NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Smith GCS, Moraitis AA, Wastlund D, et al. Universal late pregnancy ultrasound screening to predict adverse outcomes in nulliparous women: a systematic review and cost-effectiveness analysis. Southampton (UK): NIHR Journals Library; 2021 Feb. (Health Technology Assessment, No. 25.15.)

Universal late pregnancy ultrasound screening to predict adverse outcomes in nulliparous women: a systematic review and cost-effectiveness analysis.
Show detailsIntroduction
This study was commissioned to evaluate the current evidence base on the costs and clinical effectiveness of performing a routine ultrasound scan in late pregnancy in all nulliparous women combined with appropriate management plans, to identify evidence gaps, and to predict whether or not future research to fill those gaps is likely to be a cost-effective use of health-care resources. In this analysis, we use decision modelling to assess the likely outcomes from universal ultrasound screening and determine whether or not its potential benefits can be clinically and economically justified.
We present a cost–utility analysis focusing on three of the main conditions detectable by ultrasound screening that may warrant intervention: breech presentation, the fetus being SGA and the fetus being LGA. The cost-effectiveness of universal ultrasound screening for each of these conditions individually has been explored previously.11,155 However, here we evaluate the cost-effectiveness of screening for all of these conditions at the same session. Furthermore, we use decision uncertainty to predict the expected return on further research. We have applied the simplified management plan outlined in Figure 11. In essence, women are first assessed for presentation. If the infant is in breech presentation, ECV is offered. If this is successful, the woman reverts to receiving expectant management, and, if it is unsuccessful, the baby is delivered by planned caesarean section. If the infant is in a cephalic presentation and the EFW is in the normal range, the woman receives expectant management. If the infant is either SGA or LGA, IOL is offered. However, we also compare combined assessment for presentation and fetal biometry with a scan simply for presentation. The rationale for this is that a presentation scan may be readily implemented and relatively inexpensive, and there is much less uncertainty about the usefulness of knowing the infant’s presentation than there is about the usefulness of estimating the infant’s size.
The structure of this chapter is as follows. In Methods, we first introduce the general methodology for our economic evaluation. We then summarise the clinical definitions used, as well as the competing strategies evaluated, in this study before introducing the structure of the economic simulation model underlying the analysis. Once the model structure and mechanics have been explained, we discuss how we populated the model with the best available data; complete technical details regarding how individual parameters were derived are presented in Appendix 6. Finally, we describe the base-case analyses, sensitivity analyses and VOI analysis to guide how future research in this area could be prioritised.
In Results, we present the results of the baseline economic evaluation and sensitivity analyses. The results of the VOI analysis are then presented, which include the results for the expected value of perfect information (EVPI), the expected value of partial perfect information (EVPPI) and, finally, the expected value of sample information (EVSI).
In Discussion, we summarise the key findings, explain the interpretation of our results and discuss what impact our methodological limitations may have had on the results.
Methods
To compare long-term health and cost outcomes associated with different strategies of screening in third-trimester pregnancy, we constructed an economic simulation model. We focused the model on two features for which late-pregnancy ultrasound is amenable to detect: fetal presentation and fetal size. We used a decision tree model consisting of four subtrees, one each for breech presentation, LGA, SGA and AGA. The model structure is based largely on previous economic analyses of screening for these conditions individually, and the development and key characteristics of these submodels’ models have previously been described11,155 (a brief summary is provided in Appendix 7). Chapter 10 dealt with the diagnostic effectiveness of ultrasound in this setting and outlined how a positive result on scan could influence subsequent care. This chapter focuses on how these submodels were incorporated into a joint framework, enabling a cost-effectiveness analysis of simultaneous screening for all of these conditions.
Scope and population
The analysis relates to nulliparous women in England with singleton pregnancies, excluding those opting for elective caesarean section for any reason except a diagnosis of breech presentation. The economic analysis uses a public sector perspective defined as NHS and special educational needs (SEN) costs. Outcomes are from the perspective of the infant.
Comparators and interventions
This analysis evaluated three different strategies for ultrasound screening in late pregnancy, defined as a scan between 36+0 weeks’ gestation and 36+6 weeks’ gestation. ‘Selective ultrasound’ (i.e. when ultrasound is performed only if clinically indicated) is the current standard in England.152 ‘Universal ultrasound for fetal size’ would mean routinely offering a third-trimester ultrasound assessment of fetal weight in every pregnancy. Given the simplicity of detecting fetal presentation during an ultrasound scan, this screening strategy would also identify breech presentation. A third option would be to offer ‘universal ultrasound for presentation only’ (i.e. a simpler ultrasound scan with the sole purpose of detecting pregnancies with breech presentation). Compared with a standard antenatal ultrasound for which, typically, multiple measurements are made, an ultrasound scan for fetal presentation alone is technically simple. We theorised that such a scan could be carried out by an attending midwife during a standard antenatal visit in primary care, using basic ultrasound equipment.
We assumed that all women identified with breech presentation would be offered an ECV unless contraindicated, in line with RCOG guidelines.156 We further assumed that pregnancies in which the fetus is identified as SGA (whether or not correctly diagnosed) would be given early IOL. However, for pregnancies in which the fetus is diagnosed as LGA, there is uncertainty about the benefits of the intervention (IOL). For this reason, expectant management of suspected LGA pregnancies was also an option. We had previously considered also including elective caesarean section for the management of macrosomia, but we ruled this out because it was inferior to IOL in our cost-effectiveness analysis of ultrasound assessment for macrosomia alone.155 This conclusion was consistent with a previous decision model analysis.157 We therefore compare six discrete strategies in the analysis (Table 9).
TABLE 9
Comparator strategies for economic simulation model
We assume that selective scanning (i.e. only where clinically indicated) with a policy of offering ECV for suspicion of breech presentation and IOL for suspicion of SGA or LGA (see strategy 2 in Table 9) represents an approximation of the status quo from which estimates of incremental net benefit are calculated.
As discussed in Chapter 10, there is more uncertainty in relation to the management of LGA than of SGA. However, performing fetal biometry will yield a percentile of EFW and, hence, a scan involving fetal biometry can yield three possible outcomes: AGA, SGA or LGA. Consequently, we considered two possible approaches to screening involving fetal biometry. Both approaches included IOL for SGA; however, one also included IOL for LGA, whereas the other dictated expectant management, given the uncertainty.
Outcomes
In the absence of any trials on third-trimester screening strategies with long enough follow-up, we could not directly estimate long-term health outcomes as a function of screening strategies alone (hence the need for this modelling study). Instead, we simulated outcomes at delivery (survival and different levels of neonatal complications/morbidity), and then simulated long-term health outcomes as a function of these short-term outcomes. Overall health gain was captured as QALYs accrued by the infant. Overall costs for each screening strategy included the cost of the ultrasound scanning, possible intervention, delivery episode, neonatal care and mortality, and long-term care.
