Diabetes in America is in the public domain of the United States. You may use the work without restriction in the United States.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Cowie CC, Casagrande SS, Menke A, et al., editors. Diabetes in America. 3rd edition. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018 Aug.
Summary
The vast majority of the U.S. adult population suffers from periodontal diseases, as about 90% suffer from the reversible form, gingivitis, whereas almost 50% of adults age ≥30 years are affected by periodontitis, which is the chronic periodontal breakdown of both soft and hard tissues that support the teeth. Diabetes prevalence is assuming epidemic proportions with 30.2 million or 12.2% of the U.S. adult population age ≥18 years having diabetes in 2015, of whom about one-quarter are unaware of their diabetes; an additional one-third (84.1 million) have prediabetes, of whom about 90% are unaware. Due to the great prevalence of both diseases, it is important for health care professionals and lay people alike to be aware of both periodontal diseases and diabetes and their two-way interactions in which they mutually and adversely affect each other to understand how to prevent, treat, and manage both diseases. Since periodontitis and diabetes share the same risk factors, any improvement in a risk factor should beneficially affect both conditions. This concept is shown by a decrease in the level of inflammation upon nonsurgical periodontal treatment, which improves both periodontal health in individuals with type 2 diabetes and those without diabetes, as well as glycemic control in type 2 diabetes and in people with prediabetes.
Gingivitis is a reversible inflammation of the soft tissues surrounding the teeth in response to dental plaque, whereas periodontitis is a chronic, inflammatory disease that in response to dental plaque causes breakdown of the soft and hard tissues surrounding the teeth in susceptible individuals. This destruction often occurs without any pain or other symptoms. Nonetheless, if left untreated, it can lead to loosening of the tooth and eventually to its total loss, with adverse effects on nutrition, self-esteem, and function. Moreover, the number of teeth lost is strongly associated with atherosclerotic cardiovascular disease, and advanced tooth loss, especially edentulism (loss of all teeth), is associated with premature all-cause mortality.
Periodontitis causes local and systemic inflammatory responses that lead to development or worsening of hyperglycemia and hence contribute to increased blood glucose levels in healthy individuals; development of prediabetes, type 2 diabetes, and gestational diabetes; decreased glycemic control in overt diabetes; and worsening of diabetes complications.
Diabetes is also a chronic, inflammation-related metabolic disease diagnosed by hyperglycemia. Such elevated blood glucose levels negatively impact the inflammatory response to dental plaque, leading to more severe gingivitis and periodontitis. Hence, periodontitis and diabetes mutually and adversely affect each other. Importantly, the risk factors are largely identical for these two diseases, so when identifying and improving risk factors related to one of the two diseases, the other could be present and its severity lessened. Such improvements could consist of quitting smoking, decreasing intake of added sugar, reducing any inflammation, and getting sufficient sleep at healthy times per the circadian rhythm. Routine, nonsurgical, periodontal treatment (“deep cleaning”) that can be performed by dental health care professionals in general dental practice or in periodontists’ specialty offices—together with proper home oral hygiene care—can lead to improved glycemic control in type 2 diabetes.
Hyperglycemia can also contribute to impaired healing of lesions around the apex (tip) of the teeth with chronic infection and inflammation persisting in the jaw bone. Extraction of teeth that suffer from chronic periodontitis or periapical periodontitis leads to decreased levels of inflammatory biomarkers. Moreover, diabetes and the use of diabetes medication can lead to dry mouth, which contributes to development of caries, periodontitis, and thrush (candidiasis). Diabetic neuropathy can lead to burning mouth syndrome (glossodynia) and taste impairment (dysgeusia) and may be involved in trigeminal nerve pain and temporomandibular joint disorders. Both periodontitis and diabetes lead to potentially severely diminished quality of life. Nonetheless, people with diabetes have fewer dental visits than their peers without diabetes.
It is important for both dental and medical care providers to keep in mind the possible coexistence of periodontitis and dysglycemia (hyperglycemia), as both diseases negatively affect each other. Proper, mutual referral is essential, as both diseases can be improved, if the informed providers collaborate in a patient-centered, interprofessional team approach in the interest of the best possible oral and systemic health for their mutual patients.
Therefore, it may make both medical and financial sense to include the attainment of a healthy mouth in diabetes management, as well as screening for diabetes in the dental office, with the potential for substantial decreases in the burden of both human disease and suffering, as well as financial costs, to the benefit of the individuals, their caregivers, and society overall.
Introduction
The purpose of this chapter is to present scientific evidence for the links between oral health and diabetes in the United States and, thereby, attract attention to the importance of these relationships and ultimately incorporate such knowledge into the practice of both dentistry and medicine. This goal is accomplished by first introducing periodontal diseases and presenting scientific evidence for the two-way associations between these diseases and diabetes/hyperglycemia, including: (a) the effect of periodontitis on glycemic control in people with and without diabetes and diabetes complications; (b) the effect of nonsurgical periodontal treatment on glycemic control in prediabetes or diabetes; (c) the effect of diabetes/hyperglycemia on periodontal health; and (d) underlying mechanisms. Second, the chapter presents evidence regarding the effect of diabetes/hyperglycemia on other oral diseases and conditions, including: (a) tooth loss and root fragments; (b) tooth eruption; (c) caries; (d) dry mouth; (e) candidiasis (thrush); (f) burning mouth syndrome; and other oral conditions. Finally, associations between dental care utilization, medical care utilization, and medical care costs in people with diabetes who have received periodontal therapy; the need for interprofessional, patient-centered collaboration in diabetes management; and the public health significance of the oral health-diabetes links are discussed.
A major focus of this chapter is periodontitis, because it is the oral disease that is most prevalent, potentially fatal, and also most closely related to diabetes in a mutually adverse (two-way, bidirectional) manner. Diabetes, especially poorly controlled diabetes, has long been considered a risk factor for periodontitis (1,2,3). In 1993, periodontitis was declared the likely sixth complication of diabetes by Harald Löe, then Director of the federal National Institute of Dental Research (4), but not until about two decades later has the medical community begun to take notice. Only since the latter half of the 1990s has scientific evidence emerged to support periodontal infection as a risk factor for higher blood glucose levels, poorer glycemic control, and certain diabetes complications and, hence, adversely affecting diabetes outcomes; that is, an effect in the opposite direction (5). Consequently, the effects are mutual and result in a two-way relationship between periodontitis and diabetes (6,7,8). Evidence from U.S. studies is described and briefly supplemented by evidence from studies conducted in other countries when U.S.-derived evidence is sparse or absent.
Illustrations that do not cite any previously published source show results from original analyses of data from the National Health and Nutrition Examination Survey (NHANES) 2009–2010 and 2011–2012 cycles conducted specifically for Diabetes in America, 3rd edition. Only dentate participants who were age ≥30 years were eligible for the NHANES oral examination. Hence, data from the edentulous (edentate, no natural teeth) and those who due to medical contraindications (e.g., pregnant women) or for other reasons did not participate in the periodontal probing examination were not included.
Source and Limitations of Data on Diabetes and Oral Health
National Health and Nutrition Examination Surveys 2009–2012
NHANES 2009–2012 data allow for analysis of relationships between diabetes status and several oral diseases and conditions, including periodontitis, tooth loss, and presence of retained root fragments. NHANES uses independent, known probability samples that produce nationally representative estimates for the civilian noninstitutionalized U.S. population. The survey is conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC) to assess the health and nutritional status of adults and children in the United States. Each year, the NHANES examines a nationally representative sample of about 5,000 persons living in 15 counties across the country. The NHANES interview includes demographic, socioeconomic, dietary, and health-related questions. The examination component consists of medical, dental, and physiological measurements, as well as collection of blood and urine samples for laboratory tests. All protocols and data are publicly available (9).
Data from the 2009–2010 and 2011–2012 2-year cycles of the NHANES, hereafter referred to as “NHANES 2009–2012,” were analyzed specifically for this chapter using the following criteria to define diabetes, prediabetes, and normal glucose levels: 1) Diabetes was defined by self-report of previously being diagnosed by a physician; otherwise having: glycated hemoglobin (A1c) ≥6.5% (≥48 mmol/mol); or fasting (8–<24 hours) plasma glucose (FPG) ≥126 mg/dL (≥6.99 mmol/L); or plasma glucose ≥200 mg/dL (≥11.10 mmol/L) at 2 hours after a 75 g glucose load (oral glucose tolerance test [OGTT]). 2) Prediabetes was defined as self-report of not being diagnosed previously with diabetes by a physician and either A1c 5.7%–6.4% (39–46 mmol/mol); or FPG 100–125 mg/dL (5.55–6.94 mmol/L); or plasma glucose level of 140–199 mg/dL (7.77–11.04 mmol/L) at a 2-hour OGTT. 3) Participants were classified as having normal glucose levels, i.e., having normal glucose tolerance (NGT) or being normoglycemic, if they had A1c <5.7%, FPG <100 mg/dL, and plasma glucose <140 mg/dL at a 2-hour OGTT.
Major strengths of the NHANES 2009–2012 are that (a) each 2-year cycle includes a large, nationally representative sample of the civilian noninstitutionalized population in the United States and (b) remarkably, the protocol for these cycles of the NHANES includes for the first time a comprehensive clinical periodontal assessment at six sites around all (maximally 28) natural teeth, except for the often abnormally shaped or partly erupted wisdom teeth (third molars) that prevent exact measurements and often are removed or congenitally missing. Such comprehensive periodontal probing data allow application of periodontitis case definitions to calculate the most accurate prevalence estimates in the history of the NHANES (and population-based examinations globally). Prior NHANES protocols used periodontal probing at only two or three sites and probed only the buccal side (facing the face or cheeks, not the tongue or roof of the mouth) of the teeth in half of the upper and half of the lower jaw. Therefore, substantial numbers of participants have been misclassified due to disease missed. In fact, the analyses of NHANES data collected prior to the 2009–2010 cycle underestimated the true prevalence of periodontitis in the U.S. population by up to 54% (10). The reason that any disease recorded in only part of the mouth cannot reliably be multiplied by any factor to represent the entire mouth is that periodontal breakdown does not follow any symmetric pattern in a dentition (the full complement of teeth in an individual). Periodontal breakdown occurs at specific, individual sites, with much variation among the teeth in a person and even among different sites around the same tooth.
Limitations are: 1) the cross-sectional survey design limits the ability to make causal inferences; 2) the NHANES data do not permit distinction between the various types of diabetes; because type 2 is the most common, results are often interpreted as pertaining largely to type 2 diabetes, but should probably be ascribed to hyperglycemia; 3) periodontal examinations were limited to adults age ≥30 years; 4) the lack of scoring gingival bleeding on periodontal probing (BOP) prevents the assessment of gingivitis, the reversible gingival inflammation; 5) mucosal lesions were not recorded; 6) salivary function was not assessed; and 7) examination of dental caries experience, which includes both active caries and restored (filled, crowned) caries lesions, was limited to participants age 3–19 years and, hence, was not conducted in the population in whom periodontitis measures were performed. Nonetheless, the presence of root fragments of each permanent tooth was recorded in participants age ≥30 years. Such remaining root fragment usually indicates gross caries (large cavities) with near complete breakdown of the crown of the tooth, namely the part that is covered by tooth enamel and is visible above the gum line, although it is not possible to know whether such breakdown was caused by severe caries on the crown or on the root. Trauma could potentially also be the cause in rare cases.
Periodontitis: Introduction
Measurements and Definitions
Periodontal disease, referred to as “gum disease” or “pyorrhea,” is an inflammation of the soft or hard tissues surrounding the teeth that is initiated by the bacteria in the dental plaque located on the teeth near the gum line and promoted by the host immune system. Periodontal diseases comprise various forms. Gingivitis is the reversible form characterized by swollen, reddened, and possibly bleeding gums, affecting only the soft tissue around the tooth. Gingivitis is an inflammatory reaction caused by bacterial biofilm, dental plaque, that accumulates on teeth adjacent to the gums (gingiva) (11,12). Chronic periodontitis is a more severe form that irreversibly affects both soft tissue and bone support around the tooth in especially susceptible people and is a major cause of tooth loss in adults (11,12). This condition is usually referred to simply as “periodontitis.” Chronic periodontitis is a disease that needs potentially lifelong monitoring and management, similar to diabetes. Figure 31.1 illustrates the various forms of periodontal health and diseases (13).
Dental plaque is mainly an adherent biofilm of microbes and (food) debris attached to the tooth surface and to the rough surface of any calculus (calcified plaque, tartar) located above and beneath the gum line. Plaque accumulates with poor oral hygiene and causes gingivitis (11,12,14). Such plaque build-up often occurs even in people who brush their teeth, but do so incorrectly, for instance by using a toothbrush with stiff, thick, or frayed bristles or by a technique that sweeps over the groove between the tooth and the gum line and hence does not succeed in removing the plaque. In susceptible individuals, untreated gingivitis may progress to chronic periodontitis due to the host’s immune-inflammatory response to the persisting biofilm (11,12,14). A third form of periodontal disease, periapical periodontitis, affects the jaw bone around the opposite end of the tooth, namely the tip of the root (apex), and is visible only on radiographs. This condition is described separately in this chapter.
Chronic periodontitis cases can be assessed by a variety of measures. Measures of the clinical presentation are illustrated in Appendix 31.1 (15) and include: (a) periodontal probing depth (PPD); (b) clinical attachment loss (CAL); (c) redness and puffiness as a sign of inflammation; (d) spontaneous bleeding from the gingiva; (e) BOP; or (f) bleeding during tooth brushing. Radiographic assessment of bone loss is also used in patient care and research, and additional research measures include: self-report (responses to questionnaire items); presence or elevated levels of antibodies to periodontal bacteria often associated with periodontitis found in serum, saliva, or gingivo-crevicular fluid (GCF, inflammatory exudate from the gingival tissue into the pocket between the tooth and the surrounding soft tissue).
Prevalence of Chronic Periodontitis in the United States
Gingivitis is nearly ubiquitous, affecting 50%–90% of the adult population worldwide (12). Analyzing data from the NHANES 2009–2010, Figure 31.2 (16) displays the prevalence of periodontitis by age for different, commonly used periodontitis case definitions for either CAL (Panel A), PPD (Panel B), or the combination of CAL and PPD (Panel C) used in the increasingly globally accepted case definitions for periodontitis designed for periodontitis surveillance by the joint workgroup of the CDC and the American Academy of Periodontology (AAP) (17) and hereafter referred to as the CDC/AAP periodontitis case definitions (18,19). These clinical case definitions are displayed in Appendix 31.2.
Applying the CDC/AAP definitions to data from the NHANES 2009–2010 cycle, 47.2% of the U.S. population age ≥30 years had periodontitis (16), distributed as 8.7% having mild, 30% moderate, and 8.5% severe periodontitis. An update incorporating the NHANES 2011–2012 data estimated similar prevalence distributions of periodontitis, with 37.1% having mild/moderate and 8.9% severe periodontitis for a weighted total of 46.0% having some form of periodontitis among the 7,066 NHANES 2009–2012 participants, who represent 141.0 million U.S. community-dwelling persons age ≥30 years (20). About two-thirds of those age ≥65 years had periodontitis, ranging from 62.3% in Utah and New Hampshire to over 70% in New Mexico, Hawaii, and the District of Columbia (21).
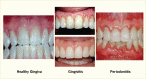
FIGURE 31.1
Periodontal Health, Gingivitis, and Periodontitis. Gingivitis is the reversible infection/inflammation of the soft tissues (gums), whereas periodontitis is a chronic, irreversible destruction of the soft and hard tissues surrounding the teeth (gums and (more...)
Because periodontitis is a chronic, irreversible, cumulative destruction of the soft and hard tissues supporting the teeth, its prevalence increases with age. Figure 31.2 shows the variation by age in the prevalence of periodontitis, based on NHANES 2009–2010 data (16). Remarkable is that the categories mild and severe periodontitis, respectively, do not increase significantly with age—a novel finding at the nationally representative population level. The increase in periodontitis prevalence by age is mostly due to the moderate severity. Although total periodontitis is highly prevalent, not everyone will necessarily experience periodontitis over a lifetime, and importantly, not all will ever develop severe periodontitis, regardless of age.
Disparities in Prevalence of Chronic Periodontitis in the United States
Race/Ethnicity
Racial and ethnic minority groups have a substantially higher prevalence of periodontitis than do non-Hispanic whites in the United States when applying the CDC/AAP case definitions (18) to the NHANES 2009–2012 data. Figure 31.3 shows the prevalence of moderate/severe periodontitis among adults age 45–74 years by race/ethnicity.
Of U.S. adults ages 45–64 years and 65–74 years, 45% and 59% were estimated to have moderate/severe periodontitis, respectively. The prevalence of moderate/severe periodontitis for both the Hispanic and non-Hispanic black groups was significantly higher than the non-Hispanic white group in both age categories. The prevalence of moderate/severe periodontitis was greater in the non-Hispanic black than in the Hispanic groups at age 45–64 years; conversely, it was higher in the Hispanic group (>80%) than in the non-Hispanic black group (~70%) at age 65–74 years. Notice the generally high prevalence (>54%) of moderate/severe periodontitis in the older age category for each racial or ethnic group shown in Figure 31.3.
Using the CDC/AAP case definitions (18) with the NHANES 2009–2012 data, the age-standardized prevalence (±standard error [SE]) among Hispanics was 68.4(±1.5)% versus the following non-Hispanic groups: 59.8(±2.0)% in blacks, 51.9(±3.8)% in Asian Americans, and 39.8(±1.8)% in whites (20). Importantly, the severe form of periodontitis affected about 16% of Hispanics and non-Hispanic blacks and 12% of Asians, but only 7% of non-Hispanic whites living in the United States (20).
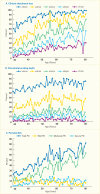
FIGURE 31.2
Prevalence of Periodontitis in Adults Age ≥30 Years, U.S., 2009–2010. (A) Prevalence by different cutoff values (3 mm, 4 mm, 5 mm, 6 mm, and 7 mm) for clinical attachment loss (CAL) by age. (B) Prevalence by different cutoff values (3 (more...)
In the Multi-Ethnic Study of Atherosclerosis (MESA) conducted in California, Illinois, Maryland, Minnesota, New York, and North Carolina, the 6,814 male and female participants age 45–84 years were asked the question: “Has a dentist ever told you that you had periodontitis or gum disease?” The highest prevalence of self-reported periodontal disease was found among the Chinese (39.8%), followed by African Americans (32.0%), whites (26.0%), and Hispanics (17.4%) (22). After adjustment for demographic and socioeconomic factors, these racial/ethnic disparities persisted, although whites and Hispanics did not differ significantly in their prevalence of self-reported periodontitis.
Socioeconomic Status
The NHANES 2009–2012 data with the CDC/AAP case definitions illustrate the stark disparity by education. For instance, those who did not graduate from high school had more than three times (17.1[SE: ±1.5]%) more severe periodontitis than those who did (5.7[SE: ±0.6]%) (20). Likewise, among those with incomes <100% of the Federal Poverty Level (FPL), 14.9(SE: ±1.2)% had severe periodontitis versus only 4.9(SE: ±0.7)% among those with ≥400% FPL incomes.
Among the oldest age groups (≥65 years), 11.0% had severe periodontitis, whereas two-thirds (68%) were affected by some form of periodontitis. However, those with the lowest income (≤130% FPL) had double the prevalence(±SE) of severe periodontitis compared to those in the highest income bracket (≥351% FPL), namely 17.7(±2.2)% versus 8.2(±1.7)% (21).
Geographic Location
The prevalence of periodontitis was not evenly distributed in the different areas of the United States. Novel methods were developed and applied to predict the periodontitis prevalence at state and local levels, using a new small area estimation (SAE) method that incorporated information from the NHANES 2009–2012, the Behavioral Risk Factor Surveillance System (BRFSS) 2012, the 2010 Census, and the American Community Survey (ACS) 2007–2011 (23). For the first time, the prevalence of total periodontitis was predicted by state, congressional district, county, and census tract levels.
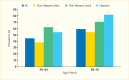
FIGURE 31.3
Prevalence of Moderate/Severe Periodontitis Among Adults Age 45–74 Years, by Age and Race/Ethnicity, U.S., 2009–2012. Periodontitis is defined using the Centers for Disease Control and Prevention/American Academy of Periodontology criteria (more...)
Figure 31.4 displays the prevalence at the state level for U.S. adults age 30–79 years of any severity of periodontitis (mild/moderate/severe) (Panel A) or severe periodontitis (Panel B) (23). At the state level, the estimated prevalence of total periodontitis ranged from 37.7% in Utah to 52.8% in New Mexico (mean 45.1%; median 44.9%), whereas the prevalence in the counties showed a greater spread, ranging from 33.7% to 68% (mean 46.6%; median 45.9%), not shown (23). At the state level, severe periodontitis ranged from 7.3% in New Hampshire to 10.3% in Louisiana (mean 8.9%; median 8.8%). Among U.S. counties, the difference was more than threefold, ranging from 5.2% to 17.9% (mean 9.2%; median 8.8%), not shown (23). The prevalence of diagnosed diabetes in adults age ≥18 years is displayed in Figure 31.4 Panel C (24) to illustrate that several states share the greater burden of both periodontitis and diabetes. The prevalence of diabetes is shown with greater prevalence indicated by darker color shades. No state had a diabetes prevalence <6%; in 8 states, 6.0%–7.4% had diabetes; in 18 states, the prevalence was 7.5%–8.9%; and in the remaining 24 states and the D.C., the prevalence of diabetes was ≥9.0% (24).
Overall, the estimated prevalence of both periodontitis and diabetes was highest for southern and southeastern states and for geographic areas in the Southeast along the Mississippi Delta, as well as along the United States-Mexico border. Aggregated model-based SAEs were consistent with national prevalence estimates from the NHANES 2009–2012. Almost one-quarter (23.8%, 7.2 million) of the U.S. population of all ages who have diabetes are not diagnosed (25), so the actual prevalence distribution could be somewhat different from that illustrated. Theoretically, knowing the estimated prevalence of periodontitis could help identify high-risk geographic areas, as well as socioeconomic population groups, because periodontitis, especially the severe category, is acknowledged as a sign of other chronic, inflammation-based diseases and conditions (26).
Periapical Periodontitis: Introduction
Whereas chronic periodontitis is located around the tooth near the crown, which is the part of the tooth that is covered by enamel and exposed in the oral cavity, a special type of periodontitis is the breakdown of the soft and hard tissues around the apex (tip) of the root, which is called apical periodontitis or periapical periodontitis. The jaw bone breakdown occurs where the root canal begins and through which the blood vessels, nerves, and connective tissue that together are known as the tooth “nerve” enter the spaces inside the tooth, namely the root canals in the roots and the pulp chamber inside the crown. This type of breakdown of the jaw bone is caused by the bacteria and their toxins generated from an infected and dying or dead “nerve.”
