Parts of this section are reproduced or adapted with permission from Wildman et al.44 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Wildman MJ, O’Cathain A, Hind D, et al. An intervention to support adherence to inhaled medication in adults with cystic fibrosis: the ACtiF research programme including RCT. Southampton (UK): NIHR Journals Library; 2021 Oct. (Programme Grants for Applied Research, No. 9.11.)

An intervention to support adherence to inhaled medication in adults with cystic fibrosis: the ACtiF research programme including RCT.
Show detailsParts of this section are reproduced or adapted with permission from Wildman et al.44 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Having integrated the identified changes into the RCT and intervention protocols, we conducted a full-scale RCT to determine the efficacy of the CFHealthHub intervention (WP 3.2). Concurrently, we undertook a process evaluation to explore implementation (including fidelity), mechanisms of action and context (WP 3.3).45 We report the independent streams of research and the triangulation of the results (WP 3.4).
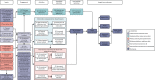
A logic model was constructed early in the programme and refined throughout to show how the intervention could affect outcomes (Figure 3).
Design
This was a two-armed, parallel-group, open-labelled, efficacy superiority RCT comparing intervention with usual care, with concurrent process evaluation. The protocol is available online.46
Methods
Objective
The objective of the RCT was to determine the effect of the CFHealthHub intervention on clinical and participant-reported outcomes.
Sample size
Sample size estimation was conducted using a between-group difference in mean exacerbations of 0.5 over the 12-month follow-up period, a standard deviation (SD) of 1.5, a design effect of 1.16 to allow for clustering, an alpha level of 5% and 90% power. After adjusting for 20% loss to follow-up, the recruitment target was 556 participants (278 per arm). The sample size was predicated on 2.0 exacerbations per year and reducing this by 0.5 to 1.5 per year. This is equivalent to an incidence rate ratio (IRR) of 0.75 (2.0 ÷ 1.5).
Participants
Potential participants were identified using the UK Cystic Fibrosis Registry. Eligible participants were aged ≥ 16 years and willing to take inhaled mucolytics and antibiotics via the eTrack nebuliser. Participants were ineligible if they were post lung transplant, on the lung transplant list, receiving palliative care, lacking capacity for informed consent or using dry-powder devices to take mucolytics or antibiotics.
Intervention and allocation
Intervention participants received the intervention described in Work package 2.2: development and refinement of the CFHealthHub intervention and Appendix 2. The intervention was delivered by full-time interventionists employed specifically for the research study to deliver both the intervention and the RCT (recruitment and some data collection). They were physiotherapists in 13 of the 19 centres and nurses, psychologists, a pharmacist and a dietitian in other centres. Some centres had two interventionists that shared the role, sometimes from different clinical disciplines. Control participants were given an eTrack controller and Qualcomm (San Diego, CA, USA) hub to enable accurate recording of inhalation data and calculation of adherence levels. They did not have access to CFHealthHub, that is, its adherence data, behaviour-change tools, educational content and visits from interventionists. Control arm participants received usual care.
Participants were allocated 1 : 1 to the intervention arm or control arm using a computer-generated pseudo-random list with random-permuted blocks of randomly varying sizes, via a central, web-based randomisation system. The allocation sequence was hosted by the Clinical Trials Research Unit at the University of Sheffield (Sheffield, UK), with the sequence created by a statistician (not otherwise involved with trial) and held on a secure server. The recruiting health-care professional logged into the server and entered basic demographic information, then the allocation was revealed. Stratification was by centre and number of past-year i.v. antibiotic days (≤ 14 days and > 14 days) – a predictor of current-year i.v. days.21 The trial statistician remained blind to treatment allocation until database freeze. Participants and health-care professionals collecting primary outcome data were not blind to treatment allocation. The trial statistician remained blind to treatment allocation until database freeze; analyses were conducted unblinded.