Model structure
As stated, the model structure is a decision tree. It was coded in R (The R Foundation for Statistical Computing, Vienna, Austria) version 3.4.1, using the packages BCEA, FinCal, ggplot2, gtools, readxl, tidyr and SAVI.158,159 The code for the model is available from the corresponding author on request.
Figure 12 shows the structure of the first stages of the decision model. The [+] indicates sub-branches that have been collapsed for clarity. Nodes are named to show their relationship to one another; nodes with the same letter have identical structures to the branches of the tree beyond, whereas a different number and/or a lower-case letter indicates a different set of probabilities. The prefixes B, L and S denote nodes with probability sets specific to breech presentation or large or small for gestational age infants, respectively.
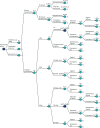
FIGURE 12
Model overview. [+], sub-branches of model collapsed for clarity.
At commencement, the scan policy can be set to selective (i.e. status quo), a universal scan for presentation only, or a universal scan for fetal biometry and presentation. The model structure is identical in each case. The difference is in the sensitivity and specificity of the scanning policies and their cost.
A fetus will be in either breech or cephalic presentation (node A1), or be LGA, SGA or AGA (node A2). For ease of modelling, we assume that all four possibilities are mutually exclusive and structured hierarchically, beginning with presentation (breech or cephalic) and followed by size (LGA, SGA or AGA). The implications of this are considered in Discussion. The probability of breech is the prevalence of breech at the time of screening (approximately 4.6%).11 If the scan policy is universal ultrasound (whether for fetal biometry or for presentation only), then, given the ease of interpretation of such a scan, we assume all breeches are detected (i.e. 100% sensitivity and specificity, node B_B). However, under the selective scan policy, approximately 45% of breeches will be undetected11 owing to the mother not having undergone a scan at all (for consistency with the rest of the model, we label these ‘false negatives’). Further outcomes relating to breech presentation are described in Outcomes relating to breech.
If the infant is in cephalic presentation, it may be LGA, SGA or AGA. The probabilities of each is the prevalence of the condition (node A2, by definition 10% for each). If an infant is LGA or SGA, the probability of detection is a function of the sensitivity of the scanning policy (nodes L_B and S_B; LGA: 26.55% under selective and presentation-only scan, 37.85% under universal scan for fetal size;138 SGA: 19.6% under selective and presentation-only scan, 56.53% under universal scan for fetal size8). The sensitivity and specificity of ultrasound for detecting SGA and LGA were derived from the POP study.8,138 The rationale for using the POP study values is that this study was conducted in NHS England, it involved nulliparous women being scanned at 36 weeks’ gestation, it is the only level 1 study of the diagnostic effectiveness of ultrasound to predict SGA and LGA (i.e. where the test result was blinded) and the values of sensitivity and specificity for SGA were similar to those in a 2019 Cochrane review of DTA.23 In addition, the DOR from the POP study for macrosomia was identical to the DOR in the meta-analysis presented in Chapter 8.
If a LGA infant is correctly diagnosed as LGA, the pregnancy is managed in accordance with the defined LGA policy of either IOL or expectant management (node ‘MGT_LGA_TP’), in either case leading to either vaginal delivery or emergency caesarean section (nodes L_C3 and L_C2a; odds ratio of emergency caesarean section compared with otherwise healthy infant, 1.79146). If a LGA infant is misdiagnosed as AGA (i.e. false-negative scan), delivery can be either vaginal or by emergency caesarean section. Further outcomes relating to LGA babies are described in Outcomes relating to large for gestational age infants.
If the infant is SGA and is correctly diagnosed as such, labour is induced, leading to either vaginal delivery or emergency caesarean section (node S_C3). False negatives may lead to vaginal delivery or emergency caesarean section (node S_C2). Further outcomes relating to SGA pregnancies are described in Outcomes relating to small for gestational age infants.
An AGA infant may be misdiagnosed as SGA or LGA (false-positive SGA and LGA, respectively), or correctly diagnosed as AGA (node B). A false-positive SGA infant will be induced unnecessarily, leading to either vaginal delivery or emergency caesarean section (node S_C4). A false-positive LGA infant will be managed in accordance with the defined LGA policy namely either IOL or expectant management (node ‘MGT_LGA_FP’). IOL and expectant management can lead to either spontaneous vaginal or emergency caesarean section delivery (nodes L_C4 and L_C1 respectively). Finally, a correctly diagnosed AGA infant (true negative) can be delivered vaginally or by emergency caesarean section (node C1).
Short- and long-term outcomes
For all parts of the model, different levels of neonatal morbidity and mortality are possible, although these outcomes are structured slightly differently between the model’s subtrees. For the breech, SGA and AGA models, delivery outcomes include no, moderate and severe neonatal morbidity, as well as perinatal death. The risks of each level of adverse outcome differ between specific branches (i.e. are affected by the true status of the infant, the mode of delivery and whether or not labour was induced early). Long-term outcomes are then modelled as a function of the level of neonatal morbidity at delivery. For the LGA model, delivery and long-term outcomes are modelled differently. This is explained in detail in Outcomes relating to large for gestational age infants.
Long-term outcomes include ‘no long-term complications’, ‘SEN’, ‘severe neurological morbidity’ (SNM) and ‘neonatal/infant mortality’. The risk of long-term complications increases with the level of neonatal morbidity (nodes E1, E2 and E3). Unlike delivery outcomes, long-term outcomes are not affected by the actual status of the infant prior to delivery, only by the level of neonatal morbidity at delivery. Importantly, this means that all screening and management options affect long-term outcomes indirectly only as a result of the impact that they have on the outcomes at delivery.
Outcomes relating to breech
Figure 13 shows the decision tree with outcomes relevant to breech expanded and the remaining branches collapsed. The prevalence of breech refers to the fetal presentation at the time of screening. We assume that sensitivity and specificity for universal ultrasound is perfect at detecting fetal presentation, whether for size or breech presentation only. The sensitivity of selective ultrasound is lower because not all women receive ultrasound screening; however, we assume that all cases of suspected breech presentation would be either confirmed or rejected by ultrasound, so false-positive diagnosis is not an option (i.e. perfect specificity).

FIGURE 13
Outcomes associated with breech. [+], collapsed sections of the decision tree.
