This is a work of the US government and distributed under the terms of the Public Domain
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
SUMMARY
Background:
Aspartame is an artificial sweetener widely used in beverages and foods. We tested if aspartame could cause cancer in two different strains of genetically modified mice.
Methods:
We fed groups of male and female Tg.AC mice, male and female p53 mice, and male and female Cdkn2a mice diets containing up to 50,000 parts per million (5%) aspartame for 9 months. Animals given feed with no sweetener added served as the control groups. Tissues from 15 sites were examined for every animal.
Results:
Exposure to aspartame had no effect on the survival of any of the animal groups. No increases in tumors were seen in males or females from either strain of mice.
Conclusions:
We conclude that aspartame did not cause cancer in the genetically modified mice used in these studies.
ABSTRACT
ASPARTAME
CAS No. 22839-47-0
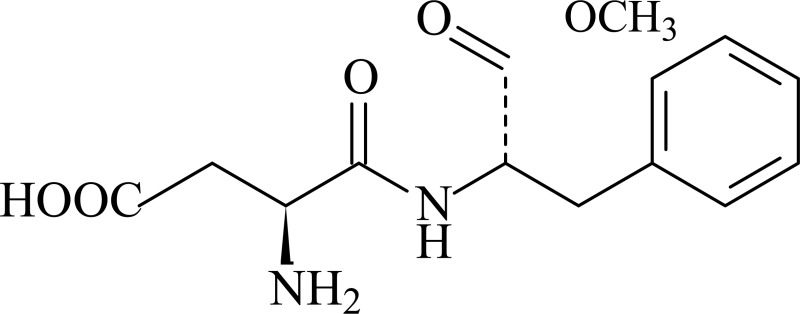
Chemical Formula: C14H18N2O5 Molecular Weight: 294.3
Synonyms: N-l-α-Aspartyl-l-phenylalanine 1-methyl ester; 3-amino-N-(α-carboxyphenethyl)succinamic acid N-methyl ester; APM; SC-18862
Trade names: Canderel, Equal, NutraSweet, Sanecta, Tri-Sweet
Aspartame is an artificial sweetener used throughout the world in food and beverages. Conventional 2-year rodent cancer studies of aspartame are considered negative, although a small number of neoplasms of the brain were observed in a rat study (Fed. Regist., 1981a,b). The NTP has explored the use of genetically altered mouse models as adjuncts to the 2-year rodent cancer assay. These models may prove to be more rapid, use fewer animals, and provide some mechanistic insights into neoplastic responses. As part of the evaluation of new mouse cancer screening models, aspartame was tested for potential toxicity and carcinogenicity in two relatively well-studied models, the Tg.AC hemizygous strain and the p53 haploinsufficient strain, and an uncharacterized model, the Cdkn2a deficient strain. Male and female Tg.AC hemizygous, p53 haploinsufficient, and Cdkn2a deficient mice were given feed containing aspartame (greater than 98% pure) for 9 months. Genetic toxicology studies were conducted in Salmonella typhimurium, rat bone marrow cells, and mouse peripheral blood erythrocytes.
9-Month Study in Tg.AC Hemizygous Mice:
Groups of 15 male and 15 female Tg.AC hemizygous mice were fed diets containing 0, 3,125, 6,250, 12,500, 25,000, or 50,000 ppm aspartame (equivalent to average daily doses of approximately 490, 980, 1,960, 3,960, or 7,660 mg aspartame/kg body weight to males and 550, 1,100, 2,260, 4,420, or 8,180 mg/kg to females) for 40 weeks. Exposure to aspartame had no effect on survival. The mean body weights of 50,000 ppm females were greater than those of the controls from week 15 until the end of the study. Feed consumption by the exposed groups was similar to that by the control groups throughout the study. There were no neoplasms or nonneoplastic lesions that were attributed to exposure to aspartame.
9-Month Study in p53 Haploinsufficient Mice:
Groups of 15 male and 15 female p53 haploinsufficient mice were fed diets containing 0, 3,125, 6,250, 12,500, 25,000, or 50,000 ppm aspartame (equivalent to average daily doses of approximately 490, 970, 1,860, 3,800, or 7,280 mg/kg to males and 630, 1,210, 2,490, 5,020, or 9,620 mg/kg to females) for 40 weeks. Exposure to aspartame had no effect on survival or mean body weights. Feed consumption by the exposed groups was similar to that by the control groups throughout the study. No neoplasms or nonneoplastic lesions were attributed to exposure to aspartame.