An intention-to-treat approach was used, with all participants included in the arm to which they were randomised and exclusions being made only in the event of insufficient data for inclusion in the model for a given outcome. In addition, per-protocol and complier average causal effect (CACE) analyses were conducted, with protocol compliers defined as participants participating in both a first intervention visit and a review visit during which adherence graphs and/or charts were accessed.
Outcome measures
The primary analysis consisted of a between-group comparison of pulmonary exacerbation rates over the 12-month period from consent, with exacerbations defined as meeting at least one of the 12 Fuchs criteria and being treated by i.v. antibiotics.47 The following sensitivity analyses were conducted to assess the robustness of the findings, applying the same model as for primary analysis: inclusion of all (including those not treated with i.v. antibiotics) exacerbations, multiple imputation for missing outcome data, best-case imputation, per-protocol analysis and CACE analysis (see Report Supplementary Material 1 and 2).
Key secondary outcomes included weekly medication adherence, forced expiratory volume in first second (per cent) (FEV1%) predicted and body mass index (BMI). To calculate numerator-adjusted normative adherence, daily doses taken were recorded, capped at the number of doses prescribed if the participant took more than the prescribed dose, divided by the appropriate daily dose given the participant’s disease status and treatment regimen, and summarised as weekly means. Lung function and BMI were measured at baseline and 12-month follow-up visits. Health-related quality of life (HRQoL), beliefs and perceived behaviours were assessed by way of the following patient-reported measures:
- generic health status – EuroQol-5 Dimensions, five-level version (EQ-5D-5L)
- Patient Activation Measure-13 item (PAM-13)
- Confusion, Hubbub and Order Scale-6 item (CHAOS-6)
- perceptions of treatment adherence – Medication Adherence Data-3 item (MAD-3)
- Self-Report Behavioural Automaticity Index (SRBAI)
- Cystic Fibrosis Questionnaire-Revised (CFQ-R)
- Generalised Anxiety Disorder-7 (GAD-7)
- specific concerns and necessities – Capability Opportunity Motivation – Behaviour Beliefs About Medicines Questionnaire (COM-BMQ)
- Patient Health Questionnaire-8 item (PHQ-8).
Patient-reported outcomes were recorded at baseline and 12 months.
Participant safety was assessed by way of adverse and serious adverse event reporting. All randomised participants were included in safety summaries.
Statistical analysis
The statistical analysis plan is detailed in Report Supplementary Material 1. Analysis is summarised in this section.
Baseline and safety data were reported using summary statistics.
For the primary outcome (and associated sensitivity analyses), pulmonary exacerbation rates were compared using the IRR from a negative binomial model adjusted for stratification factors and including an offset for follow-up time.
Weekly numerator-adjusted normative adherence data were analysed using a longitudinal mixed model with random slopes and intercepts and adjustment for stratification factors and ‘baseline’ (weeks 1 and 2 post consent) adherence. The treatment effect was quantified using the adjusted between-group difference in mean normative adherence. Other secondary outcomes were analysed using 12-month follow-up data adjusted for baseline values and stratification factors. Treatment effects were determined by adjusted between-group differences in means.
For all models, treatment effects were reported with corresponding 95% confidence intervals (CIs). No adjustments were made for multiplicity. Adjustment for multiplicity was not specified in the statistical analysis plan, which was written, in accordance with the Clinical Trials Research Unit at the University of Sheffield (Sheffield, UK) standard operating procedures, before the data were analysed and was reviewed and approved by the independent members (which included two statisticians) on the TSC. There is no consensus on what procedure to adopt to allow for multiple comparisons.48 Therefore, we followed Altman et al.’s49 recommendation of reporting unadjusted p-values (to three decimal places/significant figures) and confidence limits, with a suitable note of caution with respect to interpretation. As Perneger concludes: ‘simply describing what tests of significance have been performed, and why, is generally the best way of dealing with multiple comparisons.’50
Analyses were conducted in R v3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria) and SAS® v9.4. (SAS Institute Inc., Cary, NC, USA).
