NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Occupational Exposures of Hairdressers and Barbers and Personal Use of Hair Colourants; Some Hair Dyes, Cosmetic Colourants, Industrial Dyestuffs and Aromatic Amines. Lyon (FR): International Agency for Research on Cancer; 1993. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 57.)

Occupational Exposures of Hairdressers and Barbers and Personal Use of Hair Colourants; Some Hair Dyes, Cosmetic Colourants, Industrial Dyestuffs and Aromatic Amines.
Show details1. Exposure Data
1.1. Chemical and physical data
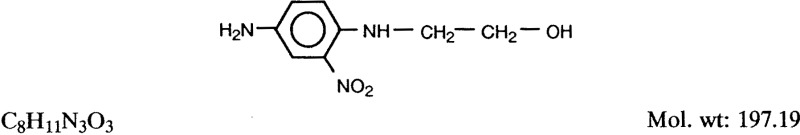
1.1.1. Synonyms, structural and molecular data
Chem. Abstr. Serv. Reg. No.: 2871-01-4
Chem. Abstr. Name: 2-[(4-Amino-2-nitrophenyl)amino]ethanol
Synonyms: 2-(4-Amino-2-nitroanilino)ethanol; HC Red 3; HC Red Number 3; 4-(2-hy- droxyethyl)amino-3-nitroaniline; N1-(2-hydroxyethyl)-2-nitro-para-phenylenediamine
1.1.2. Chemical and physical properties
From US National Toxicology Program (1986)
- (a) Description: Fine, dark-maroon crystals, with a greenish cast
- (b) Melting-point: 124–128 °C
- (c) Spectroscopy data: Infrared, ultraviolet and nuclear magnetic resonance spectral data have been reported.
- (d) Solubility: Soluble in water (0.28% w/w), ethanol and acetone
- (e) Octanol/water partition coefficient (P): 1.9
1.1.3. Trade names, technical products and impurities
HC Red No. 3 is available commercially at a purity ≥ 95%, with aminoquinoxaline, aminonaphthyridine (US National Toxicology Program, 1986) and 2-[(4-[4-amino-2-nitro-phenyl]amino-2-nitrophenyl)amino] ethanol (< 1%) as possible impurities.
1.1.4. Analysis
No data were available to the Working Group.
1.2. Production and use
1.2.1. Production
HC Red No. 3 is produced by the reaction of 4-fluoro-3-nitrobenzenamine with mono-ethanolamine. Production and use of this dye began in the late 1950s. Approximately 2300 kg are used annually in the USA, according to industry estimates.
1.2.2. Use
HC Red No. 3 is used exclusively as a dye in semi-permanent hair colour products. These products are generally shampooed into the hair, lathered and then allowed to remain in contact with the hair and scalp for 30–45 min. The concentration of HC Red No. 3 used in these preparations ranges from 0.1 to 5% (Frenkel & Brody, 1973; US National Toxicology Program, 1986).
1.3. Occurrence
1.3.1. Natural occurrence
HC Red No. 3 is not known to occur as a natural product.
1.3.2. Occupational exposure
No data were available to the Working Group.
On the basis of a survey conducted in the USA between 1981 and 1983, the US National Institute for Occupational Safety and Health estimated that a total of 42 485 workers, including 32 059 women, were potentially exposed as beauticians and cosmetologists to HC Red No. 3 (US National Library of Medicine, 1992).
1.4. Regulations and guidelines
No data were available to the Working Group.
2. Studies of Cancer in Humans
No data were available to the Working Group.
3. Studies of Cancer in Experimental Animals
3.1. Oral administration
3.1.1. Mouse
Groups of 50 male and 50 female B6C3F1 mice, eight weeks of age, were administered 0, 125 or 250 mg/kg bw HC Red No. 3 (97% pure) by gavage in corn oil on five days a week for 104 weeks and were sacrificed at 113 weeks of age. Body weight gain and survival were reduced in all groups of females because of a reproductive tract infection. Survival at the end of the study was: males—control, 30/50; low-dose, 41/50; and high-dose, 29/50; females-control, 12/50; low-dose, 8/50; and high-dose, 9/50. The incidence of hepatocellular adenomas in males was control, 11/50; low-dose, 6/50; high-dose, 16/50; the incidence of hepatocellular carcinomas was, control, 17/50; low-dose, 9/50; high-dose, 21/50. There was an increased incidence of hepatocellular adenomas and carcinomas combined in high-dose male mice (control, 25/50; low-dose, 15/50; high-dose, 35/50), which was significant (p = 0.017, incidental tumour test), but not after pair-wise comparison. The incidence in historical controls in the testing laboratory was 109/298 (37 ± 12%) (US National Toxicology Program, 1986). [The Working Group noted the poor survival of females and the unusually. high incidence of hepatocellular carcinomas in male controls.]
3.1.2. Rat
Groups of 50 male and 50 female Fischer 344/N rats, seven to eight weeks of age, were administered 0,250 or 500 mg/kg bw HC Red No. 3 (97% pure) by gavage in corn oil on five days a week for 105 weeks and were sacrificed at 113–114 weeks of age. The treatment had no effect on body weight gain throughout the study and did not reduce survival in males or females. Survival at the end of the experiment was: males—control, 34/50; low-dose, 34/50; high-dose, 32/50; females—control, 39/50; low-dose, 38/50; high-dose, 34/50. The incidence of mammary gland fibroadenomas was significantly (p = 0.019, incidental tumour test) increased in low-dose females (control, 14/50 versus low-dose, 24/50) but not in high-dose females (11/50) (US National Toxicology Program, 1986). [The Working Group noted the absence of a dose-response relationship.]