9-Month Study in Cdkn2a Deficient Mice:
Groups of 15 male and 15 female Cdkn2a deficient mice were fed diets containing 0, 3,125, 6,250, 12,500, 25,000, or 50,000 ppm aspartame for 40 weeks (equivalent to average daily doses of approximately of approximately 490, 960, 1,900, 3,700, and 7,400 mg/kg to males and 610, 1,200, 2,390, 4,850, and 9,560 mg/kg to females). Survival of all exposed groups was similar to that of the control groups. Mean body weights of 3,125 and 6,250 ppm males were less than those of the controls after weeks 29 and 16, respectively. Mean body weights of female mice were similar to those of the controls throughout the study. The incidences of minimal to mild cytoplasmic vacuolization of periportal hepatocytes were significantly greater than controls in males exposed to 6,250, 25,000, or 50,000 ppm aspartame.
Genetic Toxicology:
Aspartame was tested for induction of gene mutations in Salmonella typhimurium. No mutagenicity was detected in strains TA98, TA100, or TA1535 with or without exogenous metabolic activation (S9). In addition, a single test in TA1537 with 30% rat liver S9 gave negative results. In TA97 with 30% rat liver S9, however, a reproducible small increase in mutant colonies was observed, and this response was judged to be equivocal. No mutagenicity was detected in TA97 without S9 or with hamster liver S9.
An acute bone marrow micronucleus test was conducted with aspartame administered by gavage to male F344/N rats. No increase in micronucleated polychromatic erythrocytes was observed at any dose level.
Peripheral blood micronucleus tests were conducted after 9 months exposure of Tg.AC hemizygous, p53 haploinsufficient, and Cdkn2a deficient mice to aspartame in dosed feed. Negative results were obtained in male and female Tg.AC hemizygous and Cdkn2a deficient mice. Negative results were also obtained with male p53 haploinsufficient mice. In female p53 haploinsufficient mice, the results of the micronucleus test were judged to be positive, based on a significant trend test and a small but statistically significant increased frequency of micronucleated erythrocytes in the 50,000 ppm group.
Conclusions:
Under the conditions of this 9-month feed study, there was no evidence of carcinogenic activity* of aspartame in male or female p53 haploinsufficient mice exposed to 3,125, 6,250, 12,500, 25,000, or 50,000 ppm. Because this is a new model, there is uncertainty whether the study possessed sufficient sensitivity to detect a carcinogenic effect.
Summary of the Feed and Genetic Toxicology Studies of Aspartame in Tg.AC Hemizygous Mice
| Male | Female | |
|---|---|---|
| Concentrations in feed | 0, 3,125, 6,250, 12,500, 25,000, or 50,000 ppm | 0, 3,125, 6,250, 12,500, 25,000, or 50,000 ppm |
| Body weights | Exposed groups similar to the control group | 50,000 ppm group greater than control group |
| Survival rates | 9/15, 12/15, 8/15, 12/15, 11/15, 10/15 | 11/15, 10/15, 9/15, 9/15, 11/15, 8/15 |
| Nonneoplastic effects | None | None |
| Neoplastic effects | None | None |
| Genetic toxicology | ||
| Salmonella typhimurium gene mutations: | Equivocal in TA97 with S9; negative in TA98, TA100, TA1535, and TA1537 | |
| Micronucleated erythrocytes | ||
| Rat bone marrow in vivo: | Negative | |
| Mouse peripheral blood in vivo: | Negative in Tg.AC hemizygous males and females | |
Summary of the Feed and Genetic Toxicology Studies of Aspartame in p53 Haploinsufficient Deficient Mice
| Male | Female | |
|---|---|---|
| Concentrations in feed | 0, 3,125, 6,250, 12,500, 25,000, or 50,000 ppm | 0, 3,125, 6,250, 12,500, 25,000, or 50,000 ppm |