We followed Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines (see Report Supplementary Material 5).
Process evaluation methods
There were six components in the process evaluation. Each component was undertaken and analysed separately. The findings from each component were brought together using a triangulation protocol51 adapted for use with qualitative research and RCTs.52 Experts in process evaluation recommend that the process evaluation is analysed before the RCT results are known.53 All components, except the mediation analysis, were reported to the team before the RCT results were known, although further analysis continued on some components after the RCT results were revealed.
Fidelity
The aim was to explore the fidelity of the intervention in practice. We used the Borrelli checklist54 as the framework to assess and monitor the fidelity of the intervention delivered in the RCT. Intervention sessions were assessed for fidelity at certification and for drift. CFHealthHub data on use of different parts of the website (click analytics) were included in this assessment. Details of the fidelity assessment methods, together with the fidelity results, are available in Appendix 3.
Usual-care survey
The aim was to understand usual care in each of the RCT sites, and how it changed over the time of the RCT, to assess how different the intervention was from usual care. We used an 11-item survey at baseline and at 12 months at each RCT site. Questionnaires were completed by the site interventionist and/or other members of the MDT. Questions included a mixture of items requiring five-point nominal scale and free-text responses. Medians, interquartile ranges and percentages by response were used to summarise categorical items. Free-text responses were summarised by identifying key themes. To examine change in usual care at sites over the course of the 12-month follow-up, change scores were calculated. All sites responded to the survey. Details of methods are reported in Appendix 4.
User acceptability survey
The aim was to measure the acceptability of different components of the intervention. We asked 11 questions about the perceived helpfulness of different components of the intervention in the 12-month follow-up questionnaire for those in the intervention arm. The questionnaire was either posted and handed to PWCF who had had the intervention for completion in the presence of the interventionist. A total of 257 out of 305 (84%) participants in the intervention arm responded. Details of methods are reported in Appendix 5.
Trial monitoring data
The aim was to monitor RCT progress in terms of numbers of people approached, reasons for not agreeing to participate in the RCT and numbers withdrawing from the intervention. This allowed us to consider reach and engagement.
Qualitative research
The aim was to explore perceptions of the intervention in practice. We sampled patients purposively using a similar approach to WP 2.3. A total of 84 patients agreed to be approached for interview. We were unable to contact 37 patients, and did not approach 12, leaving a sample of 35. A total of 32 patients consented and 22 were interviewed. Some patients declined and others said that they were too busy to participate; three were unwell on the day of the interview and one died. We approached and interviewed all 26 interventionists. We approached nine MDT members and did not get a response from four, so interviewed five.
We undertook face-to-face interviews with 22 intervention users in seven CF centres, 26 interventionists (some sites had more than one) and five members of the MDT who acted as principal investigators for the study at five RCT sites. Patients comprised 10 male and 12 female patients, aged 19–58 years, across all deprivation levels and all adherence levels.
The interviews were undertaken by Sarah J Drabble (see Work packages 2.1A and 2.1B: a qualitative study – understanding the illness perceptions and treatment beliefs of people with cystic fibrosis for her credentials) and Elizabeth Lumley, a female clinically trained qualitative researcher educated to master’s level with no experience of CF research. The relationship between researchers and participants, and the approach taken, was similar to those in WPs 2.1 and 2.3. The topic guide for PWCF included questions relating to acceptability of different aspects of the intervention and what aspects of the intervention, if any, helped them to increase their adherence. The topic guide for interventionists included questions on the delivery of the intervention, the trial processes and aspects of the context (see Report Supplementary Material 2 for both topic guides). Interviews were audio-recorded and field notes taken. Interviews lasted between 17 and 83 minutes (mean 42 minutes).
We used framework analysis,31 deductively coding to the TDF,32 mechanisms of action including Vassilev’s telehealth mechanisms of action,55 different components of the intervention and its delivery, and inductively to context. Three researchers (SJD, EL, AS) coded the data in NVivo. No participant checking occurred; the findings were discussed with the PPI panel.
