NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Romieu I, Dossus L, Willett WC, editors. Energy Balance and Obesity. Lyon (FR): International Agency for Research on Cancer; 2017. (IARC Working Group Reports, No. 10.)
Food intake is a function of energy requirements as determined by body size and physical activity. Adult humans maintain a balance between their energy intake and energy expenditure, as shown by the constancy of body weight and body composition [1]. Energy balance is achieved by control of energy intake, energy expenditure, or both. Humans, however, do not balance energy intake and energy expenditure on a daily basis, as smaller animals do. They can afford to rely on their body reserves, whereas smaller species show signs of energy shortage sooner, as expressed in a lowered body temperature and reduced physical activity. Smaller species have a higher energy expenditure per kilogram of body mass as well as a relatively smaller body energy reserve [2]. Thus, a mouse cannot survive 3 days without food, whereas a normal adult human can survive more than 30 days without food.
Of course, humans can maintain a perfect energy balance in the long-term, as shown by a constant body weight in adult life [1]. An average person has an energy expenditure of 10–15 MJ/day, or 3650–5475 MJ/year. Even a weight change of 1 kg, equivalent to 30 MJ, denotes a discrepancy between intake and expenditure of only 0.6–0.8% on an annual basis. Energy intake strongly correlates with energy expenditure on a weekly basis. Discrepancies on a daily basis between intake and expenditure are especially large when days with high energy expenditure are alternated with quieter intervals. For example, military cadets did not show an increase in energy intake on days with a higher energy expenditure when they joined a drill competition. The corresponding increase in energy intake occurred about 2 days later [3].
Despite the capacity to maintain energy balance [1], there is currently a worldwide increase in the prevalence of obesity. It has been suggested that modern inactive lifestyles are the predominant factor in the increasing prevalence of overweight and obesity [4]. An analysis of measurements of daily energy expenditure by the doubly labelled water (DLW) method, as available over the past decades, suggests that physical activity levels have not declined over the period during which obesity rates have increased [5]. The data analysis included hundreds of subjects in Europe, North America, and developing countries, extending back to the 1980s. The relationship between daily energy expenditure and body mass suggests that increases in body mass are driven by increased energy intake [6].
This chapter reviews the available evidence on overall energy intake and expenditure in relation to obesity. The following questions are addressed: how to assess whether energy and macronutrient intake are different between subjects, and how overweight and obesity affect energy expenditure. Subsequently, interventions to control the obesity epidemic are reviewed, with a focus on dietary and exercise interventions.
Dietary intake and obesity
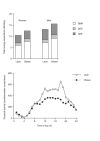
Since the application of the DLW technique for measuring energy requirements in free-living humans, it is known that reported dietary intake is generally lower than habitual dietary intake. Previously, overweight and obesity as derived from reported dietary intake were associated with a reduced energy requirement. A typical example is shown in Fig. 5.1. In women, reported energy intake is independent of body weight, whereas in men, reported intake is significantly lower in heavier subjects. For the same subjects and in both sexes, the difference between measured expenditure and reported intake is significantly higher in heavier subjects than in leaner ones. Heavier subjects tend to show more underreporting of dietary intake compared with leaner ones [10], leading to erroneous conclusions from intake as reported.
Underreporting of food intake seems to be more of a concern for specific food items, which are generally considered to be “bad for health.” An example is the inverse relationship between fat intake and obesity, called the American paradox [11]. In the adult population, the prevalence of overweight has increased while at the same time reported energy intake and percentage of energy derived from fat appear to have decreased. This is very likely to be due to selective underreporting. For example, a DLW study in obese subjects showed a negative correlation between the reported percentage of energy from fat in the diet and the amount of underreporting [9]. In the case of no underreporting, the percentage of energy from fat would be 46% ± 5%. Food supply data showed an increase in fat availability over the past 40 years [12]. Therefore, the observed decrease in reported fat intake seems to be doubtful.
Recognition of underreporting is ideally based on simultaneous DLW assessment of energy requirements. However, the cost of the DLW method limits its application in large-scale studies. A more practical alternative, which is often used to recognize underreporting, is the application of the ratio of reported energy intake to basal metabolic rate (BMR), by analogy with the ratio of daily energy expenditure to BMR known as the physical activity level (PAL). A cut-off limit for the ratio of reported energy intake to BMR is set at a minimum value, often 1.3 [13]. However, cut-off limits do not take variation in individual PAL values into account, although more recently this has become the practice in the analysis of epidemiological studies [14]. A subject with a reported intake of 10 MJ/day and a PAL value of 1.4 could be a correct reporter, whereas the energy expenditure, and thus the intake, of the same subject should be more than 14 MJ/day when the PAL value is 2.0. Therefore, to validate reported energy intake, one should use a combination of BMR and physical activity. BMR can be measured or estimated with an equation from the literature, based on the height, weight, age, and sex of the subject [15]. Physical activity can be estimated with a DLW-validated accelerometer to record body movement [16].