On diagnosis of a breech presentation, an ECV is offered (node B_ECV). If the ECV is successful (node B_ECVs) and the infant remains cephalic (node B_ECVs_rb), no further intervention will be offered (i.e. expectant management). However, the infant may spontaneously revert to breech presentation (node B_ECVs_rb). In either case, there is a probability of emergency caesarean section, which is increased if the infant has reverted to breech presentation (nodes B_C3b and B_C3a respectively). If breech presentation is not diagnosed prior to labour, delivery options include breech vaginal delivery and emergency caesarean section (node B_C2).
Following labour and delivery there is a risk of no, moderate or severe neonatal complications or perinatal death (node D1), subsequently leading to no long-term complications, SEN, SNM or perinatal mortality (node E1). Note that we assume no raised risk of neonatal morbidity associated with cephalic emergency caesarean section compared with cephalic vaginal delivery per se. We do, however, allow for a raised risk of complications with an emergency caesarean section following breech presentation compared with a vaginal breech delivery (nodes B_D2a and B_D2c). If ECV is not accepted, or fails, then elective caesarean section may be offered.
Outcomes relating to large for gestational age infants
Figure 14 shows the decision tree with outcomes relevant to LGA expanded and remaining branches collapsed. When LGA is suspected, the intervention given will be in accordance with the predetermined management strategy (IOL or expectant management) for both true-positive and false-positive LGA diagnoses. The management option will affect the likelihood of the delivery outcome, as well as the mode of delivery, which can be either vaginal or by emergency caesarean section. When LGA is not suspected, delivery can be either vaginal or by emergency caesarean section.
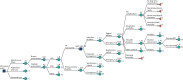
FIGURE 14
Outcomes associated with LGA. [+], collapsed sections of the decision tree.
Delivery outcomes include ‘no complications’, ‘respiratory morbidity’, ‘shoulder dystocia’, ‘other acidosis’ (i.e. acidosis not caused by shoulder dystocia) and ‘perinatal death’. The risk of each adverse outcome depends on the baseline risk, as well as on the mode of delivery, and whether or not labour was induced early.
Long-term outcomes depend on the outcome at delivery. For ‘no complications’, ‘respiratory morbidity’ and ‘other acidosis’, long-term outcomes included ‘no long-term complications’, ‘SEN’, ‘SNM’ and ‘neonatal/infant mortality’. For ‘no long-term complications’ the risk was equivalent to ‘no neonatal morbidity’ (node E1), and for ‘respiratory morbidity’ and ‘other acidosis’ the risk of long-term complications was equivalent to ‘severe neonatal morbidity’ (node E3). Shoulder dystocia (node L_E1) could result in no complications, brachial plexus injury (BPI) (node L_F1) or acidosis. BPI could be either transient or permanent (node L_G), the latter carrying the same risk of long-term outcomes as no neonatal morbidity (node E1) but with a penalty in terms of quality of life. Permanent BPI, SEN and SNM were long-term events; any other morbidity was expected to be resolved within the first year of life.
Outcomes relating to small for gestational age infants
Figure 15 shows the decision tree with the outcomes relevant to SGA expanded and the remaining branches collapsed. Labour will be induced early in suspected cases of SGA, whether based on a true or a false SGA diagnosis. Deliveries can be either vaginal or by emergency caesarean section. The probability of each mode of delivery is affected by whether or not labour was induced early. However, to avoid double counting the health effects of early labour induction, the mode of delivery affects only costs and not health outcomes.
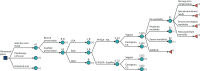
FIGURE 15
Outcomes associated with SGA. [+], collapsed sections of the decision tree.
Delivery outcomes include no, moderate and severe neonatal morbidity, as well as perinatal death. Women with correctly diagnosed SGA pregnancies (true positives) are offered early IOL, which reduces the risk of morbidity and mortality. When SGA is unsuspected (false negatives), pregnancies are managed expectantly, with no risk reduction. Note that early labour induction may also increase the risk of morbidity if initiated needlessly (i.e. in an AGA pregnancy falsely suspected of being SGA). However, in a true SGA pregnancy, early labour induction is expected to reduce the risk of morbidity. The scenario with a false-positive diagnosis is discussed further in Outcomes relating to appropriate for gestational age infants.
Long-term outcomes include ‘no long-term outcomes’, ‘SEN’, ‘SNM’ and ‘neonatal/infant mortality’. Each outcome is possible for all levels of neonatal morbidity. However, the risk of long-term complications increases for moderate and severe neonatal morbidity (nodes E2 and E3).
Outcomes relating to appropriate for gestational age infants
Figure 16 shows the decision tree with the outcomes relevant to AGA expanded and the remaining branches collapsed. An AGA fetus may be either correctly diagnosed or incorrectly diagnosed as either SGA or LGA (node B). If correctly diagnosed, the mode of delivery can be either vaginal or emergency caesarean section (node C1), after which short- and long-term outcomes will follow as described in Short- and long-term outcomes.
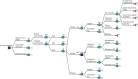
FIGURE 16
Outcomes associated with AGA. [+], collapsed sections of the decision tree.
If an AGA fetus is falsely diagnosed as SGA, early IOL is offered. Unlike in the case of a true SGA, early labour induction of AGA pregnancies increases the risk of morbidity; however, the risk of perinatal death is still reduced.160 Short- and long-term outcomes will then follow as described in Short- and long-term outcomes. If, instead, an AGA fetus is misdiagnosed as LGA, the short- and long-term outcomes depend on the management strategy. Compared with expectant management, early IOL decreases the risk of emergency caesarean section and perinatal death but increases the risk of neonatal morbidity.
Just as for other branches of the model, long-term outcomes include ‘no long-term outcomes’, ‘SEN’, ‘SNM’ and ‘neonatal mortality’. Each outcome is possible for all levels of neonatal morbidity; however, the risk of long-term complications increases for moderate and severe neonatal morbidity (nodes E2 and E3).
Data
We populated the model with data from multiple sources from the literature. Where possible, we prioritised the inclusion of good-quality systematic reviews and meta-analyses, followed by large, good-quality clinical trials or cohort studies, as appropriate. When there was no objective evidence for a parameter, we relied on expert opinion either to judge whether or not a study in a related area provided a sufficient proxy or to provide a central estimate and credible interval representing beliefs about plausible values for the parameter. Data sources were subjectively graded as high, moderate or low, where high represented directly relevant data (i.e. providing the required parameter) from a good-quality source (e.g. RCT for relative effects and high-quality epidemiological study for baseline risks). A low grade represents instances in which evidence on the required parameter was absent from the literature and so is sourced from a related parameter, used as indirect evidence and revised reflecting expert opinion as to the plausible values. Full details of the derivation of model inputs are provided in Appendix 6, Tables 25–30, and all parameters are listed in Tables 10–12.