Mediation analysis
Structural equation modelling was undertaken on the RCT data to identify the mechanisms by which the CFHealthHub intervention could have influenced medication adherence. Prior to analysis, a logic model (see Figure 3) was constructed to map the anticipated mechanistic pathway, along with potential effect moderators (including two-way interactions) from which a provisional directed acyclic graph (DAG) was created. The factors were further screened for inclusion prior to fitting the model by graphically assessing two-way associations and, for potential mediators, by calculating mean differences between the randomised arms. Factors with little apparent association (defined as an absolute correlation of < 0.1 or a mean difference < 0.1 SDs) were removed from the DAG prior to model fitting. Factors identified as potential mediator–outcome confounders were included in the model as fixed-effect covariates. Pearson’s correlation coefficients with their 95% CI (calculated using Fisher’s z transformation) were used as a guide to identify relationships between mediators. Model fit statistics comparative fit index (CFI) and root mean squared error of approximation (RMSEA) were used to select the final model. In addition, 95% bootstrap CIs were used to estimate the indirect effect of the chosen mediators as well as the direct and total effect of the intervention on medication adherence. Sensitivity analysis was carried out, removing intervention arm participants whose follow-up overlapped with the intervention system being unavailable owing to technical difficulties (i.e. from 20 March to 23 April 2019).
Results
Key results are described in this section. Further results from the statistical analysis plan are reported in Report Supplementary Material 2. Extra analyses were undertaken that were not specified in the statistical analysis plan and these are reported in Report Supplementary Material 3.
Participant flow
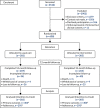
Participants were recruited from October 2017 to June 2018. Participants were followed up until trial completion in June 2019. Participant recruitment and disposition is shown in Figure 4.
Reasons for declining participation and premature discontinuation of intervention
Common reasons for declining to participate were unwillingness to change nebuliser (125/566) and that the trial would be too time-consuming (118/556). There were 54 premature discontinuations of adherence data collection (control arm, n = 29; intervention arm, n = 25) and 32 premature discontinuations of intervention delivery. Unhappiness with the device/nebuliser or a preference for a previous device was reported as a reason for discontinuation (see Report Supplementary Material 2).
Baseline characteristics
One participant withdrew prior to baseline data collection. Participant characteristics at baseline are shown in Table 3. There were no discernible between-group differences in baseline demographic characteristics. A difference was observed in ‘baseline’ numerator-adjusted normative adherence, which was measured in the first 2 weeks post consent. Participants in the intervention arm had slightly higher FEV1% predicted and fewer i.v. therapy-days in the prior year. In accordance with CONSORT reporting guidelines, we did not carry out any significance tests of baseline differences. We carried out an analysis adjusted for covariates. We describe this analysis briefly in the statistical analysis section and in more detail in the statistical analysis plan. In summary, we adjusted for baseline stratification factors (site and previous years’ i.v. therapy-days) and baseline value of the outcome (where available) in all statistical models.
TABLE 3
Participant demographics and clinical characteristics at baseline
Primary outcome
The IRRs from the primary and sensitivity analyses comparing exacerbation rates between the intervention and control arms are presented in Figure 5. The IRR for the main primary analysis was 0.96 (95% CI 0.83 to 1.12; p = 0.638). The point estimate of the IRR is < 1, which favours the intervention arm. However, the 95% CI for the treatment effect included 1, which is consistent with no overall difference in exacerbation rates between the two randomised arms. The sample size was predicted on the assumption of 2.0 exacerbations per year prior to intervention, with a reduction of 0.5 exacerbations to 1.5 per year. This is equivalent to an IRR of 0.75 (2.0 ÷ 1.5). We observed 1.77 exacerbations in the control arm and 1.63 in the intervention arm. If we are looking for a 0.5 reduction in exacerbations from 1.8 to 1.3 then this gives an IRR of 0.72 (1.8 ÷ 1.3). Based on our sample size calculation, a clinically important IRR is between 0.65 and 0.75. Because the lower limit of the estimate (i.e. 0.83) is above this, our result is not statistically significant, and not clinically significant if we believe an important IRR is ≤ 0.75. Findings from sensitivity analyses were consistent with the primary analysis, with 95% CIs encapsulating the null IRR value of 1.
