NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Division on Earth and Life Studies; Institute for Laboratory Animal Research; Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs. Necessity, Use, and Care of Laboratory Dogs at the U.S. Department of Veterans Affairs. Washington (DC): National Academies Press (US); 2020 Jul 1.

Necessity, Use, and Care of Laboratory Dogs at the U.S. Department of Veterans Affairs.
Show detailsThis chapter addresses the committee's task (see Chapter 1, Box 1-1) to explore recent past, current, and anticipated research questions directly related to the mission of the U.S. Department of Veterans Affairs (VA) to determine if laboratory dogs are or will continue to be necessary for future VA biomedical research.
The chapter begins with an overview of dog use in biomedical research in the United States. This is followed by a consideration of laboratory dog use in 10 biomedical research fields related to the VA's mission, including seven areas in which the VA currently uses laboratory dogs or has done so in the recent past (cardiovascular disease [CVD], spinal cord injury [SCI], imaging, diabetes, narcolepsy, chronic pain and osteoarthritis [OA], and experimental pharmacology and toxicology) and three areas of potential future use (cancer, infectious disease, and Alzheimer's disease). Six other areas of biomedical research in which the VA has used dogs, some in the past decade, are also discussed briefly.
The committee's efforts focused on exploring areas of biomedical research using laboratory dogs in the VA's current and recent portfolio (2016 onward) and, to a more limited extent, areas relevant to the VA's mission where dog use may be considered in the future. It would not have been feasible for this committee to cover all possible research areas of interest to the VA, and the absence of a particular field from this report should not be taken as a determination regarding the necessity of dog use in that field.
The chapter concludes with a discussion of the committee members' various interpretations of “necessary” and the committee's recommendations, including dissenting opinions, for guiding the VA's determination of when laboratory dogs are necessary for biomedical research (see Recommendations 1 and 2). The committee also provides a recommendation for improving the VA's biomedical research protocols and review processes (see Recommendation 3).
TRENDS IN DOG USE IN U.S. RESEARCH FACILITIES
Animals, including dogs, have been used in scientific demonstrations, teaching, and research since antiquity (Kinter and DeGeorge, 2016). Dog use increased dramatically in the late 19th and early 20th centuries, paralleling the development of increasingly sophisticated instrumentation for measuring physiological function, along with advances in analytic and organic chemistry that led to an explosion in the synthesis of small molecules requiring evaluation of their pharmacological properties1 (Kinter and DeGeorge, 2016). These trends in molecule synthesis and animal use continued through the mid-20th century and were amplified by new considerations, including requirements for animal safety testing in order to obtain government approvals for clinical trials and marketing of regulated products and the growth and proliferation of international biomedical research stimulated by post–World War II industrial expansion. Several members of this committee recall the large number of sophisticated dog models that supported basic human and veterinary physiological research in the postwar decades, as well as dog bioassays for pharmacological and toxicological research and product discovery and development. The advent of new molecular techniques in the 1970s and 1980s likely played a role in replacing animal research models, particularly dogs. Data from U.S. Department of Agriculture (USDA) annual reports tracking dog use from 1973 to 2018 are illustrated in Figure 3_1. (See also Appendix B for further analysis of the USDA data.)
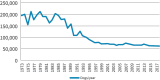
FIGURE 3-1
Annual dog usage in the United States from 1973 to 2018, based on data collected by the U.S. Department of Agriculture.
The specific factors leading to decreased dog use since the 1970s are uncertain but could include the advancement of molecular techniques, societal pressures and preferences, and the high cost of using dogs in biomedical research. It is also the case that while there has been a notable decrease in dog use since the 1970s, dog use in the past decade has been steady at around 60,000 dogs per year.
Current Distribution of Laboratory Dog Use in U.S. Research Facilities
The committee reviewed the 1,149 annual reports submitted to USDA's Animal and Plant Health Inspection Service2 from research facilities in all 50 states and territories in 2017 (the most recent year with complete publicly available data). This review indicates that a total of 60,190 dogs were used in the United States in 2017, of which 22,933 were used by 213 academic institutions and affiliated hospitals engaging in biomedical research and education (including veterinary research conducted for the benefit of dogs); 34,875 by 105 companies and private research organizations engaging in applied biomedical research and human and veterinary product development (industry), including testing required by regulatory agencies; 832 by 11 government agencies (including VA research labs) conducting basic and applied research; and 1,550 by 16 other, non-research groups. USDA data indicate that industry is currently the dominant user of dogs for biomedical research, with the use of dogs by industry exceeding the usage by academic institutions, government, and non-research groups combined.
LABORATORY DOG USE IN BIOMEDICAL RESEARCH AT THE VA
The VA provided the committee with documentation from 44 research projects involving dogs conducted by its researchers over the past 50 years. Of these projects, 30 dated from the 1960s to 2017 and 14 were active in 2018–2019 (VA, 2018a,b). Among these studies, cardiovascular/renal was the most frequently cited application (17), followed by central nervous system (15), endocrine (4), respiratory (4), gastrointestinal (3), and animal/surgical models (4). Some VA projects covered more than one research area, hence the total number of areas exceeds the number of projects. One VA study, undertaken to test a treatment for a spontaneous melanoma that occurs in both dogs and humans, used companion rather than laboratory dogs (VA, 2018b). In research performed at or funded by the VA, 99 percent of animals used in 2017 were mice or rats, while fewer than 0.05 percent were dogs (VA ORD, 2018).
In the following three sections, the committee surveys the use of dogs in 10 biomedical research areas—CVD, SCI, imaging, diabetes, narcolepsy, chronic pain and OA, experimental pharmacology and toxicology, cancer, infectious disease, and Alzheimer's disease. The purpose of this overview is to establish a context for assessing both the current need for laboratory dogs in each of these fields and the likely need for dogs in future VA biomedical research. CVD, SCI, and imaging are the current areas of laboratory dog use at the VA. Diabetes, narcolepsy, chronic pain and OA, and experimental pharmacology and toxicology are areas in which dogs were recently used (since 2016), and the remaining three fields (cancer, infectious disease, and Alzheimer's disease) were chosen based on their potential for future use (primarily in companion dogs). As stated in the introduction to this chapter, the absence of a particular field of research should not be taken as a determination regarding the necessity of dog use in that field.
Research Areas with Current Laboratory Dog Use at the VA
This section describes the state of research in three areas of current laboratory dog use at the VA—CVD, SCI, and imaging. The committee reviewed current practices and recent advancements in each of these areas of research to better understand the context for the VA's research using laboratory dogs in these areas.
Cardiovascular Disease
CVD encompasses numerous clinical entities and is the leading cause of death in the U.S. population (NCHS, 2017) and globally (Mendis et al., 2011). In a 2014 longitudinal study, the U.S. veteran population was shown to be at increased risk for the development of CVD (Assari, 2014), and veteran status was identified as a risk factor independent of other co-factors and comorbidities. A website maintained by the VA's Office of Research and Development identifies CVD as the leading cause of hospitalization in the VA health care system and as a major cause of disability (VA ORD, n.d.a). The reader may refer to this site for additional information regarding the current status of VA cardiovascular research programs as well as their historical contributions to the advancement of clinical care, discovery, and scholarship.
The laboratory dog has a long history of use as an experimental model for the investigation of cardiovascular physiology and disease. Early work in dogs led to notable advances in the understanding of the role of the autonomic nervous system in the regulation of heart rate and blood pressure (Halsey, 1917), the renin–angiotensin–aldosterone system in the control of blood pressure and circulatory volume (Goldblatt et al., 1934; Watkins et al., 1976), the pathogenesis of hypertension related to renal vascular disease (Goldblatt et al., 1934), cardiac electrophysiology and conduction disorders (Ross and Franklin, 1976), congenital and acquired heart diseases and their surgical correction (Shumway et al., 1962; Toledo-Pereyra, 2010), and congestive heart failure (Conn et al., 1966; Watkins et al., 1976).
In the development of modern cardiovascular care, from the latter half of the 20th century to the present, almost every major milestone has involved dogs. Dogs played a role in development of the pacemaker, the internal cardiac defibrillator, angioplasty, stents, congenital heart surgery, valve surgery, transplants, and transcatheter aortic valve replacement (Bernstein, 2019), and they continue to be used in efforts aimed at improving these technologies.
Nonetheless, the 1980s saw the beginning of a decline in the use of dogs in research (see Figure 3_1), including for cardiovascular research, as many disciplines shifted to other animal models, particularly rodents, which were better suited to investigations of the genetic and molecular mechanisms of disease. The miniaturization of technology and the adaptation of techniques to rodents, combined with the increased use of alternative large animal models, accelerated the decline in dog use. Over time, these alternative models—particularly the pig, goat, sheep, and non-human primate—have come to share the cardiovascular research landscape with the laboratory dog.
A survey of eight major cardiovascular journals over the past 20 years found that most studies reporting experimental models used rodents, primarily mice (Harrison, 2019). Rodents were the most commonly used animal model for studying hypertension, atherosclerosis, heart attack, heart failure, obesity and diabetes, and other vascular diseases. However, not all questions can be adequately addressed in rodents, due to their smaller size and anatomical and physiological differences from humans, and large animals frequently serve as a bridge for translating new findings from rodents to human patients.
The choice of which large animal model to use hinges on the model's similarity to the particular human condition being studied. Pigs are now favored over dogs for coronary artery disease research, for example, because of the greater similarity of the pig's coronary anatomy to that of humans (Vilahur et al., 2011; Weaver et al., 1986). Atherosclerotic coronary artery disease is readily inducible in the pig (Granada et al., 2009) and atherosclerotic plaques develop in locations relevant to the human condition (Brodala et al., 2005; Hasler-Rapacz et al., 1995) and lead to metabolic syndrome (Myers, 2019). A review of the most common large animal models for studying heart disease, which addresses the particular biological properties that may favor selection of one model over the others for a given investigation, was published recently (Camacho et al., 2016).
Despite the increase in the use of rodents, pigs, and sheep, dogs remain a preferred model for studying certain aspects of CVD. The committee's review of the scientific literature describing cardiovascular research in dogs from 2009 to mid-2019 turned up hundreds of publications, indicating that the laboratory dog continues to serve as an important animal model for CVD, both in the U.S. research enterprise and globally. Dogs were primarily being used as models for the investigation and treatment of cardiac arrhythmias and congestive heart failure and for the development and validation of devices used in the diagnosis or therapy of CVD. The following discussion summarizes the salient trends revealed by this literature survey.
Aspects of arrhythmia
The laboratory dog is used extensively for studying both atrial and ventricular arrhythmias, specifically for interrogating those physiological processes for which alternative animal models are less suitable. Rodent heart rates (500–700 bpm) and vascular shear rates differ dramatically from those of humans and dogs (Harrison, 2019; Suo et al., 2007). The ratio between the size of the myocardium and wavelength is critical for establishing the pathophysiology of arrhythmia; the animal in which that ratio is most similar to that of humans is the rabbit, followed by the dog and the pig (Efimov, 2019; Panfilov, 2006). Combined with the fact that the pig's Purkinje system differs from the human one, this similarity has supported the argument that the pig is a poor model for arrhythmia, at least in terms of modeling human pathophysiology. Nonetheless, pigs are used in many aspects of arrhythmia translational research. A recent analysis recommended pigs as the primary choice for studying myocardial ischemia and atrial tachycardia and suggested further research to characterize pigs as models for ventricular tachycardia (Clauss et al., 2019). Dogs may nonetheless be preferred for certain investigations, such as evaluation of the autonomic modulation of arrhythmias (Piktel and Wilson, 2019).
For atrial fibrillation (AF), the most common type of clinical arrhythmia in humans, the dog model involves the induction of AF through atrial tachy-pacing at a rate of 400 bpm for an extended period. This model is currently used in the investigation of diverse topics in AF, including atrial electrocardiogram analysis (Gerstenfeld et al., 2011); defibrillation therapy (Janardhan et al., 2014; Witt et al., 2018); atrial remodeling (Nakatani et al., 2013; Yamashita et al., 2019); the role of the autonomic nervous system (Ardell et al., 2014; Katsouras et al., 2009; Nishida et al., 2011); genetic, molecular, and channel biology studies (Shorofsky et al., 2009; Wakili et al., 2010; Wang and Li, 2014; Wei et al., 2018); and the evaluation of antiarrhythmic drugs (Qi et al., 2014; Sakabe et al., 2012). Research into new approach methodologies in AF has advanced in recent years and holds promise for some future cardiovascular research efforts to move away from dogs (see Chapter 4 for discussion of alternatives). Canine tissues and cells—either isolated from tachy-paced dogs or tachy-paced in vitro—are also frequently employed (Aguilar et al., 2014; Makary et al., 2011; Wiersma et al., 2017). Thromboembolism and stroke are major complications associated with atrial enlargement in AF patients, and these are managed pharmacologically with anticoagulation therapy and beta blockers. The laboratory dog is being used for the development and evaluation of nonpharmacological strategies for stroke prevention, including minimally invasive surgical procedures and new devices (Bruce et al., 2011; Fumoto et al., 2012; Hill and Guy, 2012; Kar et al., 2014; Lee et al., 2010b; Schwartz et al., 2010; Sunagawa et al., 2017).
The laboratory dog has also retained a prominent position in the study of ventricular arrhythmias, which may be attributable to several factors. The dog's Purkinje fiber network has histological (Ono et al., 2009) and electrophysiological (Huang et al., 2014) characteristics that are more similar to the human than are those of the pig (Efimov, 2019). The dog is also very sensitive to the development of electrocardiographic prolongation of the QT interval, which is regarded as an early potential predictor of Torsades de Pointes, a severe polymorphic ventricular arrhythmia that may cause sudden death. For this reason, the laboratory dog has frequently been the in vivo model of choice in the deselection of new pharmacological compounds. It should be noted, however, that the currently available bioassays for proarrhythmic activity, including the dog, are not ideal (Lee et al., 2010a). Dogs are more sensitive than humans to the development of prolonged QT, which may lead to the disqualification of potentially useful drugs. This has led some researchers to pursue new electrophysiological parameters and other approaches that may improve the predictive value of the dog model (Boulay et al., 2019; Marostica et al., 2016; van der Linde et al., 2010; Vargas, 2010).
Canine myocardial infarct models are widely used for the study of ventricular arrhythmias, where the influence of the spinal cord and autonomic nervous system on arrhythmogenesis and pursuit of new therapeutic approaches have become objects of intense focus (Baburin et al., 2018; Chen et al., 2013, 2016; del Rio et al., 2015; Lopshire et al., 2009; Nasi-Er et al., 2019a,b; Wang et al., 2015; Zhang et al., 2018; Zhou et al., 2019). The protective effects of nutritional factors (Bonilla et al., 2016), exercise (Billman, 2009; Kukielka et al., 2011), intermittent hypoxia (Estrada et al., 2016), and new compounds (Lee and Lucchesi, 2013) have been investigated in the canine myocardial infarct model.
Cardiomyopathies
Dogs are also used to model nonischemic congestive heart failure and cardiomyopathy (Camacho et al., 2016; Dixon and Spinale, 2009). Cardiomyopathy occurs naturally in several dog breeds, including Doberman pinscher, Newfoundland, Irish wolfhound, boxer, and golden retriever, and this may prove useful for future investigations of genetic, molecular, and environmental factors common to canine and human cardiac diseases (Kaplan et al., 2018; Meurs, 2017; Meurs et al., 2012; Simpson et al., 2015, 2016; Vischer et al., 2017; Wiersma et al., 2008).
Device testing
In addition to its use for the development of procedures and devices aimed at treating arrhythmias, the dog remains an important model for the development and evaluation of devices used in other areas of cardiovascular diagnosis and therapy. For testing intravascular devices, which require stable vascular dimensions over time, the dog and sheep (Joscht et al., 2016; Kalder et al., 2019) have a distinct advantage over the pig, due to the pig's continuous body growth (and commensurate changes in vasculature) throughout its life span (Myers, 2019). Dogs have been used for more than 30 years for the evaluation of intravascular stents to repair vascular defects and restore blood flow (Jeremy and Thomas, 2010; Sigwart, 2017), and there are numerous examples in the most recent decade of the contributions of the dog model to the understanding of stent design, improvements in biocompatibility, and reductions in complications (Bastijanic et al., 2014; Cho et al., 2014; Kawajiri et al., 2015a,b; Lequoy et al., 2016; Li et al., 2019; Martinez Moreno et al., 2019; Paul et al., 2012, 2013; Watanabe et al., 2014; Zhang et al., 2015). Intracardiac devices to address valvular, shunt, and septal defects have also been studied in the laboratory dog (Gruenstein and Bass, 2009; Takaseya et al., 2010). Research on the endograft model for repair of aortic aneurism switched to a sheep model as part of the general movement away from dogs. However, there have been recent reports of late failure of this device in humans, which is likely to require investigation in an animal model. This may lead to a return to dogs for studying the larger pelvic implant, which is well tolerated in dogs but causes paralysis in sheep (White, 2019; White et al., 1996; Wilson et al., 1997).
Pacemakers have been used to control heart rate in humans since their development in a laboratory dog model over half a century ago. The first pacemaker ever placed in a human, in 1958, lasted 9 hours (Bernstein, 2019). The continued advancement of this technology, as well as the development and improvement of intravascular defibrillators and the combined cardioverter–defibrillator, has relied on the laboratory dog (Bryant, 2017; Merkely et al., 2013; Sanders et al., 2011), although it has also used pigs in many instances. Dogs are also widely used to evaluate new approaches and devices for supporting the heart during cardiac failure, including ventricular assist devices and cardiac restraint devices (Clarke et al., 2015; Kakino et al., 2017; Kubota et al., 2014; Sabbah et al., 2009; Saku et al., 2016). Devices that enable an endovascular approach to denervation (Jordan et al., 2012), tissue ablation (Jilaihawi et al., 2010), central nervous system interventions (Kara et al., 2014), and protection from embolism (Kara et al., 2014) have also relied on the laboratory dog.
VA cardiovascular disease research using laboratory dogs
The VA has used dogs since the 1960s to investigate the consequences and treatment of cardiac rhythm disorders and heart failure. Early VA investigations using the dog resulted in the development and routine clinical use of the pacemaker to stabilize heart rate in humans (Chardack, 1964; Chardack et al., 1960, 1962, 1963). The size and electrophysiological similarity of the dog heart to the human heart established the dog as the preferred model for those studies. Subsequent VA researchers have cited additional similarities between human and dog collateral coronary circulation, heart geometry, heart rate, and autonomic nervous system as important features for their cardiovascular studies. Between the 1980s and 2000s VA research teams extensively studied the mechanisms for heart failure and pharmacologic approaches to intervention (Carabello et al., 1992; Ishibashi et al., 2001; Ishihara et al., 1992; Matsuo et al., 1998; Nagatsu et al., 1994, 2000; Nemoto et al., 2002; Tsutsui et al., 1994; Zile et al., 1991). Also during this period, VA researchers developed numerous techniques and equipment for the ablation of cardiac arrhythmias in humans (Antz et al., 1998, 2001; Hasdemir et al., 2003; Jackman and Zipes, 1982; Jackman et al., 1988; Nakagawa et al., 1998; Schauerte et al., 2000; Wittkampf et al., 1996).
More recently, VA researchers have relied on the dog to investigate the reasons for the failure of surgical ablation to eliminate AF in some cases (Melby et al., 2008) and to develop other novel approaches for the treatment of AF (Chinda et al., 2016; Ruaengsri et al., 2018). Clinical observations in patients also led VA researchers to investigate problems associated with premature ventricular contractions (PVCs) in the dog. These studies established that, in some cases, PVCs induce cardiomyopathy (Huizar et al., 2011), and this was subsequently recognized as a distinct clinical entity by the American Heart Association (Al-Khatib et al., 2018). A search for strategies to prevent sudden cardiac arrest found that certain electrocardiographic signals, called T-wave alternans, correlate with the onset of potentially lethal ventricular arrhythmia (Kwofie et al., 2011). Recent publications from the VA demonstrate their ongoing interest in understanding the cellular and molecular changes underpinning the development of PVC-induced cardiomyopathy as well as the neural mechanisms that might be useful for the reduction of PVCs (Gunda et al., 2019; Huizar et al., 2019; Jiang et al., 2016; Tan et al., 2016; Wang et al., 2014).
Summary
While laboratory dogs have been supplanted by rodents for most CVD research, there remains extensive ongoing research using laboratory dogs to investigate several topics relevant to human CVD. Laboratory dogs continue to be used for the study of both atrial and ventricular arrhythmias, owing to physiological traits (heart rate, vascular shear rate, myocardium/wavelength ratio, Purkinje fiber network, etc.) that model the human state better than do other large animal or rodent models. Laboratory dogs also remain important for device testing and improvement, specifically for those devices that cannot be tested in sheep or pigs owing to either physiological differences or the need for long-term stability in vivo. As described in Chapter 4, pigs are seeing increasing use for research into myocardial ischemia and atrial tachycardia, and they are beginning to be characterized as models for ventricular tachycardia. The availability of genetically engineered minipigs is likely to increase the trend away from laboratory dog research for CVD, until sufficient new approach methodologies are available.
