Abbreviations
- RSV
Respiratory syncytial virus
Context and Policy Issues
Respiratory syncytial virus (RSV) can cause respiratory illness in persons of all ages and it is the leading cause of lower respiratory tract illness in children.1,2 The virus infects almost all children prior to 2 years of age during annual epidemics which, in Northern Hemisphere locations, occur seasonally between October to May. It can cause bronchiolitis and pneumonia and is estimated to be responsible for 3.4 million hospital admissions and approximately 200,000 deaths internationally in young children.3 Data suggest that the rates of hospitalization of children with RSV related illness in northern and Arctic communities in Canada are amongst the highest rates globally.4-6 Inuit children living in circumpolar regions have higher hospital admission rates for respiratory illness compared to those living in more southern areas.7 Several patient characteristics have been identified that carry a higher risk of morbidity and mortality including premature birth, infants with chronic lung disease, hemodynamically significant congenital heart disease, immunocompromised conditions and severe neuromuscular disease.8
Palivizumab is a monoclonal antibody against RSV and was approved for use in Canada in 2002. Palivizumab is indicated for the prevention of serious lower respiratory tract disease caused by RSV in pediatric patients at high risk of RSV disease. Some Canadian Arctic and far northern jurisdictions have provided government funding for palivizumab as prophylaxis since 2005.9 Coverage criteria vary across health jurisdictions and have included such restrictions as premature birth up to 35 weeks gestation or significant cardiac or respiratory conditions.10 For example, eligible children in Quebec can receive up to 5 monthly doses of palivizumab during the RSV season. The Quebec criteria for palivizumab prophylaxis includes children who are at greatest risk for developing serious respiratory illness due to RSV such as premature infants (<33 weeks of gestation) and children with a chronic respiratory disease or a congenital heart disease.7 In 2016, criteria in Quebec were modified to include healthy Nunavik children born at term and younger than 3 months of age at the start of the RSV season or born during the RSV season.
Many Inuit infants who live in Northern regions do not have access to hospitals equipped to manage severe RSV illness and air evacuation to tertiary hospitals may be necessary. The appropriate use of palivizumab in Canadian northern and arctic communities has been the subject of debate in the scientific literature and the Canadian media.1,5,6,9-14
The purpose of this report is to determine the clinical effectiveness and cost effectiveness of universal versus high-risk palivizumab prophylaxis, and seasonal versus year-round palivizumab in Inuit children up to 4 years of age.
Research Questions
What is the clinical effectiveness of universal versus high risk palivizumab prophylaxis for respiratory syncytial virus prevention in Inuit infants?
What is the clinical effectiveness of seasonal versus year-round palivizumab prophylaxis for respiratory syncytial virus prevention in Inuit infants?
What is the cost- effectiveness of universal versus high risk palivizumab prophylaxis for respiratory syncytial virus prevention in Inuit infants?
What is the cost-effectiveness of seasonal (~6months) versus year-round palivizumab prophylaxis for respiratory syncytial virus prevention in Inuit infants?
Key Findings
No relevant literature was identified regarding the comparative clinical effectiveness of universal versus high-risk palivizumab or seasonal versus year-round palivizumab prophylaxis in Inuit children up to 4 years of age. Additionally, no relevant literature was identified regarding the comparative cost effectiveness of universal versus high-risk palivizumab or seasonal versus year-round palivizumab prophylaxis in Inuit children up to 4 years of age.
Methods
Literature Search Methods
A limited literature search was conducted by an information specialist on key resources including PubMed, the Cochrane Library, the University of York Centre for Reviews and Dissemination (CRD) databases, the websites of Canadian and major international health technology agencies, as well as a focused Internet search. The search strategy was comprised of both controlled vocabulary, such as the National Library of Medicine’s MeSH (Medical Subject Headings), and keywords. The main search concepts were Palivizumab and respiratory syncytial virus and Inuit infants. No filters were applied to limit the retrieval by study type. Where possible, retrieval was limited to the human population. A narrower search was also limited to English language documents published between January 1, 2009 and November 20, 2019. A second broader search was also limited to English language documents published between January 1, 2014 and November 20, 2019.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published before 2009. Studies in non-aboriginal populations were excluded but studies were accepted for inclusion if a majority of the study population were Inuit or First Nations.