Further details about the exacerbation analysis can be found in Report Supplementary Material 2.
Secondary outcomes
Adherence
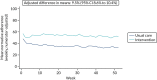
The mean weekly numerator-adjusted normative adherence over the course of the RCT is shown in Figure 6. The adjusted between-group difference in mean weekly adherence was 9.5 (95% CI 8.6 to 10.4; p < 0.001) percentage points in favour of the intervention arm. Further details about adherence can be found in Report Supplementary Material 2.
Other secondary outcomes
The adjusted mean FEV1% predicted at 12-month follow-up was 1.4 (95% CI –0.2 to 3.0; p = 0.082) percentage points higher in the intervention arm. The adjusted mean BMI was 0.3 kg/m2 (95% CI 0.1 kg/m2 to 0.6 kg/m2; p = 0.008) higher in the intervention arm. Effect sizes were modest for the remaining secondary outcomes, but all excluding the patient-reported measure of anxiety (GAD-7) showed a direction of effect favouring the intervention. Observed follow-up means and adjusted between-group differences for all outcomes are presented in Tables 4 and 5. Further detail can be found in Report Supplementary Material 2.
TABLE 4
Clinical and patient-reported outcome measures: primary outcome
TABLE 5
Clinical and patient-reported outcome measures: key secondary outcomes
Adverse event data from the 12-month follow-up period are presented in Table 6. A full list is presented in Appendix 6. There were no serious adverse events deemed related to the intervention.
TABLE 6
Non-serious and serious adverse events
Subgroup analyses
See later in Implications for randomised controlled trial subgroup and sensitivity analyses.
Extra analyses: longer-term outcomes
The above analysis is based on 12 months; however, some PWCF stayed in the RCT for up to 21 months. Analysis of the longer-term outcomes up to 21 months post consent back up the results of the primary analysis using 12-month data [no difference in exacerbations or forced expiratory volume in first second (FEV1)]. There is a difference in adherence of a similar order to the 12-month analysis.
For exacerbations, there was a total of 1326 exacerbations (control, n = 693; intervention, n = 633) during that time. The observed exacerbation rate in the extended post-consent follow-up was 1.70 per year in the control arm and 1.58 per year in the intervention arm (compared with an exacerbation rate of 1.77 per year in the control arm and 1.63 in the intervention arm in the primary analysis). The primary analysis model included adjustments for the previous year’s i.v. therapy-days and site, which were stratifying factors in the randomisation schedule. The estimated treatment effect for this analysis, the IRR, was 0.97 (95% CI 0.84 to 1.12), which is < 1, favouring the intervention arm. However, the 95% CI for the treatment effect included 1, which is consistent with no overall difference in exacerbation rates between the two arms. The estimated treatment effect for the longer-term follow-up is very similar to the primary analysis, with 12-month post-consent follow-up of 0.96 (95% CI 0.83 to 1.12). For FEV1, the estimated treatment effect was 0.6 (95% CI –0.2 to 1.4) percentage points and the time effect was –0.1 percentage points (95% CI –0.2 to –0.0 percentage points) decline in FEV1 per month. The direction of effect favoured the intervention arm, but the 95% CI included zero, consistent with there being no difference between arms. There was a small trend for decreasing FEV1% predicted over time and no significant interaction between randomised treatment arm and time. For adherence, the original mixed-effects adherence model was applied to the extended follow-up data. Increasing the follow-up time from 12 to 21 months increased the estimated treatment effect from 9.5 to 11.9 percentage points (95% CI 11.1 to 12.7 percentage points). The time coefficient was –0.2 percentage points (95% CI –0.2 to –0.1 percentage points), suggestive of a slight decreasing trend in adherence levels over time.