The natural occurrence of CVD in multiple breeds also makes companion dogs promising candidates for the investigation of the genetic and environmental factors that influence heart failure. Indeed, veterinary care of companion dogs commonly includes treatment for a range of CVDs (arrhythmia, hypertension, heart failure, etc.). Research using companion dogs stands to benefit both dogs and humans.
Spinal Cord Injury
There are 291,000 people in the United States with an SCI, 59.9 percent of which are cervical injuries resulting in tetraplegia and loss of natural breathing (NSCISC, 2019). An additional 39.5 percent are thoracic injuries that cause paraplegia (NSCISC, 2019). Health concerns of individuals with SCI are severe and include loss of hand and arm function, pressure sores, loss of bowel and bladder function, urinary tract infection, impaired breathing and cough, spasticity, neuropathic pain, and loss of sexual function (Alilain, 2019; Floyd, 2019). There is currently no cure for SCI and no therapeutic to improve outcome other than rehabilitation (although epidural stimulation has generated some promising results, as described below). The VA cares for more than 27,000 veterans afflicted with SCIs and related disorders annually (VA ORD, n.d.b).
The use of laboratory dogs as research models for SCI has a long history that goes back to the first direct experimental contusions in 1911 (Allen, 1911). Over the past half-century, however, much SCI research that previously involved laboratory dogs (as well as cats and rabbits) shifted to rodents. Rodents are now the preferred species for the initial evaluation of therapies aimed at facilitating spinal cord repair, although the human injury can be more closely approximated in large animals (Cheriyan et al., 2014). While research aimed at neuroprotection and regeneration has produced encouraging results in rodents (MacFarlane et al., 2018; Warren and Alilain, 2019; Warren et al., 2018), it has failed to generate any effective pharmacologic or stem cell approaches for treating humans to date (Alilain, 2019; Floyd, 2019), despite claims to the contrary by nonregulated stem cell “clinics” (Gabel et al., 2017).
Translational spinal cord injury research
Despite the predominance of rodents in SCI research, taking a treatment straight from rats to humans raises serious concerns. A large animal, such as a pig or dog, constitutes an intermediate model that increases confidence in the ability to safely translate a treatment from laboratory to clinic. Large animals are more similar to humans in terms of size, brain anatomy, blood flow, pharmacokinetics, and the complexity of the spinal circuitry (Floyd, 2019), and they are more amenable than rodents to detailed locomotor assessment, electrophysiology, bladder function tests, and high-quality brain imaging (Jeffery, 2019). Furthermore, not conducting large animal studies risks serious adverse events in a first-in-human study, with the potential not only to harm the patients involved but also to derail the development of an important therapy (Guest, 2019). While translational SCI research in laboratory dogs has a long history, few effective treatments for SCI currently exist, leaving significant opportunity for enhanced collaboration among researchers in this area and exploration of companion animal and new approach methodologies.
Use of companion dogs to study dog thoracic spinal cord injury
There is a growing body of research into the restoration of motor function which employs a variety of interventions in large animals. This research has seen considerable growth in the realm of veterinary medicine, aided by the relatively high incidence of SCI in companion dogs and the willingness of many pet owners to “volunteer” their affected dogs for research that may offer a therapeutic benefit for the dog. Although the treatment of the dog's SCI is the primary aim of these studies, the findings have potential to inform the treatment of analogous lesions in humans. A canine SCI consortium, CANSORT-SCI, was founded to leverage this opportunity, using clinical trials performed in companion dogs for the benefit of dogs, to facilitate the translation of results to humans (Moore, 2019; Moore et al., 2017). An international canine SCI observational registry has been established, and its founders argue that companion dogs' heterogeneity with respect to both injury and genetic backgrounds offers an advantage in the way it parallels the diversity of human injuries and genotypes (Moore et al., 2018). Veterinary clinical trials for SCI are enabling therapies that were found to be effective in rodents more than a decade ago to be tested for the first time in large animals (Granger et al., 2012; Hu et al., 2018). It is important to note, however, that the injuries studied in companion dogs are thoracic, which represent the minority of human SCI injuries.
Device testing
Another approach to treating SCI employs devices to drive essential life processes, such as breathing by selective electrical stimulation, which seeks to enable patients with cervical SCI to survive without mechanical ventilation despite the persistence of the injury. Preclinical research into device-driven breathing is carried out in large-animal models, including dogs, and was the topic of the SCI studies involving laboratory dogs at the VA published in 2018–2019 (DiMarco and Kowalski, 1985; Kowalski et al., 2019). These experiments, designed to restore respiratory muscle function, recently produced evidence of successful translation from dogs to humans (DiMarco et al., 2019a). Beyond the VA, another example of an SCI device-dependent intervention that has been moved from animal studies into humans is epidural stimulation in combination with physical therapy, which has restored some patients' ability to walk (Harkema et al., 2011; Wagner et al., 2018).
The extent to which preclinical SCI research in animals can predict human outcomes remains an open question. Numerous studies have revealed the complexity of SCI and the multiple variables that need to be addressed in order for effective interventions, aimed at repairing the damage, to have a chance at success. In addition to the obvious anatomical differences among species, there is divergence in spinal tract reorganization and functional recovery (Friedli et al., 2015). There is also a discrepancy in focus, with 81 percent of 2,209 published animal studies (1946–2016) focusing on thoracic SCI, whereas cervical SCI is the more common injury in humans (Sharif-Alhoseini et al., 2017). Of these animal SCI studies, 72.4 percent (1,599) were in rats, 2.3 percent (51) were in dogs, 2.2 percent (48) were in cats, 2.4 percent (53) were in rabbits, and 1.5 percent (33) each were in pigs and non-human primates. A review of large animal models of stem cell therapies for SCI identified 11 dog studies between 2007 and 2014, two of which used dogs with natural rather than induced injuries (Gabel et al., 2017).
Current use of dogs in spinal cord injury research
A search of the PubMed database for SCI studies involving dogs published in 2017–2019 revealed that almost all of these used companion dogs with thoracic injury, including one study that harvested cells from oral mucosa to generate stem cells for transplantation into the spinal cord (Ito et al., 2019) and another that tested low-level electrical stimulation, transplantation of adipose-derived stem cells, or a combination of the two (Krueger et al., 2019). The harvested-cell study noted wide variability in stem cell yield, while the electrical-stimulation plus adipose-derived-stem-cell study failed to demonstrate the hypothesized superiority of this combination treatment. Results from both studies indicate that much more needs to be learned before the stem cell approach can be translated into humans. A 2018 review of large animal hemisection models of SCI (Wilson et al., 2018) cited two Korean studies, performed in 2009 and 2010, that demonstrated the migration of human neural stem cells into dog spinal cord tissue and functional recovery (Kim et al., 2010; Lee et al., 2009); some of this work may have subsequently been moved to a rat model (Hong et al., 2017).
The structure of the spinal cord in the pig is close to that in humans (Guest, 2019), and recent studies aimed at optimizing techniques for delivering cells to patients with chronic SCI used the Yucatan minipig as a large animal bridge from earlier preclinical studies performed in rodents and primates (Benavides et al., 2017; Casas and Guest, 2004; Kutikov et al., 2019; Lim et al., 2010). Where the companion dog model cannot be used, such as for early drug discovery studies or for studies that require carefully timed injury intervention (Moore, 2019), the pig offers a large animal alternative to laboratory dogs. However, the continuous growth of pigs (including minipigs) throughout their life span, as well as difficulty in managing pigs, can limit their utility for long-term testing of implanted devices, while primates (non-human and human) remain essential for the testing of hand function and the role of the immune system in SCI (Floyd, 2019; Guest, 2019; Jeffery, 2019). A sheep model is also under development (Wilson et al., 2019).
VA spinal cord injury research using laboratory dogs
Recent advances in the development of new devices to stimulate breathing in humans with cervical SCI, described above, represent the culmination of more than two decades of research and rely on a dog model that was developed by the same group of VA investigators (Walter et al., 2007). The researchers noted that, although pigs were large enough for the devices, anatomical differences have prevented pigs from becoming an established model for respiratory stimulation in humans. VA research performed in the 2000s used dogs to explore methods to electrically stimulate bladder control in people with SCI (Bresler et al., 2008). The VA also supported research into a cough stimulator to reduce the incidence of respiratory infections and improve the quality of life for people with SCI (Kowalski et al., 2016). This device led to improved pulmonary function among tetraplegics enrolled in a recent clinical trial (DiMarco et al., 2019b) and is now being used by veterans with SCI. Research is continuing to refine this device to help individuals with SCI and others who have difficulty coughing such as those who experienced strokes or have amyotrophic lateral sclerosis.
Summary
The majority of SCI research is now carried out in species other than dogs or in companion dogs with thoracic injury, which stand to benefit directly from the development of new therapies. Pigs have been effective models for studying pathogenesis and translating therapies aimed at neuroregeneration, as described in Chapter 4. However, for a small number of investigations that are highly relevant to humans with SCI, such as those interrogating respiratory function in patients with cervical injury, laboratory dogs remain a model of choice, owing to specific anatomical and physiological features that cannot be recapitulated in other large animals.
Imaging
In the early days of imaging that followed the discovery of X-rays in 1895, use of the new technology often took a greater toll on human investigators, who had no awareness of the radiation to which they were exposing themselves, than on their canine research subjects (Babic et al., 2016; Barger, 1981). In time, radiation protections were implemented, and imaging modalities evolved from radiographs to ultrasound to computed tomography to magnetic resonance imaging (MRI), in some cases moving away from the use of radiation altogether. However, with increasingly sophisticated surgical techniques and other biomedical interventions constantly under development, dogs have been regularly called on for testing of both new treatments and new uses of imaging technology (Baumeister et al., 2016; Brooks et al., 2019; Chatal et al., 2015; Hadrian and Palmes, 2017; Millon et al., 2014).
Due to their larger size compared with rodents and to their ease of handling, laboratory dogs have frequently been used in clinical investigations to test a variety of imaging techniques. The radionucleotide rubidium-82, used to image myocardial perfusion, was first assessed in dogs, from the recognition of its similarities to potassium in 1954 through the first human injections in the early 1980s (Chatal et al., 2015). Laboratory dogs have also been used to investigate a number of other imaging techniques, including the following: cardiac MRI for the detection of reperfusion hemorrhage following experimentally induced myocardial infarction (Kumar et al., 2011), the use of MRI and digital subtraction angiography to evaluate a model of subarachnoid hemorrhage (Mori, 2014), and the role of MRI in tracking the development of atherosclerotic plaques in experimentally induced atherosclerosis (Millon et al., 2014). Dogs are also being eyed as potential contributors to comparative neuroscience using functional MRI, where they have begun to offer insight into the way human language is represented in a non-primate mammalian brain (Andics and Miklosi, 2018).
Dogs are not, however, the model of choice for all imaging studies. There is significant interspecies diversity with respect to the safety and efficacy of imaging agents which varies with the specific class of compounds, sometimes unexpectedly. Rabbits, for example, were preferred for the development of contrast-enhanced subharmonic ultrasound (Eisenbrey et al., 2015), and pigs are useful for thrombectomy modeling and imaging (Chueh, 2013). Studies involving the inter-atrial septum of the heart are preferentially done in swine or sheep, as the canine inter-atrial septum lacks sufficient anatomic similarity to humans (Jalal et al., 2018). Efforts at imaging the lymphatic system (lymphoscintigraphy) have moved from canine to rodent models, where the circumferential excision of lymphatic tissue can induce the required secondary lymphedema (Hadrian and Palmes, 2017).
VA imaging research using laboratory dogs
The narrowing of the arteries supplying blood to the kidneys can cause hypertension and kidney damage. Prior to the 1980s, the measurement of blood flow through renal arteries required a flowmeter to be surgically placed on the artery. VA research conducted on dogs in the 1980s helped validate the accuracy of blood flow measurements using non-invasive ultrasound imaging (Avasthi et al., 1984; VA, 2018b). Ultrasound is now accepted as the standard clinical method for assessing renal artery stenosis, which is estimated to affect 70,000–400,000 veterans (VA, 2018b).
Summary
Although laboratory dogs were critical for the development of many imaging techniques now taken for granted and still may have a role to play in studying brain function, they no longer constitute a default model for the development or testing of novel imaging modalities, particularly in those cases where an alternative is likely to exist. Nonetheless, given the great diversity of response to imaging compounds among laboratory species, the possibility that dogs may be required for specific imaging needs in the future cannot be ruled out.
Research Areas with Recent Laboratory Dog Use at the VA
This section describes the state of research in four areas of recent laboratory dog use at the VA—diabetes, narcolepsy, OA and chronic pain, and experimental pharmacology and toxicology.
Diabetes
Diabetes has been a human scourge since antiquity (Eknoyan and Nagy, 2005). Two types of diabetes are recognized: a devastating form that primarily affects youth and a milder form that primarily affects overweight adults, termed type 1 and type 2, respectively. More than one-third of the global population is at risk of developing type 2 diabetes (Kleinert et al., 2018). Type 1 diabetes is characterized by the absence of insulin, a key regulator of body glucose utilization, whereas type 2 diabetes is characterized by cells' inability to respond appropriately to circulating insulin. Today, type 2 diabetes is associated with a constellation of dysfunctions labeled the “metabolic syndrome” in both adults and youth. Diabetes was the seventh leading cause of death in the United States in 2017, with 1.5 million new cases diagnosed that year (CDC, 2020). Diabetes affects nearly 25 percent of VA patients and is the leading cause of blindness, end-stage renal disease, and amputation at the VA (VA ORD, n.d.c).
Trends in dog use for diabetes research
Dogs have played a central role in diabetes research from its earliest days. Dogs are susceptible to heritable (Cai et al., 2019), autoimmune (O'Kell et al., 2017), and environmentally stimulated diabetes (Kleinert et al., 2018) and could benefit from the same treatment strategies used in human patients. The discovery of insulin may be one of the most consequential biomedical achievements of the 20th century, not only for its impact on diabetes management in human and veterinary medicine, but for launching a century of far-ranging advances in the chemistry, biology, therapeutics, and synthesis of peptide hormones, proteins, and drugs; as well as in the manufacture of delivery systems.
With the advent of human insulins and more sophisticated technologies for insulin administration, the focus has shifted to type 2 diabetes. Dogs are susceptible to developing overweight/obesity related to diet/overfeeding but are more resistant than humans to the development of full-blown type 2 diabetes (Kleinert et al., 2018) and therefore have seen relatively little research use for this disorder compared to type 1 diabetes. Most current experiments using animals in diabetes research are carried out in rodents, with a focus on rodent models of metabolic syndrome and the glucose tolerance test (e.g., Brott et al., 2013). Nonetheless, larger animals are still used for some pharmaceutical safety studies and studies directed at veterinary care (Kleinert et al., 2018; Kumar et al., 2012).
VA diabetes research using laboratory dogs
Research performed in the century since the discovery of insulin has elucidated the role played by pancreatic islet cells in the control of blood glucose and tissue glucose use. In the 1980s and 1990s the VA funded basic research on the microstructure of the islets; this work used dogs because islet microstructure in humans is similar to that in dogs and quite different from rodents (VA, 2018b). In 1992 a VA researcher helped demonstrate the successful reversal of diabetes in dogs by the intraperitoneal implantation of microencapsulated islet cells (Soon-Shiong et al., 1992; VA, 2018b). One result of this work is that the dog is now viewed as the translational model for pancreatic islet transplantation in humans (Adin and Gilor, 2017).
Insulin administration was an immediate improvement in the lives of diabetes patients. However, the injection of insulin is complicated, with patients risking hypo- or hyperglycemia if the amount given is too high or too low. The need to better regulate insulin administration stimulated research into “artificial pancreas” technology. VA research into basic physiological mechanisms of insulin regulation of blood glucose, going back to the 1980s, laid the groundwork for the development of insulin control strategies (Benthem et al., 2001; Havel et al., 1996; VA, 2018b). In the intervening years the emphasis has shifted from transplants to miniaturized mechanical insulin pumps, wearable glucose sensors, and microcomputer algorithms for the continuous control of glucose levels (Bekiari et al., 2018), circumventing the safety issues associated with implanted tissues. Collaboration between the VA and private industry (VA, 2018a) led to the U.S. Food and Drug Administration's approval of the MiniMed 670G, the first device to automatically monitor blood glucose and provide appropriate levels of insulin, in September 2016 (FDA, 2016). This device is now used by VA patients.
Summary
Dogs played a central role in the understanding of diabetes and the development of insulin therapies for at least 100 years, and it may be surmised that without the unique contributions of dogs, knowledge of the disease's pathobiology would have been delayed along with the development of effective treatments for patients. However, rodents have been the primary species for experimental diabetes research for many years, and the genetic engineering of pigs (described in Chapter 4) has yielded strains with increased similarity to the human disease. Genetically engineered pig models have begun to show promising results for research into pathogenesis, treatment, and transplantation. Going forward, given the current understanding of diabetes pathophysiology and treatment as well as of the comparative biology of endocrine pancreatic function, there appears to be limited biological justification for the continued use of laboratory dogs, compared with rodents or other non-rodent species.
There are nonphysiological factors, such as growth rate or tractability for specific experimental procedures, that may impinge on species selection and justify the use of laboratory dogs in limited situations. However, given that companion dogs are affected by diabetes, their study offers significant opportunities to advance the understanding of the roles played by genetic, epigenetic, and environmental factors in the development of diabetes. Furthermore, companion dogs with diabetes could directly benefit from research aimed at developing new treatments for the disorder.
Narcolepsy
Narcolepsy is a disruption of normal sleep/wakefulness cycles characterized by daytime “sleep attacks” that affects 1 in 2,000 people in the United States (Mignot, 1997; Zeitzer et al., 2006) and confers a significantly increased risk of injury from motor vehicle accidents (Sakurai, 2013). Narcoleptic patients often suffer from cataplexy, an acute weakening of postural muscles while remaining conscious, as well as hypnagogic hallucinations (Mieda, 2017; Sakurai, 2013). Since the first human studies were performed in the 1960s (Mignot, 2014), investigators have expanded the molecular and clinical understanding of narcolepsy using a variety of model systems, including dogs (Ripley et al., 2001; Tafti et al., 1996), in addition to humans (Sakurai and Mieda, 2011).
Dogs as models of narcolepsy
Dogs were first identified as suffering from a form of narcolepsy—and thus of interest as a model for the human disease—in 1972, and this was followed by failed attempts to establish breeding colonies of affected poodles and beagles (Mignot, 2014). It was in the Doberman pinscher that the first genetic transmission of canine narcolepsy was demonstrated in 1976. Canine narcolepsy was well studied from 1977 through 1997, with the majority of research performed on colonies of Dobermans and Labrador retrievers. During that period, numerous sleep studies investigated the clinical manifestations of narcolepsy while genetic studies linked the trait to a single autosomal recessive gene (Baker et al., 1982; Foutz et al., 1979). Dogs also contributed to the understanding of narcolepsy through pharmacologic studies and the exploration of monoaminergic and cholinergic systems as they contributed to sleep states (Karczmar et al., 1970; Mignot, 2014).
As the understanding of narcolepsy advanced, however, the preferred animal model shifted from dogs to rodents. In 1998, two separate research teams independently identified neuropeptides, now known as orexins, that appeared to be deficient in both the dog and human forms of the disease (Hoyer and Jacobson, 2013; Sakurai, 2013). Additional studies revealed that narcolepsy in dogs was caused by a mutation at the level of the orexin receptor, whereas the human disease was due to a deficiency in orexin-producing neurons (reviewed in Hoyer and Jacobson, 2013; Mahoney et al., 2019). With a better understanding of the role of orexins in sleep/wakefulness states, investigators developed knockout mice lacking either orexin-producing neurons (orexin/ataxin-3-transgenic) or orexin receptors, thus creating models of either narcolepsy or narcolepsy/cataplexy (Sakurai, 2013). Transgenic rodent models have enabled targeted research into pharmacologic interventions for narcolepsy (Neubauer, 2010; Sakurai, 2013).