TABLE 10
Model inputs for diagnostic performance
TABLE 12
Model inputs for costs and related probabilities
TABLE 11
Model inputs for probabilities
Probabilities
Where possible, probabilities were expressed as a baseline (beta or Dirichlet) for an otherwise healthy infant (i.e. neither breech nor LGA or SGA), they were then modified by odds ratios or relative risks, depending on the statistic either reported in, or calculable from, the literature. Odds ratios were selected in preference to risk ratios, as the former are independent of the baseline risk. Where no relative quantities were identified in the literature, probabilities are reported as independent beta distributions. Sampled values for probabilities were inspected to ensure that they were bounded between 0 and 1. Where out-of-range values were sampled, resampling was repeated until within-bounds values were generated.
Where relative effects were expressed as means and 95% CIs, standard error of the log of the mean was estimated by dividing the absolute difference between the log-mean and log-lower or -upper 95% CI by 1.96.
Costs
The price year used in the analysis is 2016/17. The majority of costs were sourced from the English national schedule of reference costs.175 The national schedule of reference costs reports different costs depending on how the service was delivered (e.g. elective inpatient, non-elective inpatient, outpatient procedures). We used costs from total Healthcare Resource Groups (i.e. weighted by each category by the number of yearly activities), except for cases in which only one or a few categories made logical sense. In all categories in the schedule costs were reported as mean and interquartile range. To obtain parameter estimates of costs, we fitted a gamma distribution using these data points. Where multiple cost categories were used, we first calculated a weighted average of the mean and interquartile range by the number of yearly activities in each category before fitting the gamma distribution.
Where no directly applicable cost could be identified from the reference schedule, we first attempted to obtain resource use from literature, and assign costs to this using the reference costs. When insufficient data on resource usage were available, we adopted the costs directly from the literature. Costs reported in currencies other than Great British pounds or in 2016/17 prices were converted to Great British pounds at the exchange rate of the year that the source was published and inflated to 2016/17 prices using the Hospital & Community Health Services (HCHS) index.184 Where no credible estimates could be identified from the literature, we estimated the costs ourselves, assigning a wide credibility interval to represent the uncertainty. Full details on the derivation of all cost parameters are presented in Appendix 6.
All costs presented in Great British pounds and updated to the cost-year of 2016–17 using the Hospital & Community Health Services Index:184 quality of life
We estimated age-specific quality of life for healthy neonates using EuroQol data for a general UK population.185 Age-specific health state utilities were multiplied by age-specific survival,186 the discounted sum over the time horizon of the model yielding the expected QALYs gained for an otherwise healthy neonate. Per definition, the quality of life following mortality is zero, and we made the simplifying assumption that all deaths during a particular year of life occurred on the first day of the year. In the absence of suitable evidence of how SEN affect quality of life, we assumed for our base-case scenario that SEN would affect costs only. In the case of SNM, we adjusted the baseline quality of life with a relative decrease following the methodology of Leigh et al.,187 using cerebral palsy (CP) as a proxy for SNM. Full details on the derivation of quality-of-life parameters are presented in Appendix 6.
Analysis
The model was analysed via Monte Carlo simulation, capturing the overall uncertainty in cost-effectiveness as a function of the uncertainty of the input parameters. Health outcomes were from the fetal perspective only and ultimately presented as QALYs. Cost-effectiveness was explored through incremental cost-effectiveness ratios (ICERs) and net monetary benefits (NMBs), using a WTP threshold of £20,000 per QALY. All costs and QALYs were discounted by 3.5% per annum.188 All costs were from a third-party (payer) perspective (i.e. NHS England plus SEN costs) and the reference case time horizon was 20 years (varied in sensitivity analysis).
Stability testing was conducted to quantify (and, therefore, minimise) Monte Carlo error as a function of the number of simulations. The model was run 30 times with a given number of simulations. The coefficients of variation of the estimates of the mean and standard error of the mean cost and QALYs for each comparator were calculated. The mean of all of these was used as a summary measure of the Monte Carlo error. We used an arbitrary 2% cut-off point to declare the results stable.
Cost-effectiveness: reference case
For each of the six discrete strategies, we present mean and 95% credibility intervals for cost and QALYs gained, net benefit at a WTP of £20,000 per QALY, and incremental net monetary benefit (INMB) relative to the assumed status quo (selective scanning with IOL for macrosomia or SGA, offer of ECV for breech). The option with the highest expected NMB was identified as the most cost-effective. Decision uncertainty was expressed as the probability that each decision would be cost-effective at the reference case threshold (i.e. £20,000/QALY). The cost-effectiveness acceptability curve plots decision uncertainty as a function of WTP per QALY (see Figure 17).
Cost-effectiveness: sensitivity and scenario analyses
In addition to the primary analysis, we report a number of scenario analyses and one-way sensitivity analyses to explore specific uncertainties in more detail. Specifically:
- Time horizon.
- The base-case analysis assumes a 20-year time horizon. We vary this from 1 to 100 years.
- Cost of scan to assess fetal presentation only.
- The cost of a presentation-only scan is dependent on whether it is feasible to incorporate the scan into a routine antenatal visit, with a midwife conducting it using a hand-held unit, or if it can be done only during a dedicated visit by an ultrasonographer in a secondary care setting.
- The baseline risks of perinatal death, moderate and severe neonatal morbidity.
- The baseline risks of each of these were estimated from different sources, yet they are mutually exclusive events. Ideally, these should be modelled as a Dirichlet distribution, but because the data were from different sources we modelled them as independent betas. We thus explore these further in a one-way sensitivity analysis.
In addition, because of concerns over the validity of input data, we also explore the difference in:
- the risk of acidosis and respiratory morbidity associated with vaginal delivery of a LGA infant (vs. AGA)
- the odds ratio of perinatal death resulting from delivery by emergency caesarean section of a breech infant (vs. vaginal delivery)
- the relative risk of an emergency caesarean section from IOL for a SGA infant (vs. expectant management of an AGA infant)
- the relative risk of SEN as a result of inducing labour (vs. expectant management), and the impact that IOL has on health-related quality of life, and the sensitivity of ultrasound scanning at detecting SGA.
Value-of-information analysis
Uncertainty in cost-effectiveness results (i.e. decision uncertainty) was used to conduct a VOI analysis.189 Decision uncertainty arises from parameter uncertainty. The EVPI is the expected value of eliminating all decision uncertainty, which by definition implies eliminating all parameter uncertainty. This therefore provides an upper bound for the value of all research into the decision question. The EVPPI is the expected value of eliminating uncertainty in a single parameter or group of parameters. The EVSI is the expected value of a study of sample size n. The EVSI of a study of size n less the cost of conducting it provides a measure of the expected return on investment in that research project [expected net gain of sampling (ENGS)].190–192 An EVPPI above the plausible cost of a research project is a necessary condition for future research to be economically viable. A positive ENGS is the sufficient condition. The efficient sample size of a study is that which maximises the ENGS.