Evidence for the prevalence of periapical periodontitis in the United States is scant and does not exist on a population basis because it is unethical to expose study participants to irradiation inherent in taking X-rays for the purpose of surveillance or seeking such epidemiologic prevalence evidence.
Peri-Implant Diseases: Introduction
A condition similar to periodontitis that pertains to the soft and hard tissues around a natural tooth can occur around an implant. Briefly, the peri-implant diseases around dental implants are called peri-implant mucositis and peri-implantitis and correspond to the periodontal diseases gingivitis and periodontitis around the natural teeth.
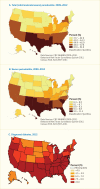
FIGURE 31.4
Age-Adjusted Prevalence of Total and Severe Periodontitis in Adults Age 30–79 Years, by State, U.S., 2009–2012 and Age-Adjusted Prevalence of Diagnosed Diabetes in Adults Age ≥18 Years, by State, U.S., 2012. Periodontitis is defined (more...)
Periodontitis: Effect on Diabetes
The first systematic review of noninterventional epidemiologic studies of the effect of periodontal infection on diabetes was published in 2013 and included 17 eligible reports from 16 studies that demonstrated an adverse effect (27); narrative reviews have also described evidence for this link (28,29,30,31,32). The biologic plausibility of periodontitis adversely affecting glucose regulation and diabetes outcomes is described in the section on underlying mechanisms.
Prediabetes (Impaired Fasting Glucose, Impaired Glucose Tolerance) and Insulin Resistance
Cross-sectional Studies
National Health and Nutrition Examination Surveys
Analyses of data from the NHANES III (1988–1994) investigated the association between chronic periodontitis (designated as exposure) and impaired fasting glucose (IFG) and diabetes prevalence (designated as outcomes) among 12,254 adults age ≥20 years. Participants were grouped into quintiles of increasing severity of periodontitis based on mean CAL and PPD, irrespective of their diabetes status (33). Glycemic outcome categories were defined as: normal (glucose <100 mg/dL), IFG (glucose 100–125 mg/dL), and diabetes (glucose ≥126 mg/dL or self-reported diabetes, affirmative to: “Has a doctor ever told you that you have diabetes?”). Chronic periodontitis was significantly associated in a dose-response manner with both the prevalence of IFG and overt diabetes, regardless of whether the periodontal status was assessed by quintiles of PPD or CAL in multivariable logistic regression models that adjusted for age, sex, education, income, race/ethnicity, smoking, alcohol intake, missing teeth, last dental visit, body mass index (BMI), central adiposity, and physical activity. Compared with participants in the lowest quintile (i.e., the least severe periodontitis category), those in the most severe CAL category had 55% greater odds (odds ratio [OR] 1.55, 95% confidence interval [CI] 1.16–2.07) of having IFG and nearly five times greater odds of having diabetes (OR 4.77, 95% CI 2.69–8.46). The corresponding findings for PPD were 39% (OR 1.39, 95% CI 1.00–1.92) and 63% greater odds (OR 1.63, 95% CI 1.10–2.42), respectively (33). This study provided evidence that chronic periodontitis was positively associated in a dose-response manner with increased prevalence of IFG and diabetes in a representative sample of U.S. adults.
A similar cross-sectional study investigating the relationship between periodontitis (designated as exposure) and insulin resistance (designated as outcome) analyzed data from 3,616 diabetes-free participants age 20–85 years in the continuous NHANES 1999–2004 (34), while considering whether systemic inflammation either mediated or modified this association. Periodontitis was specified using quartiles and continuous measures of mean PPD and CAL, as well as by the original 2007 CDC/AAP periodontitis case definitions (19). The homeostasis model assessment of insulin resistance (HOMA-IR) (35) was used to create the outcome variable of insulin resistance using a dichotomous variable with the 75th percentile of HOMA-IR (HOMA-IR ≥75th percentile) as the cutpoint. Systemic inflammation was classified by quartiles of white blood cell (WBC) count and C-reactive protein (CRP) (36). The adjusted logistic regression analyses identified an association between periodontitis assessed by PPD and increased risk for insulin resistance: for each 1 mm increase in mean PPD, the risk of HOMA-IR ≥75th percentile increased by 24% (risk ratio [RR] 1.24, 95% CI 1.03–1.48), whereas the mean CAL was not associated with greater risk for insulin resistance when controlling for age, sex, race/ethnicity, education level, smoking status, physical activity level, total energy intake, BMI, poverty income ratio, systolic blood pressure, total cholesterol-to-high density lipoprotein (HDL) cholesterol ratio, triglycerides, WBC, and high-sensitivity CRP. Moreover, 6% of the total association between mean PPD and HOMA-IR ≥75th percentile was found to be mediated by WBC. There was an interaction with systemic inflammation (effect modification) as mean PPD was not related to insulin resistance for individuals with WBC ≤6.4x109; however, the fourth quartile of mean PPD was associated with insulin resistance among participants with WBC >7.9x109 (RR 2.6, 95% CI 1.36–4.97). Among participants with CRP >3.0 mg/L, the findings were similar. While the cross-sectional design of this study precludes drawing the causal inference that periodontitis contributes to the development of insulin resistance, these results support the biologic plausibility of such a causal relationship and suggest a synergistic interaction between periodontitis and systemic inflammation (34).
A third NHANES study investigated the relationship between periodontal infection (designated as exposure) and prediabetes (IFG and impaired glucose tolerance [IGT], designated as outcomes) by analyzing data from the continuous NHANES 2009–2010 (37) collected from 1,165 diabetes-free adults age 30–80 years. Periodontitis was classified as no/mild, moderate, or severe using the original 2007 AAP/CDC periodontitis case definitions (19) and also dichotomously using the 75th percentiles for mean PPD and CAL as cutpoints. Prediabetes was defined as: IFG (FPG 100–125 mg/dL) or IGT (2-hour plasma glucose 140–199 mg/dL) (38). Periodontitis was positively associated with prevalent IGT. After adjustment, the odds of IGT in participants with severe periodontitis were almost double those for no/mild periodontitis (OR 1.93, 95% CI 1.18–3.17) and double for those in the 4th quartile (OR 2.05, 95% CI 1.24–3.39) versus those for the 1st–3rd quartiles of mean PPD. However, the odds ratios for IFG were not significant (37). This is in contrast to the study using data from the NHANES III where both CAL and PPD were associated with significantly greater odds of IFG, even in a linear, dose-response manner (33). The differences in findings could possibly be due to application of different examination protocols and periodontitis case definitions.
Longitudinal Studies
Longitudinal studies observe individuals with periodontitis over time and allow assessment of the extent to which periodontitis increases the risk for diabetes incidence (new development), severity, progression, or complications. No longitudinal studies from the United States have evaluated the association between the exposure of periodontitis and the development of prediabetes. However, a quasi-longitudinal Japanese study evaluated the relationship between changes in glucose levels over 10 years in 415 men and women initially age 40–69 years and with NGT at baseline (39) who had periodontitis assessed only at the end. The outcomes were: development of IFG; IGT; an absolute increase of A1c ≥0.2%; or development of overt type 2 diabetes. Periodontal health status was classified into three categories using mean PPD and mean CAL, respectively: 1) highest 20% (most severe category, i.e., poor periodontal health), 2) lowest 30% (periodontally healthy); and 3) the remaining 50% intermediate group. After 10 years, those in the most severe and intermediate categories of mean PPD had significantly greater odds of developing IGT than the periodontally healthy (OR 3.1, 95% CI 1.4–6.9 and OR 2.1, 95% CI 1.0–4.2, respectively). Likewise, the most severe periodontitis category had more than double the odds (OR 2.4, 95% CI 1.2–4.6) than those with healthy periodontium of experiencing A1c increases. All analyses were adjusted.
A German population-based prospective cohort study classified 2,312 dentate and initially diabetes-free adults age 20–81 years into four groups by severity of periodontitis at baseline. After 5 years, there was an adjusted and statistically significant fivefold higher absolute increase in A1c level in people with the poorest baseline periodontal status compared to those with the healthiest periodontal status (40). This A1c increase was significantly greater (p=0.003) in individuals with both poor baseline periodontal health and further deterioration (A1c change over 5 years: 0.143%) compared to those with the healthiest periodontal status at both baseline and follow-up (A1c change over 5 years: 0.005%). Importantly, the A1c change was greatest in those with elevated high-sensitivity CRP levels at baseline.
Glycemic Control
Cross-sectional Studies
U.S. Population-Based Studies
National Health and Nutrition Examination Surveys 2009–2012
Analyses of the NHANES 2009–2012 data evaluated the age-standardized prevalence of poorer glycemic control by periodontitis status among the 1,239 U.S. adults age ≥30 years who had diabetes. Poorer glycemic control was defined using two thresholds: A1c >7% (>53 mmol/mol) and A1c >8% (>64 mmol/mol). Periodontitis status was defined by combining the CDC/AAP periodontitis case definitions (18) into no/mild and moderate/severe, respectively. Both overall and within the majority of the sociodemographic and health-related subgroups, the prevalence of poor glycemic control defined as A1c >8% was consistently greater in participants with moderate/severe periodontitis compared to those with no/mild periodontitis (Table 31.1). For example, among participants with no/mild periodontitis only 14.7(SE: 2.2)% had poorly controlled diabetes (A1c >8.0%) versus 27.5(SE: 3.5)% of those with moderate/severe periodontitis.
The consistent pattern of a significantly higher prevalence of poor glycemic control in those with moderate/severe periodontitis than in those with no/mild periodontitis was found in subgroups of persons with diabetes, as summarized in Table 31.1 and illustrated in Figure 31.5 by age (Panel A); sex (Panel B); racial/ethnic groups (Panel C); and BMI (Panel D). Only among non-Hispanic whites, the difference in prevalence of A1c >8% between moderate/severe and no/mild periodontitis was not statistically significant (Panel C). Moreover, the prevalence of A1c >8% in both periodontitis categories among non-Hispanic whites is clearly lower than in the other three racial/ethnic categories.
As well, poor glycemic control (A1c >8%) in the youngest age group (30–44 years) was found in 18.9(SE: 4.1)% of those with no/mild periodontitis compared to 45.6(SE: 8.2)% of those with moderate/severe periodontitis. In the older age group (≥65 years), the corresponding figures were 3.5(SE: 1.2)% versus 12.6(SE: 2.0)%. Nonetheless, it is noteworthy that there is a diminishing gradient for prevalence of A1c >8% in both periodontitis categories as age increases. That is, among participants with moderate/severe periodontitis, roughly 45% of those age 30–44 years, decreasing to about 22% of those age 45–64 years and further decreasing to about 12% in the oldest age group had A1c >8% (Figure 31.5 Panel A).
Longitudinal Studies
Longitudinal studies observe individuals with periodontitis over time and allow quantification of the extent that periodontitis increases the risk for diabetes incidence (new development), severity, or progression. The first systematic literature review to explore the effect of periodontitis on glycemic control was published in 2013 and identified four studies exploring whether periodontitis is associated with a worsening of glycemic control over time (27). Only one of the studies was conducted in the United States, namely among the Gila River Indian Community dentate members age 18–67 years who had type 2 diabetes, defined as having plasma glucose ≥200 mg/dL after a 2-hour OGTT (5). Enrollment required a baseline A1c ≥9% (≥75 mmol/mol), at the time considered as poor control. Severe periodontitis classification required baseline CAL ≥6 mm or radiographic bone loss of ≥50% at ≥1 tooth. After a mean of 2.4 years (range 2–4 years), 80 participants had clinical and 88 had radiographic examinations. Participants with severe clinical periodontitis at baseline had a significant sixfold greater risk of developing A1c ≥9% (OR 6.2, 95% CI 1.5–25.3) than those without severe periodontitis.
The biologic plausibility for chronic periodontal infection potentially adversely affecting blood glucose levels is briefly described in the section Mechanisms Underlying the Bidirectional Relationship Between Periodontitis and Diabetes.
TABLE 31.1
Crude and Age-Standardized Prevalence of A1c Categories in Dentate Adults Age ≥30 Years With Diagnosed or Undiagnosed Diabetes and No/Mild Periodontitis (Section A) or Moderate/Severe Periodontitis (Section B), U.S., 2009–2012.
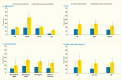
FIGURE 31.5
Prevalence of A1c >8% Among Dentate Adults Age ≥30 Years With Diabetes, by Periodontitis Status and Age, Sex, Race/Ethnicity, and Body Mass Index, U.S., 2009–2012. Periodontitis is defined using the Centers for Disease Control (more...)
Intervention Studies: Effect of Periodontal Treatment on Glycemic Control in Persons With Diabetes
Type 1 Diabetes
Few treatment studies have been conducted exclusively in people with type 1 diabetes and none in the United States. However, with chronic periodontitis affecting mostly adults, the low prevalence of type 1 diabetes among adults, and the overpowering effect of insulin, the effect of periodontal treatment on A1c in type 1 diabetes is inconclusive.
Type 2 Diabetes
Numerous smaller intervention studies conducted in many different countries and several systematic reviews and meta-analyses of various subsets thereof have explored whether routine nonsurgical periodontal therapy (“deep cleaning”) that can be provided in general dental offices can improve glycemic control in people with type 2 diabetes. Given the ethical issues of withholding treatment known to be efficacious once chronic periodontitis is diagnosed for any longer than patients would not usually see a dental care provider, combined with the prohibitive costs of conducting such studies, intervention studies are usually of short duration; hence, it is not known whether any observed effect is sustainable.
Effect of Nonsurgical Periodontal Therapy on Glycemic Control in Type 2 Diabetes
Meta-analyses of randomized controlled trials (RCTs) exploring the effect of nonsurgical periodontal therapy on glycemic control in type 2 diabetes are summarized in Table 31.2 (41,42,43,44,45,46,47,48,49,50,51,52,53,54), and four overviews of such meta-analyses are also published (55,56,57,58). The statistically significant A1c improvements range from an absolute improvement of 0.27 percentage points (95% CI −0.46 to −0.07) to 1.21 percentage points (95% CI −1.68 to −0.75). This improvement is of the same order of magnitude as that expected from adding a second oral diabetes medication to metformin, namely from 0.5 to 2.5 percentage points (59,60). Therefore, such improvement would be of potential clinical significance in diabetes management.
As of August 2017, only seven RCTs in which all participants had diabetes and periodontitis were conducted in the United States (61,62,63,64,65,66,67). These studies included an experimental group that immediately received nonsurgical periodontal treatment (i.e., scaling and root planing) with or without adjunctive local or systemic antibiotics or antimicrobial mouthwashes and at least one “comparison” group that initially received no or less intensive periodontal treatment, but received “delayed” treatment 6–9 months later. The reason that treatment cannot be withheld from any true control group is that it is unethical not to treat persons with diagnosed periodontitis for more than about 6–9 months because effective treatment is known. Of the seven mostly small RCTs conducted in the United States, four found no significant improvement in A1c (62,63,65,67), while three demonstrated significant improvement in A1c levels (61,64,66).
TABLE 31.2
Effect of Nonsurgical Periodontal Treatment on Glycemic Control in Adults With Diabetes: Meta-Analyses of Randomized Controlled Trials Published Through August 1, 2017.
The largest U.S. multicenter RCT (62,68) that was included in four of the meta-analyses (49,50,51,54) was halted early due to futility. However, this $18 million RCT received considerable criticism (69,70,71,72,73). The major areas of criticism included: baseline levels of A1c for participants already being close to the goal for good glycemic control, thus limiting the chance for the effect of periodontal treatment to significantly lower A1c; conclusions that could be drawn being limited because periodontal treatment failed to reach a successful therapeutic endpoint as per standard practice and which was demonstrated to be clinically achievable in prior studies; and the high prevalence of obesity that could have masked any anti-inflammatory effect of even successful periodontal therapy (69). Notably, at the end of this intervention study, 72.1% of all the sites had plaque (causing inflammation), 41.6% of the sites bled upon probing (sign of inflammation), and 30.7% of the participants had diseased PPD of ≥4 mm (69). Consequently, it is unknown whether successful periodontal therapy leading to acceptable periodontal health would have had an effect on A1c level. Nonetheless, the authors reported there was no significant improvement in A1c level following nonsurgical periodontal therapy.
A large, prospective cohort study of data from 126,805 people with type 2 diabetes who received periodontal treatment during 2005–2012 at all medical facilities in the U.S. Veterans Administration concluded that such care improved A1c (74). Periodontal treatment increased the likelihood of reaching the A1c <7% or <9% targets, and the effect of treatment at follow-up was greatest, namely 0.25% in absolute A1c reduction, among patients with an initial A1c >9% (74).
In conclusion, based on the body of evidence, it is prudent to recognize a potentially beneficial role for nonsurgical periodontal therapy contributing to improved glycemic control in people with diabetes, although future, solid evidence from large randomized trials remains warranted.
Adjuvant Antibiotics/Antimicrobials
There is much debate regarding whether to supplement mechanical, nonsurgical periodontal treatment (“deep cleaning”) with antibiotics/antimicrobials delivered locally or systemically, especially with the current attention to the urgent need for severely restricting the use of antibiotics. Based on 13 eligible studies, a 2016 systematic review of such treatment in type 2 diabetes concluded that systemic adjuvant antimicrobials did not enhance the outcome for most periodontitis measures (75). Only the PPD benefitted statistically significantly, but the mean difference of 0.15 mm reduction favoring those receiving antibiotics compared to those who did not seemed clinically insignificant. However, another systematic review and meta-analysis of six RCTs concluded that both PPD and CAL improved more with application of local antimicrobials, especially in deep periodontal pockets in patients with well-controlled types 1 and 2 diabetes (76).
Effect of Nonsurgical Periodontal Therapy on Inflammatory Markers in Type 2 Diabetes
Importantly, the level of systemic inflammatory biomarkers decreases upon nonsurgical periodontal treatment in people with type 2 diabetes and periodontitis (77,78). The importance of decreasing the general inflammatory response is described in the section on underlying mechanisms.
Development of Type 2 Diabetes in Persons With Periodontitis
U.S. Population-Based Studies
National Health and Nutrition Examination Surveys
Three longitudinal (cohort) studies have investigated whether periodontitis at baseline is associated with the development of new type 2 diabetes (27). Data were analyzed from 9,296 (7,168 dentate) persons age 25–74 years and initially diabetes-free who participated in the NHANES I and its Epidemiologic Follow-up Study (NHEFS) by completing a baseline dental examination (1971–1976) and having at least one follow-up evaluation between 1982 and 1992 (79). Diabetes on follow-up was ascertained by a self-reported physician diagnosis requiring pharmacological treatment; a discharge diagnosis of diabetes from a health care facility stay; or death certificate. Periodontitis status was specified in six categories for dentate participants using the Periodontal Index, with the referent group specified as periodontally healthy and the remaining five categories designated as quintiles of increasing periodontitis severity. A seventh group consisted of the 2,128 edentulous participants.
After a mean follow-up of 17 (range 1–22) years, the adjusted odds ratios for incident diabetes in the two quintiles of less severe periodontitis were similar to the referent group. The remaining three quintiles of increasingly greater periodontitis severity experienced about twice the risk of incident diabetes, with an odds ratio of 2.26 (95% CI 1.56–3.27) in the third most severe quintile with the fourth and fifth quintiles not being statistically different from the third quintile. The edentulous group had 30% greater odds of developing diabetes than the periodontally healthy group (OR 1.30, 95% CI 1.00–1.70), and dentate participants with advanced tooth loss had 70% greater odds (p<0.05) of incident diabetes than those with minimal tooth loss (79). The authors concluded that baseline periodontitis independently predicted incident diabetes over almost two decades of follow-up after adjusting for important covariates.
Non-U.S. Non-Population-Based Studies
Two longitudinal studies were conducted in Japan. A 5-year follow-up study of employed adults age 30–69 years found participants with more severe periodontitis at baseline to have a significant 3.5-fold greater risk of having A1c ≥6.5% (cutpoint for diagnosing diabetes) than those who were periodontally healthy at baseline, after adjusting for other covariates (80). The other study conducted periodontal examinations at baseline in employees age 30–59 years. After on average 7 years of follow-up, diabetes-free females with moderate periodontitis at baseline had an adjusted significant 2.3-fold greater risk for developing diabetes than their periodontally healthy counterparts (81).
Gestational Diabetes
Pregnant women with periodontitis seem to have a significantly elevated risk for developing gestational diabetes compared to those with healthy periodontal tissues. Based on 44 reports on 10 studies involving 5,724 participants, including 624 gestational diabetes cases, a 2016 systematic review and meta-analysis calculated that women with periodontitis had a significantly increased risk for gestational diabetes of 66% (OR 1.66, 95% CI 1.17–2.36, p<0.05) (82). When the meta-analysis was restricted to case-control studies of high quality with 1,176 participants, including 380 cases with periodontitis, the odds ratio for gestational diabetes in periodontitis cases was increased by 85% (OR 1.85, 95% CI 1.03–3.32). A meta-analysis of studies adjusting for potential confounders calculated a twofold higher risk for gestational diabetes among pregnant women with periodontitis (OR 2.08, 95% CI 1.21–3.58) compared to those without periodontitis.
A total of 39 women (19 with a history of gestational diabetes, plus 20 without) from a previous study conducted in Louisiana (83) were followed for 22 months postpartum. Women with periodontitis showed greater insulin resistance and lower beta cell function compared to participants without periodontitis (84). Participants with both a history of gestational diabetes and current periodontitis had the most impaired glucose metabolism with the insulin secretion-sensitivity index being significantly lower compared to women with neither past gestational diabetes nor periodontitis, namely 208.20(SE: ±2.60) versus 742.93(SE: ±1.78) (p<0.05). Hence, periodontitis may contribute to impaired glucose metabolism and future risk of developing overt diabetes.
In another U.S. study in 265 predominantly Hispanic (83%) women in New York, high vaginal levels of the periodontal bacteria Tannerella forsythia were significantly associated (p=0.01) with gestational diabetes when comparing the 22 gestational diabetes cases (8.3%) to women without gestational diabetes (85). The prevalence of PPD ≥3 mm was greater in women with gestational diabetes (50.0%) than in women without gestational diabetes (37.3%), but this difference did not reach statistical significance (p=0.38).
Diabetes Complications in Persons With Periodontitis
People with types 1 or 2 diabetes who have periodontitis, especially severe periodontitis, or are edentulous have higher risk for diabetes-related complications than those with no/mild periodontitis (27,86). Several studies have reported a dose-response effect between severity of periodontitis and risk for diabetes complications.
Cross-sectional Studies
A 2015 review that applied systematic literature review searches identified a gap in the body of evidence from U.S. studies regarding links between oral health and diabetic neuropathy (87). Only two studies, conducted in India (88) and Iran (89), were identified and reported significant associations in type 2 diabetes between periodontitis and neuropathy (88) and retinopathy (89), respectively.