VA narcolepsy research using laboratory dogs
Narcolepsy affects an estimated 10,000–20,000 veterans (VA, 2018b [protocol no. 10]). Researchers at the VA used laboratory dogs to study narcolepsy in the 1980s, at a time when dogs were the only known model for the disorder. In conjunction with work performed by other research groups, VA scientists were able to identify the parts of the brain responsible for narcolepsy and explore possible treatment approaches (Boehmer et al., 2004). Subsequent work using murine models and donated tissue from affected humans revealed that the same brain regions involved in narcolepsy are also involved in opioid use disorder, which affects an estimated 131,000 veterans (Baimel et al., 2015; Thannickal et al., 2018). Continuing research into improving treatment for narcolepsy, now performed in mice, has the potential to contribute to the understanding of both narcolepsy and opioid use disorder. The use of dogs to study human narcolepsy at the Los Angeles VA facility, a review of which led some members of the U.S. Congress to question the transparency of VA-supported canine research, as described in Chapter 1, was ended in October 2017 after the principal investigator determined that scientific goals of the study could be met with mice.
Summary
Dogs played a significant early role in elucidating the behavior, electrophysiology, and genetics of narcolepsy (Danek et al., 2017; Mignot, 2014; Sakurai, 2013). With improved molecular techniques and the availability of knockout mice that mimic the orexin deficiencies seen in human narcolepsy and narcolepsy/cataplexy, many useful non-dog models are now available to study this debilitating condition (Mignot, 2014; Neubauer, 2010; Sakurai and Mieda, 2011; Tsujino and Sakurai, 2013).
Osteoarthritis and Chronic Pain
Affecting approximately one-third of the U.S. population (more than 100 million people), chronic musculoskeletal pain leads to an economic burden estimated at $600 billion per year, with many currently available analgesics either failing over time or carrying serious risks of side effects (Babatunde et al., 2017; Lascelles et al., 2018). Laboratory dogs have often been used to investigate novel approaches to pain management in concert with rodent or cellular models (Larsen et al., 2009; Pleticha et al., 2015; Wiese et al., 2013; Yaksh et al., 2014), and several recent investigations have turned to companion dogs to study chronic pain and its management (Brown and Agnello, 2013; Carapeba et al., 2016; Cimino Brown, 2017; Hayashida, 2013; Lascelles et al., 2018; Zeira et al., 2018).
An estimated 20 percent of dogs in the United States suffer from OA (Shah et al., 2018). As with people, the most common symptom of OA in dogs is persistent pain, and the spontaneous development of OA is strikingly similar in dogs and humans, causing the same type of damage to bone and cartilage in affected joints (Cimino Brown, 2017). This high disease prevalence and similar pathogenesis, along with the genetic diversity of companion dogs and their shared environments with their human owners, combine to create a relevant model for OA in humans (Cimino Brown, 2017; Lascelles et al., 2018; Zeira et al., 2018). Given the extensive clinical knowledge of OA in dogs and the existence of validated instruments for assessing pain, including the Canine Brief Pain Inventory, the Helsinki Chronic Pain Index, and the Liverpool Osteoarthritis in Dogs owner questionnaire, companion dogs are helping to increase the understanding of how best to treat OA in both dogs and humans (Carapeba et al., 2016; Cimino Brown, 2017; Lascelles et al., 2018; Walton et al., 2013).
Dogs are also informing the treatment of pain from cancer, which they develop at twice the rate of humans (Hayashida, 2013). Investigators are increasingly using companion dogs to assess novel pain management techniques for both arthritis and cancer pain, including the implantation of mesenchymal stem cells; the intra-articular injection of micro-fragmented adipose tissue, hyaluronic acid, or resiniferatoxin; and the intrathecal delivery of substance P-saporin; and they have seen improved outcomes with some of these novel techniques as compared with standard-of-care analgesic treatment (Brown and Agnello, 2013; Carapeba et al., 2016; Iadarola et al., 2018; Shah et al., 2018; Zeira et al., 2018).
There may be a select few circumstances in which a small number of laboratory dogs are needed to provide the homogeneity and controlled circumstances required for a specific investigation focused on translating results to humans (Larsen et al., 2009; Pleticha et al., 2015; Wiese et al., 2013; Yaksh et al., 2014), but an increasing number of investigators are turning to companion dogs to better assess how best to control chronic pain (Carapeba et al., 2016; Cimino Brown, 2017; Hayashida, 2013; Vainio, 2012; Zeira et al., 2018). Researchers have begun to build multi-disciplinary teams focused on translating the results from companion dog studies into effective analgesics for humans, including through a National Institutes of Health (NIH)-supported consortium of veterinary schools and medical schools, discussed in greater detail in Chapter 4 (COHA, 2020). Promising work is also being done in the realm of using companion animals as a treatment adjunct to more traditional analgesic techniques, documenting the pain- and stress-reducing effects on humans of interactions with dogs (Marcus et al., 2012; Pedrosa et al., 2017).
VA osteoarthritis and chronic pain research using laboratory dogs
Medications required for effective pain relief or surgical anesthesia can have the side effect of slowing breathing, sometimes to a dangerous degree. As part of an ongoing effort to develop pain management techniques that do not negatively affect respiratory function, VA researchers used laboratory dogs to identify the precise region of the brain responsible for the effects of opioids on breathing (Prkic et al., 2012). The aim of this research is to understand the function of the cells in this region well enough to enable development of alternative medications capable of controlling pain without depressing respiration, to benefit veterans needing long-term pain relief or facing painful surgeries. According to the VA, the meticulous study of individual brain cells that this research required could not have been accomplished in smaller animals (VA, 2018b).
Many veterans experience back pain, often related to their service. In the 2000s the VA used dogs, whose intervertebral discs are similar in size to human discs and undergo disc degeneration, to test a treatment involving the transplantation of autologous cultured cells (Ganey et al., 2003). The results indicated that autologous cell transplant was feasible. Clinical trials based on this research are currently under way (Schol and Sakai, 2019; VA, 2018b).
Summary
Companion dogs with OA or cancer offer the opportunity to study and develop treatments for pain that may benefit both dogs and humans. However, when alternatives are not possible, there may be select instances in which a small number of laboratory dogs are needed to provide the homogeneity and controlled circumstances required for a specific investigation focused on translating results to humans.
Experimental Pharmacology and Toxicology
The investigation of pharmacologically active substances is arguably the oldest of the biomedical sciences, originating more than 3,500 years ago. Until the past century pharmaceutical preparations were tested almost exclusively in humans; animal testing occurred infrequently (Kinter and DeGeorge, 2016). The rapid increase in the synthesis of small molecules, which was enabled by advances in organic and analytical chemistry beginning in the late 1800s, was a strong driver in the move toward animal testing. For example, in 1909 Ehrlich and Hata made more than 600 organoarsenical compounds and tested these in animals to identify “drug 606” (Salvarsan), a treatment for syphilis (Bosch and Rosich, 2008). Ehrlich's early animal experiments, which demonstrated the toxicity of most of the earlier arsenicals, illustrated the strong ties linking chemistry, pharmacology, and toxicology. As more sophisticated animal models became necessary to evaluate efficacy using new technologies (catheters, pressure transducers, electrocardiograms, etc.), an increased reliance on anesthesia and surgery placed a priority on larger species. Dogs and cats became the most commonly used animal models in pharmacological investigations and remained so through the 1970s.3
To obtain a snapshot of dog usage in pharmacological research over the past decade, the committee conducted a literature search for studies published in 2010–2019 that mentioned dogs in the context of pharmacology (see the literature search criteria in Appendix A). This search identified 256 unique publications describing the use of dogs for basic research or the preclinical research and development (R&D) of human therapeutics. The areas of pharmacological investigation included cancer, respiratory, cardiovascular (CV)/renal, bone/dental, endocrine (including diabetes), central nervous system, gastrointestial, musculoskeletal, and experimental surgery. CV/renal was the most frequently cited area (64 citations), followed by bone/dental (40 citations) and central nervous system musculoskeletal (31 citations). Citations associated with product development activities, including pharmacokinetic and toxicity studies, accounted for 85 percent of the total. It bears noting that much product development work involving dogs or any other species is performed to satisfy regulatory requirements and is proprietary, with results seldom published in the public domain, so the published studies may represent a fraction of all product development activities.
VA pharmacology and toxicology research using laboratory dogs
The VA is not currently performing primary pharmacology or toxicology research on dogs. However, two projects from the VA's past were noted in communications from the VA to the committee (VA, 2018b). Work performed by VA researchers used dogs to establish the link between smoking and lung cancer, which afflicts veterans at a higher rate than non-veterans (Auerbach et al., 1967). This research has been credited with helping fuel the antismoking movement of the 1970s and saving hundreds of thousands of lives (Burkhart, 1997). In the 1980s, VA research with dogs helped establish a new class of drugs, including ciprofloxacin (“Cipro”), as improved standard treatments for urinary tract infections (UTIs) (Gasser et al., 1987). Dogs were used in order to obtain fluid samples of sufficiently large volume for analysis. This treatment was particularly important for veterans with SCI, who experience a high rate of UTIs.
Currently, the VA only performs pharmacological research on laboratory dogs to support its other studies—specifically, to optimize anesthetic regimens for studying cardiac function. In summary, while laboratory dogs continue to be used for pharmacology and toxicology research, in fields outside of product development they have been largely eclipsed by rodents. The VA currently does not perform primary pharmacological research on laboratory dogs.
Summary
The laboratory dog is not currently used as the model of choice for primary pharmacological research unrelated to product development.
Research Areas with Potential Future Companion Dog Use at the VA
This section describes three areas for possible future VA biomedical research involving companion dogs—cancer, infectious disease, and Alzheimer's disease.
Cancer
Cancer is the second leading cause of death in the United States and was responsible for an estimated 599,108 deaths in 2017 (Heron, 2019). The National Cancer Institute (NCI) predicted 1,735,350 new cancer diagnoses in the United States and 609,640 deaths from the disease in 2018 (NCI, n.d.). The incidence and distribution of cancers in veterans, who are predominantly male, are comparable to those in the general U.S. male population (Zullig et al., 2017). The VA strategic plan highlights those areas of medical R&D likely to have the greatest impact on veterans and eligible beneficiaries (Ramoni, 2019; VA, 2019); areas of active interest include environmental exposures during deployment that cause DNA damage leading to cancer as well as the disproportionate impact on veterans of certain lifestyle choices known to cause cancer, such as smoking (VA, 2017). The VA strategic plan also contains a performance goal, anticipating future improvements in the approach to personalized medicine, which would apply clinical genomics to tailor treatment to the needs of the individual. In the area of cancer research, the pursuit of this goal may involve the use of animal models.
Dogs as models in cancer research
Historically the laboratory dog played a very limited role in advancing the understanding of cancer biology, particularly when compared to rodents. Rats and mice afforded the benefits of small body size; short life span; genetically defined (and later genetically engineered) inbred strains; extensively studied immune responses; rapid, reproducible tumor induction; and a wide variety of transplantable and inducible tumor models. In contrast, the dog lacked all these characteristics and offered few transplantable tumors, with the exception of transplantable canine glioma (Barker et al., 1993; Salcman et al., 1982) and the transmissible venereal tumor (Bloom et al., 1951; Frampton et al., 2018; Karlson and Mann, 1952; Prier and Brodey, 1963). Efforts at transplanting other spontaneous tumors of the dog required sustained immunosuppression, as in the cases of melanoma (Betton and Owen, 1976) and induced uroepithelial carcinoma (Harzmann et al., 1980). Experimental tumor induction in the dog appears to have little relevance to human cancer and marginal applicability.
Despite the significant limitations of the laboratory dog as an animal model for most cancers, scientists recognized early on that research aimed at understanding spontaneous cancers of the dog might shed light on both human and canine cancer biology (Prier and Brodey, 1963). With the completion of both the human and the dog genome projects (NHGRI, 2019), the genetics underpinning the development of cancers in humans and dogs became available for study and increasingly well understood. These developments have brought the comparative study of cancer in humans and dogs to fruition (NCI CCR, n.d.), and a recent national workshop on this subject drew wide support from veterinary colleges, veterinary and research communities, federal agencies, the pharmaceutical industry, and a variety of patient advocacy and other nonprofit organizations (NASEM, 2015). As noted during this workshop and elsewhere, efforts at translating cancer studies from rodents to humans have encountered significant problems, many of which may be addressed by studying cancer in the companion dog (Gordon and Khanna, 2010; Gordon et al., 2009; Khanna et al., 2009; Paoloni and Khanna, 2007; Paoloni et al., 2014). Advantages of the companion dog include its genetic heterogeneity, large body size, and competent immune system as well as the fact that it has anatomic and physiologic characteristics and genetic/molecular pathways similar to those of humans. Spontaneous cancers of the dog are comparable to the spectrum cancers seen in humans (including histologically) but progress more quickly. Furthermore, dogs and humans have shared the same environment for millennia, potentially shaping similarities in cancer susceptibility through convergent evolution.
Currently, two federal agencies and one nonprofit organization are involved in the coordination and funding of clinical trials and applied cancer research in the companion dog population (ACF, n.d.; NCI CCR, n.d.; VA, 2018b). NCI's Center for Cancer Research's Comparative Oncology Trials Consortium encompasses 20 U.S. veterinary schools and colleges with academic comparative oncology programs that collaborate in cancer research study design, patient recruitment, and implementation (NCI CCR, n.d.). The National Human Genome Research Institute's Dog Genome Project (NHGRI, 2019) has pursued clinical research on bladder cancer, histiocytic sarcoma, and squamous cell carcinoma within its broad portfolio of research on the canine genome. Since the release of the canine genome sequence in 2005, companion dogs with spontaneous cancers have become a valuable source of biological samples associated with detailed clinical, pathological, and outcome data. In addition to funding new and innovative approaches to cancer therapy, the Animal Cancer Foundation is collaborating with the Canine Comparative Oncology & Genomics Consortium (CCOGC, 2020) to complete tumor genome mapping of all the common canine cancers, to create a resource for researchers investigating canine and human cancer biology.
VA cancer research using companion dogs
Melanoma is the most dangerous form of skin cancer and the fifth most commonly diagnosed cancer among veterans, who have a higher rate of melanoma than the general U.S. population (Riemenschneider et al., 2018; VA, 2018b). Research groups in the VA and elsewhere have observed that spontaneous dog melanomas and human melanomas produce analogous antigens, offering hope that the two species could benefit from similar vaccine strategies (Zuleger et al., 2017). VA research, which is performed on companion dogs with spontaneous melanoma, has contributed to the development of experimental vaccines against melanoma for both dogs and humans (VA, 2018b; Zuleger et al., 2017). The VA is continuing to fund this research, which is currently focused on developing new protocols to optimize effectiveness of the vaccines. More recently, VA researchers undertook a clinical trial of a new treatment for bladder cancer in companion dogs. This trial was approved for continuation in March 2018 but was listed as inactive in November 2018 (VA, 2018a).
Summary
There is now a well-organized network for scientific collaboration dedicated to advancing the understanding of cancer biology and patient care in humans and companion dogs simultaneously. Research conducted to date through this network, which includes a variety of cancer models with potential relevance to the veteran population, illustrates the validity of this approach. With companion dog patients standing to benefit directly from any improvement in clinical outcome resulting from this research, the adoption of this strategy should significantly mitigate concerns over dog use. In regard to laboratory animal use, while mice remain the predominant model for cancer, the genetically engineered pig—in particular, the Oncopig Cancer Model described in Chapter 4—offers a strong platform for studying cancer and its treatment in a large laboratory animal.
Infectious Disease
Dogs have long contributed to infectious disease research for both human and canine microbial threats, as they are useful for studying pathogens for which dogs are natural hosts (e.g., Lyme disease), reservoir hosts (e.g., leishmaniasis), or models of human disease (Petersen, 2019; Toepp et al., 2019). In their role as companion animals, dogs may transmit infectious organisms to humans as intermediate hosts or pass along antimicrobial-resistant bacteria (Luo et al., 2018; Pomba et al., 2017). One aim of infectious disease research in dogs is therefore to protect humans by preventing zoonotic diseases, such as rabies and leishmaniasis, in dogs (Huang et al., 2015; Toepp et al., 2019).
Laboratory dogs are used to investigate a variety of infectious diseases, with research ranging from basic investigations aimed at understanding pathogenicity (e.g., avian-origin H3N2 canine influenza virus) to vaccine development (e.g., rabies and Lyme disease) (Huang et al., 2015; Lafleur et al., 2010; Loría-Cervera and Andrade-Narváez, 2014; Luo et al., 2018). In recent years, researchers have demonstrated the utility of companion dogs for infectious disease research. Companion dogs are being used to study inflammatory myopathy associated with Leishmania infection, the prevalence of American trypanosomiasis in rural Brazil, and the spread of avian-origin canine influenza virus in the United States (Paciello et al., 2009; Perez et al., 2016; Voorhees et al., 2018). The dog immune response to Leishmania infection, studied in companion animals, has been found to resemble the human response more closely than that of commonly used mouse strains (Boggiatto et al., 2010; Petersen, 2019; Scorza et al., 2017).
Infectious disease research opportunities using companion or military service dogs
While the VA is not currently engaged in canine infectious disease research, this field was included for consideration by the committee due to the presence of U.S. military personnel in areas endemic for visceral leishmaniasis, trypanosomiasis, and additional pathogens not common to the United States (VA, 2020). Leishmaniasis has been described as an emerging infection among deployed U.S. military and civilian workers, with nearly 20 percent of tested soldiers who had been deployed to leishmaniasis-endemic areas of Iraq in 2015–2017 turning up positive for Leishmania infection (as did many deployed dogs) (Mody et al., 2019; Petersen, 2019; Weina et al., 2004). This suggests a possible future role for canine infectious disease research at the VA. Infectious disease research can be designed to benefit military working dogs exposed to unique risk factors as well as humans.
It bears noting that the VA has successfully used dogs to investigate a genetic disorder with relevance to infectious disease. Leukocyte adhesion deficiency (LAD) is an immune deficiency disorder that affects both humans and dogs, leading them to experience life-threatening bacterial and fungal infections and a shortened life expectancy. Studying laboratory dogs with canine leukocyte adhesion deficiency (CLAD), researchers at the VA identified the genetic mutation that causes CLAD and found it to be the same as in some humans with LAD (Kijas et al., 1999). This work was the foundation for an effective gene therapy to treat CLAD in dogs and is being used to develop treatments for LAD in humans (VA, 2018b).
Summary
Recent investigations have begun to demonstrate the utility of studying infectious disease in companion dogs, which can benefit both dogs and humans. In some cases, infectious disease research in dogs can include hunt club dogs living in kennels, as opposed to the typical pet dog. With increasing rates of antimicrobial resistance and expanding ranges of potential vectors due to climate change, dogs are likely to continue to play a key role in the study of infectious disease prevention and control, particularly with regard to emerging vector-borne diseases (Pomba et al., 2017; Regier et al., 2016; Uminski et al., 2018).
Alzheimer's Disease
As the sixth leading cause of death in the United States, Alzheimer's disease has long been a topic of interest to VA researchers. Studies performed at the VA have contributed to the understanding, detection, and development of treatments to delay functional decline in Alzheimer's disease; summaries of ongoing research and major accomplishments are available online (VA ORD, n.d.d).
Dogs as models of Alzheimer's disease
As the U.S. population ages, with the number of individuals over age 65 expected to nearly double by 2050, the anticipated prevalence of Alzheimer's disease is also increasing, highlighting the urgent need to develop effective therapies and interventions (Alzheimer's Association, 2019).
Alzheimer's disease, the most commonly diagnosed form of dementia in the elderly, is characterized by a variety of destructive neuropathologies, including the deposition of beta-amyloid protein, neuronal loss, and the formation of neurofibrillary tangles, leading to progressive memory loss and cognitive decline (reviewed in Fan et al., 2020). Intriguingly, a similar neuropathology leads to cognitive dysfunction in dogs, pointing to canines as a possible model for the study of Alzheimer's disease (González-Martínez et al., 2011; Head, 2011, 2013; Triani et al., 2018; Vasilevko and Head, 2009; Yu et al., 2011).
Although much animal research to better understand Alzheimer's disease uses transgenic mice or species in which elements of Alzheimer's disease pathology in humans can be introduced (e.g., beta amyloid-infusion rodent models, beta amyloid-expressing nematodes, and even zebrafish) (Jäkel et al., 2017; Van Dam and De Deyn, 2011), investigators have noted several distinctive features of dogs that argue for their special potential in Alzheimer's disease research (Head, 2013; Mazzatenta et al., 2017). Older dogs spontaneously develop neuropathology and cognitive deficits similar to those observed in humans with early Alzheimer's disease, and these deficits can be studied within the context of the dog's unique adaptation for communication with humans and human-like social skills (Mazzatenta et al., 2017). Companion dogs share an environment with their owners that confers similar exposures to pollutants and infectious agents and also share similar activity levels, and thanks to millennia of co-evolution, dogs and humans are able to consume and digest a similar high-starch diet (González-Martínez et al., 2011; Head, 2013). Dogs age rapidly in comparison with people (Kaeberlein et al., 2016), and the key proteins involved in the development of Alzheimer's disease in humans are remarkably similar to those in dogs, with 100 percent homology of beta-amyloid and 98 percent of amyloid precursor protein (González-Martínez et al., 2011). These features make the dog an appealing model for testing candidate Alzheimer's disease interventions (Chapagain et al., 2018; Cotman and Head, 2011). Post-mortem studies of the brains of dogs with cognitive decline, however, do not show the same prevalence of tau-containing neurofibrillary tangles as is seen in humans or other features such as TAR DNA-binding protein 43 inclusions (Smolek et al., 2016).