In conclusion, before data on reported intake are interpreted, misreporters should be identified. This will result in the exclusion of much, if not most, of the data, especially for studies in overweight and obese subjects. Therefore, it was recently advised that the scientific and medical communities should discontinue reliance on self-reported energy intake as a measure of energy intake [17].
Energy expenditure and obesity
The main determinants of total energy expenditure, and thus of energy requirement, are body size and physical activity. Body size and body composition determine maintenance metabolism (BMR), which is the largest of the three components, making up 50–70% of total energy expenditure. Body movement or physical activity determines activity-induced energy expenditure, the most variable component of total daily energy expenditure. The third component, diet-induced energy expenditure, is generally assumed to be 10% of total daily energy expenditure in subjects who consume the average mixed diet and are in energy balance [18].
Maintenance metabolism is determined mainly by fat-free body mass. Overweight and obese subjects typically have a larger fat mass but also a larger fat-free mass compared with lean subjects [19]. Excess energy during weight gain in adult subjects is stored as fat mass and fat-free mass in an energy ratio of 95:5 or in a mass ratio of 75:25 [20]. The larger fat-free body mass in overweight and obese subjects implies a higher maintenance metabolism. Maintenance metabolism in morbidly obese subjects is generally higher than total daily energy expenditure in lean subjects, possibly limiting activity-induced energy expenditure and resulting in a lower PAL [21].
Activity-induced energy expenditure is determined by body movement and body mass. In the same environment, body movement as measured with an accelerometer is higher in lean subjects than in overweight subjects. A study of adolescents attending the same school showed similar activity-induced energy expenditure for lean subjects and obese subjects, whereas body movement was lower in obese subjects than in lean subjects (Fig. 5.2). Overweight implies less physical activity, i.e. less body movement, but because of the larger body weight, the decreased body movement still results in similar or even higher activity-induced energy expenditure.
In conclusion, due to a larger body size, overweight induces a higher maintenance requirement, and obese subjects are less physically active than normal-weight subjects, although activity-induced energy expenditure is not necessarily lower.
Dietary and exercise interventions to control the obesity epidemic
Interventions to induce weight loss in overweight and obese subjects include a reduction of energy intake, an increase in energy expenditure through exercise, or both, aiming for a negative energy balance. Whatever the intervention, the success rate for long-term weight loss is low [23, 24]. First, compliance with an energy-restricted diet is low, resulting in less weight loss than expected. Second, compensatory mechanisms reduce the discrepancy between energy intake and energy expenditure required to induce weight loss [25].
Energy restriction induces a reduction in all three components of daily energy expenditure: maintenance metabolism, diet-induced energy expenditure, and activity-induced energy expenditure. The reduction in maintenance metabolism is larger than that expected from the loss of fat-free mass and fat mass and is positively related to the amount of weight lost [26]. The reduction in diet-induced energy expenditure is a direct consequence of the reduction in the amount of food consumed. The reduction in activity-induced energy expenditure is not only through a reduction of body weight but also through an energy restriction-induced reduction of body movement as measured with an accelerometer [27].
The potential of exercise programmes to induce weight loss is limited by several factors. Overweight and obesity reduce the exercise capacity of the body. In addition, it is difficult to comply with an exercise programme without compensating for an exercise-induced increase in energy expenditure by increasing energy intake [28]. Finally, exercise-induced energy expenditure is compensated for by a reduction in non-exercise activity thermogenesis, especially when subjects are in a negative energy balance [29, 30].
In conclusion, adaptive responses limit the effect of energy restriction and exercise on energy balance in overweight and obese subjects. Therefore, weight loss is lower than expected, and very few people are successful in maintaining weight loss to achieve a healthier weight.
Key points
- •
Before data on reported intake are interpreted, misreporters should be identified.
- •
Overweight and obese subjects have a higher energy requirement than lean subjects, because of a higher maintenance requirement.
- •
Obese subjects move less than normal-weight subjects, although activity-induced energy expenditure is not necessarily lower.
- •
Adaptive responses limit the effect of energy restriction and exercise on energy balance in overweight and obese subjects.
- •
Eating less is the most effective method for preventing weight gain.
Research needs
• Studies are needed on determinants of energy intake, to develop strategies for successful long-term weight maintenance by limiting energy intake, in an environment where food availability stimulates the majority of the population to overeat.
References
- 1.