National Health and Nutrition Examination Survey III
In the NHANES 1988–1994, participants with gingival bleeding upon periodontal probing at ≥5 sites had a significantly increased risk (OR 1.57, 95% CI 1.26–1.94) of also having retinal hemorrhaging; 51% of the association was explained by A1c level (90). This finding illustrates the association of each of the two diseases with blood glucose concentrations, the possibility that simultaneous presence of gingivitis and retinal hemorrhaging support the hypothesis of microvascular injury in hyperglycemia, and that even gingivitis—in addition to more severe chronic periodontitis—can be a marker for such injury in elevated blood glucose levels.
Using data from the same NHANES also demonstrated an association between periodontal disease and age-related macular degeneration among 8,208 adults age ≥40 years with retinal photographs (91). With overall prevalence of 52.3% for periodontitis and 11.5% for macular degeneration, periodontitis was independently associated with twice the risk (OR 1.96, 95% CI 1.22–3.14) for age-related macular degeneration for participants age ≤60 years, but not for the older group (age >60 years).
The Atherosclerosis Risk in Communities Study
Retinal vascular diameters measured among 457 study participants age ≥52 years from the Atherosclerosis Risk in Communities (ARIC) study showed a significant association between periodontitis defined by the CDC/AAP criteria (19) and the central retinal venular diameter in participants with type 2 diabetes, but not in diabetes-free participants (92).
Dental Atherosclerosis Risk in Communities Study
Periodontitis seems to increase the risk for subclinical atherosclerotic heart disease and coronary heart disease (CHD) in people with diabetes, as observed among 6,048 participants age 52–74 years in the Dental Atherosclerosis Risk in Communities (DARIC) study (93). Those with both diabetes and severe periodontitis had more than double the risk for increased carotid artery intimal-medial wall thickness (IMT >1 mm) (OR 2.2, 95% CI 1.4–3.5), atherosclerotic plaque calcification (acoustic shadowing) (OR 2.5, 95% CI 1.3–4.6), and CHD (OR 2.6, 95% CI 1.6–4.2) compared to those with neither diabetes nor periodontitis. For comparison, in those with diabetes but no periodontitis, the odds ratio for IMT >1 mm was 1.3 (95% CI 0.8–2.9), for atherosclerotic plaque calcification 0.9 (95% CI 0.5–1.8), and for CHD 1.4 (95% CI 0.9–2.4); none of the odds ratios were statistically significant. In those without diabetes but with severe periodontitis, the corresponding odds ratios were 1.2 (95% CI 0.9–1.6), 1.2 (95% CI 0.8–1.7), and 1.1 (95% CI 0.8–1.6), respectively; again, none of the odds ratios were statistically significant. All odds ratio calculations were adjusted for age, sex, race/field center, BMI, smoking, income, education, HDL and low density lipoprotein (LDL) cholesterol, hypertension, and triglycerides (93).
Longitudinal Studies
Pima Indians With Type 2 Diabetes
Studies among the Pima Indians and closely related Tohono O’okham (Papago) Indian residents of a geographically defined part of the Gila River Indian Community in southern Arizona have made major contributions to the body of evidence regarding relationships between type 2 diabetes and periodontitis in the United States. The Pima Indians have one of the world’s highest reported prevalence of type 2 diabetes (94). Between 1983 and 1990, a total of 3,219 Pima Indians age ≥5 years received periodic dental examinations that included a panoramic radiograph of the entire dentition, a clinical periodontal probing examination, and a tooth count.
After a median follow-up of 11 (range 0.3–16) years, the age- and sex-adjusted death rates from all natural causes, expressed as the number of deaths per 1,000 person-years of follow-up, were: 3.7 (95% CI 0.7–6.6) for no/mild periodontitis; 19.6 (95% CI 10.7–28.5) for moderate periodontitis; and 28.4 (95% CI 22.3–34.6) for severe periodontitis (95). Periodontitis significantly predicted death from ischemic heart disease (ptrend=0.04) and diabetic nephropathy (ptrend<0.01). Those with severe periodontitis had 3.2 times greater risk (95% CI 1.1–9.3) of cardio-renal mortality (ischemic heart disease and diabetic nephropathy combined) than those with no, mild, or moderate periodontitis combined (95). Similarly, during up to 22 years of follow-up, incidence of macroalbuminuria was 2.0, 2.1, and 2.6 times higher in participants who had moderate periodontitis, severe periodontitis, or were edentulous, respectively, compared to those with no/mild periodontitis (p=0.01) (96). Comparing the same groups, incidence of end-stage renal disease was 2.3, 3.5, and 4.9 times higher (p=0.02), respectively. Hence, moderate and severe periodontitis, as well as edentulousness, significantly predicted both macroalbuminuria and end-stage renal disease among Pima Indians in a dose-dependent manner.
Non-U.S. Longitudinal Studies
A multinational, 5-year follow-up study among participants with type 2 diabetes reported significant associations between tooth loss and cardiovascular disease, cerebrovascular events, and cardiovascular disease mortality (97). A Japanese prospective study of 73 eyes in 73 consecutive diabetes patients identified a significant association between severity of radiographically assessed periodontitis and the risk of proliferative diabetic retinopathy (98). Similarly, the level of the inflammatory marker interleukin-6 (IL-6) in the vitreous fluid was significantly correlated with the severity of periodontitis. In a case-control study among Swedish adults with type 1 diabetes followed for 6 years, severe periodontitis was associated with renal disease (proteinuria) and cardiovascular complications (stroke, transient ischemic attacks, angina, myocardial infarct, and intermittent claudication) (99).
Systematic Reviews and Meta-analyses
A major complication of diabetes is atherosclerotic cardiovascular disease; and a meta-analysis that pooled 17 case-controls studies involving 3,456 myocardial infarction patients and 3,875 control subjects also reported a signifi-cant association with periodontitis (100). Compared to the control group, cases had a significant 2.5-fold higher risk for periodontitis (OR 2.53, 95% CI 1.93–3.32) and a fourfold higher risk for missing teeth (OR 4.12, 95% CI 2.01–6.23).
In addition, two 2016 systematic reviews and meta-analyses concluded that periodontitis might be associated with a twofold to threefold greater risk for erectile dysfunction (101,102). However, the former was based on only four case-control studies involving 213,006 participants (OR 2.28, 95% CI 1.50–3.48) and the latter also on four studies, involving 38,111 cases and 174,807 controls (OR 3.07, 95% CI 1.87–5.05). Both reports call for future high-quality longitudinal studies. The underlying mechanism is thought to be mainly inflammatory reactions involved in chronic inflammation.
Periapical Periodontitis: Effect on Diabetes
A case report illustrates the need for diagnosing and treating infections, such as periapical periodontitis, as part of diabetes management: a diabetes patient suddenly experienced decreased insulin sensitivity and required increasing insulin doses over a period of 3 weeks of exacerbation of periapical infection. However, only 1 day after endodontic treatment (i.e., “root canal”) that stopped the outflow of infectious agents, the insulin demand dropped about 50% to the patient’s usual insulin dosage (103).
In addition, a systematic review concluded that apical periodontitis is associated with elevated levels of systemic inflammation biomarkers, such as CRP, interleukins, IgA, and IgG, that are not confined to the local lesion (104). For example, a Finnish study found elevated serum levels of the subgingival bacterium Porphyromonas endodontalis and its corresponding serum IgG and lipopolysaccharides in patients undergoing angiography, especially in those with untreated endodontic lesions (105).
Peri-Implant Diseases: Effect on Diabetes
Since dental implants have become more common only in this century, the literature does not yet provide any evidence regarding any effect on blood glucose levels or diabetes complications. Nonetheless, because the pathologic mechanisms are similar to those in periodontal diseases, it is likely that peri-implant diseases will cause systemic effects similar to periodontal diseases, including elevation of blood glucose levels due to the inflammation.
Diabetes: Effect on Periodontitis
Epidemiologic evidence of diabetes being adversely associated with periodontal health is derived from abundant cross-sectional and longitudinal studies over decades, including several population-based studies. Usually, “diabetes” is described as a risk factor for periodontitis despite early studies by Harrison and Bowen in 1983 (106), Genco in 1996 (107), and Kinane and Chestnutt in 1997 (108) that stated that especially poorly controlled diabetes affected periodontal health. Nonetheless, only from about 2010 do studies report that it is the level of hyperglycemia, often in a dose-response manner, that is important, not the mere diagnosis of diabetes (28). Microbiologic research illustrating the role of hyperglycemia severity is briefly mentioned in the Mechanisms section.
Overt Diabetes
Cross-sectional Studies
U.S. Population-Based Studies
National Health and Nutrition Examination Surveys
A study of data from NHANES III explored the association of diabetes, glycemic control, and periodontitis in 4,343 adults age 45–90 years who underwent a dental examination, of whom 502 (11.6%) had type 2 diabetes defined by FPG >126 mg/dL. Poorly controlled diabetes was defined as A1c >9% and better-controlled as A1c ≤9%. Severe periodontitis was defined as ≥2 sites with ≥6 mm CAL with ≥5 mm PPD at ≥1 at these sites. Participants with poorly controlled type 2 diabetes had a significant threefold higher odds of severe periodontitis (OR 2.90, 95% CI 1.40–6.04), and those with better glycemic control had a nonsignificant tendency toward greater odds of severe periodontitis (OR 1.56, 95% CI 0.90–2.68) compared to those without diabetes, after controlling for other risk indicators (109).
A new analyses of NHANES 2009–2012 data conducted for Diabetes in America from 3,575 dentate U.S. adults age ≥30 years investigated the age-adjusted (except age groups) association between presence of diabetes, prediabetes, and degree of glycemic control using an A1c cutpoint of 7% (designated as exposures) and prevalence of moderate/severe periodontitis, using CDC/AAP case definitions (18) designated as outcome (Table 31.3). Results show a dose-response increase in periodontitis prevalence from normoglycemic through prediabetes to diabetes with good control (A1c ≤7%), and finally to diabetes with poorer glycemic control (A1c >7.0%).
As displayed in Table 31.3, the majority of the comparisons of the prevalence of moderate/severe periodontitis among individuals with either prediabetes or diabetes are statistically significantly greater than for those with normal glucose levels. Notably, the prevalence of moderate/severe periodontitis in every subgroup with poorly controlled diabetes defined as A1c >7% is statistically significantly greater than in the normoglycemic group, whereas this is the case in only some of the subgroups with well-controlled diabetes (A1c ≤7%) or prediabetes (A1c 5.7%–6.4%). This pattern of diabetes status-related gradients of prevalence of moderate/severe periodontitis when stratified by selected covariates is illustrated in Figures 31.6 and 31.7 and suggests that better blood glucose control may mitigate periodontitis.
Another report also used data from the NHANES 2009–2012 and applied the CDC/AAP periodontitis case definitions (18), but from 7,042 U.S. adults age 30–80 years (110). Diabetes status was determined by the participants’ self-report of being told by a health professional that they had diabetes. Self-reported diabetes was not associated with periodontitis prevalence. However, A1c as a linear predictor was significantly associated with 14% increased odds of having periodontitis for each unit increase in A1c. Importantly, when participants were classified by degree of glycemic control, each of the A1c categories defining poorer glycemic control in separate models were associated with significantly greater odds of having periodontitis compared to those without diabetes after adjusting for other important covariates. The statistically significant odd ratios for having periodontitis increased linearly from 1.33 to 2.22 with increasing A1c cutpoints of 7.0%, 7.5% (58 mmol/mol), 8.0%, 8.5% (69 mmol/mol), and 9.0%.
Studies in the Gila River Indian Community (1990–1991)
One cross-sectional analysis reported that the prevalence and severity of periodontitis (assessed by the frequency distributions of median CAL or radiographic bone loss) among 2,878 Pima Indians were significantly greater in each of three age groups (5–24, 25–44, and ≥45 years) for participants with diabetes than in those without diabetes (111). The investigators also observed an earlier onset of alveolar bone loss in those with diabetes.
U.S. Non-Population-Based Studies
Type 2 Diabetes in Adults
Another cross-sectional study investigated the association of diabetes (as the exposure) and the prevalence of advanced periodontitis (defined as the outcome) in 2,273 participants age ≥15 years (595 with diabetes and 1,553 without) (112). Advanced periodontitis was defined as having <24 teeth present, at least 6 teeth with ≥25% bone loss, or having any tooth with ≥50% bone loss. The age- and sex-adjusted prevalence of advanced periodontitis was significantly higher in participants with diabetes (60%, 95% CI 55%–65%) compared to those without diabetes (36%, 95% CI 34%–38%).
A third cross-sectional study of data from 1,342 participants age ≥15 years (254 with diabetes and 1,088 diabetes-free) found that participants with diabetes had an approximately threefold greater adjusted odd ratios for destructive periodontitis (OR 2.81, 95% CI 1.91–4.13, using CAL; and OR 3.43, 95% CI 2.28–5.16, using radiographic bone loss) (94).
Type 1 Diabetes in Adults
Population-based investigation of the association of type 1 diabetes and periodontitis in U.S. adults is limited; as mentioned, NHANES data do not permit distinction between various types of diabetes. A unique study of 320 predominantly non-Hispanic white (98%) adults (mean age 32.1 years) with type 1 diabetes (median duration 24.0 years) was conducted among participants of the University of Pittsburgh Epidemiology of Diabetes Complications (EDC) study, who were selected from the Children’s Hospital of Pittsburgh registry and had been demonstrated to be representative of the residents in Allegheny County with type 1 diabetes. Participants were categorized by having CAL ≥4 mm in ≥10% of sites probed as extensive periodontitis (designated as outcome) (113). Extensive periodontitis was more likely in those with longer diabetes duration (OR 3.4 in those with diabetes for >8.5 years). There was a nonsignificant trend toward greater prevalence of extensive periodontitis in participants with poor glycemic control (12.4% at A1c >10.1% [>87 mmol/mol] vs. 6.4% at A1c ≤10.1%).
TABLE 31.3
Crude and Age-Standardized Prevalence of Moderate/Severe Periodontitis in Adults Age ≥30 Years, by Diabetes and Glycemic Control Status, and Other Characteristics, U.S., 2009–2012.
Type 1 Diabetes in Children and Adolescents
Several cross-sectional studies have reported a statistically significant association between type 1 diabetes (designated as exposure) and poorer periodontal health in children and adolescents in the United States compared to their peers without diabetes (106,114,115,116,117,118,119). An extensively studied group in New York consisted of children and adolescents age 6–18 years of whom 350 had diabetes (93% type 1 diabetes) and 350 were controls without diabetes (114,118,119,120,121). The children and adolescents with type 1 diabetes had significantly greater plaque accumulation and poorer periodontal health (both gingivitis and periodontitis). Importantly, periodontal tissue destruction was shown to start much earlier in life than known previously, as early as age 6 years. Gingival bleeding was not related to the amount of dental plaque, however, as children with diabetes exhibited stronger inflammatory responses to the same amount of dental plaque and subgingival bacterial challenge than normoglycemic children. This observation suggests that an adverse, diabetes-related modification of the host’s biologic response to dental biofilm accelerates periodontal breakdown (118,122). Furthermore, gingival bleeding at primary teeth was directly associated with bleeding at permanent teeth, indicating that gingival bleeding in primary teeth could predict future risk of periodontitis in children with diabetes. Finally, the mean A1c level over the 2-year period prior to a participant’s inclusion in the study was significantly associated with presence of periodontitis, suggesting that poorer metabolic control is associated with greater odds of periodontitis in children and adolescents.
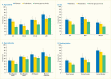
FIGURE 31.6
Prevalence of Moderate/Severe Periodontitis Among Dentate Adults Age ≥30 Years, by Diabetes Status and Age, Sex, Race/Ethnicity, and Smoking Status, U.S., 2009–2012. Periodontitis is defined using the Centers for Disease Control and Prevention/American (more...)
Non-U.S. Non-Population-Based Studies
Diabetes Complications in Adults With Type 2 Diabetes
Although the reason for the effect most likely is hyperglycemia, some studies conclude that diabetes complications may affect periodontal health status. For example, a Brazilian study of 122 patients with type 2 diabetes (mean±standard deviation [SD] A1c 9.3±2.2%, range 4.0%–14.7% [20–137 mmol/mol]) reported that having neuropathic foot ulcerations was significantly and independently associated with moderate/severe periodontitis and edentulism compared to no/mild periodontitis (123).
Longitudinal Studies
U.S. Non-Population-Based Studies: Type 2 Diabetes in Adults
Longitudinal studies observe individuals with diabetes over time and allow assessment of the extent to which diabetes increases the risk for periodontitis incidence (new development), severity, and progression. Evidence from longitudinal cohort studies supports greater risk for incident periodontitis or progression of periodontitis in people with existing type 2 diabetes, especially in poorly controlled diabetes, than among people without type 2 diabetes.
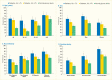
FIGURE 31.7
Prevalence of Moderate/Severe Periodontitis Among Dentate Adults Age ≥30 Years With Diabetes With Poor (A1c >7%) and Good (A1c ≤7%) Glycemic Control Compared to Normal Glucose Levels, by Age, Sex, Race/Ethnicity, and Smoking Status, (more...)
Studies in the Gila River Indian Community (1990–1991)
Three different papers report results of analyses of longitudinal data collected among residents of the Gila River Indian Community to assess the association between type 2 diabetes and risk for periodontitis incidence or progression, using radiographic bone loss as the outcome (112,124,125). The first report investigated the incidence of advanced periodontitis in 701 participants age 15–54 years with ≥23 teeth without advanced periodontitis at baseline over a mean of 2.6 years. The estimated adjusted incidence rate of advanced periodontitis for people with diabetes was 2.6 (95% CI 1.0–6.6) times greater than for those without diabetes, despite including participants with IGT in the diabetes-free category (112).
The second report investigated the severity of radiographic alveolar bone loss progression over 2 years in 362 participants, age 15–57 years, with and without type 2 diabetes. The majority (n=338) had <25% bone loss for all teeth at baseline and did not develop diabetes or lose any teeth during the study. The remaining 24 participants had type 2 diabetes and had a fourfold adjusted greater risk of more severe alveolar bone loss progression (OR 4.23, 95% CI 1.80–9.92) (124).
The third analysis included 359 subjects age 15–57 years with <25% radiographic bone loss at any tooth at baseline; 338 did not have diabetes, 14 had diabetes with better glycemic control (A1c ≤9%), and 7 had diabetes with poor control (A1c >9%) (125). Those with poorly controlled diabetes had an 11.4-fold (95% CI 2.5–53.3) greater risk for alveolar bone loss and more severe bone loss progression than those without diabetes. A dose-response gradient was observed as those with poor glycemic control also had 5.3-fold (95% CI 0.8–53.3) greater risk than those with better control, who again had a twofold greater risk than those without diabetes (95% CI 0.7–6.5).
Taken together, the results of these three studies in the Pima Indians suggest that poorer glycemic control leads to both greater risk for alveolar bone loss and more severe progression of periodontitis; in addition, there may be a gradient of risk, determined by degree of glycemic control.
Sea Island Gullah African Americans
A study of another homogeneous population, the Sea Island Gullah African Americans of coastal South Carolina and Georgia, another population with high genetic risk for type 2 diabetes, investigated progression of periodontitis by glycemic control status in 88 participants age 34–77 years over a mean of 3 years (126). Baseline glycemic control status was specified as better control (A1c <7.0%, n=28) and poorer control (A1c ≥7.0%, n=60). Progression of periodontitis was defined as the proportion of tooth sites with ≥2 mm of PPD or CAL. The odds of PPD and CAL progression were significantly greater for participants with poorer control. Moreover, this association was modified by baseline severity of PPD with deeper PPD predicting greater progression for both PPD and CAL.
Health Professionals Follow-Up Study
The Health Professionals Follow-Up Study (HPFS) enrolled 51,529 U.S. male health professionals age 40–75 years using a mailed questionnaire in 1986 and biennially followed participants by mailed questionnaires until 2006 (127). Diabetes status was determined by an affirmative response to: “Any professional diagnosis of diabetes mellitus?” Periodontitis was assessed by the question “Have you been professionally diagnosed with periodontitis with bone loss?” At baseline, 35,247 dentate men had not been diagnosed with periodontitis and 32,962 (94%) were free of diabetes, whereas 2,285 (6%) had type 2 diabetes. The adjusted risk for developing periodontitis over 20 years was 29% greater in men with type 2 diabetes than their diabetes-free counterparts (hazard ratio [HR] 1.29, 95% CI 1.13–1.47). Interestingly, this association between type 2 diabetes and periodontitis varied by level of fruit and vegetable intake. Men with diabetes whose fruit and vegetable intake was below the population median had a 49% greater risk of periodontitis compared to participants without diabetes (HR 1.49, 95% CI 1.23–1.80). However, there was no association between diabetes and periodontitis among participants with diabetes who had fruit and vegetable consumption above the population’s median.
Type 1 Diabetes in Children and Adolescents
A systematic review of 26 case-control studies concluded that there was greater plaque accumulation and poorer periodontal health status in children age ≤16 years with type 1 diabetes compared to those without diabetes, although three longitudinal prospective studies also reviewed did not report any significant difference (128).
Non-U.S. Population-Based Studies
Types 1 and 2 Diabetes in Adults
There are no reports from longitudinal studies of increased risk for development of new or progression of existing periodontitis in U.S. adults with type 1 diabetes. However, the German population-based Study of Health in Pomerania (SHIP) studied 2,626 dentate men and women age 20–81 years and found that the level of hyperglycemia had an adverse effect on progression of periodontitis as seen in poorly controlled (A1c >7.0%) participants with either type 1 or type 2 diabetes (129).
The Metabolic Syndrome
The American Diabetes Association defines metabolic syndrome as having at least three of the following five conditions: abdominal obesity, high triglyceride levels, low HDL cholesterol level, high blood pressure, and high fasting blood glucose level.
U.S. Non-Population-Based Studies
A prospective study followed a cohort of 760 male U.S. veterans participating in the Department of Veterans Affairs Dental Longitudinal Study among a subset of participants in the Normative Aging Study for up to 33 years (130). The metabolic syndrome significantly increased the hazard for periodontitis (PPD ≥5 mm) progression (HR 1.37, 95% CI 1.14–1.65). Also, the impact on periodontitis increased with the number of metabolic conditions present. Hence, the authors concluded that the metabolic syndrome may affect the development and progression of periodontitis.
Another U.S. study examined 1,097 new patients at the College of Dental Medicine at the University of Columbia who were unaware of having diabetes or prediabetes, and 591 received chairside A1c testing (131). Based on the chairside A1c testing, those with newly diagnosed potential prediabetes had periodontitis to a degree between those with potential diabetes and those without diabetes (132). These findings may be used by medical and dental practitioners as early signs of (undiagnosed) hyperglycemia (133,134).