Extensive research has detailed the behavior and neurobiology of aging dogs (Cotman and Head, 2011; González-Martínez et al., 2011; Head, 2009, 2011; Rusbridge et al., 2018). Efforts to prevent or slow the onset of cognitive decline in dogs have included antioxidant-enriched diets, behavioral enrichments, the drug rapamycin, and additional measures that could potentially serve as models for Alzheimer's disease treatment in humans (Kaeberlein et al., 2016; Mazzatenta et al., 2017; Neumann et al., 2018; Triani et al., 2018). Although there has been some research focused on Alzheimer's disease pathogenesis in laboratory dogs (generally beagles) (Head, 2009), as noted above, more recent studies argue against laboratory dogs as best suited for studies of specific pathologic processes. There remain arguments for testing candidate Alzheimer's disease interventions in companion dogs, whose areas of pathology similar to that observed in humans are further enhanced by the environmental exposures they share with their human owners (Chapagain et al., 2018; González-Martínez et al., 2011; Kaeberlein et al., 2016; Mazzatenta et al., 2017). Dogs therefore offer sufficient age-associated neuropathological overlaps with human Alzheimer's disease to entertain the possibility that a treatment for neurodegeneration in dogs might not only benefit the millions of aging companion dogs but also apply to the treatment of neurodegeneration in humans (González-Martínez et al., 2011; Kaeberlein et al., 2016; Mazzatenta et al., 2017).
Summary
Use of aging dogs to study Alzheiemer's disease is a relatively new development, and the VA has not engaged in this research to date. Given recent developments in Alzheimer's disease research, companion and possibly laboratory dogs may play a role in future studies aimed at testing Alzheimer's disease interventions.
Research Areas Previously Investigated by the VA Using Laboratory Dogs
In addition to the research areas described above, the VA submitted to the committee information on six additional research areas in which laboratory dogs had been used. These are discussed in Box 3-1. As described earlier in this chapter, the committee reviewed evidence related to current and recent (since 2016) areas of biomedical research using laboratory dogs at the VA and did not evaluate the necessity of dog use in the areas mentioned in Box 3-1. Nonetheless, it should be noted that several of these studies were active within the past decade and could be revisited by the VA in the future.
NEXT STEPS FOR THE USE OF LABORATORY DOGS IN BIOMEDICAL RESEARCH RELATED TO THE VA'S MISSION
Historically and across disease type, from the understanding and treatment of diabetes to the development of cardiac pacemakers and valve replacement, it would be challenging to find an area of biomedical research that has not benefitted from the involvement of laboratory dogs. The use of dogs in biomedical research peaked in the 1970s, with more than 200,000 dogs per year being used at USDA-regulated academic and industrial institutions. Since the 1970s, however, as molecular techniques and rodents have gained prominence, some biomedical research has moved away from using laboratory dogs. The focus has shifted toward a variety of alternatives, including rodents, pigs, companion dogs, and even sheep. Today, the majority of dog use in the United States is by companies and private research organizations engaging in applied biomedical research and product development, including testing that is required by regulatory agencies.
Nevertheless, a few areas remain in which laboratory dogs offer the potential for medically important discoveries that cannot currently be obtained elsewhere. These include subsets of CVD research, most notably cardiac rhythm disorders, that depend on anatomical and physiological features that humans share with dogs but not with other laboratory species or on the implantation of devices that rely on restricted growth in addition to some of these other features. They may also include a number of the complex treatments for SCI, particularly cervical injuries resulting in quadriplegia with its many systemic and life-altering effects, that cannot be modeled effectively in other animal or non-animal systems. Even in these limited fields, however, it will be crucial to remain vigilant for non-dog and non-animal alternatives and actively work to promote the development and use of alternatives, as described in Chapter 4.
Given the committee's inability to predict future biomedical research needs, it is not inconceivable that VA research in other fields may require use of laboratory dogs due to unique aspects of their physiology or behavior.
Conclusion 3-1: The laboratory dog is scientifically necessary for only a few areas of current U.S. Department of Veterans Affairs (VA) biomedical research. Based on the request from the VA to review areas of research from 2016 onward, the committee concludes that laboratory dogs currently remain scientifically necessary in these areas of active biomedical research at the VA:
- mechanistic insights of premature ventricular contraction-induced cardiomyopathy;
- autonomic nerve activity and cardiac arrhythmias;
- cardiovascular disease requiring functional modeling of the human Purkinje system; and
- development and testing of implantable devices to stimulate respiration and cough in spinal cord injury.
Laboratory dogs are no longer the preferred model for studies of diabetes or narcolepsy, for most imaging studies, or for primary pharmacological research. Responsibility lies with the principal investigator, scientific review committee, and institutional animal care and use committee to know the literature and accurately determine whether the laboratory dog is still the best model for any particular study.
Conclusion 3-2: A potential new approach or treatment may be developed that, for biological reasons, can only be tested in dogs. As yet unknown, new, or reemerging diseases or disorders may not be reproducible in non-dog models and could require the limited use of laboratory dogs to advance their prevention, treatment, or control. Conversely, alternatives may develop in the future that would make the laboratory dog unnecessary.
Conclusion 3-3: The U.S. Department of Veterans Affairs (VA) has an opportunity to expand the study of companion dogs in clinical trials. Companion dogs experience many of the same naturally occurring diseases as humans and stand to benefit from the results of the research in which they participate. Companion dogs are promising models for a range of disorders, including obesity, diabetes, infectious disease, Alzheimer's disease, osteoarthritis, hereditary glaucoma, cardiomyopathy, thoracic spinal cord injury, and cancer. While companion dog clinical trials can be challenging to conduct due, in part, to the financial and time costs of collecting an appropriate population of companion animals for a particular trial, these studies are possible and deserve priority consideration by VA researchers and leaders.
THE VA'S BIOMEDICAL RESEARCH REVIEW PROCESS
VA-funded biomedical research using laboratory dogs takes place either in VA medical centers or through partnerships with local academic centers (e.g., the St. Louis VA Medical Center no longer has an animal facility on the premises but partners with Washington University and St. Louis University to conduct animal research). VA funds support VA clinician–researchers, who initiate research projects based on the health care needs of veterans. Each laboratory dog research protocol undergoes several stages of VA review, as illustrated in Figure 3_2.

FIGURE 3-2
The U.S. Department of Veterans Affairs' (VA's) intramural canine research review process. NOTE: CRADO = Chief Research and Development Officer; CVMO = Chief Veterinary Medical Officer; eRA= electronic Research Administration; IACUC = institutional animal (more...)
For VA-funded research, the investigator submits a proposal using the same technology infrastructure that is employed by NIH (Grants.gov, n.d.). The proposal is reviewed by a panel of experts who are recruited by the Office of Research and Development of the relevant VA service (primary review) and who score the proposal for scientific merit (see Figure 3_2, bottom left). Completed reviews are sent to the director of the service, who makes the final funding decision. Each facility then carries out a process of regulatory approval. This includes review by a local R&D committee and its subcommittees, which include the institutional animal care and use committee (IACUC) for animal research, the institutional review board for human research, and other committees, depending on the exact requirements of the study. For research with dogs, a secondary veterinary review is carried out by the Office of the Chief Veterinary Medical Officer. The Chief Research and Development Officer; the VA National Center for Ethics in Health Care; the Deputy Undersecretary for Discovery, Education, and Affiliate Networks; and the Undersecretary for Health also review the project before it is submitted to the Secretary of the VA for final approval before the work may begin. The secondary veterinary review was instituted in the 1970s for all VA-funded research involving animals and has been required for all proposed research involving dogs at the VA, regardless of funding source, since 2017 (VHA ORD, 2017). Only after all the regulatory requirements are met is the research permitted to begin. For canine research to be supported by non-VA sources, the same secondary review process applies, with the only difference being that the external funding source, and not the VA, is responsible for the primary review for scientific merit (Bever, 2019). Recent legislative developments related to oversight of VA biomedical research using laboratory dogs are discussed in Chapter 1 and later in this chapter.
Assessment of the VA's Current Review and Oversight Practices
The committee was charged with recommending new or revised scientific parameters for how and when to use dogs for biomedical research at the VA. In order to gain an understanding of the VA's current review and oversight practices, the committee considered the following information sources from the VA:
- Fourteen animal component of research protocols (ACORPs) for VA-funded research projects involving laboratory dogs that were approved or active as of June 1, 2017 (VHA ORD, 2017);
- Four research plans or original grant applications submitted by researchers to receive funding;
- Discussion with VA officials during public meetings to understand the VA research award and review process; and
- Two site visits (Richmond, Virginia, and St. Louis, Missouri) to observe animal laboratories with dogs in VA-funded projects and hold discussions with researchers and IACUC members. Committee members participating in the Richmond site visit on August 20, 2019, were Chris Newcomer and David Powell. Committee members participating in the St. Louis site visit on November 14, 2019, were Chris Green, Nancy Marks, and David Powell. Site visit notes and observations from each site visit were shared and discussed with the full committee.
In considering this information, the committee gained insights into the written materials required from researchers seeking to justify the use of laboratory dogs in a biomedical research study. Site visits and discussions with VA officials provided context and clarification. The VA and its investigators satisfied all requests for information. Nonetheless, the committee's work did not include an organizational analysis of the VA's large, complex research organization and the culture that accompanies it. In the context of launching a biomedical research study with laboratory dogs there are many conversations, decisions, revisions, and requests that do not appear on paper or in the limited discussions that took place between the committee and VA officials and investigators.
Inadequacies in Justifying the Use of Laboratory Dogs in VA Protocols
The majority of the committee's analysis of justifications for using dogs in VA studies relied on the 14 ACORPs for approved or active protocols provided by the VA. Among the 14 protocols, the 2 most common, often jointly occurring, justifications for dog use were (1) their large size and—sometimes—anatomical and physiological similarity with humans and (2) the existence of historical data or the researcher's previous experience with dogs. The quality and extent of justification for using dogs varied across protocols. In some ACORPs the rationale for exclusion of other large animal species, such as sheep or pigs, was their lack of suitable properties (e.g., differences between human and pig Purkinje systems). Some ACORPs only provided a rationale for excluding a single species, and some failed to provide any explanation as to why other large animals were not viable. Most protocols lacked a robust description of a serious attempt to exclude other species or explore alternatives to the laboratory dog.
Inadequacies in Justifying the Number of Laboratory Dogs Used
Among those ACORPs that contained a rationale for the number of dogs used, the committee found the sample size justification to have varying levels of adequacy. Several ACORPs did not have a clear explanation, or any explanation, for the numbers of dogs used, but this reflected the nature of the study (i.e., a pilot study or technique development study that did not require statistical analysis).
Inadequacies in Justifying the Burden and Care of Dogs in VA Protocols
Justifications for the burden and care of dogs ranged from acceptable to marginal. Of note among the protocols reviewed by the committee are two that involved multiple surgeries (two survival surgeries and a terminal procedure) and multiple sedations/anesthesia. The committee was concerned with the performance of multiple surgeries on few individual dogs (to create models of malignant arrhythmias) before an assessment of the effect of sedation and anesthesia on the dog was conducted; the committee felt that these protocols would have benefited from consultation with veterinary specialists (i.e., cardiologists, anesthesiologists, behaviorists). One protocol employed a mix of anesthetizing agents used in modern canine and human anesthesia but lacked any explanation of why these agents were chosen.
Inadequacies in Justifying the Relevance to Veterans' Health
Half of the ACORPs provided an explanation of the relevance of the study to veterans' health, with some ACORPS presenting stronger evidence than others. Some ACORPs explained the relevance of the study to humans in general or to the scientific community but not to veterans specifically.
As concluded in Chapter 2, based on documentation provided by the VA and other organizations as well as on two site visits by subgroups of the committee, the committee finds that the VA's biomedical research programs involving laboratory dogs appear to satisfy applicable laws and regulations surrounding animal research. The primary form of documentation provided to the committee was the ACORP associated with VA protocols using laboratory dogs. The ACORP satisfies legal requirements but, as the conclusions below indicate, the committee believes the VA's review and oversight process could be improved by embracing the spirit of applicable laws and ethical principles.
Conclusion 3-4: The committee was not able to fully evaluate the U.S. Department of Veterans Affairs' (VA's) scientific review process for animal research protocols based on the documents provided by the VA, but the committee's analysis of the animal component of research protocol (ACORP) forms revealed deficiencies in the justification for using dogs instead of other species and for the number of dogs used. The ACORP analysis also revealed instances where the investigators did not adequately explain the relevance of the study to veterans' health.
Conclusion 3-5: Principal investigators frequently cited previous experience with and historical data in dog models as primary justifications for using laboratory dogs. These justifications are insufficient alone and constitute a form of circular reasoning that perpetuates the use of laboratory dogs without adequate examination of alternatives.
Conclusion 3-6: The committee notes that certain protocols would have benefited from consultation with veterinary specialists (cardiologists, anesthesiologists, and animal behaviorists) to address animal welfare issues stemming from the performance of multiple surgeries and multiple sedations or anesthesia on individual dogs and to inform the choice of anesthetizing agents.
OPERATIONALIZING “NECESSARY” AREAS OF AGREEMENT AND DISAGREEMENT WITHIN THE COMMITTEE
The committee offers the following recommendation for determining when it is scientifically necessary to use laboratory dogs in biomedical research funded by or conducted at the VA.
Recommendation 1: Adopt an expanded set of criteria for determining when it is scientifically necessary to use laboratory dogs in biomedical research funded by or conducted at the U.S. Department of Veterans Affairs (VA).
In order to conduct biomedical research that will lead to meaningful outcomes to support improved health of veterans, the VA should adopt an expanded set of criteria for determining if the use of laboratory dogs is scientifically necessary:4
- 1.
The scientific question and the knowledge anticipated will advance understanding or medical practices related to veterans' health;
- 2.
Based on unique physiological and other characteristics, there is no alternative to the laboratory dog that will yield scientifically valid results that meet proposed study objectives;
- 3.
The anticipated harms experienced by the laboratory dog are outweighed by the potential benefits for veterans; and
- 4.
Both the scientific review committee and institutional animal care and use committee have provided written statements attesting that the laboratory dog is the only species that can yield scientifically valid results.
After reaching agreement on Conclusion 3-1 and Recommendation 1, the committee found itself at an impasse. Ten committee members,5 a majority, believed that according to the Statement of Task, their job was not done and that a second recommendation linked to Recommendation 1 was warranted, while five committee members6 were equally convinced that this second recommendation would not be in keeping with the Statement of Task and thus should not be included in the report. The differing opinions of the two groups turn on the meaning of three specific sentences in the Statement of Task—and, in particular, on the meaning of one word that appears multiple times in those sentences.
The crucial word is “necessary,” and the portion of the Statement of Task that contains the three sentences reads as follows (with emphasis added):
Specifically, the committee will write a report to address the following:
- 1.
Explore recent past, current, and anticipated research questions directly related to the VA's mission to determine if dogs [rather than non-rodent (excluding non-human primates) or rodent species or non-animal alternatives] are or will continue to be necessary for relevant basic and translational research. The committee will:
- a.
Make a determination as to whether dogs are or will continue to be necessary for any type of biomedical research directly related to the VA's mission. If it is determined that they are necessary, describe the unique physiological and other characteristics of dogs that currently make it the necessary animal model for use in these types of research.
In reading this portion of the Statement of Task, the majority and minority groups interpret the word “necessary” in different ways and, as a result, end up with differing opinions as to whether the report should include the recommendation in question. While there are other issues that the two groups do not agree on, the crux of their disagreement can be traced to that one word.
To understand the disagreement, some background is useful. Throughout the study process, the committee debated at great length how scientific, legal, ethical, and social considerations factor into the determination of the laboratory dog's necessity in VA biomedical research. In March 2018, prior to the VA's request that the National Academies of Sciences, Engineering, and Medicine undertake this study, the federal government enacted new restrictions on the VA's use of laboratory dogs, mandating that no federal funds “may be used to conduct research using canines unless: the scientific objectives of the study can only be met by research with canines.”7 Section 254 of the Consolidated Appropriations Act of 2018 further required the Secretary of the VA to “directly approve” any such studies, and to submit to the U.S. Congress within 180 days “a detailed report outlining under what circumstances canine research may be needed if there are no other alternatives.”8
In December 2019, as the committee neared the end of its deliberative process, the Further Consolidated Appropriations Act of 20209 was enacted into law. This legislation reiterated the language from the 2018 Act and expanded it to include cats and non-human primates.10 The 2020 Act also added a new requirement that such scientific objectives must be “directly related to an illness or injury that is combat-related.”11 Furthermore, the 2020 legislation now requires the Secretary of the VA to submit a report to the U.S. Congress for any such approved research “not later than 30 days before the commencement of such research.”12 That report must describe the nature of the research and include “the justification for the determination of the Secretary that the scientific objectives of such research could only be met using canines, felines, or non-human primates.”13
In considering the Statement of Task in the context of this legislation, the members of the majority and minority disagreed about the scientific and ethical implications of this legislation and about its relevance to the committee's recommendations to the VA.
As noted, all members of the committee agreed on Recommendation 1, which addresses the Statement of Task—at least partially—by laying out a set of scientific criteria that provides the VA with a framework for deciding when it is scientifically necessary to use laboratory dogs in a proposed protocol. All of the committee members are satisfied that Recommendation 1 provides a framework for scientific grounds for determining whether the laboratory dog should be used. The issue that separates them is whether addressing the scientific grounds for using dogs in research is sufficient to satisfy the Statement of Task.
The five committee members in the minority assert that it is indeed sufficient, and they believe that the inquiry should end there. Referring to the definition of “necessary” from the Oxford English Dictionary—“required to be achieved” or “essential”—they assert that determining the use of dogs to be necessary in a line of VA research is equivalent to finding that the laboratory dog is the only animal model that can satisfy an important study objective. In short, they believe that the word “necessary” in the Statement of Task should be read as “scientifically necessary” and, in particular, that it was the VA's intention in its Statement of Task to ask only about scientific necessity. Thus, because Recommendation 1 addresses that aspect of the Statement of Task, the minority concludes that no further recommendations are necessary or warranted.
By contrast, the 10 committee members in the majority believe that the discussion of when the laboratory dog is “necessary” should not end with scientific or technical considerations related to the unique physiological and other characteristics of dogs and that parameters for guiding how and when to use laboratory dogs must include ethical, legal, and animal welfare concepts as well. In making this argument, they refer to another part of the Statement of Task, which specifically asks the committee to address other issues than the scientific ones:
- 1.
Identify ethical considerations, regulatory requirements, and currently accepted standards for the care, use and welfare of dogs in biomedical research, and make recommendations to enhance their well-being while achieving the research objectives.
If policy making on dog research is to be ethical, the ten members of the majority believe, a broader conceptualization of “necessary” than just “scientifically necessary” is required, and specifically they believe that if policy on dog research is to be ethical it must also take into consideration the implications for members of other species that might be substituted for dogs. In particular, the majority argues that while the Oxford English Dictionary provides a concise definition of “necessity,” that definition lacks sufficient conceptual or analytical clarity to guide action in this case. Instead they hold that the concept of “necessity” entails specifying at least two factors—the goals invoked and the conditions attached to such goals. The goal of the VA is to restore or improve the health and well-being of veterans, which is done in part by advancing scientific knowledge and the understanding of how diseases (e.g., SCI) affect veterans. Science is not the end goal, but rather science is the means of advancing the goals embedded in the mission of the VA. For research using laboratory dogs to be “necessary,” the research must not only advance a set of goals but do so under a set of conditions that include factors like time efficiency, law, and ethics. As described in Chapter 2, any policy that restricts research on dogs may transfer the burden of that research to other large animal models (e.g., pigs and sheep), and the alternative species could potentially experience greater harm than laboratory dogs, a scenario that could be considered unethical by some and a violation of the Three Rs—a set of well-established ethical principles that have also been embedded in many regulatory documents guiding the ethical use of animals in research.