Summary of Evidence
Quantity of Research Available
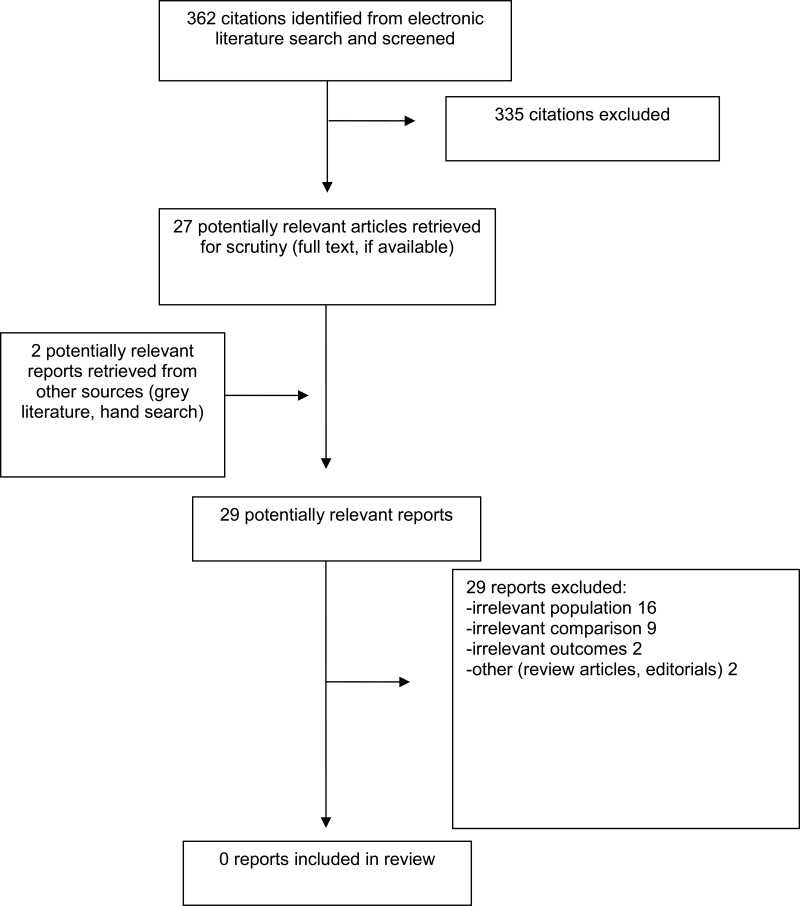
A total of 362 citations were identified in the literature search. Following screening of titles and abstracts, 335 citations were excluded and 27 potentially relevant reports from the electronic search were retrieved for full-text review. Two potentially relevant publications were retrieved from the grey literature search for full text review. Of these potentially relevant articles all were excluded for various reasons; no publications met the inclusion criteria and therefore none were included in this report. Appendix 1 presents the PRISMA15 flowchart of the study selection.
Limitations
This report is limited by the timeframe used for literature searches (from 2009 onwards) and by restricting the search to English language articles.
Conclusions and Implications for Decision or Policy Making
No relevant literature was identified regarding the comparative clinical effectiveness of universal versus high-risk palivizumab or seasonal versus year-round palivizumab prophylaxis in Inuit children up to 4 years of age. Additionally, no relevant literature was identified regarding the comparative cost effectiveness of universal versus high-risk palivizumab or seasonal versus year-round palivizumab prophylaxis in Inuit children up to 4 years of age. Therefore, no conclusions regarding relative clinical effectiveness and cost effectiveness can be provided.
Two reports that were evaluated for inclusion in this review assessed the clinical effectiveness7 and cost-effectiveness9 of palivizumab in infants residing in Canadian far north or Arctic communities, but did not address the comparisons of interest. Glica et al. evaluated the impact of palivizumab prophylaxis policies in Nunavik infants.7 Banerji et al. compared the cost effectiveness of palivizumab prophylaxis relative to no prophylaxis in term infants residing in the Canadian arctic.9 These reports did not address the comparisons of interest and hence did not satisfy the inclusion criteria for this current report and were not critically appraised or included in the summary of findings. However, as these reports may provide some relevant analyses, they are mentioned briefly here.