Process evaluation results
Fidelity (work package 2.3)
For both certification and drift, two persons independently assessed each intervention and the level of agreement between assessors was high. All interventionists were successfully certified as competent to deliver the intervention, including the first visit, review visit and phase review. A total of 110 assessments were assessed to explore drift in fidelity over the duration of the trial and a pass mark threshold of 80% was set for drift assessments. Among all paired assessments during the RCT, there was 97.2% agreement when comparing pass/fail decisions at the 80% threshold (207/213 assessments in agreement). That is, the RCT had good fidelity (overall fidelity by site, range 79–97%), with only one site not achieving over the mean threshold (> 80%) on drift assessments. See Appendix 3 for further details of the fidelity assessment methods and findings.
Usual-care survey
Although most CF centres reported using objective adherence data at baseline, this was described as ad hoc or infrequent at most sites, indicating that our intervention’s systematic approach to measurement was different from usual care. Change scores indicated that usual care within the sites was consistent from baseline to follow-up. There was variation in usual care between RCT sites. See Appendix 4 for further details.
User satisfaction
Among those intervention users completing the satisfaction survey, most rated the following intervention components as ‘very helpful’: the first intervention meeting (77.4%), adherence graphs/tables (68.5%), interventionist support to solve problems (60%) and telephone (58.7%) and face-to-face (67.2%) follow-up visits with the interventionist (Table 7). Videos of other PWCF were rated as less helpful. There was variation between RCT sites. See Appendix 5 for further details.
TABLE 7
Item-by-item questionnaire response summaries
Trial monitoring
Among the participants approached for the RCT, 48% were recruited. A total of 566 declined recruitment, citing reasons such as not wanting to change the type of nebuliser they used and being too busy (see Report Supplementary Material 2).
The CFHealthHub digital platform was taken down for emergency technical work for 5 weeks (from 20 March to 23 April 2019). It was not available to participants in the RCT in that period. This affected the delivery of the intervention for a minority of PWCF in the intervention arm. This was taken into consideration in the mediation analysis and in a sensitivity analysis for the RCT.
Qualitative research
Process evaluations focus on mechanisms of action, implementation/delivery of the intervention and context.45 The qualitative research focused on these issues. We report the findings in three parts: one focusing on a single mechanism of action (‘objective adherence data as proof’) (see Appendix 7), one focusing on the range of mechanisms of action operating in practice in the intervention, including some that were not identified in the feasibility study (see Appendix 8), and one focusing on variation in context and implementation between RCT sites/CF centres (see Appendix 9).
During data collection one of the qualitative researchers noted that PWCF and interventionists sometimes talked about the adherence data as ‘proof’. This mechanism of action was explored in detail by analysing codes related to mechanisms of action and identifying different aspects of this mechanism. The objective adherence data were described as offering proof to both self and others about adherence behaviour. PWCF perceived that this could offer benefits, including improving their relationships with their clinical team and their families, if objective adherence was higher than believed by these external parties (see Appendix 7 for further details about the role of proof in improving adherence).