The 10 committee members contend that there are no clear moral grounds for arguing that dogs have a “higher” moral status than other large animal species like pigs. Societal preferences, without additional ethical principles, do not confer greater moral value. Therefore, a rule that requires that scientists be more permissive about research on pigs than dogs or similar large animal species is not ethical. What matters most in selecting a particular species, from an ethical standpoint, is that (1) the biological characteristics of the non-human animal indicate that it will provide valuable knowledge in the context of the proposed research, and (2) the harm experienced by the animals is minimized. Some animal research protocols may be less harmful when conducted in dogs than in other species like pigs. Others might be the reverse. The majority of the committee believes these ethical considerations regarding minimizing the harm experienced by animals are part of evaluating scientific necessity.
In support of this contention the majority point to the 2011 Institute of Medicine report on chimpanzees, where the committee found itself in a similar position and took a very similar step. In particular, that committee wrote:
Neither the cost of using chimpanzees in research nor the ethical implications of that use were specifically in the committee's charge. Rather, the committee was asked for its advice on the scientific necessity of the chimpanzee model for biomedical and behavioral research. The committee agrees that cost should not be a consideration. However, the committee feels strongly that any assessment of the necessity for using chimpanzees as an animal model in research raises ethical issues, and any analysis of necessity must take these ethical issues into account. (IOM, 2011, p. 2)
The committee majority also point to similar ethical considerations being described in the Guide for the Care and Use of Laboratory Animals (the Guide) (NRC, 2011), which is an internationally accepted primary reference on animal care and use whose use is required in the United States by the U.S. Public Health Service (PHS) Policy. Many institutions, including the VA, are legally obligated to follow PHS Policy and therefore the recommendations in the Guide.
In light of such ethical considerations, the majority believes that where multiple species, including the laboratory dog, can be adequately used to answer the scientific question, the species that will incur the fewest burdens should be selected and that such considerations must play a role in determining when a proposed protocol is “necessary.”
The committee majority recognizes that this ethical consideration may at times be in conflict with the current law. Specifically, if the dog is the non-primate laboratory species that would incur the fewest burdens in a proposed protocol, ethical considerations would require its use, but that would be prohibited by law. In this case—or in any situation where the animal that would incur the fewest burdens cannot be selected, because of legal or funding restrictions or any other reason—the majority believes that the VA cannot ethically proceed and should consider forgoing the research. In some situations, this limitation might encourage researchers to find an alternative method or combination of methods that would accomplish the goals of the study and reduce harms to an acceptable level. It may require that the research be done by a different entity. It is also possible that if the current legal restrictions on the VA's dog research program are deemed to be hampering the advancement of science, research, and treatments for veterans, lawmakers will choose to remove or alter the current legal restrictions.
With this rationale the 10 committee members in the majority offer the following recommendation:
Recommendation 2: Adopt an expanded set of criteria for determining when to use laboratory dogs in U.S. Department of Veterans Affairs' (VA's) biomedical research when the dog is not scientifically necessary.14,15
In order to conduct biomedical research that will lead to meaningful outcomes to support improved health of veterans, the following criteria should be met before approving the use of laboratory dogs when other animal models are also scientifically appropriate:
- 1.
The scientific question and the knowledge expected to be gained will advance understanding or medical practices related to veterans' health;
- 2.
The research objective cannot be adequately addressed using new approach methodologies or ethically using human subjects or companion animals;
- 3.
Where multiple species [excluding non-human primates],16 including the laboratory dog, can be used to adequately answer the scientific question, the non-primate species that will incur the fewest burdens should be selected. If the species that will incur the fewest burdens cannot be selected for any reason, including legal and/or funding restrictions (e.g., the laboratory dog), the VA cannot ethically proceed and should consider forgoing the research; and
- 4.
The expected harms experienced by the selected animals are sufficiently outweighed by the expected benefits for veterans. Both the institutional animal care and use committee and the VA's central office ethics review should concur in this assessment.
The five member committee minority dissents from this recommendation because they believe the recommendation strays beyond the Statement of Task. Where the majority grounds its arguments for the recommendations in ethical considerations, the minority points to the ethical, legal, regulatory, and institutional context in which the Statement of Task was prepared and argues that the VA clearly intended the committee to address only the scientific necessity of using dogs in research and that ethics-based recommendations are outside the scope of what the committee was asked to do.
To support its position, the minority offers several observations. First, turning to the Statement of Task, the minority points to the sentence “If it is determined that they [dogs] are necessary, describe the unique physiological and other characteristics of dogs that currently make it the necessary animal model for use in these types of research.” The implicit message in this sentence, the minority argue, is that the VA wanted the committee to focus on objective scientific criteria, such as physiological characteristics, in the determination of whether dogs are necessary in particular research projects. A second sentence in the Statement of Task asks the committee to “Provide recommendations for any new or revised scientific parameters to guide how and when to use dogs for biomedical research rather than non-rodent (excluding non-human primates) or rodent species or non-animal alternatives.” Again, only scientific parameters, not ethical ones, are mentioned. Thus, the minority believes that the VA's intention in its Statement of Task was that the committee address only the scientific necessity of using dogs in research. The minority also believes that the ethical considersations (#2 in the Statement of Task) were specific to the dog (rather than to a comparative ethical analysis).
More broadly, the minority contends that Recommendation 2 is in conflict with both existing VA policy and existing federal legislation. For example, during the public meeting held in Washington, DC, on February 14, 2019, the VA representative confirmed that the definition of “necessary” used by the VA holds that the VA should only continue using dogs in biomedical research when there is no other alternative that will yield scientifically valid results that meet proposed study objectives of importance to veterans (i.e., the definition it uses of “necessary” is essentially “scientifically necessary”). Furthermore, the federal appropriations legislation that places restrictions on the use of dogs in VA-funded research uses the same definition of necessity. Beyond that Recommendation 2 contradicts accepted policies of various other federal agencies, such as NIH, many of which treat some animals, such as the chimpanzee, differently from others, and the Animal Welfare Act privileges any “dog, cat, monkey (nonhuman primate mammal), guinea pig, hamster, rabbit, or such other warm-blooded animal” over every other type of sentient species, including rats and mice, birds used for research, and farmed animals, such as horses, livestock, and poultry.17
Thus, the minority members believe that while it is certainly important to grapple with the broad ethical issues surrounding animal research, this report is not the proper venue, as it was intended to address scientific questions and ethical and regulatory positions taken by the VA and the federal government specific to the laboratory dog. Furthermore, the minority contends that if a full ethical analysis of using various species of animals in biomedical research were to be undertaken, it would require a deeper analysis than this committee was equipped to perform and likely would require convening a committee that was not composed predominantly of scientists. If the dissenting minority believed a comparative ethical analysis had been requested in the Statement of Task, the minority would have proposed the use of a broader ethical framework that acknowledges the rapid changes in science (e.g., the sophistication of non-animal models), is inclusive of societal values (particularly when the research is publicly funded), is ethically defensible, and is ultimately useful to researchers.18 Regardless, the minority members still conclude that such comparative ethical questions remain outside the scope of the committee's Statement of Task. For these reasons they dissent from Recommendation 2.
To sum up, the disagreement between the majority and minority over Recommendation 2 is essentially a disagreement about whether that recommendation comports with the Statement of Task. The majority, taking a broad view of the meaning of “necessary,” believes it does. The minority, holding a more restricted view of the meaning of “necessary,” believes it does not. The practical effect of that definitional disagreement is that the majority believes that the interests of other laboratory animals than the dog must be taken into consideration when determining the necessity of research on laboratory dogs, while the minority believes that the question the committee was asked dealt not with other research animals but only with laboratory dogs.
OPPORTUNITIES TO IMPROVE BIOMEDICAL RESEARCH PROTOCOLS AND REVIEW PROCESSES AT THE VA
The committee is concerned that the current culture of justifying biomedical research in laboratory dogs favors the continued use of dogs, given that the previous experience of principal investigators and technical support staff, along with the availability of extensive historical data for dogs, are sometimes the sole justifications provided for the continued use of laboratory dogs in research. The current system has considerable momentum around scientific review committee and IACUC acceptance of the principal investigator's judgment regarding whether laboratory dogs are necessary. In the experience of some committee members, in general, if an investigator states that the harm to laboratory dogs used in a particular study would be less than the harm incurred by pigs or sheep because more pigs or sheep would be required to build the knowledge base to a level comparable to dogs, it is possible that scientific review committees and IACUCs will accept that logic. Perpetuating this reasoning favors the continued use of laboratory dogs in biomedical research, at the VA and elsewhere.
The committee could not fully evaluate or understand the institutional momentum at the VA for or against this reasoning for the continued use of laboratory dogs. Rather, this concern is based on review of ACORPs and the opinion and experience of some on the committee. The VA Chief Research and Development Officer, Rachel Ramoni, told the committee that the VA was committed to moving away from sensitive species, including dogs, even if that meant an additional investment of time and money (Ramoni, 2019). Indeed, the committee learned that a pilot study in pigs was under way at a VA center to potentially replace the use of laboratory dogs. Also during the course of this study, several VA protocols planned in laboratory dogs were switched to mice, pigs, or humans.
Justifying the use of laboratory dogs at the VA today requires significant paperwork and sign-offs but, in the committee's judgment, does not always require a thorough examination of alternatives nor an accurate accounting of harm and burden to study animals. For example, evidence from the ACORPs reviewed by the committee suggests that literature searches for relevant alternatives are of poor quality and seem to be primarily box-checking exercises (i.e., the decision to use laboratory dogs having been made prior to the literature search for alternatives). Also, some ACORPs lacked a written descripton of the perceived harm to laboratory dogs. A few ACORPs also seemed to misapply the Three Rs by prioritizing reduction in the number of dogs used over refinement.
Tracking Impact of Laboratory Dog Research Through Prospective Registration: A Strategy for Improving Quality and Reducing Animal Use
In recent years, many commentators have encouraged the prospective registration of preclinical studies, particularly those designed to test disease interventions and toxicology in animals (Anderson and Kimmelman, 2012; Heinl et al., 2020). This process would require that full experimental design and results from preclinical studies be deposited in publicly accessible databases regardless of the studies' outcome. Prospective registration can improve the quality and refinement of animal studies (Wieschowski et al., 2016). Full disclosure of experimental procedures would be likely to increase the reproducibility of research, while the disclosure of results would reduce animal use by avoiding unnecessary duplication of studies, because failed experiments—which rarely see publication in the scientific literature—would not be repeated.
These calls echo those that motivated the establishment of clinical trial registries, such as clinicaltrials.gov, for research involving human beings (De Angelis et al., 2004). Clinical trial registries were established following several episodes in which information adverse to particular pharmaceutical products was found to have been withheld from publication.
While there are currently no requirements in the United States for the preregistration of preclinical trials, establishing this as standard procedure for studies that use laboratory dogs could both enhance the knowledge gained from this research and improve its harm–benefit ratio. Though no gold-standard registration portal has yet emerged, several options exist; these include the Open Science Framework (osf.io) and two international registries, preclinicaltrials.eu and animalstudyregistry.org (Bert et al., 2019).
Recommendation 3: Improve biomedical research protocols and review processes, and track the impact of research.
The U.S. Department of Veterans Affairs (VA) should enhance its scientific and ethical review process so that it better integrates the assessment of harm and burden with assessments of value and impact associated with biomedical research using laboratory dogs. There should be an explicit and strong connection between scientific review and institutional animal care and use committee (IACUC) consideration so that all reviewers understand the study objectives, harm–benefit assessment, and anticipated value and impact of the study on human health. The VA should focus efforts on improving the following areas:
- Protocol Development. Specifically, the VA should implement measures to ensure that:
- The principal investigator starts prior to submission to a funding agency to:
- Develop the biomedical research question and fully describe its value to the VA's mission, veterans, and the nation;
- Engage with an independent literature research group to ensure thorough and transparent evaluation of possible new approach methodology (NAM) alternatives (discussed in Chapter 4);
- Consult with the attending veterinarian to determine whether the requisite veterinary expertise is present in the VA. If additional expertise is needed, the VA's principal investigator should be supported in engaging with veterinary specialists outside the VA to develop protocols and refine procedures necessary to meet study objectives. Examples include newer imaging techniques to measure anatomical and functional parameters of tissues; minimally invasive surgical and interventional radiographic techniques for device placement; and contemporary pain assessment and relief, including current measures of inappetence, weight loss, and other clinical parameters;
- Engage with independent statisticians to ensure appropriate study design and statistical power analysis; and
- Submit the research protocol to funding agency (the VA or other) and IACUC simultaneously.
- Protocol Development and Review Processes. Specifically, the VA should:
- Emphasize the replacement of laboratory dogs and the refinement of procedures and techniques over a reduction in animal numbers in order to reduce the burden on individual dogs, even if more animals (including alternative species) will be used;
- Improve literature searches for alternatives to laboratory dogs. The VA should fund an independent party to conduct literature searches designed to yield objective, independent analyses of the need to perform proposed research in laboratory dogs versus alternative animal models, NAMs, humans, or human tissues; and
- Engage with board-certified and other experts in canine medicine and research to review research goals and ensure optimal study design, including estimates of the sample size needed to ensure adequate statistical power. Consider spontaneous clinical conditions of relevance and the possibility of clinical trials in companion dogs to complement or replace laboratory dog studies.
- Track Impact of Research. Specifically, the VA should:
- Establish a mechanism for tracking the impact and translation of research using dogs. Such a retrospective reporting mechanism should use objective and state-of-the-art methods (e.g., bibliometrics or citation in regulatory documents and patents) to track the relationship between dog experiments and translated interventions for veterans. Such performance assessment should be required to establish and, if need be, correct risk–benefit and welfare assessments used in the authorization of research.
- Take steps to encourage the prospective registration of all studies involving laboratory dogs.
Recommendations 1, 2, and 3, if adopted and enforced, would become part of the culture and process of scientific and ethical review at the VA. Recommendations 1 and 2 would create the expectation that principal investigators consider, early in the study proposal process, all possible alternatives (non-animal or animal) and the relative harm that the proposed study would bring to the candidate subjects. Scientific review committees and IACUCs would be conducting simultaneous reviews of the analysis of harm and benefit, such that all three parties—principal investigator, scientific review committee, and IACUC—would develop an agreed-upon understanding of “scientific necessity,” reconcile any differences of perspective related to the proposed study, and generally pool accountability for decisions related to the use of laboratory dogs.
REFERENCES
NOTE: An asterisk before the first author's name denotes that the publication was based on research conducted at or funded by the VA. For such references, details about the VA connection are appended after the reference.
- ACF (Animal Cancer Foundation). Home page. n.d. [March 2, 2020]. http://acfoundation
.org. - Adin CA, Gilor C. The diabetic dog as a translational model for human islet transplantation. Yale Journal of Biology and Medicine. 2017;90(3):509–515. [PMC free article: PMC5612193] [PubMed: 28955189]
- Aguilar M, Qi XY, Huang H, Comtois P, Nattel S. Fibroblast electrical remodeling in heart failure and potential effects on atrial fibrillation. Biophysical Journal. 2014;107(10):2444–2455. [PMC free article: PMC4241450] [PubMed: 25418313]
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2018;72(14):1677–1749. [PubMed: 29097294]
- Alilain WJ. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs Public Workshop on the Uses of Dogs in Biomedical Research, March 27. Washington, DC: 2019.
- Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column: A preliminary report. JAMA. 1911;57:878–880.
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimer's & Dementia. 2019;15(3):321–387.
- Anderson JA, Kimmelman J. Should preclinical studies be registered? Nature Biotechnology. 2012;30(6):488–489. [PMC free article: PMC4516408] [PubMed: 22678379]
- Andics A, Miklosi A. Neural processes of vocal social perception: Dog-human comparative fMRI studies. Neuroscience and Biobehavioral Reviews. 2018;85:54–64. [PubMed: 29287629]
- Antz M, Scherlag BJ, Otomo K, Pitha J, Tondo C, Patterson E, Jackman WM, Lazzara R. Evidence for multiple atrio-AV nodal inputs in the normal dog heart. Journal of Cardiovascular Electrophysiology. 1998;9(4):395–408. [PubMed: 9581955]
- *Antz M, Otomo K, Nakagawa H, Yamanashi WS, Jackman WM, Kuck KH. Radiofrequency catheter ablation with the split-tip electrode in the temperature-controlled mode. Pacing and Clinical Electrophysiology. 2001;24(12):1765–1773. [This study included research performed at the VA Health Care System in Oklahoma City, OK.] [PubMed: 11817810]
- Ardell JL, Cardinal R, Beaumont E, Vermeulen M, Smith FM, Armour JA. Chronic spinal cord stimulation modifies intrinsic cardiac synaptic efficacy in the suppression of atrial fibrillation. Autonomic Neuroscience: Basic and Clinical. 2014;186:38–44. [PMC free article: PMC4607028] [PubMed: 25301713]
- Assari S. Veterans and risk of heart disease in the United States: A cohort with 20 years of follow up. International Journal of Preventive Medicine. 2014;5(6):703–709. [PMC free article: PMC4085922] [PubMed: 25013689]
- *Auerbach O, Hammond EC, Kirman D, Garfinkel L. Emphysema produced in dogs by cigarette smoking. JAMA. 1967;199(4):241–246. [Two of the authors conducted their research at the VA Medical Center in East Orange, NJ.] [PubMed: 6071181]
- *Avasthi PS, Greene ER, Voyles WF, Eldridge MW. A comparison of echo-doppler and electromagnetic renal blood flow measurements. Journal of Ultrasound in Medicine. 1984;3(5):213–218. [The first author was affiliated with the Raymond G. Murphy VA Medical Center in Albuquerque, NM.] [PubMed: 6233429]
- Babatunde OO, Jordan L, Van der Windt DA, Hill JC, Foster E, Protheroe J. PLOS ONE 12. 6. 2017. Effective treatment options for musculoskeletal pain in primary care: A systematic overview of current evidence; p. e0178621. [PMC free article: PMC5480856] [PubMed: 28640822]
- Babic RR, Stankovic Babic G, Babic SR, Babic NR. 120 years since the discovery of x-rays. Medicinski Pregled. 2016;69(9-10):323–330. [PubMed: 29693857]
- Baburin I, Varkevisser R, Schramm A, Saxena P, Beyl S, Szkokan P, Linder T, Stary-Weinzinger A, van der Heyden MAG, Houtman M, Takanari H, Jonsson M, Beekman JHD, Hamburger M, Vos MA, Hering S. Dehydroevodiamine and hortiamine, alkaloids from the traditional Chinese herbal drug Evodia rutaecarpa, are IKr blockers with proarrhythmic effects in vitro and in vivo. Pharmacological Research. 2018;131:150–163. [PubMed: 29477480]
- Baimel C, Bartlett SE, Chiou L-C, Lawrence AJ, Muschamp JW, Patkar O, Tung L-W, Borgland SL. Orexin/hypocretin role in reward: Implications for opioid and other addictions. British Journal of Pharmacology. 2015;172:334–348. [PMC free article: PMC4292951] [PubMed: 24641197]
- Baker TL, Foutz AS, McNerney V, Mitler MM, Dement WC. Canine model of narcolepsy: Genetic and developmental determinants. Experimental Neurology. 1982;75(3):729–742. [PubMed: 7199479]
- Barger AC. New technology for a new century: Walter B. Cannon and the invisible rays. American Journal of Roentgenology. 1981;136(1):187–195. [PubMed: 6779567]
- Barker PB, Blackband SJ, Chatham JC, Soher BJ, Samphilipo MA, Magee CA, Hilton JD, Strandberg JD, Anderson JH. Quantitative proton spectroscopy and histology of a canine brain tumor model. Magnetic Resonance in Medicine. 1993;30(4):458–464. [PubMed: 8255193]
- Bastijanic JM, Etscheidt J, Sattiraju M, Bonsignore C, Kopchok G, White R, Sarac TP. Fatigue and in vivo validation of a peritoneum-lined self-expanding nitinol stent-graft. Journal of Endovascular Therapy. 2014;21(5):735–746. [PMC free article: PMC5621711] [PubMed: 25290804]
- Baumeister RG, Mayo W, Notohamiprodjo M, Wallmichrath J, Springer S, Frick A. Microsurgical lymphatic vessel transplantation. Journal of Reconstructive Microsurgery. 2016;32(1):34–41. [PubMed: 26165882]
- Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, Haidich A-B, Hovorka R, Tsapas A. Artificial pancreas treatment for outpatients with type 1 diabetes: Systematic review and meta-analysis. BMJ (Clinical Research Edition). 2018;361:k1310. [PMC free article: PMC5902803] [PubMed: 29669716]
- Benavides FD, Santamaria AJ, Bodoukhin N, Guada LG, Solano JP, Guest JD. Characterization of motor and somatosensory evoked potentials in the Yucatan micropig using transcranial and epidural stimulation. Journal of Neurotrauma. 2017;34(18):2595–2608. [PubMed: 27251314]
- *Benthem L, Mundinger TO, Taborsky GJ Jr. Parasympathetic inhibition of sympathetic neural activity to the pancreas. American Journal of Physiology: Endocrinology and Metabolism. 2001;280(2):E378–E381. [VA research was conducted at the VA Puget Sound Health Care System in Seattle, WA.] [PubMed: 11158944]
- Bernstein SA. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs Public Workshop on the Uses of Dogs in Biomedical Research, March 27. Washington, DC: 2019.