Non-U.S. Large Studies
Elegantly designed large Japanese longitudinal studies have provided evidence for the two-way causal effects of the metabolic syndrome and periodontitis (80,135). Two parallel studies were reported together. Among 5,856 men and women without periodontitis at baseline and another group of 6,125 with A1c <6.5% at baseline, the risk of developing periodontal disease was associated with A1c levels, and the risk of elevations of A1c concentration was associated with developing periodontal pockets (≥4 mm) after 5 years (80). Another study among 1,023 adult employees (727 males and 296 females; mean age 37.3 years) demonstrated that over 4 years, the risk for a positive conversion to having one metabolic component was 40% (OR 1.4, 95% CI 1.0–2.1) and the risk for having ≥2 components was more than twice as high (OR 2.2, 95% CI 1.1–4.1) among those with periodontitis at baseline compared to those without periodontitis at baseline (135).
Prediabetes
Non-U.S. Non-Population-Based Studies
Other Japanese longitudinal studies have provided evidence for the effect of prediabetes leading to more severe clinically assessed periodontitis in community dwellers of both sexes (39), among women with clinically assessed periodontitis (136), and radiographically diagnosed periodontitis in men (137).
Even prediabetic FPG concentrations of 100–125 mg/dL were found to be significantly associated with alveolar bone loss in 815 healthy Israeli men (mean age 38.1 years) (138).
Gestational Diabetes
A systematic review identified 190 studies exploring associations between gestational diabetes and periodontitis, of which eight were eligible for inclusion, including five cross-sectional and three case-control studies (139). Significant associations between periodontitis and gestational diabetes were calculated by meta-analyses of four cross-sectional studies (OR 1.67, 95% CI 1.20–2.32) and two case-control studies (OR 2.66, 95% CI 1.52–4.65). However, the studies were deemed too heterogeneous in their methodological, clinical, and statistical design and execution to be conclusive.
Cross-sectional Studies
U.S. Population-Based Studies
National Health and Nutrition Examination Survey III
The cross-sectional association of gestational diabetes (the exposure) with periodontitis prevalence was explored among 4,344 women age 20–59 years who participated in the NHANES III (140). There were 113 women who reported a history of gestational diabetes and 174 women who had current diabetes based on their self-report or by having FPG ≥126 mg/dL. Periodontitis was defined as having ≥1 tooth with ≥1 site with PPD ≥4 mm, CAL ≥2 mm, and BOP. Women with a history of gestational diabetes had a significant adjusted eightfold greater odds of periodontitis compared to women with a history of pregnancy without gestational diabetes or overt diabetes.
Another analysis of the NHANES III data used a different approach to explore the relationship of gestational diabetes and periodontitis. The investigators studied 4,490 women of childbearing age (15–44 years), of whom 256 were pregnant and 4,234 were not (141). Based on self-report, the participants were further classified into those with gestational diabetes and pregnant (n=11), history of gestational diabetes only during a previous pregnancy (n=70), current type 1 or 2 diabetes (n=70), or no diabetes (n=4,339). Periodontitis was defined as having ≥1 tooth with ≥1 site with PPD ≥4 mm or with CAL ≥4 mm. Women who were pregnant with gestational diabetes at the time of the NHANES III examination had a significant adjusted ninefold greater odds of having periodontitis than those who were pregnant without gestational diabetes.
National Health and Nutrition Examination Surveys 2009–2012
Women who were pregnant were ineligible for periodontal probing per NHANES 2009–2012 protocol. So we defined gestational diabetes status by self-report of having a history of diabetes first diagnosed during pregnancy or delivering a baby weighing >9 pounds. Current diabetes status was defined as either self-report of having been told by a physician they had diabetes other than during pregnancy or having A1c ≥6.5% or FPG ≥126 mg/dL or 2-hour OGTT ≥200 mg/dL.
A total of 831 women reported having been pregnant in the past. The 89 women with current diabetes and with a history of gestational diabetes had the highest prevalence(±SD) of severe periodontitis (12.6[±5.1]%). Almost 40% of the women with past pregnancies without gestational diabetes and either current prediabetes (n=181; 38.4[±4.9]%) or overt diabetes (n=118; 38.9[±7.9]%) had moderate/severe periodontitis, whereas the prevalence among the 281 normoglycemic women with gestational diabetes-free past pregnancies was 18.8(±2.3)%, and among 90 counterparts with past gestational diabetes, it was 14.9(±3.7)%.
The prevalence (±SD) of moderate/severe periodontitis in women with prior gestational diabetes increased from 14.9(±3.7)% in normoglycemia (n=90) to 29.1(±5.0)% in prediabetes (n=72) to 35.2(±6.5)% in overt diabetes (n=89). This evidence supports the existence of a gradient of increasing periodontitis prevalence by increasing severity of diabetes status in a dose-response manner.
U.S. Non-Population-Based Studies
A case-control study included 53 women with gestational diabetes and 106 pregnant women without any diabetes who were age 25–35 years (83). The primary periodontitis case definition was that which the investigators used with the NHANES III data (141), where periodontitis was defined a priori as the presence of any site exhibiting PPD ≥4 mm or CAL ≥4 mm. Periodontitis prevalence was significantly higher in the pregnant women with gestational diabetes than in those without gestational diabetes, 77.4% versus 57.5%, respectively (OR 2.5, 95% CI 1.2–5.3).
Diabetes: Effect on Periapical Periodontitis
Endodontic therapy (i.e., “root canal” treatment) cleans the root canal by removing soft tissue from inside the tooth (the “nerve”) and subsequently filling it with gutta-percha, an inert natural latex material; the bone lesion is expected then to heal in the absence of further leakage of bacteria and their toxins from the root canal. The healing of the inflamed and often infected jaw bone around the apex of the tooth (i.e., root tip) may especially in hyperglycemic states be impaired and can be delayed or remain a source of chronic inflammation. When such teeth are extracted, an improvement in glycemic control is seen, although the body of evidence for this situation is scant. Radiographs are necessary to diagnose such healing, but in the United States, it is unethical to submit a population to radiographic exposures of all teeth to search for periapical lucencies in the alveolar bone to study their epidemiology in the population.
Soft pulp tissue (the “nerve”) is located inside the tooth, consists of blood vessels, connective tissue, and nerves, and is shown to be different in individuals with diabetes compared to those without diabetes (142). In addition, the hyperglycemia triggers loss of tooth support and causes histologic changes inhibiting osteoblastic differentiation and also stimulating bone resorption, which in turn enhances the progression of periapical lesions (142).
As in chronic periodontitis that occurs at the gingival margin toward the crown of the tooth, periapical periodontitis (around the root tip) represents a chronic challenge with mostly anaerobic bacteria and their toxins that elicit inflammatory local responses that spread system ically. A review concluded that in hyperglycemic states, the prevalence of periapical lesions is higher, the bone lesions are larger, the likelihood of asymptomatic lesions is greater, and the prognosis for healing and survival of the tooth is lower than in normoglycemic individuals (143). A study specifically exploring periapical lesions in type 2 diabetes compared with people without diabetes found apical periodontitis around ≥1 tooth in 81.3% of the former versus 58% of the latter (OR 3.2, 95% CI 1.1–9.4) (144). Using teeth as the unit, the chance for teeth having apical periodontitis was almost double in those with diabetes compared to those without (OR 1.8, 95% CI 1.2–2.8) as 7% versus 4% of teeth were affected.
A 2016 systematic review included seven eligible studies reporting on 1,593 root-filled teeth in 582 patients with diabetes and 1,011 patients without diabetes (145). The overall prevalence of radiolucent periapical lesions was 36% in those with diabetes versus 31% in those without. The authors of the meta-analysis calculated that root-filled teeth in people with diabetes had a significant 42% higher risk of periapical lesions than among those without diabetes (OR 1.42, 95% CI 1.11–1.80).
Diabetes: Effect on Peri-Implant Diseases
Due to the relatively short history of using osseointegrated dental implants to replace missing teeth for functional and esthetic reasons, it is premature to evaluate their use in diabetes/hyperglycemia, which until recently was considered a relative contraindication for placing such implants. Lack of global case definitions for the peri-implant diseases also hampers high-quality synthesis of the current evidence.
Nonetheless, in a systematic review and meta-analyses, the main question was whether diabetes/hyperglycemia affects the prevalence of the two peri-implant diseases of peri-implant mucositis (reversible) and peri-implantitis (irreversible). From 401 identified citations, results from 13 studies were included in the qualitative synthesis and 9 in the meta-analyses. Even though the evidence is fairly scant, a tendency toward greater prevalence in hyperglycemic conditions was suggested for both conditions in meta-analyses that included studies applying the same case definitions (146). In another review, a dose response was identified with the prevalence of peri-implantitis increasing with poorer glycemic control, as reported in the only two studies reporting on glycemic control, which involved 204 implants in 85 participants with diabetes. However, due to scarce evidence and poor similarity between case definitions used, a meta-analysis failed to show that more implants failed in people with diabetes compared to their normoglycemic peers (147).
Risk Factors for Periodontitis and Diabetes (Hyperglycemia)
The two-way association between diabetes/hyperglycemia and periodontitis has been well established for years (6), and hence, these two conditions may be mutual risk factors (or determinants or indicators) for each other or share the same risks. What is realized is that the two conditions share many common risk factors. A large body of evidence supports the role of risk factors for periodontitis (14,148,149), and several of these are also risk factors or risk markers for diabetes (14,148). Two extensive reviews described and categorized these risk factors as “nonmodifiable” and “modi fiable.” The nonmodifiable risk factors, that are not as unmodifiable as formerly assumed, include age, sex, race/ethnicity, socioeconomic status, genetic polymorphism (inflammatory hypo- or hyper-response, elevated susceptibility), epigenetic, and other systemic conditions (genetic, dermatological, hematological, granulomatous, immunosuppressive, and neoplastic disorders) (148,149), as summarized in Figure 31.8 (148).
Modifiable risk factors include periodontal bacteria, fungi, and virus in the dental plaque; poor oral hygiene; cigarette smoking; alcohol consumption; hyperglycemia (diabetes, prediabetes); obesity; osteoporosis/dietary calcium/vitamin D; HIV/AIDS; psychosocial factors (stress, anxiety, psychiatric conditions, such as depression, and inadequate coping skills); and diet/nutrition (14,149).
Of special interest is the identification of risk indicators for periodontitis (using the CDC/AAP periodontitis case definitions) (18,19) based on the NHANES 2009–2012 data that pointed to cigarette smoking, especially current smoking, as an important modifiable risk for all levels of periodontitis severity. Also, advanced age is an important risk determinant, along with being male and having uncontrolled diabetes (150).
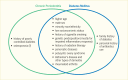
FIGURE 31.8
“Nonmodifiable” Risk Factors for Chronic Periodontitis and Diabetes Mellitus Are Largely Identical.
Mechanisms Underlying the Bidirectional Relationship Between Periodontitis and Diabetes
While a detailed explanation of the biologic, microbiologic, and inflammatory processes underlying the relationship between periodontal infection and diabetes is outside the scope of this chapter, a brief overview of some of the mechanisms considered in this bidirectional relationship follows.
The biologic plausibility of periodontitis adversely affecting glucose regulation and diabetes outcomes is based on evidence that chronic systemic inflammation is involved in the development of insulin resistance and the pathogenesis of diabetes and that chronic periodontal infection contributes to systemic inflammation, which in turn adversely affects insulin sensitivity and increases glucose dysregulation.
In the opposite direction, hyperglycemia causes microvascular and macrovascular changes and delayed wound healing. Wound healing is necessary to help the periodontal soft tissues regain homeostasis upon responding to the chronic injuries caused by the microbiota in the dental plaque found adhering to the surface of the teeth and in the periodontal pockets. While the usual response to plaque accumulation is gingival inflammation, those with diabetes develop an exaggerated and earlier inflammatory response than do normoglycemic individuals (151). The nonenzymatic glycation and oxidation of proteins and lipids lead to formation of advanced glycation endproducts (AGEs). For example, glycated albumin has been shown to stimulate secretion of inflammatory cytokines by monocytes, especially when the latter were incubated with lipopolysaccharides (LPS) from periodontal bacteria associated with periodontitis (152).
Figure 31.9 shows conceptually the two-way adverse effects periodontal infection and hyperglycemia exert on each other (122). Periodontal infection and diabetes influence each other in a bidirectional relationship with wide-ranging activation of the innate immune system causing chronic low-grade systemic inflammation. These inflammatory responses are integrally involved in the pathogenesis of type 2 diabetes and its complications, through an ongoing cytokine-induced acute-phase response. They are also implicated in the pathogenesis of periodontitis, in which proinflammatory cytokines play a central role in the host’s response to the periodontal biofilm. Periodontal infection contributes to the low-grade systemic inflammation associated with diabetes and thereby contributes to insulin resistance, IFG, IGT, poorer control of diabetes, and possibly the development of diabetes and its hyperglycemia-related complications. These mechanisms are illustrated conceptually in Figure 31.10, in which each arrow is supported by scientific evidence (87).
Helicobacter Pylori
The bacterium Helicobacter pylori (H. pylori) is found as a transient or permanent inhabitant of the oral cavity, which may act as a reservoir and aid in recurrence of H. pylori gastric infection upon eradication—or even be part of the mechanism for initial infection of the stomach (153). This could have relevance in glycemic control, according to the conclusion of a meta-analysis of six studies involving 325 participants with type 2 diabetes that there is a significant difference between H. pylori-positive and H. pylori-negative participants regarding their FPG levels (154).

FIGURE 31.9
A “Two-Hit” Model for the Pathogenesis of Accelerated Periodontal Destruction in Patients With Diabetes. A “two-hit” model for the pathogenesis of accelerated periodontal destruction in patients with diabetes. Bacterial (more...)
Gestational Diabetes: Periodontal Bacteria and Systemic Inflammation
A study in Turkey that compared groups consisting of various combinations of women with and without gingivitis and gestational diabetes demonstrated that gingivitis may increase the cumulative systemic inflammatory response during gestational diabetes (155). Having gestational diabetes was also independently associated with increased abundance of individual and multiple oral pathogens (all p<0.05). Both gingivitis and gestational diabetes contribute to elevated levels of CRP, a risk marker for adverse pregnancy outcomes and cardiovascular events.
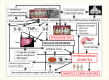
FIGURE 31.10
Mechanisms Underlying the Associations Between Periodontitis and Hyperglycemia/Diabetes and Its Complications: Conceptual Model. The oral microbiome in dental plaque (biofilm) causes infection in the gingival tissues that respond with inflammation. In (more...)
Diabetes: Associated Oral Conditions Other Than Current Periodontitis
While periodontitis is the most widely studied diabetes-related oral disease, diabetes is also associated with several other oral health conditions.
Tooth Loss/Missing Teeth
Partial tooth loss is present in individuals who retain some, but not all, of their 28 non-third-molar natural dentition. In epidemiologic studies among adults, edentulism or edentulousness means that all 28 permanent non-third-molar teeth are absent by clinical examination. Numbers of missing or present teeth concern only those 28 teeth. The four third molars, known as “wisdom teeth,” are not taken into consideration because they are often extracted by choice, not due to disease; may be buried in the jaws; or one or more of them may be congenitally missing (156). Because severe periodontitis is a major cause of tooth loss in adults, the number of missing teeth is often used as a proxy for a history of periodontitis. However, among both younger and older individuals, dental caries can also be a significant cause of loss of teeth (157,158).
Actually, because the term “tooth loss” implies that the tooth has been present, the term “missing teeth” is more correct, because that expression includes both teeth that have been extracted, as well as those that are congenitally lacking, and possibly some that may still be embedded in the jaw bone and are not visible on a clinical examination.
Based on five national U.S. surveys from 1957–1958 to 2009–2012, the rate of edentulism in the population age ≥15 years is declining dramatically and is predicted to reach 2.6% by 2050, with 30% fewer edentulous in 2050 (8.6 million) than in 2010 (12.2 million) (159). Of great importance is that the vast majority of the population have natural teeth that will need to be cared for.
Cross-sectional Studies
U.S. Population-Based Studies
National Health and Nutrition Examination Surveys
Evidence from U.S. population-based studies suggests that people with diabetes consistently are missing more teeth than people without diabetes.
Trend analyses of nine waves of national survey data over a 40-year period—NHANES I (1971–1975), NHANES III (1988–1994), and seven continuous NHANES surveys from 1999 to 2012—from 37,609 dentate (i.e., ≥1 permanent tooth) adults age ≥25 years showed that people with self-reported diabetes were missing almost twice as many teeth as their nondiabetic counterparts (156). During 1971–2012, the mean number of missing teeth decreased from 11.2 to 6.6 among people with diabetes and from 9.4 to 3.4 in those without diabetes.
There are great disparities in number of missing teeth by race/ethnicity that persist over time. Among dentate adults with diabetes, non-Hispanic blacks were missing significantly more teeth than their white peers, and the number of such missing teeth increased more by age in non-Hispanic blacks, despite a decrease over the decades in number of missing teeth in both races. On the contrary, no decreasing trend was seen in Mexican Americans regardless of diabetes status. Nonetheless, Mexican Americans were missing fewer teeth than non-Hispanic whites. These racial differences persisted in the strata by age group, survey period, and birth cohort (156).
Among 11,761 dentate U.S. adults age ≥20 years participating in the NHANES 1999–2004, people with self-reported diabetes were missing significantly more teeth than the total population (160). The age- and sex-standardized mean numbers of permanent teeth missing were: 4.1 teeth among all dentate (with natural teeth); 5.7 teeth among all dentate participants with diabetes (including uncontrolled diabetes); and 6.0 missing teeth among dentate participants with uncontrolled diabetes. The mean numbers of missing teeth were significantly higher for participants with diabetes than those without diabetes.
A report analyzing NHANES 2003–2004 data from 2,508 participants age ≥50 years with oral examinations and self-reported diabetes status investigated the prevalence of edentulism and partial tooth loss (161). The unadjusted prevalence of edentulism was significantly twofold higher in the participants with diabetes than those without diabetes, 28.4% versus 14.1%, respectively (p<0.001). Similarly, dentate participants with diabetes had a significantly greater unadjusted mean number of missing teeth than those without diabetes, 9.8 versus 6.7, respectively (p<0.001). Those with diabetes were more than twice as likely to be edentulous than those without diabetes (OR 2.25, 95% CI 1.19–4.21). Diabetes was estimated to be associated with 18% of the cases of edentulism in U.S. adults age ≥50 years, and the statistically significant greater mean number of missing teeth among the dentate participants with diabetes persisted in multiple linear regression analysis after adjustment (161).
In 5,511 U.S. adults participating in the NHANES 2005–2008, the number of natural teeth was inversely associated with FPG and insulin concentrations (p<0.01 for both) (162). Results of new analyses of the NHANES 2009–2012 data exploring the prevalence of edentulism in 4,209 U.S. adults age ≥30 years with various categories of diabetes in different demographic and smoking status groups are displayed in Table 31.4.
The age-standardized (except for the age groups) weighted prevalence of edentulism, independent of any other characteristics, was significantly greater in participants with diabetes than in those without diabetes (8.9% vs. 5.3%, p<0.05). The prevalences of edentulism in participants with prediabetes (6.6%) and with poorer controlled (A1c >7%) diabetes (9.2%) were higher than in those without diabetes, but the differences were not significantly different statistically.
TABLE 31.4
Crude and Age-Standardized Prevalence of Edentulism in Adults Age ≥30 Years, by Diabetes Status, Glycemic Control Status, and Other Characteristics, U.S., 2009–2012.
Table 31.4 also presents results of analyses for the prevalence of edentulism by diabetes status. The prevalence of edentulism was greater for participants with diabetes and poorer controlled diabetes in most of the categories of subgroups, although not all differences reached statistical significance.
Figures 31.11 and 31.12 show the consistent pattern of differences in prevalence of edentulism by diabetes status and categories of population subgroups. Overall, the data displayed in Table 31.4 and illustrated in Figures 31.11 and 31.12 support the prevalence of edentulism being greater in people with diabetes, in accord with findings using the NHANES 2003–2004 data (161).
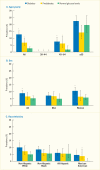
FIGURE 31.11
Prevalence of Edentulism Among Adults Age ≥30 Years, by Diabetes Status and Age, Sex, and Race/Ethnicity, U.S., 2009–2012. Edentulism is defined as missing all teeth. Diabetes is defined by self-report of previously being diagnosed by (more...)
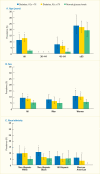
FIGURE 31.12
Prevalence of Edentulism Among Adults Age ≥30 Years With Diabetes With Poor (A1c >7%) and Good (A1c≤7%) Glycemic Control Compared to Normal Glucose Levels, by Age, Sex, and Race/Ethnicity, U.S., 2009–2012. Edentulism is (more...)
The new analyses of NHANES 2009–2012 data also evaluated tooth loss as the mean number of missing teeth in dentate individuals by diabetes and glycemic control status, as shown in Table 31.5. The age-standardized (except for the age groups) weighted mean number of missing teeth was significantly higher for people with overt diabetes (5.2), in poorer controlled (A1c >7%) diabetes (5.3), and in better controlled (A1c ≤7%) diabetes (5.2) than among those without diabetes (3.1).
Table 31.5 also presents results of analyses of the mean number of missing teeth by diabetes status. The pattern of statistically significantly greater numbers of missing teeth for people with diabetes and poorer controlled diabetes is consistent across the majority of categories of all population subgroups. Non-Hispanic black individuals with diabetes and poorer controlled diabetes have noticeably higher numbers of missing teeth than their counterparts in other racial/ethnic categories. Table 31.5 and Figures 31.13 and 31.14 also show the expected linear gradients for increasing numbers of missing teeth as age and smoking (and serum cotinine content) increase, as well as decreasing numbers of missing teeth for individuals as education and poverty income ratio increase.
TABLE 31.5
Crude and Age-Standardized Mean Number of Missing Teeth, Among Dentate Adults Age ≥30 Years, by Diabetes Status, Glycemic Control Status, and Other Characteristics, U.S., 2009–2012.
Behavioral Risk Factor Surveillance System
Among 155,280 U.S. dentate adults who participated in the BRFSS 2004 telephone survey and reported having had a dental visit in the past year, those with self-reported diabetes had significantly more teeth extracted because of decay or periodontitis than those without diabetes (163). Participants with diabetes were almost 50% more likely to have any teeth extracted (OR 1.46, 95% CI 1.30–1.64) and three times more likely to have ≥6 teeth extracted, after adjustment for confounders. This independent association between diabetes and tooth loss was stronger among the younger age group (18–44 years). Among 70,363 older U.S. adults (age ≥65 years) taking part in the BRFSS 2006, 2008, and 2010, those with diabetes were more likely to have lost teeth to caries or periodontitis than respondents without diabetes (82.3% vs. 74.3%, p<0.001) (164).