| Body weights | Exposed groups similar to the control group | Exposed groups similar to the control group |
| Survival rates | 14/15, 15/15, 13/15, 15/15, 14/15, 14/15 | 14/15, 14/15, 14/15, 15/15, 15/15, 15/15 |
| Nonneoplastic effects | None | None |
| Neoplastic effects | None | None |
| Level of evidence of carcinogenic activity | No evidence | No evidence |
| Genetic toxicology | ||
| Micronucleated erythrocytes | ||
| Mouse peripheral blood in vivo: | Positive in p53 haploinsufficient females and negative in p53 haploinsufficient males | |
Summary of the Feed and Genetic Toxicology Studies of Aspartame in Cdkn2a Deficient Mice
| Male | Female | |
|---|---|---|
| Concentrations in feed | 0, 3,125, 6,250, 12,500, 25,000, or 50,000 ppm | 0, 3,125, 6,250, 12,500, 25,000, or 50,000 ppm |
| Body weights | 3,125 ppm and 6,250 ppm groups less than control group | Exposed groups similar to the control group |
| Survival rates | 14/15, 14/15, 15/15, 14/15, 14/15, 15/15 | 13/15, 15/15, 13/15, 15/15, 15/15, 14/15 |
| Nonneoplastic effects | Liver: hepatocyte, cytoplasmic periportal vacuolization (6/15, 11/15, 14/15, 8/14, 13/15, 13/15) | None |
| Neoplastic effects | None | None |
| Genetic toxicology | ||
| Micronucleated erythrocytes | ||
| Mouse peripheral blood in vivo: | Negative in Cdkn2a deficient males and females | |
- *
Explanation of Levels of Evidence of Carcinogenic Activity is on page 9. A summary of the Technical Report Review Subcommittee comments and public discussion on this report appears on page 11.
Contents
- FOREWORD
- CONTRIBUTORS
- EXPLANATION OF LEVELS OF EVIDENCE OF CARCINOGENIC ACTIVITY
- NATIONAL TOXICOLOGY PROGRAM BOARD OF SCIENTIFIC COUNSELORS TECHNICAL REPORTS REVIEW SUBCOMMITTEE
- SUMMARY OF TECHNICAL REPORTS REVIEW SUBCOMMITTEE COMMENTS
- INTRODUCTION
- MATERIALS AND METHODS
- RESULTS
- DISCUSSION AND CONCLUSIONS
- REFERENCES
- APPENDIX A. SUMMARY OF LESIONS IN MALE Tg.AC HEMIZYGOUS MICE IN THE 9-MONTH FEED STUDY OF ASPARTAME
- APPENDIX B. SUMMARY OF LESIONS IN FEMALE Tg.AC HEMIZYGOUS MICE IN THE 9-MONTH FEED STUDY OF ASPARTAME
- APPENDIX C. SUMMARY OF LESIONS IN MALE p53 HAPLOINSUFFICIENT MICE IN THE 9-MONTH FEED STUDY OF ASPARTAME
- APPENDIX D. SUMMARY OF LESIONS IN FEMALE p53 HAPLOINSUFFICIENT MICE IN THE 9-MONTH FEED STUDY OF ASPARTAME
- APPENDIX E. SUMMARY OF LESIONS IN MALE Cdkn2a DEFICIENT MICE IN THE 9-MONTH FEED STUDY OF ASPARTAME
- APPENDIX F. SUMMARY OF LESIONS IN FEMALE Cdkn2a DEFICIENT MICE IN THE 9-MONTH FEED STUDY OF ASPARTAME
- APPENDIX G. GENETIC TOXICOLOGY
- APPENDIX H. ORGAN WEIGHTS AND ORGAN-WEIGHT-TO-BODY-WEIGHT RATIOS
- APPENDIX I. CHEMICAL CHARACTERIZATION AND DOSE FORMULATION STUDIES
- APPENDIX J. FEED AND COMPOUND CONSUMPTION IN THE 9-MONTH FEED STUDIES OF ASPARTAME
About the Series
- NLM CatalogRelated NLM Catalog Entries
- NTP report on the toxicology studies of dichloroacetic acid (CAS No. 79-43-6) in genetically modified (FVB Tg.AC hemizygous) mice (dermal and drinking water studies) and carcinogenicity studies of dichloroacetic acid in genetically modified [B6.129-Trp53(tm1Brd) (N5) haploinsufficient] mice (drinking water studies).[Natl Toxicol Program Genet Mod...]NTP report on the toxicology studies of dichloroacetic acid (CAS No. 79-43-6) in genetically modified (FVB Tg.AC hemizygous) mice (dermal and drinking water studies) and carcinogenicity studies of dichloroacetic acid in genetically modified [B6.129-Trp53(tm1Brd) (N5) haploinsufficient] mice (drinking water studies).National Toxicology Program. Natl Toxicol Program Genet Modif Model Rep. 2007 Apr; (11):1-168.