- James WPT (1985). Appetite control and other mechanisms of weight homeostasis. In: Blaxter K, Waterlow JC, editors. Nutritional adaptations. London, UK: John Libbey; pp. 141–54.
- 2.
- Schmidt-Nielsen K (1972). Animal physiology: adaptation and environment. London, UK: Cambridge University Press.
- 3.
- Edholm OG, Fletcher JG, Widdowson EM, McCance RA (1955). The energy expenditure and food intake of individual men. Br J Nutr. 9(3):286–300. 10.1079/BJN19550040 [PubMed: 13250128] [CrossRef]
- 4.
- Prentice AM, Jebb SA (1995). Obesity in Britain: gluttony or sloth? BMJ. 311(7002):437–9. 10.1136/bmj.311.7002.437 [PMC free article: PMC2550498] [PubMed: 7640595] [CrossRef]
- 5.
- Westerterp KR, Speakman JR (2008). Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obes. 32(8):1256–63. 10.1038/ijo.2008.74 [PubMed: 18504442] [CrossRef]
- 6.
- Swinburn BA, Sacks G, Lo SK, Westerterp KR, Rush EC, Rosenbaum M, et al. (2009). Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am J Clin Nutr. 89(6):1723–8. 10.3945/ajcn.2008.27061 [PMC free article: PMC3738432] [PubMed: 19369382] [CrossRef]
- 7.
- Meijer GAL, Westerterp KR, van Hulsel AMP, ten Hoor F (1992). Physical activity and energy expenditure in lean and obese adult human subjects. Eur J Appl Physiol Occup Physiol. 65(6):525–8. 10.1007/BF00602359 [PubMed: 1483440] [CrossRef]
- 8.
- Westerterp KR, Verboeket-van de Venne WPHG, Bouten CVC, de Graaf C, van het Hof KH, Weststrate JA (1996). Energy expenditure and physical activity in subjects consuming full-or reduced-fat products as part of their normal diet. Br J Nutr. 76(6):785–95. 10.1079/BJN19960086 [PubMed: 9014648] [CrossRef]
- 9.
- Goris AHC, Westerterp-Plantenga MS, Westerterp KR (2000). Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr. 71(1):130–4. 10.1093/ajcn/71.1.130 [PubMed: 10617957] [CrossRef]
- 10.
- Westerterp KR, Goris AHC (2002). Validity of the assessment of dietary intake: problems of misreporting. Curr Opin Clin Nutr Metab Care. 5(5):489–93. 10.1097/00075197-200209000-00006 [PubMed: 12172471] [CrossRef]
- 11.
- Heini AF, Weinsier RL (1997). Divergent trends in obesity and fat intake patterns: the American paradox. Am J Med. 102(3):259–64. 10.1016/S0002-9343(96)00456-1 [PubMed: 9217594] [CrossRef]
- 12.
- Balanza R, García-Lorda P, Pérez-Rodrigo C, Aranceta J, Bonet MB, Salas-Salvadó J (2007). Trends in food availability determined by the Food and Agriculture Organization’s food balance sheets in Mediterranean Europe in comparison with other European areas. Public Health Nutr. 10(2):168–76. 10.1017/S1368980007246592 [PubMed: 17261226] [CrossRef]
- 13.
- Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, et al. (1991). Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr. 45(12):569–81. [PubMed: 1810719]
- 14.
- Black AE (2000). Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord. 24(9):1119–30. 10.1038/sj.ijo.0801376 [PubMed: 11033980] [CrossRef]
- 15.
- Sabounchi NS, Rahmandad H, Ammerman A (2013). Best-fitting prediction equations for basal metabolic rate: informing obesity interventions in diverse populations. Int J Obes. 37(10):1364–70. 10.1038/ijo.2012.218 [PMC free article: PMC4278349] [PubMed: 23318720] [CrossRef]
- 16.
- Goris AH, Meijer EP, Kester A, Westerterp KR (2001). Use of a triaxial accelerometer to validate reported food intakes. Am J Clin Nutr. 73(3):549–53. 10.1093/ajcn/73.3.549 [PubMed: 11237930] [CrossRef]
- 17.
- Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sørensen TI, et al.; Energy Balance Measurement Working Group (2015). Energy balance measurement: when something is not better than nothing. Int J Obes. 39(7):1109–13. 10.1038/ijo.2014.199 [PMC free article: PMC4430460] [PubMed: 25394308] [CrossRef]
- 18.
- Westerterp KR (2004). Diet induced thermogenesis. Nutr Metab (Lond). 1(1):5. 10.1186/1743-7075-1-5 [PMC free article: PMC524030] [PubMed: 15507147] [CrossRef]
- 19.
