use in an individual, and tests are readily available in the medical setting, whereas other types of testing are not (Jaffe et al., 2016). Urine screens are relatively inexpensive, with five-panel tests (i.e., tests for five different drugs) costing on average $4 and 14-panel tests costing about $7 (Jaffe et al., 2016). Both qualitative and quantitative urine assays are usually needed to verify use and time/amount taken. Repeated assays can be used to track elimination of stimulants from the system if large amounts have been detected.
Because no standard set of substances is tested in a urine substance screen, medical personnel should make certain that assays for suspected substances are included. Also, no toxicology screen can determine with certainty whether someone used any particular substance—or any substances at all. The detection limitations may be too broad or the specific substance may have been completely metabolized before a urine specimen was collected. A positive report will not necessarily indicate when the substance was last used. Metabolites for some substances are detectable for days or weeks after last use but take some time after substance administration to be detectable in urine (K. E. Moeller et al., 2017).
MA can be detected in urine for approximately 48 hours following use, and cocaine metabolites may be detected for as long as 2 to 4 days following use (K. E. Moeller et al., 2017). Many prescription and over-the-counter medications (e.g., diet aids, cold remedies) contain phenylpropanolamine or ephedrine that may yield positive EMIT or radioimmunoassay tests for amphetamines. Certain agents (e.g., phenylpropanolamine, ephedrine) can produce cross-reactivity in amphetamine tests, causing immunoassays for the analysis of amphetamine-type substances to potentially produce false positives (K. E. Moeller et al., 2017). Urine screening tests are not confirmation of patient substance use but rather are one piece of information to help guide clinical decision making. Many substances may interact with an amphetamine screening test. For this reason, the preferred method for determination of stimulant substance use is confirmatory urine testing in the form of gas chromatography/mass spectrometry.
Physical Signs and Mental Status
Signs and symptoms of cocaine use can include extreme happiness or being very energetic; hypersensitivity to sight, sound, and touch; irritability; paranoia; and, in large amounts, bizarre and violent behavior (NIDA, 2016a). Signs of MA use can include increased attention, decreased fatigue, increased activity and wakefulness, decreased appetite, euphoric mood, (NIDA, 2019a) and, in large amounts, fever, sweating, tremors, a rapid heart rate, stroke, aggression, and paranoia (Radfar & Rawson, 2014). Increased sensitivity to noise, nervous physical activity like scratching, irritability, dizziness, confusion, extreme anorexia, convulsions, and blood pressure are also potential harmful effects of MA use (SAMHSA, 2018c). Prescription stimulant misuse can manifest as feelings of euphoria, but in large amounts can result in restlessness, tremors, overactive reflexes, rapid breathing, confusion, aggression, hallucinations, panic, high fever, muscle pains, and weakness (NIDA, 2018b).
Data acquired from monitoring vital signs (temperature, blood pressure, pulse rate, respiration rate) can be used to document physical indicators of stimulant use. In addition, observations of physical manifestations related to acute or chronic stimulant use and to withdrawal can be documented. Similarly, a variety of instruments exists to determine mental status, although observational data regarding psychological and mental status may be adequate (see Appendix B of TIP 42, Substance Use Disorder Treatment for People With Co-Occurring Disorders at https://store.samhsa.gov/product/tip-42-substance-use-treatment-persons-co-occurring-disorders/PEP20-02-01-004. [SAMHSA, 2020l]).
Box
ASSESSING FOR COGNITIVE DEFICITS.
Differential Diagnosis
In the diagnostic process, other disorders and conditions with similar or identical presentations must be considered. Many people with stimulant use disorder have coexisting mental illnesses such as bipolar disorder and borderline personality disorder, which share some symptoms with stimulant use disorders (SAMHSA, 2020l). A heart attack, seizure, or other type of adverse medical event that can be brought on by stimulant toxicity may instead have a different cause. The cause of the symptoms or adverse events must be determined for optimal continuing care and medical management.
Before a differential diagnosis of a coexisting mental disorder can be made, the patient must be abstinent for at least 4 weeks following cessation of withdrawal or severe intoxication (APA, 2013). The presenting psychiatric syndrome and symptoms can be treated meanwhile, and a diagnosis of unspecified schizophrenia spectrum and other psychotic disorders can be given. More information regarding the diagnostic process for patients with symptoms that indicate coexisting substance use and mental disorders can be found in TIP 42, Substance Use Disorder Treatment for People With Co-Occurring Disorders (https://store.samhsa.gov/product/tip-42-substance-use-treatment-persons-co-occurring-disorders/PEP20-02-01-004).
New forms of brain imaging techniques could offer a promising approach for making certain differential diagnoses—for example, if current research determines that these techniques are useful for distinguishing among drug-induced and other forms of psychosis.
Summary
Research has shown how stimulants such as cocaine, MA, and prescription stimulants exert their effects on the nervous system and affect feelings, emotional response, and behavior. There is now a greater understanding of neurologic systems related to reward and reinforcement, the development of stimulant use disorders, and the roles that craving and memory play in sustaining SUDs. Existing research can also help guide treatment approaches. Although more research is needed on the long-term neurologic, medical, psychiatric, and neurocognitive effects of stimulants in humans, animal studies have demonstrated cocaine's and MA's ability to disrupt normal brain function and cause long-lasting and perhaps permanent neurologic impairments. Continuing research and emerging imaging technologies will assist in the development of new and improved approaches for treating stimulant use disorders. Assessment of people with stimulant use should involve taking a thorough history, complemented by urine toxicology and data from physical observations. For less severe stimulant use, clinicians can use screening, brief intervention, and referral to treatment techniques to help guide patients toward behavior change. Patients with severe stimulant use disorder should be referred for specialized SUD treatment.
Publication Details
Copyright
This is an open-access report distributed under the terms of the Creative Commons Public Domain License. You can copy, modify, distribute and perform the work, even for commercial purposes, all without asking permission.
Publisher
Substance Abuse and Mental Health Services Administration (US), Rockville (MD)
NLM Citation
Treatment for Stimulant Use Disorders: Updated 2021 [Internet]. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 1999. (Treatment Improvement Protocol (TIP) Series, No. 33.) Chapter 2—How Stimulants Affect the Brain and Behavior.