4. Other Relevant Data
4.1. Absorption, distribution, metabolism and excretion
No data were available to the Working Group.
4.2. Toxic effects
4.2.1. Humans
No data were available to the Working Group.
4.2.2. Experimental systems
In the studies reported above, no compound-related toxic effect was reported (US National Toxicology Program, 1986).
HC Red No. 3 was present at low concentrations (0.02%) in semi-permanent hair colouring formulations evaluated in a two-year feeding study in dogs (Wernick et al., 1975) and in a 20-month study of dermal toxicity in mice (Jacobs et al., 1984, 0.3%), described in detail on p. 97. No treatment-related adverse effect was detected. [The Working Group noted that the dose of each component of the formulations was very low and unlikely to have been toxic]
4.3. Reproductive and developmental effects
4.3.1. Humans
No data were available to the Working Group.
4.3.2. Experimental systems
No data were available to the Working Group on the reproductive and developmental effects of HC Red No. 3 alone. The compound was present at low concentrations in semi-permanent hair colouring formulations evaluated in a study of fertility and reproductive performance in rats (Wernick et al., 1975, 0.02%; see p. 99), in a study of heritable translocation in rats (Burnett et al., 1981, 0.01 %; see p. 104), and in studies of teratogenesis in rats and rabbits (Wernick et al., 1975) (see p. 100). No treatment-related adverse effect was detected. [The Working Group noted that the dose of each component of the formulations was very low and unlikely to have been toxic]
5. Summary of Data Reported and Evaluation
5.1. Exposure data
HC Red No. 3 is used as a semi-permanent hair dye.
5.2. Human carcinogenicity data
No data were available to the Working Group.
5.3. Animal carcinogenicity data
HC Red No. 3 was tested for carcinogenicity by gavage in one study in mice and in one study in rats. There was a significant increase in the incidence of hepatocellular adenomas and carcinomas combined in male mice administered the high dose; poor survival precluded evaluation of the females. No increase in the incidence of treatment-related tumours was seen in rats of either sex.
5.4. Other relevant data
HC Red No. 3 was mutagenic to bacteria.
5.5. Evaluation1
There is inadequate evidence in humans for the carcinogenicity of HC Red No. 3.
There is inadequate evidence in experimental animals for the carcinogenicity of HC Red No. 3.
Overall evaluation
HC Red No. 3 is not classifiable as to its carcinogenicity to humans (Group 3).
6. References
- Burnett C., Loehr R., Corbett J. Heritable translocation study on two hair dye formulations. Fundam. appl. Toxicol. 1981;1:325–328. [PubMed: 7185580]
- Frenkel E.P., Brody F. Percutaneous absorption and elimination of an aromatic hair dye. Arch. environ. Health. 1973;27:401–404. [PubMed: 4752703]
- US National Library of Medicine (1992) Registry of Toxic Effects of Chemical Substances (RTECS No.KJ6500000), Bethesda, MD.
- US National Toxicology Program (1986) Toxicology and Carcinogenesis Studies of HC Red No. 3 (CAS No. 2871-01-4) in F344/N Rats and B6C3F1 Mice (Gavage Studies) (NTP Technical Report 281; NIH Publ. No. 86–2537), Research Triangle Park, NC. [PubMed: 12748698]
- Wernick T., Lanman B.M., Fraux J.L. Chronic toxicity, teratologic and reproduction studies with hair dyes. Toxicol. appl. Pharmacol. 1975;32:450–460. [PubMed: 1154407]
- Zeiger E., Anderson B., Haworth S., Lawlor T., Mortelmans K. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ. mol. Mutag. 1988;11(12):1–158. [PubMed: 3277844]
Footnotes
- 1
For definition of the italicized terms, see Preamble, pp. 26–30.
- PubMedLinks to PubMed
- Review HC Blue No. 2.[IARC Monogr Eval Carcinog Risk...]Review HC Blue No. 2.. IARC Monogr Eval Carcinog Risks Hum. 1993; 57:143-51.
- Review HC Blue No. 1.[IARC Monogr Eval Carcinog Risk...]Review HC Blue No. 1.. IARC Monogr Eval Carcinog Risks Hum. 1993; 57:129-42.
- Review 1,4-Diamino-2-nitrobenzene (2-nitro-para-phenylenediamine).[IARC Monogr Eval Carcinog Risk...]Review 1,4-Diamino-2-nitrobenzene (2-nitro-para-phenylenediamine).. IARC Monogr Eval Carcinog Risks Hum. 1993; 57:185-200.
- Review HC Yellow No. 4.[IARC Monogr Eval Carcinog Risk...]Review HC Yellow No. 4.. IARC Monogr Eval Carcinog Risks Hum. 1993; 57:159-65.
- Review 2-Amino-4-nitrophenol.[IARC Monogr Eval Carcinog Risk...]Review 2-Amino-4-nitrophenol.. IARC Monogr Eval Carcinog Risks Hum. 1993; 57:167-76.
- HC RED NO. 3 - Occupational Exposures of Hairdressers and Barbers and Personal U...HC RED NO. 3 - Occupational Exposures of Hairdressers and Barbers and Personal Use of Hair Colourants; Some Hair Dyes, Cosmetic Colourants, Industrial Dyestuffs and Aromatic Amines
Your browsing activity is empty.
Activity recording is turned off.
See more...