We estimated that there are approximately 196,297 singleton births at ≥ 37 weeks’ gestation to nulliparous women that are not delivered by elective caesarean section each year. Assuming a time horizon for which the decision question remains valid of 10 years yields a (discounted) beneficial population of 1,689,663. If it is reasonable to assume that our analyses are generalisable to all births in England, the beneficiary population is 5,477,940.
We report the per-patient (i.e. per mother/infant dyad) and population EVPI at a WTP of £20,000 per QALY. We then report the per-patient and population EVPPI for each parameter individually, calculated using the Sheffield Accelerated Value-of-information (SAVI) tool.159 Parameters with a positive EVPPI were grouped into those that could logically be collected in one research study, and the EVPPI for that group of parameters was calculated (also with the SAVI tool159). The EVSI for any parameters or groups of parameters is then calculated using the method of Heath et al.193 Population values are presented as a ‘conservative’ estimate, assuming that the information is of value only to singleton nulliparous pregnancies (i.e. using the 1,689,663 beneficiary population) and a broader estimate that assumes the information is of value to all pregnancies in England (5,477,940 population).
Results
Stability testing
Our analyses showed that we were able to achieve extremely stable results (coefficient of variation of < 0.01%) with 100,000 simulations, at a ‘reasonable’ run time of around 30 seconds (Table 13). We therefore ran our cost-effectiveness analyses with 100,000 simulations. However, because of the need for repeated loops, the EVSI calculations are based on 10,000 simulations.
TABLE 13
Results from stability testing
Cost-effectiveness results
Table 14 shows the overall costs, QALYs, net benefit and incremental net benefit for each of the six screening management strategies. Net benefit is calculated assuming a WTP of £20,000 per QALY gained. INMB is shown relative to the status quo (assumed selective ultrasound scanning and IOL for both suspected SGA and LGA). Strategies are ordered in terms of increasing cost.
TABLE 14
Cost-effectiveness results (per woman scanned)
Given current evidence, and assuming a WTP of £20,000 per QALY, the strategy associated with the highest net benefit is a presentation-only scan for all women (where women with relevant indications also get a full scan). When LGA is suspected, the recommended management is IOL; on average, IOL is associated with a small improvement in QALYs compared with expectant management (SGA is assumed managed with IOL). Universal ultrasound screening for fetal size is not supported by this analysis as its added benefits do not justify its added cost. Decision uncertainty suggests that there is a 44.19% probability that this is the most cost-effective strategy (Table 14 and Figure 17).
One-way and scenario analyses
Cost-effectiveness conclusions were sensitive only to the time horizon, the cost of an ultrasound scan for fetal presentation only, the background risk of stillbirth, moderate and severe perinatal complications, and the risk of SEN associated with IOL.189
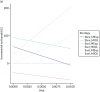
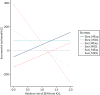
With respect to the time horizon, universal ultrasound for fetal presentation is the most cost-effective option only as long as the time horizon of the analysis is < 45 years (Figure 18). Beyond this time horizon, universal ultrasound for size and presentation becomes the most cost-effective option. With respect to the cost of a presentation scan, a presentation-only scan remains the most cost-effective option, provided that this costs no more than £90. Above this cost, status quo is the most cost-effective (Figure 19).
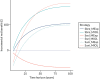
FIGURE 18
One-way sensitivity analysis of model time horizon. MExp, expectant management; MIOL, IOL; Sbre, universal ultrasound for fetal presentation only; Ssel, selective ultrasound; Suni, universal ultrasound for fetal biometry plus presentation.
As the background risks of perinatal mortality, moderate and severe perinatal complications rise, the net benefit of a detailed universal scan rises (Figure 20). This is because the risks of complications from SGA and LGA infants are modelled relative to the baseline risks; as the baseline risk rises, the risks for SGA and LGA infants rises more than proportionately, thus the benefit from detection and intervention rises. A breech-only scan remains the most cost-effective option so long as the baseline risk of perinatal death remains < 0.28% and the risk of moderate and severe complications is < 4.8% and < 1.12%, respectively. Above these values, universal screening becomes the cost-effective option.
Our base-case analysis assumed a linear progression through the model whereby long-term outcomes were dependent on perinatal outcomes, which were dependent on mode of delivery alone [vaginal vs. caesarean section (emergency or elective)]. However, there is evidence to suggest that IOL may increase the risk of SEN in later life.172 We therefore explored the impact on the results via a one-way sensitivity analysis. We found that our results remained the same as long as the relative risk of SEN as a result of IOL is between approximately 0.95 and 1.3 and the estimated risk at 38 weeks’ gestation was within this range.172 Below this risk, the most cost-effective strategy is to perform universal screening for both presentation and EFW, and to induce labour when SGA or LGA is suspected. Above this risk, then while the recommended scan remains a presentation-only scan, the most cost-effective intervention for suspected SGA or LGA is expectant management (i.e. IOL ceases to be the appropriate intervention; Figure 21). Given this, although not captured in our formal VOI analysis (because of structural assumptions), it may be worthwhile exploring the impact that inducing labour has on long-term risk of SEN in future research.
Figure 18 shows the expected INMB for different strategies compared with current practice (selective ultrasound with IOL for suspected LGA) as a function of the model’s time horizon (years). Calculations are based on a WTP (i.e. valuation of one additional QALY) of £20,000.
Figure 19 shows the expected INMB for different strategies compared with current practice (selective ultrasound with IOL for suspected LGA) as a function of the cost of an ultrasound for fetal presentation only. Calculations are based on a WTP (i.e. valuation of one additional QALY) of £20,000.
Figure 20 shows the expected INMB for different strategies compared with current practice (selective ultrasound with IOL for suspected LGA) as a function of the baseline risk of perinatal mortality (see Figure 20a), severe neonatal morbidity (see Figure 20b) and moderate neonatal morbidity (see Figure 20c). Calculations are based on a WTP (i.e. valuation of one additional QALY) of £20,000.
Figure 21 shows the expected INMB for different strategies compared with current practice (selective ultrasound with IOL for suspected LGA) as a function of the relative risk of SEN if labour is induced early (compared with expectant management). Calculations are based on a WTP (i.e. valuation of one additional QALY) of £20,000.