- Toxicology studies of bromodichloromethane (CAS No. 75-27-4) in genetically modified (FVB Tg.AC Hemizygous) mice (dermal, drinking water, and gavage studies) and carcinogenicity studies of bromodichloromethane in genetically modified [B6.129-Trp53(tm1Brd) (N5) haploinsufficient] mice (drinking water and gavage studies).[Natl Toxicol Program Genet Mod...]Toxicology studies of bromodichloromethane (CAS No. 75-27-4) in genetically modified (FVB Tg.AC Hemizygous) mice (dermal, drinking water, and gavage studies) and carcinogenicity studies of bromodichloromethane in genetically modified [B6.129-Trp53(tm1Brd) (N5) haploinsufficient] mice (drinking water and gavage studies).National Toxicology Program. Natl Toxicol Program Genet Modif Model Rep. 2007 May; (5):1-227.
- NTP report on the toxicology studies of dicyclohexylcarbodiimide (CAS No. 538-75-0) in F344/N rats, B6C3F 1 mice, and genetically modified (FVB Tg.AC hemizygous) mice and carcinogenicity study of dicyclohexylcarbodiimide in genetically modified [B6.129-Trp53 tm1Brd (N5) haploinsufficient] mice (dermal studies).[Natl Toxicol Program Genet Mod...]NTP report on the toxicology studies of dicyclohexylcarbodiimide (CAS No. 538-75-0) in F344/N rats, B6C3F 1 mice, and genetically modified (FVB Tg.AC hemizygous) mice and carcinogenicity study of dicyclohexylcarbodiimide in genetically modified [B6.129-Trp53 tm1Brd (N5) haploinsufficient] mice (dermal studies).National Toxicology Program. Natl Toxicol Program Genet Modif Model Rep. 2007 Sep; (9):1-138.
- Review Toxicology and carcinogenesis studies of milk thistle extract (CAS No. 84604-20-6) in F344/N rats and B6C3F1 mice (Feed Studies).[Natl Toxicol Program Tech Rep ...]Review Toxicology and carcinogenesis studies of milk thistle extract (CAS No. 84604-20-6) in F344/N rats and B6C3F1 mice (Feed Studies).National Toxicology Program. Natl Toxicol Program Tech Rep Ser. 2011 May; (565):1-177.
- Review Toxicology and carcinogenesis studies of androstenedione (CAS No. 63-05-8) in F344/N rats and B6C3F1 mice (gavage studies).[Natl Toxicol Program Tech Rep ...]Review Toxicology and carcinogenesis studies of androstenedione (CAS No. 63-05-8) in F344/N rats and B6C3F1 mice (gavage studies).. Natl Toxicol Program Tech Rep Ser. 2010 Sep; (560):1, 7-31,33-171 passim.
- NTP Genetically Modified Model Report on the Toxicology Studies of Aspartame (CA...NTP Genetically Modified Model Report on the Toxicology Studies of Aspartame (CASRN 22839-47-0) in Genetically Modified (FVB Tg.AC Hemizygous) and B6.129-Cdkn2atm1Rdp (N2) Deficient Mice and Carcinogenicity Studies of Aspartame in Genetically Modified [B6.129-Trp53tm1Brd (N5) Haploinsufficient] Mice (Feed Studies)
Your browsing activity is empty.
Activity recording is turned off.
See more...
![Cover of NTP Genetically Modified Model Report on the Toxicology Studies of Aspartame (CASRN 22839-47-0) in Genetically Modified (FVB Tg.AC Hemizygous) and B6.129-Cdkn2atm1Rdp (N2) Deficient Mice and Carcinogenicity Studies of Aspartame in Genetically Modified [B6.129-Trp53tm1Brd (N5) Haploinsufficient] Mice (Feed Studies)](/corehtml/pmc/pmcgifs/bookshelf/thumbs/th-ntpgmmr1-lrg.png)