- Westerterp KR, Donkers JH, Fredrix EW, Boekhoudt P (1995). Energy intake, physical activity and body weight: a simulation model. Br J Nutr. 73(3):337–47. 10.1079/BJN19950037 [PubMed: 7766558] [CrossRef]
- 20.
- Schutz Y, Kyle UU, Pichard C (2002). Fat-free mass index and fat mass index percentiles in Caucasians aged 18-98 y. Int J Obes Relat Metab Disord. 26(7):953–60. 10.1038/sj.ijo.0802037 [PubMed: 12080449] [CrossRef]
- 21.
- Prentice AM, Black AE, Coward WA, Cole TJ (1996). Energy expenditure in overweight and obese adults in affluent societies: an analysis of 319 doubly-labelled water measurements. Eur J Clin Nutr. 50(2):93–7. [PubMed: 8641251]
- 22.
- Ekelund U, Aman J, Yngve A, Renman C, Westerterp K, Sjöström M (2002). Physical activity but not energy expenditure is reduced in obese adolescents: a case-control study. Am J Clin Nutr. 76(5):935–41. 10.1093/ajcn/76.5.935 [PubMed: 12399263] [CrossRef]
- 23.
- Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. (2007). Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 107(10):1755–67. 10.1016/j.jada.2007.07.017 [PubMed: 17904936] [CrossRef]
- 24.
- Anderson JW, Konz EC, Frederich RC, Wood CL (2001). Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 74(5):579–84. 10.1093/ajcn/74.5.579 [PubMed: 11684524] [CrossRef]
- 25.
- Anastasiou CA, Karfopoulou E, Yannakoulia M (2015). Weight regaining: From statistics and behaviors to physiology and metabolism. Metabolism. 64(11):1395–407. 10.1016/j.metabol.2015.08.006 [PubMed: 26362728] [CrossRef]
- 26.
- Camps SG, Verhoef SP, Westerterp KR (2013). Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 97(5):990–4. 10.3945/ajcn.112.050310 [PubMed: 23535105] [CrossRef]
- 27.
- Camps SG, Verhoef SP, Westerterp KR (2013). Weight loss-induced reduction in physical activity recovers during weight maintenance. Am J Clin Nutr. 98(4):917–23. 10.3945/ajcn.113.062935 [PubMed: 23985804] [CrossRef]
- 28.
- Thomas DM, Bouchard C, Church T, Slentz C, Kraus WE, Redman LM, et al. (2012). Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev. 13(10):835–47. 10.1111/j.1467-789X.2012.01012.x [PMC free article: PMC3771367] [PubMed: 22681398] [CrossRef]
- 29.
- Pontzer H (2015). Constrained total energy expenditure and the evolutionary biology of energy balance. Exerc Sport Sci Rev. 43(3):110–6. 10.1249/JES.0000000000000048 [PubMed: 25906426] [CrossRef]
- 30.
- Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA (2013). Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Med Sci Sports Exerc. 45(8):1600–9. 10.1249/MSS.0b013e31828ba942 [PMC free article: PMC3696411] [PubMed: 23470300] [CrossRef]
- The Obesity Epidemic: A Consequence of Reduced Energy Expenditure and the Uncoupling of Energy Intake?[Obesity (Silver Spring). 2018]The Obesity Epidemic: A Consequence of Reduced Energy Expenditure and the Uncoupling of Energy Intake?Church T, Martin CK. Obesity (Silver Spring). 2018 Jan; 26(1):14-16.
- Review Macronutrients and energy balance in obesity.[Metabolism. 1995]Review Macronutrients and energy balance in obesity.Schutz Y. Metabolism. 1995 Sep; 44(9 Suppl 3):7-11.
- Review [Energy expenditure and obesity].[Rev Med Brux. 1994]Review [Energy expenditure and obesity].Balasse E. Rev Med Brux. 1994 Jul-Aug; 15(4):255-8.
- Dietary intake, physical activity and energy expenditure of Malaysian adolescents.[Singapore Med J. 2006]Dietary intake, physical activity and energy expenditure of Malaysian adolescents.Zalilah MS, Khor GL, Mirnalini K, Norimah AK, Ang M. Singapore Med J. 2006 Jun; 47(6):491-8.
- Review The 'Fat Mass and Obesity Related' (FTO) gene: Mechanisms of Impact on Obesity and Energy Balance.[Curr Obes Rep. 2015]Review The 'Fat Mass and Obesity Related' (FTO) gene: Mechanisms of Impact on Obesity and Energy Balance.Speakman JR. Curr Obes Rep. 2015 Mar; 4(1):73-91.
- How are overall energy intake and expenditure related to obesity? - Energy Balan...How are overall energy intake and expenditure related to obesity? - Energy Balance and Obesity
Your browsing activity is empty.
Activity recording is turned off.
See more...