Hispanic Community Health Study/Study of Latinos (HCHS/SOL)
Among Hispanic/Latino adults age 18–74 years (N=15,945), those with uncontrolled diabetes (A1c ≥7%) had about twice the risk of missing more than nine, but not all, teeth (OR 1.92, 95% CI 1.44–2.55) or being edentate (OR 1.73, 95% CI 1.22–2.46) than their normoglycemic counterparts (165). However, there was no difference in number of missing teeth between normoglycemic participants and those with prediabetes or well-controlled diabetes.
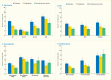
FIGURE 31.13
Mean Number of Missing Teeth Among Dentate Adults Age ≥30 Years, by Diabetes Status and Age, Sex, Race/Ethnicity, and Smoking Status, U.S., 2009–2012. Number of missing teeth is the number of the 28 non-wisdom teeth that are not present (more...)
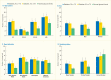
FIGURE 31.14
Mean Number of Missing Teeth Among Dentate Adults Age ≥30 Years With Diabetes With Poor (A1c >7%) and Good (A1c ≤7%) Glycemic Control Compared to Normal Glucose Levels, by Age, Sex, Race/Ethnicity, and Smoking Status, U.S., 2009–2012. (more...)
Tooth Loss in Type 1 Diabetes
Reports specifically investigating associations between type 1 diabetes and tooth loss in adults are sparse, and there are no nationwide population-based studies of U.S. adults with type 1 diabetes. A study of 406 adults who were representative of residents of Allegheny County, Pennsylvania, included primarily white non-Hispanic (98%) persons who were diagnosed with type 1 diabetes (166). This study did not include a comparison group without diabetes, but the analysis included a comparison with NHANES III data, in which approximately 95% of the participants did not have diabetes. This study did not identify a significant difference in the age-adjusted mean number of teeth lost or prevalence of edentulism between the study participants. Partial tooth loss in the type 1 diabetes subjects was significantly associated with the following diabetes-related factors: extensive periodontitis in remaining teeth (OR 7.35, 95% CI 2.49–27.28), BOP (OR 1.82, 95% CI 1.03–3.23), duration of diabetes >24 years (OR 5.32, 95% CI 2.96–9.84), and diabetic neuropathy (OR 2.29, 95% CI 1.15–4.55). Other risk factors significantly associated with partial tooth loss in the model included household income <$20,000, not using dental floss, and multiple teeth with coronal decay or fillings.
The German population-based SHIP study investigated the association between diabetes and tooth loss in 145 participants with type 1 diabetes compared to 2,647 participants without diabetes, age 20–59 years (167). Tooth loss was specified as a dichotomous variable to define cases with the top quartile of missing teeth in each 5-year age group in males and females, respectively. An adjusted multivariable logistic regression estimated significant twofold higher odds for belonging to the top quartile of number of missing teeth in participants with type 1 diabetes compared to those without diabetes (OR 1.93, 95% CI 1.37–2.71) overall. However, when stratified by age groups, the odds of being in the highest quartile of tooth loss were statistically significant for participants ages 40–49 and 50–59 years, but not significant for those ages 20–29 and 30–39 years.
Longitudinal Studies
U.S. Health Professionals Follow-Up Study
As mentioned, the HPFS enrolled U.S. male health professionals age 40–75 years at baseline by questionnaires mailed every other year (127); diabetes status was self-reported. Participants reported the number of natural teeth at baseline and the number of natural teeth lost for each subsequent biennial questionnaire. The follow-up question to determine tooth loss was: “How many natural teeth have you lost since January 1 (of the previous questionnaire cycle)?” The multivariable models estimated that among the 35,247 originally dentate men, those with type 2 diabetes had an adjusted modest, but significant, 10% higher risk of experiencing tooth loss over 20 years than those without diabetes.
U.S. Women’s Health Initiative Observational Study
A study of 1,021 postmenopausal women participating in the Women’s Health Initiative Observational Study investigated incident tooth loss during 5 years of follow-up (168). Presence of teeth was assessed by clinical examination, whereas diabetes status was determined by self-report. Overall, nearly 30% experienced tooth loss during the study, and participants with diabetes had a statistically significant almost 2.5-fold greater risk of any tooth loss than those without diabetes.
Study of Health in Pomerania
The population-based SHIP cohort study of German men and women (mean age[±SD] 46[±14] years, range 21–80 years) provides evidence that uncontrolled diabetes (A1c >7%) increases the risk for incident tooth loss during 5 years of follow-up, regardless of whether the participants had type 1 or type 2 diabetes (129). Overall, one-third (34%) experienced tooth loss. The significant risk ratios for incident tooth loss among those with uncontrolled diabetes compared with no diabetes were 1.36 (95% CI 1.11–1.67) and 1.93 (95% CI 1.55–2.39) for type 2 and type 1 diabetes, respectively, whereas the risks for tooth loss in controlled diabetes of either type were not statistically significant compared to those without diabetes.
U.S. Non-Population-Based Studies
In the aforementioned prospective study following a cohort of 760 male U.S. veterans for up to 33 years, suffering from metabolic syndrome significantly increased the adjusted hazard for tooth loss (HR 1.39, 95% CI 1.08–1.79). Also, its impact on tooth loss increased with the number of metabolic syndrome conditions present (130).
Among 1,097 point-of-care A1c-tested adults age ≥40 years (≥30 years, if Hispanic) in Manhattan, New York, 494 were newly diagnosed with potential hyperglycemia; the number of missing teeth increased in a dose-response manner with increasing level of hyperglycemia. Statistically significantly more teeth (±SD) were missing in the overt diabetes group (9.3±7.1) than in the prediabetes group (7.4±6.7) that again had more missing teeth than the normoglycemic group (5.8±6.2) (132). However, the higher number of missing teeth in the diabetes group than in those with prediabetes did not reach statistical significance.
Root Fragments
A root fragment is defined as any permanent residual tooth structure, predominantly composed of dental root structure, with more than 90% of the coronal structure (crown of the tooth) destroyed by caries, and occupying the position of a tooth within the dental arch (169). The presence of such root remnants may be a marker for lack of access to dental care for its removal or for treatment (or prevention) of the severe caries on the root or the crown of the tooth that could prevent the occurrence of root fragments. While the oral examination in the NHANES 2009–2012 did not include examination for coronal or root caries in adults age ≥30 years, the presence of root remnants was assessed. The age-standardized (except for results by age groups) and age-stratified prevalences of any root fragments by diabetes status and glycemic control status are presented in Table 31.6 and illustrated in Figures 31.15 and 31.16.
As shown in Table 31.6, there was consistent evidence of a gradient of increased prevalence of any root fragments in the categories of diabetes status from normal glucose levels to prediabetes to diabetes. This consistent gradient of increased root fragment prevalence also occurs from normal glucose levels to better controlled diabetes (A1c ≤7%) to poorer controlled diabetes (A1c >7%). Additionally, in almost every subpopulation category, the prevalence of any root fragments was greater for individuals with diabetes and for individuals with poorer controlled diabetes than for those without diabetes. The consistency of these patterns of prevalence of root fragments associated with diabetes and glycemic control status is illustrated in Figures 31.15 and 31.16 for the subgroups displayed in Table 31.6.
Potential Consequences of Tooth Loss
Abundant evidence exists for the sequela of tooth loss, especially edentulism, which is briefly summarized in the following. Diminished quality of life in all its dimensions (physical, functional, psychological, social, etc.) is the greatest overarching result of missing teeth. In the title of a study that sought to understand the varying perceptions of tooth loss and replacement, British health sociologists summed it up as follows: “Your whole life is lived through your teeth” (170).
The adverse effects include, but are not limited to: difficulty in brushing and other cleaning of the remaining dentition; difficulty in biting and chewing, leading to lower consumption of dietary fiber, fruits, and vegetables and higher consumption of soft foods containing cholesterol, saturated fat, and added sugar; speaking; esthetic dissatisfaction; poorer self-rated oral health, as well as general health; diminished self-esteem; depression; social stigma; and possibly difficulties in gaining employment. Physically, tooth loss is associated with incident type 2 diabetes and decreased glycemic control; bone stiffness and osteoporotic fracture risk; cardiovascular disease and events; hypertension; (silent) ischemic stroke; cognitive decline and dementia; kidney disease; cancer (head and neck, esophagus, stomach, pancreas, lung); decreased longevity, and all-cause and cardiovascular mortality. Finally, having lost some teeth, but not all, is the most important risk factor for losing more teeth. The negative impact of missing teeth is potentially accentuated in people with diabetes who already are burdened by a chronic health issue.
TABLE 31.6
Crude and Age-Standardized Prevalence of Any Root Fragments Among Adults Age ≥30 Years, by Diabetes Status, Glycemic Control Status, and Other Characteristics, U.S., 2009–2012.
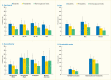
FIGURE 31.15
Prevalence of Any Root Fragments Among Adults Age ≥30 Years, by Diabetes Status and Age, Sex, Race/Ethnicity, and Periodontitis Status, U.S., 2009–2012. Any root fragment is defined as the presence of one or more pieces of a root of a (more...)
Tooth Eruption
Children with diabetes tend to experience accelerated tooth eruption, especially in girls and possibly in cases with simultaneous obesity, as demonstrated by a study of 270 children age 6–14 years with diabetes and 320 comparison children without diabetes examined in New York City (171).
The permanent first molars erupt—most often unnoticed—behind the deciduous (primary, “baby”) teeth, usually at age 5–6 years, and possibly earlier in children with diabetes. It is important for health care providers and parents/guardians to be alert to this eruption to accomplish proper tooth cleaning and also in order for the occlusal (biting) surface to be professionally sealed to prevent development of caries.
Caries
Dental caries is seen at two different locations: coronal caries on the crown portion of the tooth that is covered by tooth enamel and visible in the oral cavity; and root caries that occurs on the portion of the tooth imbedded in the alveolar (jaw) bone and covered by the gums and hence not visible in periodontal health.
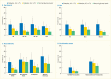
FIGURE 31.16
Prevalence of Any Root Fragments Among Adults Age ≥30 Years With Diabetes With Poor (A1c >7%) and Good (A1c ≤7%) Glycemic Control Compared to Normal Glucose Levels, by Age, Sex, Race/Ethnicity, and Periodontitis Status, U.S., 2009–2012. (more...)
Evidence regarding the occurrence of coronal caries in both children and adults with type 1 diabetes (128,172,173,174) and type 2 diabetes (175,176) is inconsistent. Reports include results showing increased (173,175,176,177), decreased, and no difference (172,174) in coronal caries prevalence or incidence in people with diabetes compared to those without diabetes. Noteworthy are a systematic review (128) and a comprehensive review (178) that both conclude the evidence for associations between type 1 diabetes and caries in children is inconclusive. However, there is greater consistency in the evidence for adults with diabetes to have greater occurrence of root caries than people without diabetes (174,179).
National Health and Nutrition Examination Surveys
In the NHANES 1999–2004 representing the U.S. population age ≥20 years, 14,213 individuals (representing a population of 172.4 million) had oral examinations and 11,761 were dentate (160). Remarkably, almost three-quarters (72.7%) of the participants with diabetes received a referral for treatment from the oral examiner for the most pressing treatment needs, caries (35.8%) and periodontitis (36.6%). Corresponding percentages for all participants (including those with diabetes) were 59.3% total, with 25.6% for caries and 24.3% for periodontitis. The study also reported that among participants with diabetes, 57.2% rated their oral health “poor” versus 39.1% of all dentate participants (160). However, due to the cross-sectional design of the NHANES, no causality can be deducted, so it was not possible to know whether having diabetes or some other factors in the people with diabetes led to the poor oral health status.
Non-U.S. Study
Japanese researchers followed 117,175 neonates and concluded that the newborn conditions of macrosomia (birthweight ≥4,000 g) and large for gestational age (LGA) both were associated with increased risk of caries at 3 years of follow-up (180). This finding is relevant because babies born to women with diabetes often are macrosomic or LGA.
Dry Mouth
The term “dry mouth” can be used for both xerostomia and hyposalivation, but the two conditions do not necessarily occur simultaneously. Xerostomia is the subjective feeling of mouth dryness, whereas hyposalivation refers to salivary hypofunction with diminished saliva secretion. Evidence is consistent that reported dry mouth is more frequent in people with type 1 (181) and type 2 diabetes at all ages than in those without diabetes. However, the reported relationship of this association to other measures of diabetes status, such as metabolic control and diabetic neuropathy, is not consistent (87); some studies, however, have reported that neuropathy in people with type 1 diabetes is often associated with dry mouth (181). Diabetic neuropathy can affect the nerves that control the salivary production (87). Consequences of hyposalivation include diminished quality of life; increased risk of dental caries, gingivitis, and periodontitis; and oral mucosal infections, especially Candida albicans; taste and olfactory diminution; difficulties in eating and speaking; development of denture-related mucosal ulcers; and difficulties in retention of upper complete dentures (87). Moreover, people suffering from diabetes and all patients undergoing hemodialysis are likely to use several medications, almost all of which can cause dry mouth and thereby greatly affects their quality of life (182).
Candidiasis
Infection by the fungus Candida albicans (thrush) or dormant carriage is one of the most commonly reported “non-dental” oral complications of diabetes. Poor glycemic control, with concomitant increased levels of glucose in the blood, saliva, and tissues, in combination with diminished polymorphonuclear neutrophil phagocytosis and killing capacity have been proposed to explain Candida over-growth in people with diabetes. Studies indicate Candida albicans is the species most frequently identified. There are several risk factors associated with the presence of Candida pseudohyphae in adults with diabetes, including presence of removable dentures, poor glycemic control, and current cigarette smoking. The largest study on the association between diabetes and oral Candida infection and Candida lesions conducted in the United States reported that adults with type 1 diabetes had significantly greater prevalence of Candida pseudohyphae (a measure of Candida carriage) than the comparison group without diabetes, namely 23% and 5.7%, respectively (183). Individuals with type 1 diabetes also had significantly greater prevalence of Candida-related clinical mucosal lesions, including atrophy of the tongue dorsum papillae, median rhomboid glossitis, angular cheilitis, and denture stomatitis.
Studies outside the United States have further elaborated on the presence of Candida species. Among persons age 21–70 years with type 2 diabetes, higher prevalence was found among those with A1c >9%, age >40 years, men, and those with periodontitis (184), whereas another study found the highest prevalence in women of all ages and in those with poor glycemic control. Eight different Candida species were identified with Candida albicans most frequently present, more often on the dorsum of the tongue than in subgingival plaque (185). Even in prediabetes, Candida carriage was shown to be more prevalent than in normoglycemic adults (186). Candida species were found in most persons age 3–18 years, but resulted in candidiasis only in children with type 1 diabetes or with nephrotic syndrome undergoing treatment with immunosuppressive medication (187). More information about Candida infections in persons with diabetes is available in Chapter 30 Infections Associated With Diabetes.
Burning Mouth and Other Conditions Caused by Diabetic Neuropathy
Burning mouth syndrome, or glossodynia, is characterized by a chronic sensation of burning or irritation of the oral mucosa and/or tongue (87). It is classified as “primary” if there is no clear clinical cause for the symptoms (188). The “secondary” form of burning mouth syndrome involves presence of an identifiable cause. The evidence suggests diabetes and diabetes-related conditions of oral candidiasis, xerostomia, and poor glycemic control may be associated with burning mouth syndrome. Burning mouth syndrome is more prevalent in women who are experiencing or have experienced menopause. For instance, in the Pittsburgh EDC study, the investigators found being female and having diabetes neuropathy were significantly associated with symptoms of burning mouth syndrome (189). Synthesis of the literature supported diabetic neuropathy as an underlying cause of glossodynia in people with diabetes (87).
Additionally, this review explored the association of three other oral conditions with diabetic neuropathy: taste impairment (dysgeusia), trigeminal nerve pain, and temporomandibular joint disorders (87). Evidence consistently supported an association of diabetic neuropathy with dysgeusia, but data were sparse to deduce this association with the two other disorders (87).
Dental Care Utilization in People With Diabetes
Cross-Sectional Studies
U.S. Population-Based Studies
National Health and Nutrition Examination Surveys
Analysis of the NHANES 1999–2004 data investigated the influence of periodontitis, as well as female sex, on the association between diabetes status and dental care visits among 8,451 dentate adults age ≥25 years (190). Unlike the finding from the National Health Interview Survey (NHIS) 2003 of a female-specific association between diabetes status and dental care visits (191), described later in this section, this study did not identify such effect. A significantly lower percentage of dentate adults with diabetes had a dental visit the prior year than those without diabetes, namely 56.8% versus 64.7%, respectively, but this difference was not limited to women. Similarly, having periodontitis was not an independent predictor of dental care in dentate people with diabetes. The adjusted odds ratio for individuals with diabetes having a dental visit the preceding year were 29% lower than those without diabetes (OR 0.71, 95% CI 0.5–1.00, p<0.05).
Behavioral Risk Factor Surveillance System
Data from 105,718 dentate individuals age ≥25 years including 4,605 individuals with diabetes from 38 states who participated in the BRFSS 1995–1998 provided several findings: 1) dentate adults with diabetes were less likely than those without diabetes to have seen a dentist within the preceding 12 months (65.8% vs. 73.1%, p<0.0001); 2) dentate adults with diabetes were less likely to have seen a dentist than to have seen a health care provider for diabetes care (65.8% vs. 86.3%, respectively), and 3) for participants with diabetes, the disparity in dental visits among racial or ethnic groups and among socioeconomic groups was greater than that for any other type of health care visit, with particularly smaller proportions of Hispanic and African Americans having had dental visits (192). However, this study found the percentage who saw a dentist was comparable with the percentage who had their feet examined (67.7%) or had a dilated eye examination (62.3%). Similarly, the estimated median percentage of BRFSS 2004 participants age ≥18 years with diabetes who reported having a dental visit during the preceding 12 months was 67% (193). The proportions seeing a dentist were lower among non-Hispanic blacks, persons with lower education and income, those who lacked health insurance, and those who had never attended a class in diabetes self-management.
Another study analyzed combined data from the BRFSS 2006, 2008, and 2010 to investigate the association between health-related quality of life (HRQOL), dentate status, and receipt of dental care in adults age ≥65 years with diabetes (164). The study included 70,363 older adults with diabetes and 308,658 without diabetes. A higher proportion of participants with diabetes reported a significantly longer time interval since their last dental visit or dental cleaning than those without diabetes. For example, within the year prior to the survey, significantly fewer participants with diabetes (57.1%) visited the dentist compared to those without diabetes (69.5%). Similarly, significantly fewer participants with diabetes reported having a dental cleaning in the previous year than those without diabetes (65.0% vs. 75.5%).
National Health Interview Survey
Analysis of data from the NHIS 2003 also investigated the relation between diabetes status and dental care visits and compared diabetes care, foot care, eye care, and dental care visits among 24,189 dentate adults age ≥25 years with diabetes (191). Overall, 60.7% of adults with diabetes had a dental visit the preceding year compared to 68.0% of adults without diabetes. Among dentate men, there was no significant association between diabetes status and dental care visits, whereas dentate women with diabetes were significantly 37% less likely to have had a dental care visit than peers without diabetes. Of the four types of health care visits compared—visits to providers for diabetes care, foot care, eye care, and dental care—dentate adults with diabetes were least likely to have had a dental care visit in the preceding year. The investigators also found that disparities in health care visit rates across race/ethnicity, poverty status, education, and private insurance categories were most pronounced for dental care.
Additionally, an analysis of the NHIS 2008 found 52% of respondents with diabetes versus 60.9% of those without diabetes reported having a dental visit within the preceding year; adults with diabetes were almost twice as likely as adults without diabetes not to have had a dental visit in more than 5 years (19% vs. 11%, respectively) (194).
Medicare Current Beneficiary Survey
Self-reported receipt of preventive dental care in 2002 (N=8,725) and in 2011 (N=7,425) as reported in the Medicare Current Beneficiary Survey among fee-for-service Medicare beneficiaries (age ≥65 years), increased from 42.9% in 2002 to 45.5% in 2011 overall (195). However, among respondents with diabetes, the corresponding rates rose from only 28.8% to 36.0%, thus leaving two-thirds (64.0%) without any dental checkup.
U.S. Non-Population-Based Studies
Another study investigated the association of dental insurance and annual dental visits among 20,188 dentate adults age 30–75 years with diabetes (196). Overall, a significantly higher percentage of participants with diabetes who had dental insurance reported annual dental visits than those without dental insurance (82% vs. 61%, respectively). There were modest social disparities in dental visits reported by race/ethnicity (73% of African Americans and Latinos vs. 85% for Chinese and Filipinos). There were larger differences by socioeconomic status, with a higher percentage of participants with diabetes who earned college degrees reporting annual dental visits than those without a high school diploma (86% vs. 66%), as well as a higher percentage of participants with diabetes living in households with annual incomes of ≥$65,000 than those with annual household incomes of ≤$15,000 (85% vs. 59%) (196).
Periodontal Care, Glycemic Control, Medical Care Utilization, and Costs: Insurance Claims Data
In addition to population-based survey data describing differences in dental care utilization in individuals with and without diabetes, a small number of studies have investigated dental and medical care administrative claims data from insurance companies. Even though these data were not collected for the purpose of research, and data on important confounders were not collected, these studies have provided some additional perspectives on the impact of dental care utilization on systemic health, medical care utilization, and medical care costs in people with diabetes.
A 2-year retrospective insurance claims study among 116,306 enrollees with simultaneous medical and dental coverage concluded that insureds who received periodontitis treatment (proxy for having periodontitis) incurred significantly higher per member per month medical costs for diabetes care compared to those receiving only gingivitis treatment or no such treatment (proxy for not having periodontitis) (197).
A cross-sectional study linking 5 years of electronic medical record and dental insurance claims data for patients with diabetes investigated A1c levels among 5,103 adults age 40–70 years, comparing those who had to those who had not received periodontal treatment (198). The study reported that 38% of participants received periodontal care (i.e., had been diagnosed with periodontitis) during the previous 5 years. The average A1c level was 0.08% higher in patients who received periodontal care than in those who did not (p=0.02). The authors attributed this difference to likely reflect the effect of periodontitis on A1c levels. In stratified analyses, this association was present for women (0.18 percentage points higher for those receiving periodontal care, p<0.001), but was not significant for men (0.008 percentage points lower, p=0.86). Individuals who received more intensive periodontal treatment had lower A1c levels. Intensive periodontal treatment was defined as receiving one and two or more surgical treatments or having a total number of nonsurgical periodontal visits above the median. Individuals with more than the median number of nonsurgical visits had a mean A1c level of 0.13 (p=0.01) percentage points lower, and those with one and two or more surgical treatments had A1c levels that were 0.25 (p=0.04) and 0.36 percentage points lower, respectively, than individuals without periodontal surgeries. The investigators suggested their observation of lower A1c associated with greater intensity of periodontal treatment was consistent with meta-analyses supporting the concept that periodontal treatment improves glycemic control (198).