During the feasibility study, we explored the range of mechanisms of action of the intervention. During the evaluation phase we continued to be interested in this. We had coded data to expected mechanisms of action and mechanisms of action associated with effective telehealth intervention and analysed these codes. There was evidence to support expected mechanisms of action around self-monitoring and self-regulation. Other mechanisms of action were also apparent, for example being monitored by others, which some interventionists believed affected control participants as well as intervention participants who mistakenly believed that clinicians could see their adherence data. The relationship between interventionists and PWCF in the intervention arm appeared to be an important mechanism, as found in the feasibility study. Open communication, home visits, continuity of relationship and time helped to build trust between interventionists and PWCF. This trust helped PWCF to talk openly and honestly about the challenges that they faced adhering to treatment. This meant that the interventionists understood more about the real-life challenges faced by PWCF and could help them to find ways to address those challenges. PWCF with high levels of baseline adherence reported gaining reassurance from the intervention. PWCF with very low levels of baseline adherence had challenging life situations that made improvement difficult. PWCF with low to moderate levels of adherence could improve adherence, with action plans to help establish treatment habits, especially if the time was right in their lives. PWCF found the components of the intervention acceptable, but some did not like the patient video clips and some struggled with setting formal action plans. (See Appendix 8 for further details about mechanisms of action).
There was considerable variation between the different RCT sites/CF centres in terms of the backgrounds of the interventionists delivering the intervention at each site, and the way in which the MDT engaged with the intervention and interventionist. That is, the context in which the intervention was delivered varied in the RCT, with the potential to affect implementation. See Appendix 9 for further details.
Mediation analysis
Awareness, habit, concerns, self-efficacy and effort were selected as mediators from the logic model (see Figure 3) because these were associated with both intervention and normative medication adherence. The standardised mean differences of the mediator between the intervention and control arms ranged from 0.6 to 0.2 and mediator–medication adherence correlation ranged from 0.1 to 0.5. Two-way interaction graphs showed that treatment effect had a stronger effect on awareness as baseline prescription increased, and treatment effect on effort was different for men and women. Hence, baseline prescription and gender were included as moderators. Age, gender and baseline measures of FEV1, number of nebulisers prescribed, depression score, i.v. therapy-days (binary), medication adherence, awareness, motivation and chaos were included as fixed-effect covariates. All selected mediators except awareness had a statistically significant association between each other.
The final mediation analysis model included all the selected mediators, moderators and fixed-effect covariates, demonstrating a good model fit (CFI 1.00, RMSEA 0.00, 95% CI 0.00 to 0.01). The total effect of the intervention on mean normative medication adherence was 13.3 (95% CI 9.6 to 17.0). The direct effect on adherence (i.e. not explained via the selected mediators) was 6.1 (95% CI 2.7 to 9.5). The overall indirect effect of awareness was 5 (95% CI 3.2 to 6.8) and mediated 37.3% of the total effect, but interacted linearly with mean baseline prescription: awareness mediated 18% of the total effect for patients using a nebuliser on alternate days and mediated 58% of the effect for those using six per day. The indirect effect of habit was 1.3 (95% CI 0.4 to 2.1; 9.4% mediated), of self-efficacy was 0.2 (95% CI –0.3 to 0.7; 1.6% mediated) and of concerns was 0.2 (95% CI –0.3 to 0.7; 1.5% mediated). The indirect effect of ease of effort was 0.2 (95% CI –0.1 to 0.5; 1.4% mediated) but interacted with sex: effort had some mediation for women at 0.4 (95% CI –0.2 to 1.0; 2.8% mediated) but little mediation for men at –0.03 (95% CI –0.3 to 0.3; –0.2% mediated). Results from the sensitivity analysis showed a similar trend but with a slightly higher mediating effect as mean baseline prescription increased (14% to 63% for the baseline prescription range 0.5 to 6.0).
The results from the mediation analysis suggests that the intervention helped improve patient’s awareness of their medication usage. This increased awareness contributed to an increased medication adherence, with some evidence to suggest that this effect was more pronounced for patients who used several nebulisers at baseline. Another pathway in which the intervention could have affected medication adherence was by facilitating habit formation, resulting in decreased effort required to take medication, thereby reducing concerns and improving self-efficacy. The total mediated effect of all these mediators was 51%.
See Appendix 10 for further information.
Triangulation for process evaluation
The triangulation grid bringing together all the components of the process evaluation is reported in Appendix 11. This grid was used to draw key conclusions from the process evaluation. The synthesised conclusions were as follows:
- Implementation was very good. The intervention was delivered with good fidelity at all RCT sites with the exception of one. There was one period of 5 weeks towards the end of the RCT when the CFHealthHub platform was not available; this was considered in both the mediation analysis and the RCT sensitivity analysis.
- The intervention was acceptable. The majority of PWCF who completed the survey found it very helpful, particularly the graphs of adherence and the interventionists’ visits. Some caution is appropriate because not all PWCF completed the survey and PWCF may have wanted to please the interventionist, who might have been present at the time the questionnaire was completed.
- The expected mechanisms of action were evident (e.g. self-monitoring), and further mechanisms of action were identified for improvements in adherence to nebulisers. Changes in people’s calibration of their perceived adherence rates affected improvements in objectively measured adherence rates, showing the importance of the objective adherence data.
- The intervention was different from usual care.
- The context in which the intervention was delivered differed by RCT site owing to the differing strengths of the interventionists and the different levels of engagement of MDTs with the intervention.
Implications for randomised controlled trial subgroup and sensitivity analyses
Our proposed RCT subgroup analyses identified from the process evaluation were specified prior to analysis of the RCT data. Based on the process evaluation, we were keen to understand if RCT results differed by:
- good versus poor implementation at different sets of sites
- whether or not the CFHealthHub intervention was available (i.e. where it was not available for 5 weeks owing to technical difficulties)
- levels of baseline adherence.
In practice it was not possible to identify a set of RCT sites with poorer implementation because contextual issues varied greatly between sites. Therefore, we did not undertake this subgroup analysis. The issue about the lack of availability of the intervention for a few weeks was addressed in a sensitivity analysis and the mediation analysis (see Report Supplementary Material 2). The lack of availability of the intervention for 5 weeks did not affect the RCT results.
The baseline adherence subgroup analysis had been specified in our set of a priori RCT subgroup analyses but these planned subgroup analyses were undertaken on the primary outcome only. After our process evaluation we were interested in the relationship between baseline adherence and the key secondary outcomes of change in adherence rates and FEV1.
Subgroup analyses are shown in Report Supplementary Material 2. There were no statistically significant subgroup differences in number of exacerbations (primary outcome). Post hoc additional subgroup analyses are also shown in Report Supplementary Material 2. The only statistically significant subgroup difference was related to improvement in adherence rates differing by baseline objective adherence (p < 0.001). PWCF with low and moderate levels of baseline adherence had the biggest improvement (18% and 15%, respectively) and high-level adherers had the least (3%) improvement and very low-level adherers had a 10% improvement. Adherence graphs by baseline adherence are displayed in Report Supplementary Material 2.
Triangulation of randomised controlled trial results and process evaluation findings (work package 3.4)
Work package 3.4 brings together the findings from the RCT and process evaluation related to outcomes. See Appendix 12 for more details. The process evaluation cannot identify changes in outcomes compared with control but can offer insights to support (or otherwise) RCT results, as well as explain how outcomes might have been achieved. The overall conclusions of this triangulation process were:
- The intervention did not statistically significantly reduce the primary outcome of numbers of exacerbations. This was not due to implementation problems because the intervention was implemented with high levels of fidelity.
- The intervention increased adherence rates. This finding was supported by multiple components of the process evaluation, showing the importance of the objective adherence data to this improvement as well as habit forming and the relationship with interventionists. Improvements in adherence rates were greater among those with low to moderate levels of baseline adherence than among those with high levels of baseline adherence (where ceiling effects may have operated) or those with very low levels of baseline adherence (who may have struggled with complex lives).
Parts of this section are reproduced or adapted with permission from Wildman et al.44 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
- Work packages 3.2, 3.3 and 3.4: full-scale randomised controlled trial with conc...Work packages 3.2, 3.3 and 3.4: full-scale randomised controlled trial with concurrent process evaluation - An intervention to support adherence to inhaled medication in adults with cystic fibrosis: the ACtiF research programme including RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...