Gilca et al. (2018) attempted to evaluate the impact of the extension of palivizumab prophylaxis criteria on Nunavik Inuit infants <3 months of age born at term, since the reason for broadening the criteria in 2016 was based upon expert opinion and not based on empirical evidence.7
Gilca et al. reported that their analysis of data following the first year of implementing the broader prophylaxis criteria was inconclusive and that a longer observation period was required to evaluate the impact of palivizumab prophylaxis in Nunavik.7 Banerji et al. reported that there is great variability in incremental cost effectiveness ratios for palivizumab prophylaxis compared to no prophylaxis across several regions of the Canadian arctic.9
Further research using well-designed studies is needed to provide evidence to evaluate the comparative clinical and cost-effectiveness in Inuit populations with respect to seasonal versus year-round administration and with respect to universal versus high risk prophylaxis.
References
- 1.
Paes
BA, Mitchell
I, Banerji
A, Lanctot
KL, Langley
JM. A decade of respiratory syncytial virus epidemiology and prophylaxis: translating evidence into everyday clinical practice.
Can Respir J. 2011;18(2):e10–19. [
PMC free article: PMC3084427] [
PubMed: 21499597]
- 2.
- 3.
Nair
H, Nokes
DJ, Gessner
BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis.
Lancet. 2010;375(9725):1545–1555. [
PMC free article: PMC2864404] [
PubMed: 20399493]
- 4.
Borse
RH, Singleton
RJ, Bruden
DT, Fry
AM, Hennessy
TW, Meltzer
MI. The Economics of strategies to reduce respiratory syncytial virus hospitalizations in Alaska.
J Pediatric Infect Dis Soc. 2014;3(3):201–212. [
PubMed: 26625383]
- 5.
Banerji
A, Greenberg
D, White
LF, et al. Risk factors and viruses associated with hospitalization due to lower respiratory tract infections in Canadian Inuit children : a case-control study.
Pediatr Infect Dis J. 2009;28(8):697–701. [
PubMed: 19461554]
- 6.
Banerji
A, Panzov
V, Young
M, et al. Hospital admissions for lower respiratory tract infections among infants in the Canadian Arctic: a cohort study.
CMAJ Open. 2016;4(4):E615–e622. [
PMC free article: PMC5173479] [
PubMed: 28018874]
- 7.
- 8.
- 9.
Banerji
A, Ng
K, Moraes
TJ, Panzov
V, Robinson
J, Lee
BE. Cost-effectiveness of palivizumab compared to no prophylaxis in term infants residing in the Canadian Arctic.
CMAJ Open. 2016;4(4):E623–e633. [
PMC free article: PMC5396468] [
PubMed: 28443266]
- 10.
Banerji
A, Panzov
V, Young
M, et al. The real-life effectiveness of palivizumab for reducing hospital admissions for respiratory syncytial virus in infants residing in Nunavut.
Can Respir J. 2014;21(3):185–189. [
PMC free article: PMC4128465] [
PubMed: 24367792]
- 11.
- 12.
- 13.
Banerji
A, Lanctot
KL, Paes
BA, et al. Comparison of the cost of hospitalization for respiratory syncytial virus disease versus palivizumab prophylaxis in Canadian Inuit infants.
Pediatr Infect Dis J. 2009;28(8):702–706. [
PubMed: 19461555]
- 14.
Tam
DY, Banerji
A, Paes
BA, Hui
C, Tarride
JE, Lanctot
KL. The cost effectiveness of palivizumab in term Inuit infants in the Eastern Canadian Arctic.
J Med Econ. 2009;12(4):361–370. [
PubMed: 19900071]
- 15.
Liberati
A, Altman
DG, Tetzlaff
J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
Appendix 1. Selection of Included Studies
Tables
Table 1Selection Criteria
View in own window
| Population | Q1-4: Infants living in the Arctic or Northern communities or who are Inuit or First Nations aged 0 to 4 years |
|---|
| Intervention | Q1,3: Universal Palivizumab (brand name: Synagis) administration Q2,4: Palivizumab administered during RSV season/ 6 months |
|---|
| Comparator | Q1,3: Palivizumab administered only to high risk infants; No prophylaxis Q2,4: Palivizumab administered year round |
|---|
| Outcomes | Q1: Clinical effectiveness: number of hospital admissions, number of medical evacuations/transfers, incidence respiratory infection (e.g. respiratory syncytial virus [RSV] infection, bronchiolitis), morbidity Q2: Cost-effectiveness |
|---|
| Study Designs | Health technology assessments, systematic reviews, meta-analyses, economic evaluations, randomized controlled trials, non-randomized studies |
|---|
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Palivizumab for infection prevention in inuit infants: a review of the clinical effectiveness and cost-effectiveness. Ottawa: CADTH; 2019 Dec. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.