A retrospective cohort study investigated the effect of regular receipt of dental care on diabetes control, diabetes-specific emergency department visits, and diabetes-specific hospital admissions among adult members of a health maintenance organization with type 2 diabetes who had medical and dental insurance benefits continuously during the 36-month study period (199). Participants included 493 adults age 18–80 years who received regular dental care (defined as ≥2 dental cleaning visits, periodontal treatment visits, or both during a 12-month period) who were matched with 493 people with diabetes who did not receive regular dental care. Receipt of regular dental care was associated with lower diabetes-specific emergency room utilization (OR 0.61, 95% CI 0.40–0.92) and hospital admissions (OR 0.61, 95% CI 0.39–0.95). The analyses did not indicate a statistically significant association between receipt of regular dental care and glycemic control, but the authors suggested receipt of dental care may reduce diabetes-specific health care utilization (199).
A comprehensive literature search concluded there is almost no evidence exploring associations between perio-dontal disease and medical expenditures related to diabetes in which periodontal health status is assessed applying an objective index (200). Regrettably, the only study identified by the search was a flawed study (201,202). But the authors also reported their findings regarding 359 Japanese adults age 80 years participating in the longitudinal Niigata Study. Based on clinical periodontal examinations, they calculated the inflamed periodontal surface area, which, in a dose-response manner, was correlated with diabetes-related medical expenses (200).
Interprofessional Collaboration
Given the important mutual effects of oral health and diabetes/hyperglycemia, it is crucial for all health professionals to collaborate in an interprofessional, patient-centered team approach that includes dental care professionals for the patient with hyperglycemia. Potentially, such dental care providers could even play important managerial roles in such teams (203,204). The National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases focused on such interprofessional efforts in a comprehensive report “Redesigning the Health Care Team: Diabetes Prevention and Lifelong Management” (205) that includes a description of practical approaches in the section “Collaborative care in practice” (206).
Both dental health care providers (207) and dental patients may be ready to accept screening for hyperglycemia/diabetes in the dental setting (208). In the future, such glucose screening and monitoring might occur by using a noninvasive sampling of the GCF found in the space between the teeth and the gingiva in patients with periodontitis, because glycemic levels in GCF and in blood are closely correlated (0.991) (209).
In a medical-dental research collaboration in which a simple, nine-item questionnaire was developed (and supplemented by random capillary glucose levels for validation), a study was conducted in 13 private general dental offices in Michigan among 1,013 adult, dentate patients (mean age[±SD] 52.8[±12.7] years) who stated they did not have diabetes. However, 30% had hyperglycemia, with more than one-quarter (28.7%) having prediabetes and 1.3% having type 2 diabetes (210).
Due to the shared risk factors for periodontitis and diabetes, management of such risks is proposed to also occur in the dental office (14,148,211), with a major goal being reduction of the cumulative systemic inflammatory burden (212). Medical care providers are gradually being introduced to the notion of incorporating awareness of the health of the oral cavity in diabetes management as exemplified by a review paper with practical examples targeting nurse practitioners (213). Primary care physicians reportedly regard medical screening in dental offices as both valuable and worthwhile (214), whereas organizations and other authorities were found to have a positive attitude, but need more information and elevated awareness of the concept of dental chairside medical screening (215).
In a novel study among 143,212 Saudi adults (mean age[±SD] 57[±14.2] years, range 40–84 years), the presence of a periapical abscess was identified as a potential sign of undiagnosed diabetes, with recurrent periapical abscesses occurring specifically in uncontrolled diabetes in a dose-response relationship with A1c levels (216). Thus, both dental and medical care providers could use this sign to look for undiagnosed diabetes, especially in older patients in whom undiagnosed diabetes could cause severe health consequences. Likewise, the fact that people with diabetes consistently are reported to have fewer teeth than their normoglycemic counterparts (161) could be used by all health care providers to suspect potentially undiagnosed diabetes and other chronic diseases, especially cardiovascular disease.
The degree of missing teeth has been associated with all-cause mortality and cardiovascular mortality, as concluded by a systematic review of cohort studies (217). This was also shown among 500 participants in the Baltimore Longitudinal Study of Aging (BLSA) (218). Knowing the sign of having few or no natural teeth could aid health professionals to identify frailty and look closer for various health issues related to premature mortality or a poor longevity prognosis.
Public Health Significance of the Oral Health-Diabetes Links
Oral health-related quality of life is repeatedly found to be lower in people with diabetes. An analysis of BRFSS 2006, 2008, and 2010 data from 70,363 U.S. inhabitants age ≥65 years with diabetes and 308,658 without diabetes found that tooth loss and lack of dental care were associated with more days with low levels of general health in the preceding month (164).
Both periodontitis and diabetes are chronic, inflammation-related diseases that share virtually the same risk factors and often occur in the same individuals, with the two diseases mutually and adversely affecting each other in a two-way relationship. Therefore, any prevention or treatment provided for one of the diseases would be expected to also positively impact the other.
In the case of periodontitis, relatively simple measures are available to prevent or manage the development or progression of the periodontal tissue breakdown. Evidence exists that nonsurgical periodontal therapy supported by effective home oral hygiene measures, such as tooth brushing, decreases the amount of dental plaque and its inflammatory responses in type 2 diabetes. In addition to treatment by periodontists who are dentists with several years of specialty education, such treatment and oral hygiene instruction can be provided by dental hygienists, dental therapists, or general dentists in dental offices or clinics.
Because periodontitis and diabetes share the same risk factors, the same individuals often have both diseases. The prevalence pattern of both diseases shows severe disparities, as they affect mostly the social groups and minorities with the least resources to cope with these diseases and their consequences.
Screening and monitoring of diabetes in a dental setting could be valuable for early detection, prevention, or early treatment and monitoring (219) and, hence, improve public health beyond the health of the oral cavity.
Actual cost savings for the U.S. health care system were estimated for screenings in the dental setting of dental patients age ≥40 years for diabetes, hypertension, and hypercholesterolemia. Savings from $42.4 million ($13.51 per person screened) to $102.6 million ($32.72 per person screened) over 1 year were estimated (220). Over the longer term, greater savings could be realized by prevention and monitoring.
For 3 years, Mosen et al. followed 493 participants with type 2 diabetes who received regular dental care and 493 with type 2 diabetes who did not have regular dental visits and discovered that the former group received less diabetes-related medical care in the form of visits to the emergency room, as well as hospitalization (199).
In another 3-year study, the Mosen team followed 5,216 adults who had regular dental care and 5,216 who did not and concluded that those who received at least one annual dental visit had better adherence to 7 of 11 measures contained in the Healthcare Effectiveness Data and Information Set (HEDIS) (221), including diabetes-related screening for retinopathy and better A1c levels.
People with diabetes are consistently shown to have less frequent dental visits than their diabetes-free counterparts (190,191,192,193,194), which is especially pronounced among those who also have chronic kidney disease (222). Hence, such patients might benefit if medical care providers include attainment of a “healthy mouth as free of infection and inflammation as possible” in the management of diabetes and properly refer the patient for dental assessment and treatment if needed, complete with monitoring. Likewise, the providers of dental care can enhance the health of their patients by being aware of the possibility that their patients might have undiagnosed or poorly controlled diabetes and refer them to medical care providers for proper assessment and follow-up.
Since controlling the cumulative load of bacteria and its subsequent inflammatory responses in a person’s body by cleaning the teeth is a relatively simple and inexpensive endeavor and because periodontitis can have adverse impact on various potentially fatal systemic diseases as cardiovascular diseases and events, such periodontal treatment could have a potentially large impact on the health of the public.
The modeling approach applied to create the first estimation of periodontitis prevalence at state and local levels in the United States aids public health surveillance efforts to identify areas with a high burden of periodontitis (23). Thus, population groups to whom public health efforts should be targeted can now be better identified for preventive, therapeutic, and management purposes. Periodontitis is regarded as the “canary in the coal mine” (26) namely a sign of potentially grave danger, and can alert medical care professionals to identify and manage or treat chronic diseases and conditions early and thereby save both human suffering and societal expenses.
It is advisable that patient-centered, interprofessional collaboration be conducted by all health care providers engaged in the health and wellbeing of their mutual patients with hyperglycemia or at risk thereof for the benefit of the patients, their caregivers, and society as a whole, with potentially immense savings in human suffering and financial burdens for the individuals and for the country.
List of Abbreviations
- A1c
glycated hemoglobin
- AAP
American Academy of Periodontology
- AIDS
acquired immunodeficiency syndrome
- BMI
body mass index
- BOP
bleeding on probing (bleeding from the pocket or gingival margin after periodontal probing)
- BRFSS
Behavioral Risk Factor Surveillance System
- CAL
clinical attachment loss
- CDC
Centers for Disease Control and Prevention
- CHD
coronary heart disease
- CI
confidence interval
- CRP
C-reactive protein
- EDC
Epidemiology of Diabetes Complications study
- FPG
fasting plasma glucose
- FPL
Federal Poverty Level
- GCF
gingivo-crevicular fluid
- HDL
high density lipoprotein
- HIV
human immunodeficiency virus
- HOMA-IR
homeostasis model assessment of insulin resistance
- HPFS
Health Professionals Follow-Up Study
- HR
hazard ratio
- IFG
impaired fasting glucose
- IgA/IgG
immunoglobulin A/G
- IGT
impaired glucose tolerance
- IMT
intimal-medial wall thickness
- LGA
large for gestational age
- NGT
normal glucose tolerance
- NHANES
National Health and Nutrition Examination Survey
- NHIS
National Health Interview Survey
- OGTT
oral glucose tolerance test
- OR
odds ratio
- PPD
periodontal probing depth
- RCT
randomized controlled trial
- RR
risk ratio
- SAE
small area estimation
- SD
standard deviation
- SE
standard error
- SHIP
Study of Health in Pomerania
- WBC
white blood cell count
References
- 1.
- Genco RJ: Assessment of risk of periodontal disease. Compend Suppl S678–S683, 1994 [PubMed: 8039205]
- 2.
- Genco RJ, Löe H: The role of systemic conditions and disorders in periodontal disease. Periodontol 2000 2:98–116, 1993 [PubMed: 9673184]
- 3.
- Safkan-Seppälä B, Ainamo J: Periodontal conditions in insulin-dependent diabetes mellitus. J Clin Periodontol 19:24–29, 1992 [PubMed: 1732306]
- 4.
- Löe H: Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 16:329–334, 1993 [PubMed: 8422804]
- 5.
- Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ: Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol 67(10 Suppl):1085–1093, 1996 [PubMed: 8910827]
- 6.
- Grossi SG, Genco RJ: Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol 3:51–61, 1998 [PubMed: 9722690]
- 7.
- Mealey BL: Periodontal disease and diabetes. A two-way street. J Am Dent Assoc 137(Suppl):26S–31S, 2006 [PubMed: 17012733]
- 8.
- Taylor GW: Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol 6:99–112, 2001 [PubMed: 11887478]
- 9.
- National Center for Health Statistics: About the National Health and Nutrition Examination Survey [article online], 2017. Available from https://www
.cdc.gov/nchs /nhanes/about_nhanes.htm. Accessed 16 Jun 2018 - 10.
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA: Accuracy of NHANES periodontal examination protocols. J Dent Res 89:1208–1213, 2010 [PubMed: 20858782]
- 11.
- Bartold PM, Van Dyke TE: Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontol 2000 62:203–217, 2013 [PMC free article: PMC3692012] [PubMed: 23574467]
- 12.
- Pihlstrom BL, Michalowicz BS, Johnson NW: Periodontal diseases. Lancet 366:1809–1820, 2005 [PubMed: 16298220]
- 13.
- Xiong X, Buekens P, Vastardis S, Yu SM: Periodontal disease and pregnancy outcomes: state-of-the-science. Obstet Gynecol Surv 62:605–615, 2007 [PubMed: 17705886]
- 14.
- Borgnakke WS: Modifiable risk factors for periodontitis and diabetes. Curr Oral Health Rep 3:254–269, 2016
- 15.
- Clerehugh V, Tugnait A, Genco RJ: Periodontology at a Glance. 1st ed. Oxford, U.K., Wiley-Blackwell, 2009
- 16.
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance Workgroup: Beck J, Douglass G, Page R, Slade G, Taylor GW, Borgnakke WS; American Academy of Periodontology: Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914–920, 2012 [PubMed: 22935673]
- 17.
- Eke PI, Genco RJ: CDC Periodontal Disease Surveillance Project: background, objectives, and progress report. J Periodontol 78(7 Suppl):1366–1371, 2007 [PubMed: 17610396]
- 18.
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ: Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83:1449–1454, 2012 [PMC free article: PMC6005373] [PubMed: 22420873]
- 19.
- Page RC, Eke PI: Case definitions for use in population-based surveillance of periodontitis. J Periodontol 78(7 Suppl):1387–1399, 2007 [PubMed: 17608611]
- 20.
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ: Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol 86:611–622, 2015 [PMC free article: PMC4460825] [PubMed: 25688694]
- 21.
- Eke PI, Wei L, Borgnakke WS, Thornton-Evans G, Zhang X, Lu H, McGuire LC, Genco RJ: Periodontitis prevalence in adults ≥65 years of age, in the USA. Periodontol 2000 72:76–95, 2016 [PMC free article: PMC8223257] [PubMed: 27501492]
- 22.
- Weatherspoon DJ, Borrell LN, Johnson CW, Mujahid MS, Neighbors HW, Adar SD: Racial and ethnic differences in self-reported periodontal disease in the Multi-Ethnic Study of Atherosclerosis (MESA). Oral Health Prev Dent 14:249–257, 2016 [PMC free article: PMC4970861] [PubMed: 26870845]
- 23.
- Eke PI, Zhang X, Lu H, Wei L, Thornton-Evans G, Greenlund KJ, Holt JB, Croft JB: Predicting periodontitis at state and local levels in the United States. J Dent Res 95:515–522, 2016 [PMC free article: PMC6092742] [PubMed: 26848071]
- 24.
- Centers for Disease Control and Prevention; Division of Diabetes Translation; United States Diabetes Surveillance System: Maps of trends in diagnosed diabetes [article online], 2017. Available from https://www
.cdc.gov/diabetes /statistics/slides /maps_diabetes_trends.pdf. Accessed 16 Jun 2018 - 25.
- Centers for Disease Control and Prevention: National Diabetes Statistics Report, 2017. Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2017
- 26.
- Borgnakke WS, Glick M, Genco RJ: Periodontitis: the canary in the coal mine. J Am Dent Assoc 144:764–766, 2013 [PubMed: 23813251]
- 27.
- Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ: Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol 40(Suppl 14):S135–S152, 2013 [PubMed: 23627324]
- 28.
- Borgnakke WS: Chapter 6: Hyperglycemia/diabetes mellitus and periodontal infection adversely affect each other. In Periodontal Disease and Overall Health: A Clinician’s Guide. 2nd ed. Genco RJ, Williams RC, Eds. Yardley, PA, Professional Audience Communications, 2014, p. 99–122
- 29.
- Borgnakke WS, Genco RJ: Chapter 68: Periodontal disease and diabetes mellitus. In International Textbook of Diabetes Mellitus. 4th ed. DeFronzo RA, Ferrannini E, Zimmet P, Alberti KGMM, Eds. Chichester, West Sussex, U.K., John Wiley & Sons, Ltd, 2015, p. 988–1004
- 30.
- Lamster IB, Lalla E, Borgnakke WS, Taylor GW: The relationship between oral health and diabetes mellitus. J Am Dent Assoc 139(Suppl):19S–24S, 2008 [PubMed: 18809650]
- 31.
- Taylor GW, Borgnakke WS: Relationship of periodontal infection to diabetes: glycemic control, complications and occurrence. J Dent Hlth 61:170–177, 2011
- 32.
- Taylor GW, Borgnakke WS: Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis 14:191–203, 2008 [PubMed: 18336370]
- 33.
- Choi YH, McKeown RE, Mayer-Davis EJ, Liese AD, Song KB, Merchant AT: Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care 34:381–386, 2011 [PMC free article: PMC3024353] [PubMed: 21216848]
- 34.
- Demmer RT, Squillaro A, Papapanou PN, Rosenbaum M, Friedewald WT, Jacobs DR, Jr., Desvarieux M: Periodontal infection, systemic inflammation, and insulin resistance: results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care 35:2235–2242, 2012 [PMC free article: PMC3476901] [PubMed: 22837370]
- 35.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419, 1985 [PubMed: 3899825]
- 36.
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr., Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association: Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511, 2003 [PubMed: 12551878]
- 37.
- Arora N, Papapanou PN, Rosenbaum M, Jacobs DR, Jr., Desvarieux M, Demmer RT: Periodontal infection, impaired fasting glucose and impaired glucose tolerance: results from the continuous National Health and Nutrition Examination Survey 2009–2010. J Clin Periodontol 41:643–652, 2014 [PMC free article: PMC4072528] [PubMed: 24708451]
- 38.
- American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 35(Suppl 1):S64–S71, 2012 [PMC free article: PMC3632174] [PubMed: 22187472]
- 39.
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, Koga T: The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J Dent Res 83:485–490, 2004 [PubMed: 15153457]
- 40.
- Demmer RT, Desvarieux M, Holtfreter B, Jacobs DR, Jr., Wallaschofski H, Nauck M, Völzke H, Kocher T: Periodontal status and A1c change: longitudinal results from the Study of Health in Pomerania (SHIP). Diabetes Care 33:1037–1043, 2010 [PMC free article: PMC2858171] [PubMed: 20185742]
- 41.
- Teeuw WJ, Gerdes VE, Loos BG: Effect of periodontal treatment on glycemic control of diabetic patients: a systematic review and meta-analysis. Diabetes Care 33:421–427, 2010 [PMC free article: PMC2809296] [PubMed: 20103557]
- 42.
- Simpson TC, Needleman I, Wild SH, Moles DR, Mills EJ: Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database Syst Rev 5:CD004714, 2010 [PubMed: 20464734]
- 43.
- Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A: Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol 84:958–973, 2013 [PubMed: 23106512]
- 44.
- Engebretson S, Kocher T: Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Periodontol 84(4 Suppl):S153–S169, 2013 [PMC free article: PMC4100543] [PubMed: 23631575]
- 45.
- Liew AK, Punnanithinont N, Lee YC, Yang J: Effect of non-surgical periodontal treatment on HbA1c: a meta-analysis of randomized controlled trials. Aust Dent J 58:350–357, 2013 [PubMed: 23981218]
- 46.
- Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD: Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J Diabetes Investig 4:502–509, 2013 [PMC free article: PMC4025114] [PubMed: 24843701]
- 47.
- Sun QY, Feng M, Zhang MZ, Zhang YQ, Cao MF, Bian LX, Guan QB, Song KL: Effects of periodontal treatment on glycemic control in type 2 diabetic patients: a meta-analysis of randomized controlled trials. Chin J Physiol 57:305–314, 2014 [PubMed: 25575518]
- 48.
- Wang TF, Jen IA, Chou C, Lei YP: Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta-analysis. Medicine (Baltimore) 93:e292, 2014 Dec 2 [Epub] doi: 10.1097/MD.0000000000000292 [PMC free article: PMC4603101] [PubMed: 25526470] [CrossRef]
- 49.
- Li Q, Hao S, Fang J, Xie J, Kong XH, Yang JX: Effect of non-surgical periodontal treatment on glycemic control of patients with diabetes: a meta-analysis of randomized controlled trials. Trials 16:291, 2015 Jul 3 [Epub] doi: 10.1186/s13063-015-0810-2 [PMC free article: PMC4490675] [PubMed: 26137892] [CrossRef]
- 50.
- Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, Stevenson B, Furness S, Iheozor-Ejiofor Z: Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev 11:CD004714, 2015 [PMC free article: PMC6486035] [PubMed: 26545069]
- 51.
- Teshome A, Yitayeh A: The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health 17:31, 2016 Jul 30 [Epub] doi: 10.1186/s12903-016-0249-1 [PMC free article: PMC4967318] [PubMed: 27473177] [CrossRef]
- 52.
- Janket SJ, Wightman A, Baird AE, Van Dyke TE, Jones JA: Does periodontal treatment improve glycemic control in diabetic patients? A meta-analysis of intervention studies. J Dent Res 84:1154–1159, 2005 [PMC free article: PMC1797067] [PubMed: 16304446]
- 53.
- Darré L, Vergnes JN, Gourdy P, Sixou M: Efficacy of periodontal treatment on glycaemic control in diabetic patients: a meta-analysis of interventional studies. Diabetes Metab 34:497–506, 2008 [PubMed: 18948050]
- 54.
- Wang X, Han X, Guo X, Luo X, Wang D: The effect of periodontal treatment on hemoglobin A1c levels of diabetic patients: a systematic review and meta-analysis. PLOS One 9:e108412, 2014 Sep 25 [Epub] doi: 10.1371/journal.pone.0108412 [PMC free article: PMC4177914] [PubMed: 25255331] [CrossRef]
- 55.
- Botero JE, Rodriguez C, Agudelo-Suarez AA: Periodontal treatment and glycaemic control in patients with diabetes and periodontitis: an umbrella review. Aust Dent J 61:134–148, 2016 [PubMed: 26815303]
- 56.
- D’Aiuto F, Gable D, Syed Z, Allen Y, Wanyonyi KL, White S, Gallagher JE: Evidence summary: the relationship between oral diseases and diabetes. Br Dent J 222:944–948, 2017 [PubMed: 28642531]
- 57.
- Faggion CM, Jr., Cullinan MP, Atieh M: An overview of systematic reviews on the effectiveness of periodontal treatment to improve glycaemic control. J Periodontal Res 51:716–725, 2016 [PubMed: 26913689]
- 58.
- Hasuike A, Iguchi S, Suzuki D, Kawano E, Sato S: Systematic review and assessment of systematic reviews examining the effect of periodontal treatment on glycemic control in patients with diabetes. Med Oral Patol Oral Cir Bucal 22:e167–e176, 2017 [PMC free article: PMC5359698] [PubMed: 28160589]
- 59.
- Ibrahim M, Abu Al Magd M, Annabi FA, Assaad-Khalil S, Ba-Essa EM, Fahdil I, Karadeniz S, Meriden T, Misha’l AA, Pozzilli P, Shera S, Thomas A, Bahijri S, Tuomilehto J, Yilmaz T, Umpierrez GE: Recommendations for management of diabetes during Ramadan: update 2015. BMJ Open Diabetes Res Care 3:e000108, 2015 Jun 16 [Epub] doi: 10.1136/bmjdrc-2015-000108 [PMC free article: PMC4477152] [PubMed: 26113983] [CrossRef]
- 60.
- Kurukulasuriya LR, Sowers JR: Therapies for type 2 diabetes: lowering HbA1c and associated cardiovascular risk factors. Cardiovasc Diabetol 9:45, 2010 Aug 30 [Epub] doi: 10.1186/1475-2840-9-45 [PMC free article: PMC2940872] [PubMed: 20804556] [CrossRef]
- 61.