- Bert B, Heinl C, Chmielewska J, Schwarz F, Grune B, Hensel A, Greiner M, Schönfelder G. Refining animal research: The Animal Study Registry. PLOS Biology. 2019;17(10):e3000463. [PMC free article: PMC6793840] [PubMed: 31613875]
- Betton GR, Owen LN. Allogeneic grafts of spontaneous canine melanomas and their cell culture strains in neonatal immunosuppressed dogs. British Journal of Cancer. 1976;34(4):374–380. [PMC free article: PMC2025258] [PubMed: 974003]
- Bever C. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs meeting open session, March 28. Washington, DC: 2019.
- Billman GE. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: Effect of endurance exercise training. American Journal of Physiology: Heart and Circulatory Physiology. 2009;297(4):H1171–H1193. [PubMed: 19684184]
- Bloom F, Paff GH, Noback CR. The transmissible venereal tumor of the dog: Studies indicating that the tumor cells are mature end cells of reticulo-endothelial origin. American Journal of Pathology. 1951;27(1):119–139. [PMC free article: PMC1937229] [PubMed: 14799618]
- *Boehmer LN, Wu M-F, John J, Siegel LM. Treatment with immunosuppressive and anti-inflammatory agents delays onset of canine genetic narcolepsy and reduces symptom severity. Experimental Neurology. 2004;188:292–299. [Research was conducted at the VA Greater Los Angeles Healthcare System.] [PMC free article: PMC8788643] [PubMed: 15246829]
- Boggiatto PM, Ramer-Tait AE, Metz K, Kramer EE, Gibson-Corley K, Mullin K, Hostetter JM, Gallup JM, Jones DE, Petersen CA. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clinical and Vaccine Immunology. 2010;17(2):267–273. [PMC free article: PMC2815526] [PubMed: 20032217]
- Bonilla IM, Nishijima Y, Vargas-Pinto P, Baine SH, Sridhar A, Li C, Billman GE, Carnes CA. Chronic omega-3 polyunsaturated fatty acid treatment variably affects cellular repolarization in a healed post-MI arrhythmia model. Frontiers in Physiology. 2016;7:225. [PMC free article: PMC4906012] [PubMed: 27378936]
- Bosch F, Rosich L. The contributions of Paul Ehrlich to pharmacology: A tribute on the occasion of the centenary of his Nobel Prize. Pharmacology. 2008;82(3):171–179. [PMC free article: PMC2790789] [PubMed: 18679046]
- Boulay E, Abernathy MM, Chui R, Friedrichs GS, Gendron-Parra N, Greiter-Wilke A, Guillon JM, Koerner JE, Menard A, Steidl-Nichols J, Pierson J, Pugsley MK, Rossman EI, Strauss D, Troncy E, Valentin JP, Wisialowski T, Authier S. A proof-of-concept evaluation of JTPc and Tp-Tec as proarrhythmia biomarkers in preclinical species: A retrospective analysis by an HESI-sponsored consortium. International Journal of Toxicology. 2019;38(1):23–32. [PubMed: 30567462]
- *Bresler L, Walter JS, Jahoda A, Wheeler JS, Turk T, Wurster RD. Effective methods of pelvic plexus nerve and bladder stimulation in anesthetized animal model. Journal of Rehabilitation Research and Development. 2008;45:627–638. [VA research was performed at the Edward J. Hines Jr. VA Hospital in Hines, IL.] [PubMed: 18712648]
- Brodala N, Merricks EP, Bellinger DA, Damrongsri D, Offenbacher S, Beck J, Madianos P, Sotres D, Chang YL, Koch G, Nichols TC. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arteriosclerosis Thrombosis and Vascular Biology. 2005;25:1446–1451. [PubMed: 15845905]
- Brooks OW, King RM, Nossek E, Marosfoi M, Caroff J, Chueh JY, Puri AS, Gounis MJ. A canine model of mechanical thrombectomy in stroke. Journal of Neurointerventional Surgery. 2019;1(12):1243–1248. [PubMed: 31103992]
- Brott DA, Diamond M, Campbell P, Zuvich A, Cheatham L, Bentley P, Gorko MA, Fikes J, Saye J. An acute rat in vivo screening model to predict compounds that alter blood glucose and/or insulin regulation. Journal of Pharmacological and Toxicological Methods. 2013;68(2):190–196. [PubMed: 23835094]
- Brown DC, Agnello K. Intrathecal substance P-saporin in the dog: Efficacy in bone cancer pain. Anesthesiology. 2013;119(5):1178–1185. [PMC free article: PMC3868346] [PubMed: 24195949]
- Bruce CJ, Stanton CM, Asirvatham SJ, Danielsen AJ, Johnson SB, Packer DL, Friedman PA. Percutaneous epicardial left atrial appendage closure: Intermediate-term results. Journal of Cardiovascular Electrophysiology. 2011;22(1):64–70. [PubMed: 20662983]
- Bryant RM. How implantable cardioverter-defibrillators work and simple programming. Cardiology in the Young. 2017;27(S1):S121–S125. [PubMed: 28084970]
- Burkhart F. The New York Times, January 16. 1997. [September 19, 2019]. Oscar Auerbach, 92, dies; linked smoking to cancer. https://www
.nytimes.com /1997/01/16/nyregion /oscar-auerbach-92-dies-linked-smoking-to-cancer.html. - Cai SV, Famula TR, Oberbauer AM, Hess RS. Heritability and complex segregation analysis of diabetes mellitus in American Eskimo dogs. Journal of Veterinary Internal Medicine. 2019;33(5):1926–1934. [PMC free article: PMC6766479] [PubMed: 31318104]
- Camacho P, Fan H, Liu Z, He JQ. Large mammalian animal models of heart disease. Journal of Cardiovascular Development and Diseases. 2016;3(4):30. [PMC free article: PMC5715721] [PubMed: 29367573]
- Carabello BA, Zile MR, Tanaka R, Cooper G 4th. Left ventricular hypertrophy due to volume overload versus pressure overload. Amerian Journal of Physiology. 1992;263(4 Pt 2):H1137–H1144. [PubMed: 1415762]
- Carapeba GO, Cavaleti P, Nicácio GM, Brinholi RB, Giuffrida R, Cassu RN. Intra-articular hyaluronic acid compared to traditional conservative treatment in dogs with osteoarthritis associated with hip dysplasia. Evidence-Based Complementary and Alternative Medicine. 2016;2016:2076921. [PMC free article: PMC5101385] [PubMed: 27847523]
- Casas CE, Guest JD. Percutaneous endoscopic cellular transplantation into the lower lumbar spinal cord. Neurosurgery. 2004;54(4):950–955. discussion 955. [PubMed: 15046663]
- CCOGC (Canine Comparative Oncology & Genomics Consortium). Home page. 2020. [March 2, 2020]. https://ccogc
.net. - CDC (Centers for Disease Control and Prevention). National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. [March 6, 2020]. https://www
.cdc.gov/diabetes /pdfs/data/statistics /national-diabetes-statistics-report.pdf. - Chapagain D, Range F, Huber L, Virányi Z. Cognitive aging in dogs. Gerontology. 2018;64(2):165–171. [PMC free article: PMC5841136] [PubMed: 29065419]
- Chardack WM. Heart block treated with an implantable pacemaker: Past experience and current developments. Progress in Cardiovascular Disease. 1964;6:507–537. [PubMed: 14171950]
- *Chardack WM, Gage AA, Greatbatch W. A transistorized, self-contained, implantable pacemaker for the long-term correction of complete heart block. Surgery. 1960;48:643–654. [Research was conducted at the VA Medical Center in Buffalo, NY.] [PubMed: 13692461]
- Chardack WM, Gage AA, Greatbatch W. Treatment of complete heart block with an implantable and self-contained pacemaker Bulletin de la Société Internationale Chirurgie. 1962;21:411–432. [PubMed: 14020217]
- Chardack WM, Gage AA, Schimert G, Thomson NB, Sanford CE, Greatbatch W. Two years' clinical experience with the implantable pacemaker for complete heart block. Diseases of the Chest. 1963;43:225–239. [PubMed: 14020218]
- Chatal JF, Rouzet F, Haddad F, Bourdeau C, Mathieu C, Le Guludec D. Story of rubidium-82 and advantages for myocardial perfusion PET imaging. Frontiers in Medicine (Lausanne). 2015;2:65. [PMC free article: PMC4566054] [PubMed: 26442267]
- Chen M, Zhou X, Yu L, Liu Q, Sheng X, Wang Z, Wang S, Jiang H, Zhou S. Low-level vagus nerve stimulation attenuates myocardial ischemic reperfusion injury by antioxidative stress and antiapoptosis reactions in canines. Journal of Cardiovascular Electrophysiology. 2016;27(2):224–231. [PubMed: 26546374]
- Chen SL, Zhang YJ, Zhou L, Xie DJ, Zhang FF, Jia HB, Wong SS, Kwan TW. Percutaneous pulmonary artery denervation completely abolishes experimental pulmonary arterial hypertension in vivo. EuroIntervention. 2013;9(2):269–276. [PubMed: 23466961]
- Cheriyan T, Ryan DJ, Weinreb JH, Cheriyan J, Paul JC, Lafage V, Kirsch T, Errico TJ. Spinal cord injury models: A review. Spinal Cord. 2014;52:588–595. [PubMed: 24912546]
- Chinda K, Tsai WC, Chan YH, Lin AY, Patel J, Zhao Y, Tan AY, Shen MJ, Lin H, Shen C, Chattipakorn N, Rubart-von der Lohe M, Chen LS, Fishbein MC, Lin SF, Chen Z, Chen PS. Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm. 2016;13(3):771–780. [PMC free article: PMC4762711] [PubMed: 26607063]
- Cho H, Nango M, Sakai Y, Sohgawa E, Kageyama K, Hamamoto S, Kitayama T, Yamamoto A, Miki Y. Neointimal hyperplasia after stent placement across size-discrepant vessels in an animal study. Japanese Journal of Radiology. 2014;32(6):340–346. [PubMed: 24715330]
- Chueh J. Experimental models of vascular occlusions for evaluation of thrombectomy devices. Cardiovascular Engineering and Technology. 2013;4(4):309–322.
- Cimino Brown D. What can we learn from osteoarthritis pain in companion animals? Clinical and Experimental Rheumatology 35 Suppl. 2017;107(5):53–58. [PubMed: 28967360]
- Clarke SA, Goodman NC, Ailawadi G, Holmes JW. Effect of scar compaction on the therapeutic efficacy of anisotropic reinforcement following myocardial infarction in the dog. Journal of Cardiovascular Translational Research. 2015;8(6):353–361. [PMC free article: PMC4527936] [PubMed: 26077797]
- Clauss S, Bleyer C, Schüttler D, Tomsits P, Renner S, Klymiuk N, Wakili R, Massberg S, Wolf E, Kääb S. Animal models of arrhythmia: Classic electrophysiology to genetically modified large animals. Nature Reviews Cardiology. 2019;16(8):457–475. [PubMed: 30894679]
- COHA (Clinical and Translational Science Award One Health Alliance). Home page. 2020. [February 26, 2020]. https:
//ctsaonehealthalliance.org. - Conn HL Jr, Detweiler DK, McGiff J, Luchi RJ. Why we have gone to the dogs. Transactions of the American Clinical and Climatological Association. 1966;77:34–47. [PMC free article: PMC2441100] [PubMed: 5894832]
- Cotman CW, Head E. The canine model of human aging and disease. Advances in Alzheimer's Disease. 2011;1:15–38.
- Danek M, Danek J, Araszkiewicz A. Large animals as potential models of human mental and behavioral disorders. Psychiatria Polska. 2017;51(6):1009–1027. [PubMed: 29432500]
- De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJPM, Schroeder TV, Sox HC, Van Der Weyden MB. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. Canadian Medical Association Journal. 2004;171(6):606–607. [PMC free article: PMC516198] [PubMed: 15367465]
- del Rio CL, Clymer BD, Billman GE. Myocardial electrotonic response to submaximal exercise in dogs with healed myocardial infarctions: evidence for beta-adrenoceptor mediated enhanced coupling during exercise testing. Frontiers in Physiology. 2015;6:25. [PMC free article: PMC4318283] [PubMed: 25698976]
- DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation in a subacute animal model of spinal cord injury. Journal of Applied Physiology. 1985;127(1):98–102. [PMC free article: PMC6692743] [PubMed: 31095462]
- DiMarco AF, Geertman RT, Tabbaa K, Kowalski KE. Complete restoration of respiratory muscle function in three subjects with spinal cord injury: Pilot interventional clinical trial. American Journal of Physical Medicine and Rehabilitation. 2019a;98(1):43–50. [PMC free article: PMC8883340] [PubMed: 30119089]
- DiMarco AF, Geertman RT, Tabbaa K, Nemunaitis GA, Kowalski KE. Restoration of cough via spinal cord stimulation improves pulmonary function in tetraplegics. Journal of Spinal Cord Medicine. 2019b;6:1–7. [PMC free article: PMC7534376] [PubMed: 31809251]
- Dixon JA, Spinale FG. Large animal models of heart failure: A critical link in the translation of basic science to clinical practice. Circulation: Heart Failure. 2009;2(3):262–271. [PMC free article: PMC2762217] [PubMed: 19808348]
- Efimov I. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs meeting open session, March 28. Washington, DC: 2019.
- Eisenbrey JR, Sridharan A, Liu J-B, Forsberg F. Recent experiences and advances in contrast-enhanced subharmonic ultrasound. BioMed Research International. 2015;2015:640397. [PMC free article: PMC4450275] [PubMed: 26090430]
- Eknoyan G, Nagy J. A history of diabetes mellitus, or how a disease of the kidneys evolved into a kidney disease. Advances in Chronic Kidney Disease. 2005;12:223–229. [PubMed: 15822058]
- Estrada JA, Williams AG Jr, Sun J, Gonzalez L, Downey HF, Caffrey JL, Mallet RT. δ-opioid receptor (DOR) signaling and reactive oxygen species (ROS) mediate intermittent hypoxia induced protection of canine myocardium. Basic Research in Cardiology. 2016;111(2):17. [PubMed: 26879900]
- Fan L, Mao C, Hu X, Zhang S, Yang Z, Hu Z, Sun H, Fan Y, Dong Y, Yang Shi J, Xu Y. New insights into the pathogenesis of Alzheimer's disease. Frontiers in Neurology. 2020;10:1312. [PMC free article: PMC6965067] [PubMed: 31998208]
- FDA (U.S. Food and Drug Administration). FDA approves first automated insulin delivery device for type 1 diabetes. 2016. [March 4, 2020]. https://www
.fda.gov/news-events /press-announcements /fda-approves-first-automated-insulin-delivery-device-type-1-diabetes. - Floyd CL. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs meeting open session, March 28. Washington, DC: 2019.
- Foutz A, Mitler M, Cavalli-Sforza L, Dement WC. Genetic factors in canine narcolepsy. Sleep. 1979;1(4):413–421. [PubMed: 574310]
- Frampton D, Schwenzer H, Marino G, Butcher LM, Pollara G, Kriston-Vizi J, Venturini C, Austin R, de Castro KF, Ketteler R, Chain B, Goldstein RA, Weiss RA, Beck S, Fassati A. Molecular signatures of regression of the canine transmissible venereal tumor. Cancer Cell. 2018;33(4):620–633. [PMC free article: PMC5896242] [PubMed: 29634949]
- Friedli L, Rosenzweig ES, Barraud Q, Schubert M, Dominici N, Awai L, Nielson JL, Musienko P, Nout-Lomas Y, Zhong H, Zdunowski S, Roy RR, Strand SC, van den Brand R, Havton LA, Beattie MS, Bresnahan JC, Bézard E, Bloch J, Edgerton VR, Ferguson AR, Curt A, Tuszynski MH, Courtine G. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Science Translational Medicine. 2015;7(302):302ra134. [PMC free article: PMC5669362] [PubMed: 26311729]
- Fumoto H, Gillinov AM, Saraiva RM, Horai T, Anzai T, Takaseya T, Shiose A, Arakawa Y, Vince DG, Dessoffy R, Fukamachi K. Left atrial appendage occlusion: Pilot study of a fourth-generation, minimally invasive device. Innovations (Phila). 2012;7(3):195–200. [PubMed: 22885461]
- Gabel BC, Curtis EI, Marsala M, Ciacci JD. A review of stem cell therapy for spinal cord injury: Large animal models and the frontier in humans. World Neurosurgery. 2017;98:438–443. [PubMed: 27876663]
- *Ganey T, Libera J, Moos V, Alasevic O, Fritsch K-G, Meisel HJ, Hutton WC. Disc chondrocyte transplantation in a canine model: A treatment for degenerated or damaged intervertebral disc. Spine. 2003;28(23):2609–2620. [VA research was conducted at the Atlanta VA Medical Center in Atlanta, GA.] [PubMed: 14652478]
- *Gasser TC, Graversen PH, Madsen PO. Fleroxacin (Ro 23-6240) distribution in canine prostatic tissue and fluids. Antimicrobial Agents and Chemotherapy. 1987;31(7):1010–1013. [VA research for this project was conducted at the William S. Middleton Memorial Veterans Hospital in Madison, WI.] [PMC free article: PMC174862] [PubMed: 3116916]
- Gerstenfeld EP, Lavi N, Bazan V, Gojraty S, Kim SJ, Michele J. Mechanism of complex fractionated electrograms recorded during atrial fibrillation in a canine model. Pacing and Clinical Electrophysiology. 2011;34(7):844–857. [PubMed: 21418250]
- Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. Journal of Experimental Medicine. 1934;59(3):347–379. [PMC free article: PMC2132360] [PubMed: 19870251]
- González-Martínez Á, Rosado B, Pesini P, Suárez ML, Santamarina G, García-Belenguer S, Villegas A, Monleón I, Sarasa M. Plasma β-amyloid peptides in canine aging and cognitive dysfunction as a model of Alzheimer's disease. Experimental Gerontology. 2011;46(7):590–596. [PubMed: 21377518]
- Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: Using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLOS Medicine. 2009;6(10):e1000161. [PMC free article: PMC2753665] [PubMed: 19823573]
- Gordon IK, Khanna C. Modeling opportunities in comparative oncology for drug development. ILAR Journal. 2010;51(3):214–220. [PMC free article: PMC7303812] [PubMed: 21131722]
- Granada JF, Kaluza GL, Wilensky RL, Biedermann BC, Schwartz RS, Falk E. Porcine models of coronary atherosclerosis and vulnerable plaque for imaging and interventional research. EuroIntervention. 2009;5(1):140–148. [PubMed: 19577996]
- Granger N, Blamires H, Franklin RJ, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: A randomized double-blinded trial in a canine translational model. Brain. 2012;135(Pt 11):3227–3237. [PMC free article: PMC3501977] [PubMed: 23169917]
- Grants.gov. Home page. n.d. [March 5, 2020]. https://www
.grants.gov/web/grants. - *Grozdanic SD, Kecova H, Harper MM, Nilaweera W, Kuehn MH, Kardon RH. Functional and structural changes in a canine model of hereditary primary angle-closure glaucoma. Investigative Opthalmology & Visual Science. 2010;51(1):255–263. VA research was conducted at the Iowa City VA Center. [PMC free article: PMC3258664] [PubMed: 19661222]
- Gruenstein DH, Bass JL. Experimental evaluation of a new articulated Amplatzer ductal occluder device without fabric. Catheterization and Cardiovascular Interventions. 2009;74(3):482–487. [PubMed: 19405162]
- Guest J. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs Public Webinar, May 7. 2019.
- *Gunda S, Akyeampong D, Gomez-Arroyo J, Jovin DG, Kowlgi NG, Kaszala K, Tan AY, Koneru JN, Kron J, Ellenbogen KA, Huizar JF. Consequences of chronic frequent premature atrial contractions: Association with cardiac arrhythmias and cardiac structural changes. Journal of Cardiovascular Electrophysiology. 2019;30(10):1952–1959. VA research was conducted at the Hunter Holmes McGuire VA Medical Center in Richmond, VA. [PMC free article: PMC6786912] [PubMed: 31310360]
- Hadrian R, Palmes D. Animal models of secondary lymphedema: New approaches in the search for therapeutic options. Lymphatic Research and Biology. 2017;15(1):2–16. [PubMed: 28128668]
- Halsey JT. The digitalized dog's heart as affected by amyl nitrite or atropine, studied electrocardiographically. Journal of Experimental Medicine. 1917;25(5):729–754. [PMC free article: PMC2125513] [PubMed: 19868120]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet. 2011;377(9781):1938–1947. [PMC free article: PMC3154251] [PubMed: 21601270]
- Harrison D. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs Public Workshop on the Uses of Dogs in Biomedical Research, March 27. Washington, DC: 2019.
- Harzmann R, Gericke D, Altenähr E, Bichler KH. Induction of a transplantable urinary bladder carcinoma in dogs. Investigations in Urology. 1980;18(1):24–28. [PubMed: 7410009]
- Hasdemir C, Scherlag BJ, Yamanashi WS, Lazzara R, Jackman WM. Endovascular stimulation of autonomic neural elements in the superior vena cava using a flexible loop catheter. Japanese Heart Journal. 2003;44(3):417–427. [PubMed: 12825809]
- Hasler-Rapacz J, Prescott MF, Von Linden-Reed J, Rapacz JM, Hu Z, Rapacz J. Elevated concentrations of plasma lipids and apolipoproteins B, C-III, and E are associated with the progression of coronary artery disease in familial hypercholesterolemic swine. Arteriosclerosis Thrombosis and Vascular Biology. 1995;15:583–592. [PubMed: 7749872]
- *Havel PJ, Mundinger TO, Taborsky GJ Jr. Pancreatic sympathetic nerves contribute to increased glucagon secretion during severe hypoglycemia in dogs. American Journal of Physiology: Endocrinology and Metabolism. 1996;270(1 Pt 1):E20–E26. VA research was conducted at the VA Puget Sound Health Care System in Seattle, WA. [PubMed: 8772469]
- Hayashida KI. Substance P-saporin for bone cancer pain in dogs: Can man's best friend solve the lost in translation problem in analgesic development? Anesthesiology. 2013;119(5):999–1000. [PMC free article: PMC3827733] [PubMed: 24195943]
- Head E. Oxidative damage and cognitive dysfunction: Antioxidant treatments to promote healthy brain aging. Neurochemical Research. 2009;34(4):670–678. [PMC free article: PMC4392815] [PubMed: 18683046]
- Head E. Neurobiology of the aging dog. Age. 2011;33(3):485–496. [PMC free article: PMC3168593] [PubMed: 20845082]
- Head E. A canine model of human aging and Alzheimer's disease. Biochimica et Biophysica Acta. 2013;1832(9):1384–1389. [PMC free article: PMC3937962] [PubMed: 23528711]
- *Heiner JP, Manley P, Kohles S, Ulm M, Bogart L, Vanderby R Jr. Ingrowth reduces implant-to-bone relative displacements in canine acetabular prostheses. Journal of Orthopaedic Research. 1994;12:657–664. VA research was conducted at the William S. Middleton Memorial Veterans Hospital in Madison, WI. [PubMed: 7931782]
- Heinl C, Chmielewska J, Olevska A, Grune B, Schönfelder G, Bert B. Rethinking the incentive system in science: Animal study registries: Preregistering experiments using animals could greatly improve transparency and reliability of biomedical studies and improve animal welfare. EMBO Reports. 2020;21(1):e49709. [PMC free article: PMC6945056] [PubMed: 31867805]
- Heron M. Deaths: Leading causes for 2017. Hyattsville, MD: National Center for Health Statistics; National Vital Statistics Reports. 2019;68(6):1–76. [PubMed: 32501203]
- Hill AC, Guy TS. Minimally invasive surgical implantation of the percutaneous left atrial appendage transcatheter occlusion device: initial experience in a canine model. Innovations (Phila). 2012;7(1):52–58. [PubMed: 22576036]
- Hong LTA, Kim YM, Park HH, Hwang DH, Cui Y, Lee EM, Yahn S, Lee JK, Song SC, Kim BG. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nature Communications. 2017;8(1):533. [PMC free article: PMC5599609] [PubMed: 28912446]
- Hoyer D, Jacobson LH. Orexin in sleep, addiction and more: Is the perfect insomnia drug at hand? Neuropeptides. 2013;47(6):477–488. [PubMed: 24215799]
- Hu HZ, Granger N, Pai SB, Bellamkonda RV, Jeffery ND. Therapeutic efficacy of microtube-embedded chondroitinase ABC in a canine clinical model of spinal cord injury. Brain. 2018;141(4):1017–1027. [PubMed: 29444239]
- Huang J, Dosdall DJ, Cheng KA, Li L, Rogers JM, Ideker RE. The importance of Purkinje activation in long duration ventricular fibrillation. Journal of the American Heart Association. 2014;3(1):e000495. [PMC free article: PMC3959715] [PubMed: 24584738]
- Huang Y, Gnanadurai CW, Fu ZF. Rabies virus vaccines. In: Pattnaik AK, Whitt MA, editors. Biology and pathogenesis of rhabdo- and filoviruses. Singapore: World Scientific Publishing Co Pte Ltd; 2015. pp. 387–426.
- *Huizar JF, Kaszala K, Potfay J, Minisi AJ, Lesnefsky EJ, Abbate A, Mezzaroma E, Chen Q, Kukreja RC, Hoke NN, Thacker LR 2nd, Ellenbogen KA, Wood MA. Left ventricular systolic dysfunction induced by ventricular ectopy: A novel model for premature ventricular contraction-induced cardiomyopathy. Circulation: Arrhythmia and Electrophysiology. 2011;4(4):543–549. Research was conducted at the Hunter Holmes McGuire VA Medical Center in Richmond, VA. [PMC free article: PMC3175603] [PubMed: 21576277]
- Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. Journal of the American College of Cardiology. 2019;73(18):2328–2344. [PMC free article: PMC6538508] [PubMed: 31072578]
- Iadarola MJ, Sapio MR, Raithel SJ, Mannes AJ, Brown DC. Long-term pain relief in canine osteoarthritis by a single intra-articular injection of resiniferatoxin, a potent TRPV1 agonist. Pain. 2018;159(10):2105–2114. [PMC free article: PMC8121156] [PubMed: 30015705]
- IOM (Institute of Medicine). Chimpanzees in biomedical and behavioral research: Assessing the necessity. Washington, DC: The National Academies Press; 2011. [PubMed: 22514816]
- Ishibashi Y, Rembert JC, Carabello BA, Nemoto S, Hamawaki M, Zile MR, Greenfield JC Jr, Cooper G. Normal myocardial function in severe right ventricular volume overload hypertrophy. American Journal of Physiology: Heart and Circulatory Physiology. 2001;280(1):H11–H16. [PubMed: 11123212]
- Ishihara K, Zile MR, Tomita M, Tanaka R, Kanazawa S, Carabello BA. Left ventricular hypertrophy in a canine model of reversible pressure overload. Cardiovascular Research. 1992;26(6):580–585. [PubMed: 1451137]
- Ito D, Carwardine D, Prager J, Wong LF, Kitagawa M, Jeffery N, Granger N. Methods of olfactory ensheathing cell harvesting from the olfactory mucosa in dogs. PLOS ONE. 2019;14(3):e0213252. [PMC free article: PMC6402693] [PubMed: 30840687]
- Jackman WM, Zipes DP. Low-energy synchronous cardioversion of ventricular tachycardia using a catheter electrode in a canine model of subacute myocardial infarction. Circulation. 1982;66(1):187–195. [PubMed: 7083506]
- Jackman WM, Kuck KH, Naccarelli GV, Carmen L, Pitha J. Radiofrequency current directed across the mitral anulus with a bipolar epicardial-endocardial catheter electrode configuration in dogs. Circulation. 1988;78(5 Pt 1):1288–1298. [PubMed: 3180385]
- Jäkel L, Van Nostrand WE, Nicoll JAR, Werring DJ, Verbeek MM. Animal models of cerebral amyloid angiopathy. Clinical Science (London). 2017;131(19):2469–2488. [PubMed: 28963121]
- Jalal Z, Seguela PE, Baruteau AE, Benoist D, Bernus O, Villemain O, Boudjemline Y, Iriart X, Thambo JB. Role of animal models for percutaneous atrial septal defect closure. Journal of Thoracic Disease. 2018;10(Suppl 24):S2966–S2974. [PMC free article: PMC6174152] [PubMed: 30305957]
- Janardhan AH, Gutbrod SR, Li W, Lang D, Schuessler RB, Efimov IR. Multistage electrotherapy delivered through chronically-implanted leads terminates atrial fibrillation with lower energy than a single biphasic shock. Journal of the American College of Cardiology. 2014;63(1):40–48. [PMC free article: PMC4123180] [PubMed: 24076284]
- Jeffery N. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs Public Workshop on the Uses of Dogs in Biomedical Research, March 27. Washington, DC: 2019.
- Jeremy JY, Thomas AC. Animal models for studying neointima formation. Current Vascular Pharmacology. 2010;8(2):198–219. [PubMed: 19485916]
- Jiang M, Zhang M, Howren M, Wang Y, Tan A, Balijepalli RC, Huizar JF, Tseng GN. JPH-2 interacts with Cai-handling proteins and ion channels in dyads: Contribution to premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2016;13(3):743–752. [PMC free article: PMC4762763] [PubMed: 26538326]
- Jilaihawi H, Virmani R, Nakagawa H, Ducharme A, Shi Y-F, Carter-Monroe N, Ladich E, Iyer M, Ikeda A, Asgar A, Bonan R. Mitral annular reduction with subablative therapeutic ultrasound: Pre-clinical evaluation of the ReCor device. EuroIntervention. 2010;6(1):54–62. [PubMed: 20542798]
- Jordan J, Heusser K, Brinkmann J, Tank J. Electrical carotid sinus stimulation in treatment resistant arterial hypertension. Autonomic Neuroscience: Basic and Clinical. 2012;172(1-2):31–36. [PubMed: 23146623]
- Joscht M, Martin M, Henin M, Nisolle JF, Kirschvink N, Dugdale A, Godart B, Coulon H, Simon V, Hontoir F, Graffin R, De Raeve Y, Vandeweerd JM. Angiographic anatomy of external iliac arteries in the sheep. Anatomia, Histologia, Embryologia. 2016;45(6):443–449. [PubMed: 26680154]
- Kaeberlein M, Creevy KE, Promislow DE. The dog aging project: Translational geroscience in companion animals. Mammalian Genome. 2016;27(7-8):279–288. [PMC free article: PMC4936929] [PubMed: 27143112]
- Kakino T, Saku K, Sakamoto T, Sakamoto K, Akashi T, Ikeda M, Ide T, Kishi T, Tsutsui H, Sunagawa K. Prediction of hemodynamics under left ventricular assist device. American Journal of Physiology: Heart and Circulatory Physiology. 2017;312(1):H80–H88. [PubMed: 27793856]
- Kalder J, Isfort P, Reinartz SD, Gremse F, Yamoah GG, Gesche V, Kotelis D, Tolba R, Jacobs MJ, Jalaie H. New large animal model for aortic aneurysms in the viscerorenal segment. Journal of Surgical Research. 2019;240:156–164. [PubMed: 30933829]
- Kaplan JL, Stern JA, Fascetti AJ, Larsen JA, Skolnik H, Peddle GD, Kienle RD, Waxman A, Cocchiaro M, Gunther-Harrington CT, Klose T, LaFauci K, Lefbom B, Machen Lamy M, Malakoff R, Nishimura S, Oldach M, Rosenthal S, Stauthammer C, O'Sullivan L, Visser LC, Williams R, Ontiveros E. Taurine deficiency and dilated cardiomyopathy in golden retrievers fed commercial diets. PLOS ONE. 2018;13(12):e0209112. [PMC free article: PMC6292607] [PubMed: 30543707]
- Kar S, Hou D, Jones R, Werner D, Swanson L, Tischler B, Stein K, Huibregtse B, Ladich E, Kutys R, Virmani R. Impact of Watchman and Amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC: Cardiovascular Interventions. 2014;7(7):801–809. [PubMed: 25060026]
- Kara T, Leinveber P, Vlasin M, Jurak P, Novak M, Novak Z, Chrastina J, Czechowicz K, Belehrad M, Asirvatham SJ. Endovascular brain intervention and mapping in a dog experimental model using magnetically-guided micro-catheter technology. Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czech Republic. 2014;158(2):221–226. [PubMed: 23154541]
- Karczmar AG, Longo VG, De Carolis AS. A pharmacological model of paradoxical sleep: The role of cholinergic and monoamine systems. Physiology & Behavior. 1970;5(2):175–182. [PubMed: 5525792]
- Karlson AG, Mann FC. The transmissible venereal tumor of dogs: Observations on forty generations of experimental transfers. Annals of the New York Academy of Sciences. 1952;54(6):1197–1213. [PubMed: 12977017]
- Katsouras G, Sakabe M, Comtois P, Maguy A, Burstein B, Guerra PG, Talajic M, Nattel S. Differences in atrial fibrillation properties under vagal nerve stimulation versus atrial tachycardia remodeling. Heart Rhythm. 2009;6(10):1465–1472. [PubMed: 19968926]
- Kawajiri H, Mizuno T, Moriwaki T, Ishibashi-Ueda H, Yamanami M, Kanda K, Yaku H, Nakayama Y. Development of tissue-engineered self-expandable aortic stent grafts (bio stent grafts) using in-body tissue architecture technology in beagles. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2015a;103(2):381–386. [PubMed: 24895150]
- Kawajiri H, Mizuno T, Moriwaki T, Iwai R, Ishibashi-Ueda H, Yamanami M, Kanda K, Yaku H, Nakayama Y. Implantation study of a tissue-engineered self-expanding aortic stent graft (bio stent graft) in a beagle model. Journal of Artificial Organs. 2015b;18(1):48–54. [PubMed: 25320016]
- Khanna C, London C, Vail D, Mazcko C, Hirschfeld S. Guiding the optimal translation of new cancer treatments from canine to human cancer patients. Clinical Cancer Research. 2009;15(18):5671–5677. [PMC free article: PMC2748812] [PubMed: 19737961]
- *Kijas JMH, Bauer TR Jr, Gäfvert S, Marklund S, Trowald-Wigh G, Johannisson A, Hedhammar Å, Binns M, Juneja RK, Hickstein DD, Andersson L. A missense mutation in the beta-2 integrin gene (ITGB2) causes canine leukocyte adhesion deficiency. Genomics. 1999;61(1):101–107. Two of the authors were affiliated with the VA Puget Sound Health Care System in Seattle, WA. [PubMed: 10512685]
- Kim BG, Kang YM, Phi JH, Kim YH, Hwang DH, Choi JY, Ryu S, Elastal AE, Paek SH, Wang KC, Lee SH, Kim SU, Yoon BW. Implantation of polymer scaffolds seeded with neural stem cells in a canine spinal cord injury model. Cytotherapy. 2010;12(6):841–845. [PubMed: 20629485]
- Kinter LB, DeGeorge JJ. Scientific knowledge and technology, animal experimentation, and pharmaceutical development. ILAR Journal. 2016;57(2):101–108. [PubMed: 28053064]
- Kleinert M, Clemmensen C, Hofmann S, Moore M, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schürmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristow M, Lickert H, Wolf E, Havel PJ, Müller TD, Tschöp MH. Animal models of obesity and diabetes mellitus. Nature Reviews Endocrinology. 2018;14(3):140–162. [PubMed: 29348476]
- *Kowalski KE, Romaniuk JR, Brose S, Richmond MA, Kowalski T, DiMarco AF. High frequency spinal cord stimulation—New method to restore cough. Respiratory Physiology & Neurobiology. 2016;232:54–56. VA research was performed at the Louis Stokes Cleveland VA Medical Center in Cleveland, OH. [PMC free article: PMC5012955] [PubMed: 27395446]
- Kowalski KE, Romaniuk JR, Kirkwood PA, DiMarco AF. Inspiratory muscle activation via ventral lower thoracic high-frequency spinal cord stimulation. Journal of Applied Physiology (1985). 2019;126(4):977–983. [PubMed: 30763163]
- Krueger E, Magri LMS, Botelho AS, Bach FS, Rebellato CLK, Fracaro L, Fragoso FYI, Villanova JA Jr, Brofman PRS, Popovi-Maneski L. Effects of low-intensity electrical stimulation and adipose derived stem cells transplantation on the time-domain analysis-based electromyographic signals in dogs with SCI. Neuroscience Letters. 2019;696:38–45. [PubMed: 30528708]
- Kubota Y, Miyagawa S, Fukushima S, Saito A, Watabe H, Daimon T, Sakai Y, Akita T, Sawa Y. Impact of cardiac support device combined with slow-release prostacyclin agonist in a canine ischemic cardiomyopathy model. Journal of Thoracic and Cardiovascular Surgery. 2014;147(3):1081–1087. [PubMed: 24131787]
- Kukielka M, Holycross BJ, Billman GE. Endurance exercise training reduces cardiac sodium/calcium exchanger expression in animals susceptible to ventricular fibrillation. Frontiers in Physiology. 2011;2:3. [PMC free article: PMC3059610] [PubMed: 21423413]
- Kumar A, Green JD, Sykes JM, Ephrat P, Carson JJ, Mitchell AJ, Wisenberg G, Friedrich MG. Detection and quantification of myocardial reperfusion hemorrhage using T2*-weighted CMR. JACC: Cardiovascular Imaging. 2011;4(12):1274–1283. [PubMed: 22172784]
- Kumar S, Singh R, Vasudeva N, Sharma S. Acute and chronic animal models for the evaluation of anti-diabetic agents. Cardiovascular Diabetology. 2012;11:9. [PMC free article: PMC3286385] [PubMed: 22257465]
- Kutikov AB, Moore SW, Layer RT, Podell PE, Sridhar N, Santamaria AJ, Aimetti AA, Hofstetter CP, Ulich TR, Guest JD. Method and apparatus for the automated delivery of continuous neural stem cell trails into the spinal cord of small and large animals. Neurosurgery. 2019;85(4):560–573. [PubMed: 30169668]
- *Kwofie MA, Chaudhary AK, Martins JB. Association among intracardiac T-wave alterans, ischemia, and spontaneous ventricular arrhythmias after coronary artery occlusion in a canine model. Translational Research. 2011;158(5):265–272. A co-author held an appointment at the VA Health Care System in Iowa City, IA. [PubMed: 22005265]
- Lafleur RL, Callister SM, Dant JC, Jobe DA, Lovrich SD, Warner TF, Wasmoen TL, Schell RF. One-year duration of immunity induced by vaccination with a canine lyme disease bacterin. Clinical and Vaccine Immunology. 2010;17(5):870–874. [PMC free article: PMC2863397] [PubMed: 20237200]
- Larsen M, Holm R, Jensen KG, Brodin B, Nielsen CU. Intestinal gaboxadol absorption via PAT1 (SLC36A1): Modified absorption in vivo following co-administration of L-tryptophan. British Journal of Pharmacology. 2009;157(8):1380–1389. [PMC free article: PMC2765307] [PubMed: 19594759]
- Lascelles BDX, Brown DC, Maixner W, Mogil JS. Spontaneous painful disease in companion animals can facilitate the development of chronic pain therapies for humans. Osteoarthritis and Cartilage. 2018;26(2):175–183. [PubMed: 29180098]
- Lee JY, Lucchesi BR. HBI-3000 prevents secondary sudden cardiac death. Journal of Cardiovascular Pharmacology and Therapeutics. 2013;18(5):453–459. [PubMed: 23615576]
- Lee N, Authier S, Pugsley MK, Curtis MJ. The continuing evolution of torsades de pointes liability testing methods: Is there an end in sight? Toxicolicology and Applied Pharmacology. 2010a;243(2):146–153. [PubMed: 20005885]
- Lee RJ, Bartus K, Yakubov SJ. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: Initial experience in a canine model. Circulation: Cardiovascular Interventions. 2010b;3(3):224–229. [PubMed: 20484100]
- Lee SH, Chung YN, Kim YH, Kim YJ, Park JP, Kwon DK, Kwon OS, Heo JH, Kim YH, Ryu S, Kang HJ, Paek SH, Wang KC, Kim SU, Yoon BW. Effects of human neural stem cell transplantation in canine spinal cord hemisection. Neurological Research. 2009;31(9):996–1002. [PubMed: 19138477]
- Lequoy P, Savoji H, Saoudi B, Bertrand-Grenier A, Wertheimer MR, De Crescenzo G, Soulez G, Lerouge S. In vitro and pilot in vivo evaluation of a bioactive coating for stent grafts based on chondroitin sulfate and epidermal growth factor. Journal of Vascular and Interventional Radiology. 2016;27(5):753–760. [PubMed: 27036642]
- Li CH, Gao BL, Wang JW, Liu JF, Li H, Yang ST. Construction of aneurysmal models on a curved vascular segment of a carotid siphon model for testing endovascular devices. World Neurosurgery. 2019;123:e581–e587. [PubMed: 30529529]
- Lim JH, Piedrahita JA, Jackson L, Ghashghaei T, Olby NJ. Development of a model of sacrocaudal spinal cord injury in cloned Yucatan minipigs for cellular transplantation research. Cellular Reprogramming. 2010;12(6):689–697. [PMC free article: PMC3004133] [PubMed: 21108536]
- Lopshire JC, Zhou X, Dusa C, Ueyama T, Rosenberger J, Courtney N, Ujhelyi M, Mullen T, Das M, Zipes DP. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120(4):286–294. [PubMed: 19597055]
- Loría-Cervera EN, Andrade-Narváez FJ. Animal models for the study of leishmaniasis immunology. Revista do Instituto de Medicina Tropical de Sao Paulo. 2014;56(1):1–11. [PMC free article: PMC4085833] [PubMed: 24553602]
- Luo J, Lu G, Ye S, Ou J, Fu C, Zhang X, Wang X, Huang J, Wu P, Xu H, Wu L, Li S. Comparative pathogenesis of H3N2 canine influenza virus in beagle dogs challenged by intranasal and intratracheal inoculation. Virus Research. 2018;255:147–153. [PubMed: 29860092]
- MacFarlane PM, Vinit S, Mitchell GS. Enhancement of phrenic long-term facilitation following repetitive acute intermittent hypoxia is blocked by the glycolytic inhibitor 2-deoxyglucose. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2018;314(1):R135–R144. [PMC free article: PMC5866367] [PubMed: 29021191]
- Mahoney CE, Cogswell A, Koralnik IJ, Scammell TE. The neurobiological basis of narcolepsy. Nature Reviews Neuroscience. 2019;20(2):83–93. [PMC free article: PMC6492289] [PubMed: 30546103]
- Makary S, Voigt N, Maguy A, Wakili R, Nishida K, Harada M, Dobrev D, Nattel S. Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circulation Research. 2011;109(9):1031–1043. [PubMed: 21903936]
- Marcus DA, Bernstein CD, Constantin JM, Kunkel FA, Breuer P, Hanlon RB. Animal-assisted therapy at an outpatient pain management clinic. Pain Medicine. 2012;13(1):45–57. [PubMed: 22233395]
- Marostica E, Van Ammel K, Teisman A, Gallacher D, Van Bocxlaer J, De Ridder F, Boussery K, Vermeulen A. PK/PD modelling of the QT interval: A step towards defining the translational relationship between in vitro, awake beagle dogs, and humans. AAPS Journal. 2016;18(4):1000–1012. [PubMed: 27116025]
- Martinez Moreno R, Bhogal P, Lenz-Habijan T, Bannewitz C, Siddiqui A, Lylyk P, Hannes R, Monstadt H, Henkes H. In vivo canine study of three different coatings applied to p64 flow-diverter stents: Initial biocompatibility study. European Radiology Experimental. 2019;3(1):3. [PMC free article: PMC6342750] [PubMed: 30671686]
- Matsuo T, Carabello BA, Nagatomo Y, Koide M, Hamawaki M, Zile MR, McDermott PJ. Mechanisms of cardiac hypertrophy in canine volume overload. American Journal of Physiology. 1998;275(1 Pt 2):H65–H74. [PubMed: 9688897]
- Mazzatenta A, Carluccio A, Robbe D, Giulio CD, Cellerino A. The companion dog as a unique translational model for aging. Seminars in Cell and Developmental Biology. 2017;70:141–153. [PubMed: 28803893]
- Melby SJ, Lee AM, Zierer A, Kaiser SP, Livhits MJ, Boineau JP, Schuessler RB, Damiano RJ Jr. Atrial fibrillation propagates through gaps in ablation lines: Implications for ablative treatment of atrial fibrillation. Heart Rhythm. 2008;5(9):1296–1301. [PMC free article: PMC2923579] [PubMed: 18774106]
- Mendis S, Puska P, Norrving B, editors. Global atlas on cardiovascular disease prevention and control. Geneva, Switzerland: World Health Organization; 2011.
- Merkely B, Molnar L, Geller L, Neuzil P, Skoda J, Bednarek J, Bartus K, Reddy VY, Sanders WE Jr. Chronic implantation of intravascular cardioverter defibrillator in a canine model: Device stability, vascular patency, and anchor histology. Pacing and Clinical Electrophysiology. 2013;36(10):1251–1258. [PubMed: 23952482]
- Meurs KM. Arrhythmogenic right ventricular cardiomyopathy in the boxer dog: An update. Veterinary Clinics of North America: Small Animal Practice. 2017;47(5):1103–1111. [PubMed: 28647112]
- Meurs KM, Lahmers S, Keene BW, White SN, Oyama MA, Mauceli E, Lindblad-Toh K. A splice site mutation in a gene encoding for PDK4, a mitochondrial protein, is associated with the development of dilated cardiomyopathy in the Doberman pinscher. Human Genetics. 2012;131(8):1319–1325. [PubMed: 22447147]
- Mieda M. The roles of orexins in sleep/wake regulation. Neuroscience Research. 2017;118:56–65. [PubMed: 28526554]
- Mignot EJM. Genetica of narcolepsy and other sleep disorders. American Journal of Human Genetics. 1997;60(6):1289–1302. [PMC free article: PMC1716121] [PubMed: 9199548]
- Mignot EJM. History of narcolepsy at Stanford University. Immunologic Research. 2014;58(2-3):315–339. [PMC free article: PMC4028550] [PubMed: 24825774]
- Millon A, Canet-Soulas E, Boussel L, Fayad Z, Douek P. Animal models of atherosclerosis and magnetic resonance imaging for monitoring plaque progression. Vascular. 2014;22(3):221–237. [PubMed: 24907292]
- Mody RM, Lakhal-Naouar I, Sherwood JE, Koles NL, Shaw D, Bigley DP, Co E-MA, Copeland NK, Jagodzinski LL, Mukbel RM, Smiley RA, Duncan RC, Kamhawi S, Jeronimo SMB, DeFraites RF, Aronson NE. Asymptomatic visceral Leishmania infantum infection in U.S. soldiers deployed to Iraq. Clinical Infectious Diseases. 2019;68(12):2036–2044. [PMC free article: PMC6769235] [PubMed: 30239631]
- Moore S. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs Public Workshop on the Uses of Dogs in Biomedical Research, March 27. Washington, DC: 2019.
- Moore SA, Granger N, Olby NJ, Spitzbarth I, Jeffery ND, Tipold A, Nout-Lomas Y S, da Costa RC, Stein VM, Noble-Haeusslein LJ, Blight AR, Grossman RG, Basso DM, Levine JM. Targeting translational successes through CANSORT-SCI: Using pet dogs to identify effective treatments for spinal cord injury. Journal of Neurotrauma. 2017;34(12):2007–2018. [PMC free article: PMC5467140] [PubMed: 28230415]
- Moore SA, Zidan N, Spitzbarth I, Nout-Lomas YS, Granger N, da Costa RC, Levine JM, Jeffery ND, Stein VM, Tipold A, Olby NJ. Development of an International Canine Spinal Cord Injury observational registry: A collaborative data-sharing network to optimize translational studies of SCI. Spinal Cord. 2018;56(7):656–665. [PMC free article: PMC6035082] [PubMed: 29795173]
- Mori K. Double cisterna magna blood injection model of experimental subarachnoid hemorrhage in dogs. Translational Stroke Research. 2014;5(6):647–652. [PubMed: 24986149]
- Myers DD. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs Public Workshop on the Uses of Dogs in Biomedical Research, March 27. Washington, DC: 2019.
- *Nagatsu M, Zile MR, Tsutsui H, Schmid PG, DeFreyte G, Cooper GT, Carabello BA. Native beta-adrenergic support for left ventricular dysfunction in experimental mitral regurgitation normalizes indexes of pump and contractile function. Circulation. 1994;89(2):818–826. This study included research performed at the Ralph H. Johnson VA Medical Center in Charleston, SC. [PubMed: 8313571]
- Nagatsu M, Spinale G, Koide M, Tagawa H, DeFreitas G, Cooper GT, Carabello BA. Bradycardia and the role of beta-blockade in the amelioration of left ventricular dysfunction. Circulation. 2000;101(6):653–659. [PubMed: 10673258]
- Nakagawa H, Wittkampf FH, Yamanashi WS, Pitha JV, Imai S, Campbell B, Arruda M, Lazzara R, Jackman WM. Inverse relationship between electrode size and lesion size during radiofrequency ablation with active electrode cooling. Circulation. 1998;98(5):458–465. [PubMed: 9714097]
- Nakatani Y, Nishida K, Sakabe M, Kataoka N, Sakamoto T, Yamaguchi Y, Iwamoto J, Mizumaki K, Fujiki A, Inoue H. Tranilast prevents atrial remodeling and development of atrial fibrillation in a canine model of atrial tachycardia and left ventricular dysfunction. Journal of the American College of Cardiology. 2013;61(5):582–588. [PubMed: 23273396]
- NASEM (National Academies of Sciences, Engineering, and Medicine). The role of clinical studies for pets with naturally occurring tumors in translational cancer research: Workshop summary. Washington, DC: The National Academies Press; 2015. [PubMed: 26803853]
- Nasi-Er BG, Lou X, Zhang Y, Sun H, Zhou X, Li Y, Zhou Q, Zhang J, Tang B, Lu Y. Renal sympathetic denervation improves outcomes in a canine myocardial infarction model. Medical Science Monitor. 2019a;25:3887–3893. [PMC free article: PMC6556070] [PubMed: 31127792]
- Nasi-Er BG, Wenhui Z, HuaXin S, Xianhui Z, Yaodong L, Yanmei L, Hongli W, TuEr-Hong ZL, Qina Z, BaoPeng T. Vagus nerve stimulation reduces ventricular arrhythmias and increases ventricular electrical stability. Pacing and Clinical Electrophysiology. 2019b;42(2):247–256. [PubMed: 30552763]
- NCHS (National Center for Health Statistics). Leading causes of death. 2017. [March 17, 2020]. https://www
.cdc.gov/nchs /fastats/leadingcauses-of-death.htm. - NCI (National Cancer Institute). Cancer statistics. n.d. [August 5, 2019]. https://www
.cancer.gov /about-cancer/understanding/statistics. - NCI CCR (Center for Cancer Research). Comparative oncology program. n.d. [March 2, 2020]. https://ccr
.cancer.gov /Comparative-Oncology-Program. - Nemoto S, Hamawaki M, De Freitas G, Carabello BA. Differential effects of the angiotensin-converting enzyme inhibitor lisinopril versus the beta-adrenergic receptor blocker atenolol on hemodynamics and left ventricular contractile function in experimental mitral regurgitation. Journal of the American College of Cardiology. 2002;40(1):149–154. [PubMed: 12103269]
- Neubauer DN. Almorexant, a dual orexin receptor antagonist for the treatment of insomnia. Current Opinion in Investigational Drugs (London, England: 2000). 2010;11(1):101–110. [PubMed: 20047164]
- Neumann U, Ufer M, Jacobson LH, Rouzade-Dominguez ML, Huledal G, Kolly C, Lüönd RM, Machauer R, Veenstra SJ, Hurth K, Rueeger H, Tintelnot-Blomley M, Staufenbiel M, Shimshek DR, Perrot L, Frieauff W, Dubost V, Schiller H, Vogg B, Beltz K, Avrameas A, Kretz S, Pezous N, Rondeau J-M, Beckmann N, Hartmann A, Vormfelde S, David OJ, Galli B, Ramos R, Graf A, Lopez CL. The BACE-1 inhibitor CNP520 for prevention trials in Alzheimer's disease. EMBO Molecular Medicine. 2018;10(11):e9316. [PMC free article: PMC6220303] [PubMed: 30224383]
- NHGRI (National Human Genome Research Institute). The NHGRI Dog Genome Project. 2019. [February 28, 2020]. https://research
.nhgri .nih.gov/dog_genome. - Nishida K, Maguy A, Sakabe M, Comtois P, Inoue H, Nattel S. The role of pulmonary veins vs. autonomic ganglia in different experimental substrates of canine atrial fibrillation. Cardiovascular Research. 2011;89(4):825–833. [PubMed: 20962102]
- NRC (National Research Council). Guide for the care and use of laboratory animals, 8th edition. Washington, DC: The National Academies Press; 2011. [PubMed: 21595115]
- NSCISC (National Spinal Cord Injury Statistical Center). Facts and figures at a glance. Birmingham, AL: University of Alabama at Birmingham; 2019. [January 16, 2020]. https://www
.nscisc.uab .edu/Public/Facts%20and %20Figures%202019%20-%20Final.pdf. - O'Kell AL, Wasserfall C, Catchpole B, Davison LJ, Hess RS, Kushner JA, Atkinson MA. Comparative pathogenesis of autoimmune diabetes in humans, NOD mice, and canines: Has a valuable animal model of type 1 diabetes been overlooked? Diabetes. 2017;66:1443–1452. [PMC free article: PMC5440022] [PubMed: 28533295]
- Ono N, Yamaguchi T, Ishikawa H, Arakawa M, Takahashi N, Saikawa T, Shimada T. Morphological varieties of the Purkinje fiber network in mammalian hearts, as revealed by light and electron microscopy. Archives of Histology and Cytology. 2009;72(3):139–149. [PubMed: 20513977]
- *Otterson MF, Leming SC, Fox CJ, Moulder JE. Propagation of giant migrating contractions between the small intestine, cecum and colon during radiation. Neurogasteroenterology and Motility. 2010;22(8):919–926. VA research was conducted at the Clement J. Zablocki VA Medical Center in Milwaukee, WI. [PMC free article: PMC4520439] [PubMed: 20529206]
- Paciello O, Oliva G, Gradoni L, Manna L, Foglia Manzillo V, Wojcik S, Trapani F, Papparella S. Canine inflammatory myopathy associated with Leishmania infantum infection. Neuromuscular Disorders. 2009;19(2):124–130. [PubMed: 19084398]
- Panfilov AV. Is heart size a factor in ventricular fibrillation? Or how close are rabbit and human hearts? Heart Rhythm. 2006;3(7):862–864. [PubMed: 16818223]
- *Pang Y, Wang X, Ucuzian AA, Brey EM, Burgess WH, Jones KJ, Alexander TD, Greisler HP. Local delivery of a collagen-binding FGF-1 chimera to smooth muscle cells in collagen scaffolds for vascular tissue engineering. Biomaterials. 2010;31(5):878–885. VA research was conducted at the Edward J. Hines Jr. VA Hospital in Hines, IL. [PMC free article: PMC2789982] [PubMed: 19853908]
- Paoloni MC, Khanna C. Comparative oncology today. Veterinary Clinics of North America: Small Animal Practice. 2007;37(6):1023–1032. [PMC free article: PMC2174910] [PubMed: 17950880]
- Paoloni M, Webb C, Mazcko C, Cherba D, Hendricks W, Lana S, Ehrhart EJ, Charles B, Fehling H, Kumar L, Vail D, Henson M, Childress M, Kitchell B, Kingsley C, Kim S, Neff M, Davis B, Khanna C, Trent J. Prospective molecular profiling of canine cancers provides a clinically relevant comparative model for evaluating personalized medicine (PMed) trials. PLOS ONE. 2014;9(3):e90028. [PMC free article: PMC3956546] [PubMed: 24637659]
- Paul A, Shao W, Shum-Tim D, Prakash S. The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNA(Ang1+Vegf) nanoparticles. Biomaterials. 2012;33(30):7655–7664. [PubMed: 22818986]
- Paul A, Elias CB, Shum-Tim D, Prakash S. Bioactive baculovirus nanohybrids for stent based rapid vascular re-endothelialization. Science Reports. 2013;3:2366. [PMC free article: PMC3734445] [PubMed: 23917680]
- Pedrosa S, Aguado D, Canfrán S, Torres J, Miró J. Dog-assisted therapy in the treatment of people with chronic pain: A systematic review. Revista de la Sociedad Española del Dolor. 2017;24(1):11–18.
- Perez TD, Figueiredo FB, Velho Junior AAM, Silva VL, de M, Madeira F, Brazil RP, Coura JR. Prevalence of American trypanosomiasis and leishmaniases in domestic dogs in a rural area of the municipality of São João Do Piauí, Piauí State, Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo. 2016;58:79. [PMC free article: PMC5096633] [PubMed: 27828620]
- Petersen C. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs Webinar, May 7. 2019.
- Piktel JS, Wilson LD. Translational models of arrhythmia mechanisms and susceptibility: Success and challenges of modeling human disease. Frontiers in Cardiovascular Medicine. 2019;6:135. [PMC free article: PMC6748164] [PubMed: 31552276]
- Pleticha J, Malkmus SA, Heilmann LF, Veesart SL, Rezek R, Xu Q, Yaksh TL, Beutler AS. High cerebrospinal fluid levels of interleukin-10 attained by AAV in dogs. Gene Therapy. 2015;22(2):202–208. [PMC free article: PMC5079622] [PubMed: 25354684]
- Pomba C, Rantala M, Greko C, Baptiste KE, Catry B, van Duijkeren E, Mateus A, Moreno MA, Pyörälä S, Ružauskas M, Sanders P, Teale C, Threlfall EJ, Kunsagi Z, Torren-Edo J, Jukes H, Törneke K. Public health risk of antimicrobial resistance transfer from companion animals. Journal of Antimicrobial Chemotherapy. 2017;72(4):957–968. [PubMed: 27999066]
- Prier JE, Brodey RS. Canine neoplasia: A prototype for human cancer study. Bulletin of the World Health Organization. 1963;29:331–344. [PMC free article: PMC2554964] [PubMed: 14058226]
- *Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EAE, Hopp FA, Dean C, Zuperku EJ. Pontine μ-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. Journal of Neurophysiology. 2012;108:2430–2441. VA research was conducted at the Clement J. Zablocki VA Medical Center in Milwaukee, WI. [PMC free article: PMC3545180] [PubMed: 22875901]
- Qi XY, Diness JG, Brundel BJ, Zhou XB, Naud P, Wu CT, Huang H, Harada M, Aflaki M, Dobrev D, Grunnet M, Nattel S. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation. 2014;129(4):430–440. [PubMed: 24190961]
- Ramoni R. VA Office of Research and Development: Organization, mission, and funding process. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs meeting open session, March 28. Washington, DC: 2019. [June 9, 2020]. https://www
.nationalacademies .org/event/03-27-2019 /assessment-of-the-care-and-use-of-dogs-in-biomedical-research-funded-by-or-conducted-at-the-us-department-of-veterans-affairs---public-workshop-on-the-uses-of-dogs-in-biomedical-research---public-workshop. - Regier Y, O'Rourke F, Kempf VAJ. Bartonella spp.—A chance to establish One Health concepts in veterinary and human medicine. Parasites and Vectors. 2016;9(1):261. [PMC free article: PMC4862191] [PubMed: 27161111]
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: A systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel. Journal of the American Academy of Dermatology. 2018;78(6):1185–1192. [PMC free article: PMC7262574] [PubMed: 29291955]
- Ripley B, Fujiki N, Okura M, Mignot E, Nishino S. Hypocretin levels in sporadic and familial cases of canine narcolepsy. Neurobiology of Disease. 2001;8(3):525–534. [PubMed: 11442359]
- Ross J Jr, Franklin D. Analysis of regional myocardial function, dimensions, and wall thickness in the characterization of myocardial ischemia and infarction. Circulation. 1976;53(3 Suppl):I88–I92. [PubMed: 1253375]
- *Ruaengsri C, Schill MR, Lancaster TS, Khiabani AJ, Manghelli JL, Carter DI, Greenberg JW, Melby SJ, Schuessler RB, Damiano RJ Jr. The hemodynamic and atrial electrophysiologic consequences of chronic left atrial volume overload in a controllable canine model. Journal of Thoracic and Cardiovascular Surgery. 2018;156(5):1871–1879. A co-author held an appointment at the VA St. Louis Health Care System. The VA is continuing to fund this work. [PMC free article: PMC6935371] [PubMed: 30336917]
- Rusbridge C, Salguero FJ, David MA, Faller KME, Bras JT, Guerreiro RJ, Richard-Londt AC, Grainger D, Head E, Brandner SGP, Summers B, Hardy J, Tayebi M. An aged canid with behavioral deficits exhibits blood and cerebrospinal fluid amyloid beta oligomers. Frontiers in Aging Neuroscience. 2018;10:7. [PMC free article: PMC5797595] [PubMed: 29441010]
- Sabbah HN, Wang M, Gupta RC, Rastogi S, Ilsar I, Viole T, Brewer R. Acute left ventricular unloading in dogs with chronic heart failure: Continuous aortic flow augmentation versus intra-aortic balloon pumping. Journal of Cardiac Failure. 2009;15(6):523–528. [PubMed: 19643364]
- Sakabe M, Fujiki A, