This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Show detailsContinuing Education Activity
Thyroid cancer is often described as an indolent pathology with a good prognosis; however, poorly differentiated subtypes describe an aggressive behavior with extensive metastatic disease at the time of diagnosis. PET/CT has been established as an important modality in the detection of poorly differentiated thyroid cancer. This activity reviews the biochemical basis, indications, technique, and clinical significance of PET/CT, highlighting the interprofessional team's role in evaluating and treating patients with thyroid cancer.
Objectives:
- Identify the role of PET/CT in the management of thyroid cancer.
- Review the physiological basis behind radiotracer uptake in thyroid cancer subtypes.
- Summarize imaging findings in thyroid cancer and potential pitfalls.
- Discuss current recommendations and indications for PET/CT use by the interprofessional team.
Introduction
Thyroid cancer remains the most common endocrine malignancy and comprises 2% of all cancers in the United States. Papillary and follicular types are considered differentiated carcinomas and have an excellent treatment response with survival rates of up to 93% in the first ten years.[1] Conversely, poorly differentiated carcinomas like medullary and anaplastic subtypes demonstrate aggressive behavior with survival rates ranging between 10% to 60%.[2]
Within the array of diagnostic tools available for detecting thyroid cancer, the use of ultrasound and fine-needle aspiration is the first step and gold standard modalities, especially in high-risk patients (palpable neck mass, family history of malignancy, etc.). However, additional imaging modalities such as PET/CT are often utilized as a troubleshooting diagnostic tool, particularly in metastatic disease with unknown primary and extension of disease in poorly differentiated subtypes.
Anatomy and Physiology
To discern each carcinoma subtype, Radiotracers are often used to distinguish specific biological features and as a follow-up diagnostic method for surveillance and evaluation of metastatic disease.
In the thyroid, follicular epithelial cells demonstrate avid iodide uptake mediated by the sodium-iodide symporter. The partially preserved activity of this transporter in the aberrant cells of differentiated carcinomas is the foundation for the successful therapeutic and diagnostic utility of radioiodide interventions.[3] Poorly differentiated carcinomas do not express the same behavior. This characteristic presents a challenging clinical scenario; however, the use of radiotracer like F18 fluorodeoxyglucose (18 F FDG) has been demonstrated to be a troubleshooting tool targeting the increased glucose uptake through overexpression of glucose transporter-1 (GLUT 1) in the hypermetabolic state of neoplastic cells. In some instances, previously differentiated diseases describe a “flip-flop phenomenon,” where early tissue dedifferentiation starts acting like anaplastic cells with increased FDG uptake.[4] Finally, neuroendocrine tumors like medullary carcinoma display additional structural and metabolic characteristics such as upregulation of somatostatin receptors and increased decarboxylation of dopamine, respectively, allowing for the potential use of F-DOPA and 68-gallium DOTA peptides as radiotracers in diagnostic imaging.[5]
Indications
In previously treated differentiated thyroid carcinoma, the American Thyroid Association (ATA) recommendations for the use of FDG PET/CT are almost exclusively for patients with a high risk of metastasis or recurrence presenting with increasing thyroglobulin levels (> 10 ng/ml) and negative radioiodide scan (RAI). ATA recommends further cross-sectional evaluation with calcitonin levels higher than 150 pg/ml in those with medullary carcinoma.[6]
Additional indications include surveillance and treatment response in a previously identified FDG avid tumor, suspicion of metastatic disease secondary to an identified aggressive tumor after total or near-total thyroidectomy, and second-line preoperative evaluation for possible residual disease with negative RAI uptake.[7]
Contraindications
There are no absolute contraindications to PET/CT. However, suboptimal preparation can result in rescheduling on the day of the study. For example, hyperglycemic states decrease optimal FDG uptake in the targeted regions, hence glucose levels > 200 m/dl will limit the accuracy of the test, and the study will need to be postponed until the patient can achieve glycemic control.
Equipment
The most common radiopharmaceutical is 18F FDG obtained via bombardment in cyclotrons. Their relatively long half-life (T 1/2 = 110 min) allows for production and shipping the same day of the study.[8] Additional radiotracers are available for clinical and research purposes; however, the formal indication is still debatable.
The study requires a PET/CT scanner, a hybrid system that includes a single table for sequential acquisitions of PET and CT images. Compared to the gamma camera commonly used in radioiodide scans, this modality allows for increase spatial resolution through high-energy gamma rays from the emission of positrons without requiring collimators to improve image quality. Additionally, image reconstruction with cross-sectional CT permits better characterization of increased uptake in specific anatomical structures.
Personnel
Interpretation of PET/CT images must be performed by a board-certified radiologist or nuclear medicine physician with a continuing experience and education of no less than 150 studies in a period of 3 years.
A registered radiographer can acquire images, a registered or certified nuclear medicine technologist, or a registered radiation therapist with credentials to operate CT and radiopharmaceuticals.
Finally, a designated safety radiation officer (SRO) is required in all institutions certified to use radiopharmaceuticals for healthcare purposes. The SRO's responsibilities include ensuring adequate handling of radiopharmaceuticals (shipping, receiving, securing, and disposal), maintaining calibration instruments, and promoting radiation safety in the institution.[9]
Preparation
Fasting or withholding of parenteral feedings for 4-6 hours is indicated before administration of radiotracer. For patients with a history of diabetes, glucose levels should be lower than 200 mg/dl on the study day. Exercise avoidance is recommended to decrease muscle uptake, and patients are encouraged to void just before the scan to decrease the background signal in the urinary system. A pregnancy test is indicated in females of childbearing age before administering radiopharmaceutical to avoid potential harm to the fetus.[10] Breastfeeding does not need to be interrupted before the study, and the mother should limit contact with the child in the first 12 hours after the radiotracer administration.
Technique or Treatment
The recommended intravenous dose for adults is 370-740 mBq/kg (10-20 mCi/Kg) and for children is 5.18-7.4 MBq/kg (0.14 to 20 mCi/Kg).[10] Administration of tracer should be done in the contralateral site, if possible, and image acquisition is obtained 60 minutes after the injection. A whole-body scan including skull base through feet is recommended for all tumor types, especially in cases with unknown primary and evidence of metastatic disease.
Image acquisition can be enhanced and non-enhanced. Every study must include a CT tomogram or scout views. Non-enhanced studies are done with low-dose CT followed by PET acquisition. The enhanced protocol requires a whole-body scan with a 45 to 60 second to account for the venous phase in the abdomen, followed by PET acquisition.
Both PET and CT images are necessary for accurate interpretation, especially when images are degraded due to motion. In addition, interpretation of the study must consider normal uptake and infection or inflammatory processes that can decrease avid uptake. Physiological normal uptake can be detected in the brain, myocardium, reticuloendothelial and gastrointestinal system, kidneys, muscle, uterus, ovaries, teste, and brown adipose tissue.
Complications
PET/CT is a safe procedure. Radiopharmaceuticals doses are small and not associated with adverse effects. In enhanced protocols, the major risk consideration is allergic reactions attributed to iodine contrast, including rash and anaphylactic reactions. IV access extravasation and bruising are inherent mild complications of any venipuncture procedure.
Clinical Significance
The strength of the role of PET/CT in the management of thyroid cancer resides in the evaluation of recurrent or metastatic disease, especially in poorly differentiated subtypes. Once identified by pathology, the aggressive behavior of this subtype increases the risk for metastatic disease, worsening the patient’s prognosis. PET/CT is greatly superior to radioiodide scan in identifying aggressive tumor cells with decreased or lack of iodide uptake.
Despite the limited role in the surveillance of differentiated carcinomas, there’s an identifiable benefit in patients with negative radioiodine scans. Meta-analysis studies revealed that PET/CT has a sensitivity and specificity of more than 80% in detecting extensive disease.[11][12] Authors recognized higher grades of differentiation, larger tumor size, and higher TSH levels as possible factors positively impacting the accuracy of the test. Overall, higher uptake values correlate with more aggressive tumors and worse prognosis.
The use of other radiotracers like 18 F DOPA for detection of thyroid cancer seems promising, especially in the case of well-differentiated medullary carcinoma with a sensitivity ranging between 70 and 80% and has described an improved ability to detect distant metastatic disease when compared to FDG.[13][14] However, official recommendations for routine use remain under discussion.
Finally, opportunistic detection of incidentalomas in studies aimed for other oncologic purposes is an important advantage of PET/CT. The detection of focal avid uptake has a considerable risk of 24% to 36% for malignancy and triggers warning signs for additional diagnostic workup with tissue sampling. Conversely, diffuse thyroid uptake suggests a benign inflammatory process like thyroiditis and should not prompt further investigation. (level 3, ATA guidelines).[5]
Enhancing Healthcare Team Outcomes
The initial diagnosis of thyroid cancer is based on ultrasound and cytology findings. In low-risk patients with differentiated localized or regional disease and no evidence of metastasis, surgical intervention is curative.[15] [Level 1] However, a significant number of patients will exhibit residual or recurrent disease after surgery, detectable by elevated thyroglobulin levels but without evident avid iodide uptake on the scan (“flip flop phenomenon” discussed above). Moreover, for indeterminate cytology, some studies have considered the role of PET/CT in preoperative diagnosis with promising results.[16]
ATA guidelines favored the specificity of molecular cytological testing over PET/CT for these cases. Hence, individualized interprofessional management with endocrine, pathology, radiology, and surgery departments is necessary to provide optimal patient care and minimize healthcare expenses. This interprofessional team includes specialists, clinicians, mid-level providers, nurses, and radiology technicians, working collaboratively to obtain optimal diagnostic information.
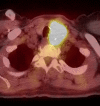
Figure
PET/CT Hurtle cell carcinoma Contributed by Daniela Garcia, MD
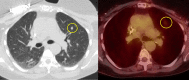
Figure
Previously treated papillary thyroid carcinoma with metastatic lung nodule without significant FDG uptake Contributed by Daniela Garcia, MD
References
- 1.
- Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol). 2010 Aug;22(6):395-404. [PubMed: 20627675]
- 2.
- Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011;2011:542358. [PMC free article: PMC3136148] [PubMed: 21772843]
- 3.
- Dohán O, Carrasco N. Advances in Na(+)/I(-) symporter (NIS) research in the thyroid and beyond. Mol Cell Endocrinol. 2003 Dec 31;213(1):59-70. [PubMed: 15062574]
- 4.
- Johnson NA, Tublin ME. Postoperative surveillance of differentiated thyroid carcinoma: rationale, techniques, and controversies. Radiology. 2008 Nov;249(2):429-44. [PubMed: 18936309]
- 5.
- Marcus C, Whitworth PW, Surasi DS, Pai SI, Subramaniam RM. PET/CT in the management of thyroid cancers. AJR Am J Roentgenol. 2014 Jun;202(6):1316-29. [PubMed: 24848831]
- 6.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016 Jan;26(1):1-133. [PMC free article: PMC4739132] [PubMed: 26462967]
- 7.
- Donohoe KJ, Aloff J, Avram AM, Bennet KG, Giovanella L, Greenspan B, Gulec S, Hassan A, Kloos RT, Solórzano CC, Stack BC, Tulchinsky M, Tuttle RM, Van Nostrand D, Wexler JA. Appropriate Use Criteria for Nuclear Medicine in the Evaluation and Treatment of Differentiated Thyroid Cancer. J Nucl Med. 2020 Mar;61(3):375-396. [PubMed: 32123131]
- 8.
- Lameka K, Farwell MD, Ichise M. Positron Emission Tomography. Handb Clin Neurol. 2016;135:209-227. [PubMed: 27432667]
- 9.
- Berry K, Elder D, Kroger L. The Evolving Role of the Medical Radiation Safety Officer. Health Phys. 2018 Nov;115(5):628-636. [PubMed: 30260854]
- 10.
- Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA, Hubner K, Stabin MG, Zubal G, Kachelriess M, Cronin V, Holbrook S. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006 May;47(5):885-95. [PubMed: 16644760]
- 11.
- Leboulleux S, Schroeder PR, Schlumberger M, Ladenson PW. The role of PET in follow-up of patients treated for differentiated epithelial thyroid cancers. Nat Clin Pract Endocrinol Metab. 2007 Feb;3(2):112-21. [PubMed: 17237838]
- 12.
- Treglia G, Muoio B, Giovanella L, Salvatori M. The role of positron emission tomography and positron emission tomography/computed tomography in thyroid tumours: an overview. Eur Arch Otorhinolaryngol. 2013 May;270(6):1783-7. [PubMed: 23053387]
- 13.
- Archier A, Heimburger C, Guerin C, Morange I, Palazzo FF, Henry JF, Schneegans O, Mundler O, Abdullah AE, Sebag F, Imperiale A, Taïeb D. (18)F-DOPA PET/CT in the diagnosis and localization of persistent medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2016 Jun;43(6):1027-33. [PubMed: 26497699]
- 14.
- Beheshti M, Pöcher S, Vali R, Waldenberger P, Broinger G, Nader M, Kohlfürst S, Pirich C, Dralle H, Langsteger W. The value of 18F-DOPA PET-CT in patients with medullary thyroid carcinoma: comparison with 18F-FDG PET-CT. Eur Radiol. 2009 Jun;19(6):1425-34. [PubMed: 19156423]
- 15.
- Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016 Dec 03;388(10061):2783-2795. [PubMed: 27240885]
- 16.
- Piccardo A, Trimboli P, Foppiani L, Treglia G, Ferrarazzo G, Massollo M, Bottoni G, Giovanella L. PET/CT in thyroid nodule and differentiated thyroid cancer patients. The evidence-based state of the art. Rev Endocr Metab Disord. 2019 Mar;20(1):47-64. [PubMed: 30900067]
Disclosure: Daniela Garcia declares no relevant financial relationships with ineligible companies.
Disclosure: Vikramjeet Singh declares no relevant financial relationships with ineligible companies.
- Nuclear Medicine PET/CT Thyroid Cancer Assessment, Protocols, and Interpretation...Nuclear Medicine PET/CT Thyroid Cancer Assessment, Protocols, and Interpretation - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...