- Engebretson SP, Hey-Hadavi J: Sub-antimicrobial doxycycline for periodontitis reduces hemoglobin A1c in subjects with type 2 diabetes: a pilot study. Pharmacol Res 64:624–629, 2011 [PMC free article: PMC3195946] [PubMed: 21782948]
- 62.
- Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, Seaquist ER, Reddy MS, Lewis CE, Oates TW, Tripathy D, Katancik JA, Orlander PR, Paquette DW, Hanson NQ, Tsai MY: The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA 310:2523–2532, 2013 [PMC free article: PMC4089989] [PubMed: 24346989]
- 63.
- Gay IC, Tran DT, Cavender AC, Weltman R, Chang J, Luckenbach E, Tribble GD: The effect of periodontal therapy on glycaemic control in a Hispanic population with type 2 diabetes: a randomized controlled trial. J Clin Periodontol 41:673–680, 2014 [PMC free article: PMC4080623] [PubMed: 24797222]
- 64.
- Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, Dunford RG, Genco RJ: Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol 68:713–719, 1997 [PubMed: 9287060]
- 65.
- Jones JA, Miller DR, Wehler CJ, Rich SE, Krall-Kaye EA, McCoy LC, Christiansen CL, Rothendler JA, Garcia RI: Does periodontal care improve glycemic control? The Department of Veterans Affairs Dental Diabetes Study. J Clin Periodontol 34:46–52, 2007 [PubMed: 17137468]
- 66.
- Taylor GW, Manz MC, Borgnakke WS, Eber RM, Pritzel SJ, Herman WH, Offenbacher S, Genco RJ: Treating periodontal infection; effects on glycemic control (Abstract). J Dent Res 89:#139739, 2010
- 67.
- Al-Mubarak S, Ciancio S, Aljada A, Mohanty P, Ross C, Dandona P: Comparative evaluation of adjunctive oral irrigation in diabetics. J Clin Periodontol 29:295–300, 2002 [PubMed: 11966926]
- 68.
- Engebretson S, Gelato M, Hyman L, Michalowicz BS, Schoenfeld E; DPTT Study Group: Design features of the Diabetes and Periodontal Therapy Trial (DPTT): a multicenter randomized single-masked clinical trial testing the effect of nonsurgical periodontal therapy on glycosylated hemoglobin (HbA1c) levels in subjects with type 2 diabetes and chronic periodontitis. Contemp Clin Trials 36:515–526, 2013 [PMC free article: PMC3885354] [PubMed: 24080100]
- 69.
- Borgnakke WS, Chapple IL, Genco RJ, Armitage G, Bartold PM, D’Aiuto F, Eke PI, Giannobile WV, Kocher T, Kornman KS, Lang NP, Madianos PN, Murakami S, Nishimura F, Offenbacher S, Preshaw PM, Rahman AU, Sanz M, Slots J, Tonetti MS, Van Dyke TE: The multi-center randomized controlled trial (RCT) published by the Journal of the American Medical Association (JAMA) on the effect of periodontal therapy on glycated hemoglobin (HbA1c) has fundamental problems. J Evid Based Dent Pract 14:127–132, 2014 [PMC free article: PMC4502578] [PubMed: 25234213]
- 70.
- Chapple IL, Borgnakke WS, Genco RJ: Hemoglobin A1c levels among patients with diabetes receiving nonsurgical periodontal treatment. JAMA 311:1919–1920, 2014 [PubMed: 24825650]
- 71.
- Engebretson SP, Hyman LG, Michalowicz BS: Hemoglobin A1c levels among patients with diabetes receiving nonsurgical periodontal treatment—reply. JAMA 311:1921–1922, 2014 [PubMed: 24825652]
- 72.
- Merchant AT: Hemoglobin A1c levels among patients with diabetes receiving nonsurgical periodontal treatment. JAMA 311:1919, 2014 [PubMed: 24825649]
- 73.
- Vergnes JN: Hemoglobin A1c levels among patients with diabetes receiving nonsurgical periodontal treatment. JAMA 311:1920–1921, 2014 [PubMed: 24825651]
- 74.
- Merchant AT, Georgantopoulos P, Howe CJ, Virani SS, Morales DA, Haddock KS: Effect of long-term periodontal care on hemoglobin A1c in type 2 diabetes. J Dent Res 95:408–415, 2016 [PMC free article: PMC4802779] [PubMed: 26701348]
- 75.
- Grellmann AP, Sfreddo CS, Maier J, Lenzi TL, Zanatta FB: Systemic antimicrobials adjuvant to periodontal therapy in diabetic subjects: a meta-analysis. J Clin Periodontol 43:250–260, 2016 [PubMed: 26790108]
- 76.
- Rovai ES, Souto ML, Ganhito JA, Holzhausen M, Chambrone L, Pannuti CM: Efficacy of local antimicrobials in the non-surgical treatment of patients with periodontitis and diabetes: a systematic review. J Periodontol 87:1406–1417, 2016 [PubMed: 27468792]
- 77.
- Borgnakke WS: Does treatment of periodontal disease influence systemic disease? Dent Clin North Am 59:885–917, 2015 [PubMed: 26427573]
- 78.
- Artese HP, Foz AM, Rabelo Mde S, Gomes GH, Orlandi M, Suvan J, D’Aiuto F, Romito GA: Periodontal therapy and systemic inflammation in type 2 diabetes mellitus: a meta-analysis. PLOS One 10:e0128344, 2015 May 26 [Epub] doi: 10.1371/journal.pone.0128344 [PMC free article: PMC4444100] [PubMed: 26010492] [CrossRef]
- 79.
- Demmer RT, Jacobs DR, Jr., Desvarieux M: Periodontal disease and incident type 2 diabetes: results from the first National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care 31:1373–1379, 2008 [PMC free article: PMC2453650] [PubMed: 18390797]
- 80.
- Morita I, Inagaki K, Nakamura F, Noguchi T, Matsubara T, Yoshii S, Nakagaki H, Mizuno K, Sheiham A, Sabbah W: Relationship between periodontal status and levels of glycated hemoglobin. J Dent Res 91:161–166, 2012 [PubMed: 22157098]
- 81.
- Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T: Periodontal disease and incident diabetes: a seven-year study. J Dent Res 90:41–46, 2011 [PubMed: 21041549]
- 82.
- Abariga SA, Whitcomb BW: Periodontitis and gestational diabetes mellitus: a systematic review and meta-analysis of observational studies. BMC Pregnancy Childbirth 16:344, 2016 Nov 8 [Epub] doi: 10.1186/s12884-016-1145-z. [PMC free article: PMC5101727] [PubMed: 27825315] [CrossRef]
- 83.
- Xiong X, Elkind-Hirsch KE, Vastardis S, Delarosa RL, Pridjian G, Buekens P: Periodontal disease is associated with gestational diabetes mellitus: a case-control study. J Periodontol 80:1742–1749, 2009 [PMC free article: PMC3011834] [PubMed: 19905944]
- 84.
- Xiong X, Elkind-Hirsch KE, Xie Y, Delarosa R, Maney P, Pridjian G, Buekens P: Periodontal disease as a potential risk factor for the development of diabetes in women with a prior history of gestational diabetes mellitus. J Public Health Dent 73:41–49, 2013 [PubMed: 23215856]
- 85.
- Dasanayake AP, Chhun N, Tanner AC, Craig RG, Lee MJ, Moore AF, Norman RG: Periodontal pathogens and gestational diabetes mellitus. J Dent Res 87:328–333, 2008 [PMC free article: PMC2561333] [PubMed: 18362313]
- 86.
- Fisher MA, Borgnakke WS, Taylor GW: Periodontal disease as a risk marker in coronary heart disease and chronic kidney disease. Curr Opin Nephrol Hypertens 19:519–526, 2010 [PMC free article: PMC3084591] [PubMed: 20948377]
- 87.
- Borgnakke WS, Anderson PF, Shannon C, Jivanescu A: Is there a relationship between oral health and diabetic neuropathy? Curr Diab Rep 15:93, 2015 [PubMed: 26374570]
- 88.
- Bajaj S, Prasad S, Gupta A, Singh VB: Oral manifestations in type-2 diabetes and related complications. Indian J Endocrinol Metab 16:777–779, 2012 [PMC free article: PMC3475903] [PubMed: 23087863]
- 89.
- Amiri AA, Maboudi A, Bahar A, Farokhfar A, Daneshvar F, Khoshgoeian HR, Nasohi M, Khalilian A: Relationship between type 2 diabetic retinopathy and periodontal disease in Iranian adults. N Am J Med Sci 6:139–144, 2014 [PMC free article: PMC3978937] [PubMed: 24741553]
- 90.
- Hujoel PP, Stott-Miller M: Retinal and gingival hemorrhaging and chronic hyperglycemia. Diabetes Care 34:181–183, 2011 [PMC free article: PMC3005455] [PubMed: 20937687]
- 91.
- Wagley S, Marra KV, Salhi RA, Gautam S, Campo R, Veale P, Veale J, Arroyo JG: Periodontal disease and age-related macular degeneration: results from the National Health and Nutrition Examination Survey III. Retina 35:982–988, 2015 [PubMed: 25627087]
- 92.
- Boillot A, Bouchard P, Moss K, Offenbacher S, Czernichow S: Periodontitis and retinal microcirculation in the Atherosclerosis Risk in Communities study. J Clin Periodontol 42:342–349, 2015 [PMC free article: PMC6524540] [PubMed: 25728988]
- 93.
- Southerland JH, Moss K, Taylor GW, Beck JD, Pankow J, Gangula PR, Offenbacher S: Periodontitis and diabetes associations with measures of atherosclerosis and CHD. Atherosclerosis 222:196–201, 2012 [PubMed: 22440543]
- 94.
- Emrich LJ, Shlossman M, Genco RJ: Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol 62:123–131, 1991 [PubMed: 2027060]
- 95.
- Saremi A, Nelson RG, Tulloch-Reid M, Hanson RL, Sievers ML, Taylor GW, Shlossman M, Bennett PH, Genco R, Knowler WC: Periodontal disease and mortality in type 2 diabetes. Diabetes Care 28:27–32, 2005 [PubMed: 15616229]
- 96.
- Shultis WA, Weil EJ, Looker HC, Curtis JM, Shlossman M, Genco RJ, Knowler WC, Nelson RG: Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care 30:306–311, 2007 [PubMed: 17259499]
- 97.
- Li Q, Chalmers J, Czernichow S, Neal B, Taylor BA, Zoungas S, Poulter N, Woodward M, Patel A, de Galan B, Batty GD; ADVANCE Collaborative Group: Oral disease and subsequent cardiovascular disease in people with type 2 diabetes: a prospective cohort study based on the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation (ADVANCE) trial. Diabetologia 53:2320–2327, 2010 [PMC free article: PMC4170775] [PubMed: 20700576]
- 98.
- Noma H, Sakamoto I, Mochizuki H, Tsukamoto H, Minamoto A, Funatsu H, Yamashita H, Nakamura S, Kiriyama K, Kurihara H, Mishima HK: Relationship between periodontal disease and diabetic retinopathy. Diabetes Care 27:615, 2004 [PubMed: 14747249]
- 99.
- Thorstensson H, Kuylenstierna J, Hugoson A: Medical status and complications in relation to periodontal disease experience in insulin-dependent diabetics. J Clin Periodontol 23:194–202, 1996 [PubMed: 8707978]
- 100.
- Shi Q, Zhang B, Huo N, Cai C, Liu H, Xu J: Association between myocardial infarction and periodontitis: a meta-analysis of case-control studies. Front Physiol 7:519, 2016 Nov 4 [Epub] doi: 10.3389/fphys.2016.00519 [PMC free article: PMC5095113] [PubMed: 27867362] [CrossRef]
- 101.
- Liu LH, Li EM, Zhong SL, Li YQ, Yang ZY, Kang R, Zhao SK, Li FT, Wan SP, Zhao ZG: Chronic periodontitis and the risk of erectile dysfunction: a systematic review and meta-analysis. Int J Impot Res 29:43–48, 2017 [PubMed: 27829669]
- 102.
- Wang Q, Kang J, Cai X, Wu Y, Zhao L: The association between chronic periodontitis and vasculogenic erectile dysfunction: a systematic review and meta-analysis. J Clin Periodontol 43:206–215, 2016 [PubMed: 26749274]
- 103.
- Schulze A, Schonauer M, Busse M: Sudden improvement of insulin sensitivity related to an endodontic treatment. J Periodontol 78:2380–2384, 2007 [PubMed: 18052712]
- 104.
- Gomes MS, Blattner TC, Sant’Ana Filho M, Grecca FS, Hugo FN, Fouad AF, Reynolds MA: Can apical periodontitis modify systemic levels of inflammatory markers? A systematic review and meta-analysis. J Endod 39:1205–1217, 2013 [PubMed: 24041380]
- 105.
- Liljestrand JM, Mäntylä P, Paju S, Buhlin K, Kopra KA, Persson GR, Hernandez M, Nieminen MS, Sinisalo J, Tjäderhane L, Pussinen PJ: Association of endodontic lesions with coronary artery disease. J Dent Res 95:1358–1365, 2016 [PubMed: 27466397]
- 106.
- Harrison R, Bowen WH: Periodontal health, dental caries, and metabolic control in insulin-dependent diabetic children and adolescents. Pediatr Dent 9:283–286, 1987 [PubMed: 3507646]
- 107.
- Genco RJ: Current view of risk factors for periodontal diseases. J Periodontol 67(10 Suppl):1041–1049, 1996 [PubMed: 8910821]
- 108.
- Kinane DF, Chestnutt IG: Relationship of diabetes to periodontitis. Curr Opin Periodontol 4:29–34, 1997 [PubMed: 9655018]
- 109.
- Tsai C, Hayes C, Taylor GW: Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol 30:182–192, 2002 [PubMed: 12000341]
- 110.
- Garcia D, Tarima S, Okunseri C: Periodontitis and glycemic control in diabetes: NHANES 2009 to 2012. J Periodontol 86:499–506, 2015 [PubMed: 25427615]
- 111.
- Shlossman M, Knowler WC, Pettitt DJ, Genco RJ: Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc 121:532–536, 1990 [PubMed: 2212346]
- 112.
- Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, Knowler WC: Periodontal disease and NIDDM in Pima Indians. Diabetes Care 13:836–840, 1990 [PubMed: 2209317]
- 113.
- Moore PA, Weyant RJ, Mongelluzzo MB, Myers DE, Rossie K, Guggenheimer J, Block HM, Huber H, Orchard T: Type 1 diabetes mellitus and oral health: assessment of periodontal disease. J Periodontol 70:409–417, 1999 [PubMed: 10328653]
- 114.
- Lalla E, Cheng B, Lal S, Tucker S, Greenberg E, Goland R, Lamster IB: Periodontal changes in children and adolescents with diabetes: a case-control study. Diabetes Care 29:295–299, 2006 [PubMed: 16443876]
- 115.
- Pinson M, Hoffman WH, Garnick JJ, Litaker MS: Periodontal disease and type I diabetes mellitus in children and adolescents. J Clin Periodontol 22:118–123, 1995 [PubMed: 7775667]
- 116.
- Cianciola LJ, Park BH, Bruck E, Mosovich L, Genco RJ: Prevalence of periodontal disease in insulin-dependent diabetes mellitus (juvenile diabetes). J Am Dent Assoc 104:653–660, 1982 [PubMed: 7042797]
- 117.
- Ringelberg ML, Dixon DO, Francis AO, Plummer RW: Comparison of gingival health and gingival crevicular fluid flow in children with and without diabetes. J Dent Res 56:108–111, 1977 [PubMed: 320239]
- 118.
- Lalla E, Cheng B, Lal S, Kaplan S, Softness B, Greenberg E, Goland RS, Lamster IB: Diabetes mellitus promotes periodontal destruction in children. J Clin Periodontol 34:294–298, 2007 [PubMed: 17378885]
- 119.
- Lal S, Cheng B, Kaplan S, Softness B, Greenberg E, Goland RS, Lalla E, Lamster IB: Gingival bleeding in 6- to 13-year-old children with diabetes mellitus. Pediatr Dent 29:426–430, 2007 [PubMed: 18027779]
- 120.
- Lalla E, Cheng B, Lal S, Kaplan S, Softness B, Greenberg E, Goland RS, Lamster IB: Diabetes-related parameters and periodontal conditions in children. J Periodontal Res 42:345–349, 2007 [PubMed: 17559632]
- 121.
- Lalla E: Periodontal infections and diabetes mellitus: when will the puzzle be complete? J Clin Periodontol 34:913–916, 2007 [PubMed: 17935499]
- 122.
- Lalla E, Papapanou PN: Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 7:738–748, 2011 [PubMed: 21709707]
- 123.
- Abrao L, Chagas JK, Schmid H: Periodontal disease and risk for neuropathic foot ulceration in type 2 diabetes. Diabetes Res Clin Pract 90:34–39, 2010 [PubMed: 20637517]
- 124.
- Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ: Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol 69:76–83, 1998 [PubMed: 9527565]
- 125.
- Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M: Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol 3:30–39, 1998 [PubMed: 9722688]
- 126.
- Bandyopadhyay D, Marlow NM, Fernandes JK, Leite RS: Periodontal disease progression and glycaemic control among Gullah African Americans with type-2 diabetes. J Clin Periodontol 37:501–509, 2010 [PMC free article: PMC2891073] [PubMed: 20507373]
- 127.
- Jimenez M, Hu FB, Marino M, Li Y, Joshipura KJ: Type 2 diabetes mellitus and 20 year incidence of periodontitis and tooth loss. Diabetes Res Clin Pract 98:494–500, 2012 [PMC free article: PMC3551264] [PubMed: 23040240]
- 128.
- Ismail AF, McGrath CP, Yiu CK: Oral health of children with type 1 diabetes mellitus: a systematic review. Diabetes Res Clin Pract 108:369–381, 2015 [PubMed: 25817182]
- 129.
- Demmer RT, Holtfreter B, Desvarieux M, Jacobs DR, Jr., Kerner W, Nauck M, Völzke H, Kocher T: The influence of type 1 and type 2 diabetes on periodontal disease progression: Prospective results from the Study of Health in Pomerania (SHIP). Diabetes Care 35:2036–2042, 2012 [PMC free article: PMC3447825] [PubMed: 22855731]
- 130.
- Kaye EK, Chen N, Cabral HJ, Vokonas P, Garcia RI: Metabolic syndrome and periodontal disease progression in men. J Dent Res 95:822–828, 2016 [PMC free article: PMC4914866] [PubMed: 27025874]
- 131.
- Lalla E, Cheng B, Kunzel C, Burkett S, Lamster IB: Dental findings and identification of undiagnosed hyperglycemia. J Dent Res 92:888–892, 2013 [PubMed: 23979781]
- 132.
- Lamster IB, Cheng B, Burkett S, Lalla E: Periodontal findings in individuals with newly identified pre-diabetes or diabetes mellitus. J Clin Periodontol 41:1055–1060, 2014 [PubMed: 25195497]
- 133.
- Lamster I: Diabetes mellitus and tooth loss. Colgate Oral Care Report Series 26:1–3, 2016
- 134.
- Diabetes Mellitus and Oral Health: An Interprofessional Approach. Lamster I, Ed. Oxford, U.K., John Wiley & Sons, Inc., 2014
- 135.
- Morita T, Yamazaki Y, Mita A, Takada K, Seto M, Nishinoue N, Sasaki Y, Motohashi M, Maeno M: A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol 81:512–519, 2010 [PubMed: 20367094]
- 136.
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, Yamashita Y: Relationship between obesity, glucose tolerance, and periodontal disease in Japanese women: the Hisayama study. J Periodontal Res 40:346–353, 2005 [PubMed: 15966913]
- 137.
- Saito T, Murakami M, Shimazaki Y, Matsumoto S, Yamashita Y: The extent of alveolar bone loss is associated with impaired glucose tolerance in Japanese men. J Periodontol 77:392–397, 2006 [PubMed: 16512753]
- 138.
- Zadik Y, Bechor R, Galor S, Levin L: Periodontal disease might be associated even with impaired fasting glucose. Br Dent J 208:E20, 2010 Mar 26 [Epub] doi: 10.1038/sj.bdj.2010.291 [PubMed: 20339371] [CrossRef]
- 139.
- Esteves Lima RP, Cyrino RM, de Carvalho Dutra B, Oliveira da Silveira J, Martins CC, Miranda Cota LO, Costa FO: Association between periodontitis and gestational diabetes mellitus: systematic review and meta-analysis. J Periodontol 87:48–57, 2016 [PubMed: 26334246]
- 140.
- Novak KF, Taylor GW, Dawson DR, Ferguson JE, 2nd, Novak MJ: Periodontitis and gestational diabetes mellitus: exploring the link in NHANES III. J Public Health Dent 66:163–168, 2006 [PubMed: 16913241]
- 141.
- Xiong X, Buekens P, Vastardis S, Pridjian G: Periodontal disease and gestational diabetes mellitus. Am J Obstet Gynecol 195:1086–1089, 2006 [PubMed: 16846573]
- 142.
- Lima SM, Grisi DC, Kogawa EM, Franco OL, Peixoto VC, Goncalves-Junior JF, Arruda MP, Rezende TM: Diabetes mellitus and inflammatory pulpal and periapical disease: a review. Int Endod J 46:700–709, 2013 [PubMed: 23442003]
- 143.
- Segura-Egea JJ, Castellanos-Cosano L, Machuca G, Lopez-Lopez J, Martin-Gonzalez J, Velasco-Ortega E, Sanchez-Dominguez B, Lopez-Frias FJ: Diabetes mellitus, periapical inflammation and endodontic treatment outcome. Med Oral Patol Oral Cir Bucal 17:e356–e361, 2012 [PMC free article: PMC3448330] [PubMed: 22143698]
- 144.
- Segura-Egea JJ, Jimenez-Pinzon A, Rios-Santos JV, Velasco-Ortega E, Cisneros-Cabello R, Poyato-Ferrera M: High prevalence of apical periodontitis amongst type 2 diabetic patients. Int Endod J 38:564–569, 2005 [PubMed: 16011776]
- 145.
- Segura-Egea JJ, Martin-Gonzalez J, Cabanillas-Balsera D, Fouad AF, Velasco-Ortega E, Lopez-Lopez J: Association between diabetes and the prevalence of radiolucent periapical lesions in root-filled teeth: systematic review and meta-analysis. Clin Oral Investig 20:1133–1141, 2016 [PubMed: 27055847]
- 146.
- Monje A, Catena A, Borgnakke WS: Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: systematic review and meta-analysis. J Clin Periodontol 44:636–648, 2017 [PubMed: 28346753]
- 147.
- Shi Q, Xu J, Huo N, Cai C, Liu H: Does a higher glycemic level lead to a higher rate of dental implant failure? A meta-analysis. J Am Dent Assoc 147:875–881, 2016 [PubMed: 27435008]
- 148.
- Borgnakke WS: “Non-modifiable” risk factors for periodontitis and diabetes. Curr Oral Health Rep 3:270–281, 2016
- 149.