Value-of-information analysis
Expected value of perfect information
At a WTP of £20,000 per QALY, the per-patient EVPI is £31.56. Given a beneficiary population of 1,689,663, the population EVPI to England is £53.3M. If the results of the analysis are assumed generalisable to all pregnancies in England, then the population EVPI is £172.9M. Figure 22 shows the per-patient EVPI as a function of the WTP threshold. The two local peaks indicate where the decision (i.e. which screening strategy is preferred) changes, and, thus, the impact of decision uncertainty is greatest around these thresholds.
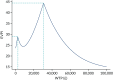
FIGURE 22
Per-patient EVPI as a function of the WTP for an additional QALY. EVPI is presented per person. WTP refers to monetary valuation of an additional QALY (£).
Expected value of perfect parameter information and expected value of sample information
Table 15 shows the parameters with an EVPPI exceeding £100,000 under the broader assumption that any future study will be of value to all births in England, not just low-risk singleton pregnancies. The most valuable parameter is difference in cost of delivery from IOL, accounting for 84% of the EVPI. Except for this cost, no other parameters individually account for > 1% of the total EVPI. The other parameters with the greatest contribution to EVSI are the relative risk (LGA vs. AGA) of acidosis from a vaginal delivery following IOL, the odds ratio of perinatal death (LGA vs. AGA) from an infant delivered vaginally without IOL, the relative risk (SGA vs. AGA) of emergency caesarean section following IOL and the odds ratio (SGA vs. AGA) of severe neonatal morbidity under expectant management.
TABLE 15
The expected value of partial perfect information for individual parameters and groups of parameters
These five parameters could naturally be collected from three separate studies:
- a costing study of the difference in cost of delivery associated with IOL compared with expectant management
- a RCT of delivery outcomes relating to LGA babies
- a RCT of delivery outcomes relating to SGA infants.
The EVPPI of the costing study is either £44.8M or £145.2M, depending on whether the results are considered applicable to singleton nulliparous pregnancies only or to all pregnant mothers, respectively. The two RCTs have EVPPIs of up to £3.9M and £1.4M under the broader applicability criteria.
The EVSI of the costing study suggests that there is scope for the study to yield a positive return on investment. For example, a two-arm study with 1000 patients in each arm has an EVSI to England of £11.3M (or £97.2M if this information is of value to all pregnancies in England, not just to low-risk nulliparous singleton pregnancies). If such a study was to cost £1M, then it would yield a net return on investment of at least £10.3M (Figure 23).
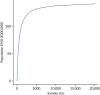
FIGURE 23
Population EVSI for a study on the cost of IOL.
We were not able to calculate non-zero EVSI estimates for studies on macrosomia or SGA outcomes as the per-patient EVPPI is too low.
Expected value of perfect parameter information under alternative scenarios
The EVPPI provides the value of obtaining perfect information for a parameter based on the magnitude at which perfect information would affect the decision outcome. This means that even parameters that have a great impact on overall cost and QALYs, and for which the value is highly uncertain, may have low EVPPI if perfect information would not change the decision (i.e. which screening strategy is most cost-effective). However, whether or not the exact value of a parameter affects the decision outcome is highly dependent on context. Through simulating alternative scenarios, we analysed how the EVPPI of key parameters was affected by model assumptions.
Given the uncertainty about the setting in which an ultrasound scan for fetal presentation only could be provided, there were some concerns that the cost was not correctly specified in the base-case scenario. We therefore simulated three alternative scenarios where we varied the assumptions underlying the cost calculations: (1) fetal presentation could be assessed through directly accessed diagnostic services (£52, 95% CI £24 to £91), (2) an antenatal standard routine ultrasound scan was required (£108, 95% CI £97 to £118) and (3) costs could range between those of either of these scenarios (£24–118). The results showed that EVPPI was highest where the cost was highest. In this scenario, the EVPPI was £6.07 per person. Depending on the beneficial population, the overall EVPPI was £10.3M (nulliparous women only) or £33.3M (all women). It is worth noting that the model’s assessment of the value of further studies is, in this case, at odds with cost-effectiveness. A higher cost for scanning means a lower chance that ultrasound for fetal presentation will be cost-effective, but the value of researching this parameter further increases.
The cost of IOL (specifically, the net difference in total cost between pregnancies that were induced early and that of expectant management) had the highest EVPPI in our base-case scenario, and hence the greatest expected benefit from future research. In the base-case scenario, the cost was £125 (95% CI –£1343 to £1594); more details are presented in Appendix 6. To test how sensitive the EVPPI was to the exact input values used, we simulated two alternative scenarios: (1) where the standard error of the mean was reduced by 50% and (2) where costs were instead obtained from the 35/39 trial,194 where the cost difference was –£236 (95% CI –£646 to £174);194 see Appendix 6 for details. When the standard error was reduced by 50%, the EVPPI fell by ≈ 80%. When costs were obtained from the 35/39 trial, the EVPPI was £6.3M for the beneficial population (i.e. nulliparous women).
Discussion
Main findings
This study has evaluated the cost-effectiveness of alternative screening strategies for ultrasound in the third-trimester in a population of low-risk nulliparous women. Based on current information, and assuming a WTP of £20,000 per QALY, offering a universal ultrasound presentation-only scan is, on average, the most cost-effective strategy. This is associated with an INMB of £87.36 (95% CI £4.88 to £205.68) per pregnancy compared with current practice. Scaled up to the English population, this equates to an added net benefit of £17.1M or 857 QALYs per annual birth cohort. This is the present value of the future flows of expected costs and benefits over a time horizon of 20 years.
Third-trimester scans for fetal size should take place only where clinically indicated. We estimate that the added benefits of including estimation of fetal weight in the scan may not justify the added cost; more health would be lost elsewhere than would be gained from the added knowledge and subsequent management from these scans. When LGA is suspected following ultrasound, early IOL is the preferred management irrespective of whether screening is offered routinely or following clinical indication.
It should be noted that the presentation-only scan policy implies an increased burden on those performing the scan, but that this is partially offset by reductions in the cost of complications from delivery. Implementation would therefore require a reallocation of resources away from delivery and towards antenatal care or ultrasonography.
Owing to uncertainties in the evidence base (parameter uncertainty), there is a only a 44% probability that this screening strategy really is the most cost-effective (i.e. there is a 56% probability that this conclusion is incorrect, in which case a loss will be incurred). The expected loss associated with this decision uncertainty is £31.56 per pregnancy. Equivalently, this is the expected gain if uncertainty were to be eliminated (EVPI). Scaled up to the population of England who could benefit from the information from any future studies, this equates to an EVPI of £53.3M. If it is assumed that the results of any future study are generalisable to all pregnancies in England, the EVPI is £172.9M.
The net difference in cost between an induced delivery and expectant management was the parameter that had the largest impact on decision uncertainty in the base-case scenario, and hence this is the parameter that should be prioritised in future research. It should be noted that this does not relate simply to the cost of a procedure to induce delivery; included in this definition is uncertainty about the timing of induction, and the impact on, for example, antenatal appointments, as well as the cost of the delivery itself. A study of ‘reasonable size’ to reduce uncertainty in this parameter is likely to yield a positive return on investment. For example, the EVSI of a study with 1000 women in each arm is worth in excess of £11M. If this was to be delivered at a cost of £1M, it would yield a > 10-fold return on investment. Alternative scenarios found that the value of future research may be less than for the base-case scenario. Nonetheless, although the exact value of future research is hard to determine, the net cost of labour induction appears influential on which screening strategy is the most cost-effective. Of note is that studies on the outcomes for SGA or LGA fetuses are unlikely to yield a positive return on investment based on the model.
Our base-case scenario showed very limited value in further researching the cost for which an ultrasound scan for fetal presentation only can be provided. However, this was because the model deemed a policy of universal ultrasound for fetal presentation so cost-effective that the cost of the scan was unlikely to change which policy is preferred; one-way sensitivity analysis showed that, all else being equal, the cost of a presentation scan would need to exceed £90 before another screening strategy was likely to be more cost-effective. In practice, the cost for which universal ultrasound for fetal presentation only could be provided is uncertain, mainly because it is unclear which type of clinical setting would be required for the scan. Therefore, prior to any roll-out, it is essential to establish whether, for example, midwives can be trained to perform the presentation-only scans and find it feasible to incorporate them into routine antenatal visits, or these scans can be carried out in a secondary care setting only.
The results described above relate to a WTP threshold of £20,000 per QALY. At a threshold of £30,600 per QALY (just above the upper threshold of NICE’s stated acceptable range of £20,000–30,000188), universal scanning becomes the most cost-effective option. Furthermore, our one-way sensitivity analyses suggest that there is scope for universal scanning to be cost-effective under other assumptions. For example, the most cost-effective option remains a breech-only scan as long as the time horizon of the analysis is below 45 years only. The ideal time horizon for an economic evaluation should be sufficient to capture all relevant differences in cost and outcomes.188 In many cases this implies a lifetime horizon;195 however, our base-case analysis was limited to 20 years. This represents a compromise between the desire for a long time horizon and the inherent uncertainties in extrapolating relatively short-term data into long-term outcomes. We therefore acknowledge the possibility that universal ultrasound scanning may be cost-effective in the long run, but we would urge caution in any recommendation of such.
Finally, all else being equal, presentation-only scan is the most cost-effective option provided that it can be accomplished for < £90 per scan. This is a higher price than we estimated in our previous work, which estimated a maximum cost-effective price of a presentation scan of approximately £20.11 This difference is due to the more detailed modelling in this analysis; where the previous analysis based QALY gains on mortalities averted and a set life expectancy, this analysis included the impact that morbidity has on costs and quality of life, and incorporates explicit survival functions.
Strengths and limitations
By incorporating several conditions detectable by ultrasound screening into one decision model, this study was able to assess the overall effect that the introduction of universal ultrasound may have on a population of nulliparous women. It also enables an assessment of the impact that introducing such a programme would have on the NHS budget and whether or not it is likely to represent good value for money. Furthermore, by incorporating a VOI analysis, this study has the potential to assess not only where the current gaps are in the evidence base for evaluating the use of universal ultrasound screening, but also for which of these gaps future research would have the greatest potential of finding meaningful results.
A key limitation of this study is that only fetal outcomes were considered, excluding the outcomes of the mother. Maternal outcomes may also be significant. Furthermore, the well-being of mother and child are sometimes at odds with each other, and clinical decisions frequently involve a trade off between the two. Incorporating maternal outcomes into the analysis, therefore, could have an impact on both the cost-effectiveness of the different strategies (in either direction) and our VOI analyses guiding where future research could be prioritised. However, as per our original protocol, maternal health consequences were not incorporated in this study. The primary justification for this is the lack of sufficiently reliable evidence of how screening outcomes may affect maternal quality of life. We have previously emphasised the need for further research in this area, particularly surrounding long-term maternal consequences from mode of delivery,11,155 and repeat that call here.
Throughout the development of the simulation model, we have attempted to capture clinical probabilities and their uncertainties as accurately as possible. However, uncertainty persists for many parameters, not only over their exact value, but also about how well suited these are for the new decision context. Essentially, this creates two separate types of uncertainties. The internal validity is well captured in the model through the incorporation of parameter uncertainty as quantified by the authors of the respective source. However, there is also the question of external validity (i.e. the extent to which that parameter is suitable for our model), which is uncaptured by the model. This means that the true uncertainty of our results is likely to be greater than that expressed in the CIs of the outputs. Although this does not invalidate the model as a tool for decision-making, it means that thoughtful interpretation of the results is needed, and that such interpretation should always acknowledge the inherent uncertainty involved in combining data from different sources.
Through its focus on breech presentation, SGA, and LGA only, this analysis may have underestimated the merits of universal ultrasound. Such a screening programme would also increase the chances of detecting otherwise unknown complications (e.g. previously undetected congenital anomalies or placenta praevia). Although these are less prevalent than the conditions included in this analysis, the potential to detect such complications could be an added benefit of introducing a universal ultrasound programme. However, it is important that subsequent management of other such complications follows protocols that have taken the diagnostic performance of ultrasound into account. If the risk of false-positive diagnoses is high, and if the consequences are severe, the introduction of universal ultrasound risks putting patients in a worse position than they would have been in without screening.
The outcomes of economic modelling and especially VOI analysis are highly sensitive to the structural assumptions that underlie the simulation model. Throughout this analysis, we have attempted to model the potential outcomes of screening using parameters for which credible data are available. Where parameter uncertainty has been wider, the expected value of future research is generally greater. However, this approach has required us to be able to incorporate a parameter into the model structure. The problem has been capturing effects that we suspect exist but for which no evidence has been available.
In this analysis, we modelled the risk of long-term outcomes, such as SEN, as a function of neonatal morbidity. This means that clinical interventions that can alleviate neonatal morbidity are also expected to alleviate the risk of SEN. Similarly, interventions that do not affect neonatal morbidity will have no impact on the risk of SEN. However, this may not accurately capture how interventions affect the risk of SEN. This model structure has been adopted because of data limitations and to avoid overestimating the effect of intervention.
There is some evidence that the risk of SEN increases with early IOL, and the perceived risk of this is often influential in the clinical decision of whether or not to induce labour early. Our model structure captures long-term effects on SEN from early IOL if it is mediated through neonatal morbidity. However, if there is a direct link between gestational age at delivery and the risk of SEN that is not mediated through neonatal morbidity, this is uncaptured in the model. One-way sensitivity analyses exploring this suggest that our results hold as long as the risk of SEN associated with IOL (vs. expectant management) is below approximately 1.34. Above this, the recommendation for a presentation-only scan holds, but inducing labour for LGA is no longer recommended. If it is plausible that the increased risk of SEN associated with IOL exceeds 34%, then it may be worthwhile exploring this in future research. However, observational data indicate that delivery at 38 weeks’ gestation is associated with < 34% increase in risk.172
Although macrosomia and SGA are mutually exclusive by definition, we assumed that breech presentation was also mutually exclusive with SGA and LGA. This simplification was used because data constraints would not allow a credible estimation of risk adjustments for fetuses who were both breech and SGA/LGA, and for structural simplicity of an already complex model. It was also considered likely that breech presentation would be a stronger determinant of possible clinical interventions than fetal size. Relaxing this assumption would, in practice, have the same effect in the model as a slight increase in the prevalence of SGA and LGA; however, the effect of this would be limited given the low prevalence of breech presentation and SGA/LGA.
The conclusions of our economic analysis, and especially of the VOI analysis, depend heavily on the exact data used to capture parameter uncertainty in the economic model. However, accurately capturing the uncertainty of a parameter in the light of all current evidence is far from straightforward. For many parameters, alternative sources were available, and the combined parameter uncertainty for multiple studies is theoretically smaller than for just the one study. Ideally, every input parameter in the model should be subject to a meta-analysis. However, because of the large number of parameters in the model, this was not feasible. Furthermore, in many cases, we suspected that the difference in parameter values between studies was the result of different clinical definitions rather than reflective of the true parameter uncertainty. To address this issue, we conducted extensive one-way sensitivity analyses.
We modelled acidosis risk as that secondary to shoulder dystocia as well as ‘other acidosis’. No sources disaggregated that attributable to shoulder dystocia from that attributable to other causes. We may therefore have overestimated the risk of acidosis as a result of double counting. However, our sensitivity analyses suggested that the base-case results were insensitive to this parameter.
Comparison with other studies
A previous review of studies of universal ultrasound assessment during late pregnancy found no clear benefit of universal ultrasound.21 In this study, we have found that universal ultrasound may be associated with better clinical outcomes. Whether or not universal screening is cost-effective, however, depends on the features included in such a scan. Our analysis shows that universal ultrasound for fetal size is unlikely to be cost-effective, unless the valuation of additional health is higher than that recommended by current UK guidelines.188 By contrast, universal ultrasound for fetal presentation alone is likely to be cost-effective, although uncertainty persists over whether or not fetal presentation can be assessed sufficiently cheaply using ultrasound to make such a screening policy feasible.
Furthermore, the findings also align with our cost-effectiveness analyses of universal ultrasound for individual complications only. When exploring the cost-effectiveness of universal ultrasound for breech presentation only, we found that whether or not such a screening programme could be cost-effective largely depended on the price at which fetal presentation could be detected.11 It seemed unlikely that screening for SGA or LGA only would be cost-effective, but we highlighted that the effectiveness of labour induction was uncertain and may warrant further research. This joint analysis confirms these findings, and has allowed us to point more specifically towards those parameters for which further research may have a meaningful impact on the decision problem.
Implementation considerations
The purpose of this study has been to make recommendations on screening policy based on our current understanding of the evidence base, to identify the current gaps in the evidence and to provide recommendations about which of these gaps should be addressed to allow future policy-making about late-pregnancy ultrasound in the relevant population. We speculate that late-pregnancy ultrasound screening for fetal presentation only could be provided by midwives as part of a routine antenatal assessment. Such a screening setting has obvious benefits for the patient, as an extra appointment (typically in a secondary care setting) could be avoided, saving time and travel costs for women and possibly their partners as well. However, an ultrasound scan in this context would not also assess fetal biometry. It is important that the introduction of such a screening programme into NHS routine care would not expand the scope of this scan beyond assessing fetal presentation, as this may lead to unnecessary intervention. Another potential problem for the NHS would be the implied relocation of budget between units. Although universal ultrasound in a primary care setting may be cost-effective for the NHS as a whole, in practice this would put extra financial strain on primary care, whereas the benefits would mostly arise from the avoidance of complications following delivery. To be successful, the implementation of such a screening policy would need to be accompanied by a suitable reallocation of budget from the benefiting units into primary care.
The consequences of future research are likely to go beyond the perspective employed in this analysis. First, our analysis focused on nulliparous women with singleton pregnancies, but, for many parameters, reducing uncertainty would be helpful to women regardless of parity. To address this, we provided two population values of information: one based on nulliparous singleton pregnancies and the other based on all pregnancies. Second, the scope of our study was limited to England, but many findings are likely to be just as applicable to the rest of the UK, and indeed to other high-income countries as well. If the VOI analyses are considered applicable to the entire UK, the EVPI, EVPPI and EVSI figures should be multiplied by approximately 25% to reflect this (England accounts for approximately 80% of the UK population). Third, the economic perspective of this study was NHS England and education services only, but many consequences would go beyond this. For instance, it has been estimated that the majority of the costs associated with stillbirth and CP are indirect (e.g. from decreased productivity, extra monitoring for subsequent pregnancies and mourning181,183,196). When considering such perspectives, both the attractiveness of universal ultrasound and the value of future research are likely to increase.
Conclusions
The remit of this work was to advise the National Institute for Health Research on the current body of evidence regarding the cost-effectiveness of late-pregnancy ultrasound screening and specifically whether or not there is value in commissioning further research in the area and, if so, what this research should focus on.
Our results suggest that universal ultrasound for fetal presentation only may be both clinically and economically justified, but implementation research is needed before it is adopted into routine care. Specifically, this must explore whether or not a scan can be conducted by a midwife during a routine antenatal visit. Universal ultrasound including estimation of fetal weight is of borderline cost-effectiveness and is sensitive to certain assumptions. Our formal VOI analysis suggests that future research should be focused on the net cost of IOL compared with expectant management.
- Economic analysis of universal versus selective ultrasound screening in late-sta...Economic analysis of universal versus selective ultrasound screening in late-stage pregnancy: cost-effectiveness and value-of-information analyses - Universal late pregnancy ultrasound screening to predict adverse outcomes in nulliparous women: a systematic review and cost-effectiveness analysis
- VISS syndromeVISS syndromeMedGen
- C5561955[conceptid] (1)MedGen
Your browsing activity is empty.
Activity recording is turned off.
See more...