- Genco RJ, Borgnakke WS: Risk factors for periodontal disease. Periodontol 2000 62:59–94, 2013 [PubMed: 23574464]
- 150.
- Eke PI, Wei L, Thornton-Evans GO, Borrell LN, Borgnakke WS, Dye B, Genco RJ: Risk indicators for periodontitis in US adults: NHANES 2009 to 2012. J Periodontol 87:1174–1185, 2016 [PubMed: 27367420]
- 151.
- Salvi GE, Kandylaki M, Troendle A, Persson GR, Lang NP: Experimental gingivitis in type 1 diabetics: a controlled clinical and microbiological study. J Clin Periodontol 32:310–316, 2005 [PubMed: 15766376]
- 152.
- Shim E, Babu JP: Glycated albumin produced in diabetic hyperglycemia promotes monocyte secretion of inflammatory cytokines and bacterial adherence to epithelial cells. J Periodontal Res 50:197–204, 2015 [PubMed: 24815722]
- 153.
- Borgnakke WS: Chapter 4: The traveling oral microbiome. In The Oral-Systemic Health Connection: A Guide to Patient Care. 1st ed. Glick M, Ed. Chicago, IL, Quintessence Publishing Company, 2014, p. 61–102
- 154.
- Dai YN, Yu WL, Zhu HT, Ding JX, Yu CH, Li YM: Is Helicobacter pylori infection associated with glycemic control in diabetics? World J Gastroenterol 21:5407–5416, 2015 [PMC free article: PMC4419082] [PubMed: 25954115]
- 155.
- Gogeneni H, Buduneli N, Ceyhan-Öztürk B, Gümüş P, Akcali A, Zeller I, Renaud DE, Scott DA, Özçaka Ö: Increased infection with key periodontal pathogens during gestational diabetes mellitus. J Clin Periodontol 42:506–512, 2015 [PMC free article: PMC4699310] [PubMed: 25959628]
- 156.
- Luo H, Pan W, Sloan F, Feinglos M, Wu B: Forty-year trends in tooth loss among American adults with and without diabetes mellitus: an age-period-cohort analysis. Prev Chronic Dis 12:E211, 2015 Dec 3 [Epub] [PMC free article: PMC4674438] [PubMed: 26632952]
- 157.
- Thorstensson H, Johansson B: Why do some people lose teeth across their lifespan whereas others retain a functional dentition into very old age? Gerodontology 27:19–25, 2010 [PubMed: 19545321]
- 158.
- Taylor GW, Manz MC, Borgnakke WS: Diabetes, periodontal diseases, dental caries, and tooth loss: a review of the literature. Compend Contin Educ Dent 25:179–184, 186–188, 190, 2004 [PubMed: 15641324]
- 159.
- Slade GD, Akinkugbe AA, Sanders AE: Projections of U.S. edentulism prevalence following 5 decades of decline. J Dent Res 93:959–965, 2014 [PMC free article: PMC4212322] [PubMed: 25146182]
- 160.
- Griffin SO, Barker LK, Griffin PM, Cleveland JL, Kohn W: Oral health needs among adults in the United States with chronic diseases. J Am Dent Assoc 140:1266–1274, 2009 [PubMed: 19797557]
- 161.
- Patel MH, Kumar JV, Moss ME: Diabetes and tooth loss: an analysis of data from the National Health and Nutrition Examination Survey, 2003–2004. J Am Dent Assoc 144:478–485, 2013 [PubMed: 23633695]
- 162.
- Zhu Y, Hollis JH: Associations between the number of natural teeth and metabolic syndrome in adults. J Clin Periodontol 42:113–120, 2015 [PubMed: 25581485]
- 163.
- Kapp JM, Boren SA, Yun S, LeMaster J: Diabetes and tooth loss in a national sample of dentate adults reporting annual dental visits. Prev Chronic Dis 4:A59, 2007 Jun 15 [Epub] [PMC free article: PMC1955413] [PubMed: 17572963]
- 164.
- Huang DL, Chan KC, Young BA: Poor oral health and quality of life in older U.S. adults with diabetes mellitus. J Am Geriatr Soc 61:1782–1788, 2013 [PMC free article: PMC3855434] [PubMed: 24001058]
- 165.
- Greenblatt AP, Salazar CR, Northridge ME, Kaplan RC, Taylor GW, Finlayson TL, Qi Q, Badner V: Association of diabetes with tooth loss in Hispanic/Latino adults: findings from the Hispanic Community Health Study/Study of Latinos. BMJ Open Diabetes Res Care 4:e000211, 2016 May 12 [Epub] doi: 10.1136/bmjdrc-2016-000211 [PMC free article: PMC4873949] [PubMed: 27239319] [CrossRef]
- 166.
- Moore PA, Weyant RJ, Mongelluzzo MB, Myers DE, Rossie K, Guggenheimer J, Hubar H, Block HM, Orchard T: Type 1 diabetes mellitus and oral health: assessment of tooth loss and edentulism. J Public Health Dent 58:135–142, 1998 [PubMed: 9729758]
- 167.
- Kaur G, Holtfreter B, Rathmann W, Schwahn C, Wallaschofski H, Schipf S, Nauck M, Völzke H, Kocher T: Association between type 1 and type 2 diabetes with periodontal disease and tooth loss. J Clin Periodontol 36:765–774, 2009 [PubMed: 19622096]
- 168.
- Bole C, Wactawski-Wende J, Hovey KM, Genco RJ, Hausmann E: Clinical and community risk models of incident tooth loss in postmenopausal women from the Buffalo Osteo Perio Study. Community Dent Oral Epidemiol 38:487–497, 2010 [PMC free article: PMC2975786] [PubMed: 20636416]
- 169.
- Centers for Disease Control and Prevention (CDC): National Health and Nutrition Examination Survey (NHANES): Oral health examiners manual 2009–2010 [article online], 2009. Available from https://www
.cdc.gov/nchs /data/nhanes/nhanes_09_10 /oralhealth_examiners.pdf. Accessed 8 Apr 2018 - 170.
- Rousseau N, Steele J, May C, Exley C: ‘Your whole life is lived through your teeth’: biographical disruption and experiences of tooth loss and replacement. Sociol Health Illn 36:462–476, 2014 [PubMed: 24720855]
- 171.
- Lal S, Cheng B, Kaplan S, Softness B, Greenberg E, Goland RS, Lalla E, Lamster IB: Accelerated tooth eruption in children with diabetes mellitus. Pediatrics 121:e1139–e1143, 2008 May [Epub] doi: 10.1542/peds.2007-1486 [PubMed: 18450858] [CrossRef]
- 172.
- El-Tekeya M, El Tantawi M, Fetouh H, Mowafy E, Abo Khedr N: Caries risk indicators in children with type 1 diabetes mellitus in relation to metabolic control. Pediatr Dent 34:510–516, 2012 [PubMed: 23265173]
- 173.
- Miralles L, Silvestre FJ, Hernandez-Mijares A, Bautista D, Llambes F, Grau D: Dental caries in type 1 diabetics: influence of systemic factors of the disease upon the development of dental caries. Med Oral Patol Oral Cir Bucal 11:E256–E260, 2006 [PubMed: 16648764]
- 174.
- Moore PA, Weyant RJ, Etzel KR, Guggenheimer J, Mongelluzzo MB, Myers DE, Rossie K, Hubar H, Block HM, Orchard T: Type 1 diabetes mellitus and oral health: assessment of coronal and root caries. Community Dent Oral Epidemiol 29:183–194, 2001 [PubMed: 11409677]
- 175.
- Sandberg GE, Sundberg HE, Fjellstrom CA, Wikblad KF: Type 2 diabetes and oral health: a comparison between diabetic and non-diabetic subjects. Diabetes Res Clin Pract 50:27–34, 2000 [PubMed: 10936666]
- 176.
- Singh I, Singh P, Singh A, Singh T, Kour R: Diabetes an inducing factor for dental caries: a case control analysis in Jammu. J Int Soc Prev Community Dent 6:125–129, 2016 [PMC free article: PMC4820571] [PubMed: 27114951]
- 177.
- Seethalakshmi C, Reddy RC, Asifa N, Prabhu S: Correlation of salivary pH, incidence of dental caries and periodontal status in diabetes mellitus patients: a cross-sectional study. J Clin Diagn Res 10:ZC12–ZC14, 2016 Mar 1 [Epub] doi: 10.7860/JCDR/2016/16310.7351 [PMC free article: PMC4843377] [PubMed: 27134992] [CrossRef]
- 178.
- Novotna M, Podzimek S, Broukal Z, Lencova E, Duskova J: Periodontal diseases and dental caries in children with type 1 diabetes mellitus. Mediators Inflamm 2015 Aug 4 [Epub] doi: 10.1155/2015/379626 [PMC free article: PMC4539482] [PubMed: 26347009] [CrossRef]
- 179.
- Tavares M, Depaola P, Soparkar P, Joshipura K: The prevalence of root caries in a diabetic population. J Dent Res 70:979–983, 1991 [PubMed: 2045579]
- 180.
- Yokomichi H, Tanaka T, Suzuki K, Akiyama T; Okinawa Child Health Study Group, Yamagata Z: Macrosomic neonates carry increased risk of dental caries in early childhood: findings from a cohort study, the Okinawa Child Health Study, Japan. PLOS One 10:e0133872, 2015 Jul 24 [Epub] doi: 10.1371/journal.pone.0133872 [PMC free article: PMC4514765] [PubMed: 26207737] [CrossRef]
- 181.
- Moore PA, Guggenheimer J, Etzel KR, Weyant RJ, Orchard T: Type 1 diabetes mellitus, xerostomia, and salivary flow rates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 92:281–291, 2001 [PubMed: 11552145]
- 182.
- Drugs that promote dental caries. Prescrire Int 24:41–42, 44, 2015 [PubMed: 25802916]
- 183.
- Guggenheimer J, Moore PA, Rossie K, Myers D, Mongelluzzo MB, Block HM, Weyant R, Orchard T: Insulin-dependent diabetes mellitus and oral soft tissue pathologies. II. Prevalence and characteristics of Candida and Candidal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89:570–576, 2000 [PubMed: 10807713]
- 184.
- Al Mubarak S, Robert AA, Baskaradoss JK, Al-Zoman K, Al Sohail A, Alsuwyed A, Ciancio S: The prevalence of oral Candida infections in periodontitis patients with type 2 diabetes mellitus. J Infect Public Health 6:296–301, 2013 [PubMed: 23806705]
- 185.
- Hammad MM, Darwazeh AM, Idrees MM: The effect of glycemic control on Candida colonization of the tongue and the subgingival plaque in patients with type II diabetes and periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol 116:321–326, 2013 [PubMed: 23953417]
- 186.
- Javed F, Ahmed HB, Mehmood A, Saeed A, Al-Hezaimi K, Samaranayake LP: Association between glycemic status and oral Candida carriage in patients with prediabetes. Oral Surg Oral Med Oral Pathol Oral Radiol 117:53–58, 2014 [PubMed: 24332327]
- 187.
- Olczak-Kowalczyk D, Pyrzak B, Dabkowska M, Panczyk-Tomaszewska M, Miszkurka G, Rogozinska I, Swoboda-Kopec E, Gozdowski D, Kalinska A, Pirog A, Mizerska-Wasiak M, Roszkowska-Blaim M: Candida spp. and gingivitis in children with nephrotic syndrome or type 1 diabetes. BMC Oral Health 15:57, 2015 May 8 [Epub] doi: 10.1186/s12903-015-0042-6 [PMC free article: PMC4425863] [PubMed: 25952029] [CrossRef]
- 188.
- National Institutes of Health; National Institute of Dental and Craniofacial Research: Burning mouth syndrome [article online], 2017. Available from https://www
.nidcr.nih .gov/sites/default/files /2017-12/burning-mouth-syndrome.pdf. Accessed 8 Apr 2018 - 189.
- Moore PA, Guggenheimer J, Orchard T: Burning mouth syndrome and peripheral neuropathy in patients with type 1 diabetes mellitus. J Diabetes Complications 21:397–402, 2007 [PubMed: 17967714]
- 190.
- Macek MD, Tomar SL: Dental care visits among dentate adults with diabetes and periodontitis. J Public Health Dent 69:284–289, 2009 [PubMed: 19552674]
- 191.
- Macek MD, Taylor GW, Tomar SL: Dental care visits among dentate adults with diabetes, United States, 2003. J Public Health Dent 68:102–110, 2008 [PubMed: 18221318]
- 192.
- Tomar SL, Lester A: Dental and other health care visits among U.S. adults with diabetes. Diabetes Care 23:1505–1510, 2000 [PubMed: 11023144]
- 193.
- Centers for Disease Control and Prevention; Eke PI, Thornton-Evans GO, Beckles GL: Dental visits among dentate adults with diabetes—United States, 1999 and 2004. MMWR Morb Mortal Wkly Rep 54:1181–1183, 2005 [PubMed: 16304554]
- 194.
- Bloom B, Simile CM, Adams PF, Cohen RA: Oral health status and access to oral health care for U.S. adults aged 18–64: National Health Interview Survey, 2008. Vital Health Stat 10:1–22, 2012 [PubMed: 23077776]
- 195.
- Wiener RC, Shen C, Sambamoorthi N, Sambamoorthi U: Preventive dental care in older adults with diabetes. J Am Dent Assoc 147:797–802, 2016 [PMC free article: PMC5045763] [PubMed: 27189741]
- 196.
- Moffet HH, Schillinger D, Weintraub JA, Adler N, Liu JY, Selby JV, Karter AJ: Social disparities in dental insurance and annual dental visits among medically insured patients with diabetes: the Diabetes Study of Northern California (DISTANCE) Survey. Prev Chronic Dis 7:A57, 2010 Apr 15 [Epub] [PMC free article: PMC2879989] [PubMed: 20394696]
- 197.
- Albert DA, Sadowsky D, Papapanou P, Conicella ML, Ward A: An examination of periodontal treatment and per member per month (PMPM) medical costs in an insured population. BMC Health Serv Res 6:103, 2006 Aug 16 [Epub] doi: 10.1186/1472-6963-6-103 [PMC free article: PMC1574303] [PubMed: 16914052] [CrossRef]
- 198.
- Spangler L, Reid RJ, Inge R, Newton KM, Hujoel P, Chaudhari M, Genco RJ, Barlow WE: Cross-sectional study of periodontal care and glycosylated hemoglobin in an insured population. Diabetes Care 33:1753–1758, 2010 [PMC free article: PMC2909057] [PubMed: 20504894]
- 199.
- Mosen DM, Pihlstrom DJ, Snyder JJ, Shuster E: Assessing the association between receipt of dental care, diabetes control measures and health care utilization. J Am Dent Assoc 143:20–30, 2012 [PubMed: 22207663]
- 200.
- Iwasaki M, Sato M, Yoshihara A, Miyazaki H: Effects of periodontal diseases on diabetes-related medical expenditure. Curr Oral Health Rep 3:7–13, 2016
- 201.
- Jeffcoat MK, Jeffcoat RL, Gladowski PA, Bramson JB, Blum JJ: Impact of periodontal therapy on general health: evidence from insurance data for five systemic conditions. Am J Prev Med 47:166–174, 2014 [PubMed: 24953519]
- 202.
- Sheiham A: Claims that periodontal treatment reduces costs of treating five systemic conditions are questionable. J Evid Based Dent Pract 15:35–36, 2015 [PubMed: 25666581]
- 203.
- Ryan ME, Raja V: Diet, obesity, diabetes, and periodontitis: a syndemic approach to management. Curr Oral Health Rep 3:14–27, 2016
- 204.
- Theile CW, Strauss SM, Northridge ME, Birenz S: The oral health care manager in a patient-centered health facility. J Evid Based Dent Pract 16(Suppl):34–42, 2016 [PMC free article: PMC4888908] [PubMed: 27236994]
- 205.
- National Diabetes Education Program; National Institutes of Health: Redesigning the Health Care Team: Diabetes Prevention and Lifelong Management. Bethesda, MD: U.S. Department of Health and Human Services, 2011
- 206.
- National Diabetes Education Program; National Institutes of Health: Collaborative care in practice. In Redesigning the Health Care Team: Diabetes Prevention and Lifelong Management. Bethesda, MD: U.S. Department of Health and Human Services, 2011
- 207.
- Greenberg BL, Glick M, Frantsve-Hawley J, Kantor ML: Dentists’ attitudes toward chairside screening for medical conditions. J Am Dent Assoc 141:52–62, 2010 [PubMed: 20045822]
- 208.
- Rosedale MT, Strauss SM: Diabetes screening at the periodontal visit: patient and provider experiences with two screening approaches. Int J Dent Hyg 10:250–258, 2012 [PMC free article: PMC3469730] [PubMed: 22284167]
- 209.
- Strauss SM, Rosedale MT, Pesce MA, Rindskopf DM, Kaur N, Juterbock CM, Wolff MS, Malaspina D, Danoff A: The potential for glycemic control monitoring and screening for diabetes at dental visits using oral blood. Am J Public Health 105:796–801, 2015 [PMC free article: PMC4358165] [PubMed: 25713975]
- 210.
- Herman WH, Taylor GW, Jacobson JJ, Burke R, Brown MB: Screening for prediabetes and type 2 diabetes in dental offices. J Public Health Dent 75:175–182, 2015 [PMC free article: PMC5053230] [PubMed: 25662777]
- 211.
- Genco RJ, Genco FD: Common risk factors in the management of periodontal and associated systemic diseases: the dental setting and interprofessional collaboration. J Evid Based Dent Pract 14(Suppl):4–16, 2014 [PubMed: 24929584]
- 212.
- Hein C, Batista EL, Jr.: Obesity and cumulative inflammatory burden: a valuable risk assessment parameter in caring for dental patients. J Evid Based Dent Pract 14(Suppl):17–26.e1, 2014 [PubMed: 24929585]
- 213.
- Darling-Fisher CS, Kanjirath PP, Peters MC, Borgnakke WS: Oral health: an untapped resource in managing glycemic control in diabetes and promoting overall health. J Nurse Pract 11:889–896, 2015
- 214.
- Greenberg BL, Thomas PA, Glick M, Kantor ML: Physicians’ attitudes toward medical screening in a dental setting. J Public Health Dent 75:225–233, 2015 [PubMed: 25760645]
- 215.
- Friman G, Hultin M, Nilsson GH, Wardh I: Medical screening in dental settings: a qualitative study of the views of authorities and organizations. BMC Res Notes 8:580, 2015 Oct 19 [Epub] doi: 10.1186/s13104-015-1543-8 [PMC free article: PMC4610051] [PubMed: 26478099] [CrossRef]
- 216.
- Alagl AS: Periodontal abscess as a possible oral clinical sign in the diagnosis of undiagnosed diabetes mellitus of elderly in a dental clinic set up—a 7-year cross-sectional study. J Investig Clin Dent 8:e12217, 2017 Aug 7 [Epub] doi: 10.1111/jicd.12217 [PubMed: 27157341] [CrossRef]
- 217.
- Polzer I, Schwahn C, Völzke H, Mundt T, Biffar R: The association of tooth loss with all-cause and circulatory mortality. Is there a benefit of replaced teeth? A systematic review and meta-analysis. Clin Oral Investig 16:333–351, 2012 [PubMed: 22086361]
- 218.
- Padilha DM, Hilgert JB, Hugo FN, Bos AJ, Ferrucci L: Number of teeth and mortality risk in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 63:739–744, 2008 [PMC free article: PMC4984838] [PubMed: 18693229]
- 219.
- Greenberg BL, Glick M: Assessing systemic disease risk in a dental setting: a public health perspective. Dent Clin North Am 56:863–874, 2012 [PubMed: 23017556]
- 220.
- Nasseh K, Greenberg B, Vujicic M, Glick M: The effect of chairside chronic disease screenings by oral health professionals on health care costs. Am J Public Health 104:744–750, 2014 [PMC free article: PMC4025684] [PubMed: 24524531]
- 221.
- Mosen D, Pihlstrom D, Snyder J, Smith N, Shuster E, Rust K: Association of dental care with adherence to HEDIS measures. Perm J 20:33–40, 2016 [PMC free article: PMC4732792] [PubMed: 26580145]
- 222.
- Grubbs V, Plantinga LC, Tuot DS, Powe NR: Chronic kidney disease and use of dental services in a United States public healthcare system: a retrospective cohort study. BMC Nephrol 13:16, 2012 Apr 2 [Epub] doi: 10.1186/1471-2369-13-16 [PMC free article: PMC3368751] [PubMed: 22471751] [CrossRef]
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONVERSIONS
Conversions for A1c and glucose values are provided in Diabetes in America Appendix 1 Conversions.
DUALITY OF INTEREST
Drs. Borgnakke, Genco, Eke, and Taylor reported no conflicts of interest.
- Summary
- Introduction
- Periodontitis: Introduction
- Periodontitis: Effect on Diabetes
- Diabetes: Effect on Periodontitis
- Risk Factors for Periodontitis and Diabetes (Hyperglycemia)
- Mechanisms Underlying the Bidirectional Relationship Between Periodontitis and Diabetes
- Diabetes: Associated Oral Conditions Other Than Current Periodontitis
- Dental Care Utilization in People With Diabetes
- Interprofessional Collaboration
- Public Health Significance of the Oral Health-Diabetes Links
- List of Abbreviations
- References
- Appendices
- Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review.[JBI Libr Syst Rev. 2012]Periodontal Disease as a Risk Factor for Rheumatoid Arthritis: A Systematic Review.Kaur S, White S, Bartold M. JBI Libr Syst Rev. 2012; 10(42 Suppl):1-12.
- Review Primary prevention of periodontitis: managing gingivitis.[J Clin Periodontol. 2015]Review Primary prevention of periodontitis: managing gingivitis.Chapple IL, Van der Weijden F, Doerfer C, Herrera D, Shapira L, Polak D, Madianos P, Louropoulou A, Machtei E, Donos N, et al. J Clin Periodontol. 2015 Apr; 42 Suppl 16:S71-6.
- Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions.[J Clin Periodontol. 2018]Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions.Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, Demirel K, de Sanctis M, Ercoli C, Fan J, et al. J Clin Periodontol. 2018 Jun; 45 Suppl 20:S219-S229.
- Review Chronic Periodontitis – Prevention, Diagnosis and Treatment: A Systematic Review[ 2004]Review Chronic Periodontitis – Prevention, Diagnosis and Treatment: A Systematic ReviewSwedish Council on Health Technology Assessment. 2004 Oct
- Review Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions.[J Periodontol. 2018]Review Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions.Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, Demirel K, de Sanctis M, Ercoli C, Fan J, et al. J Periodontol. 2018 Jun; 89 Suppl 1:S237-S248.
- Oral Health and Diabetes - Diabetes in AmericaOral Health and Diabetes - Diabetes in America
- LOC114409699 [Glycine soja]LOC114409699 [Glycine soja]Gene ID:114409699Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...

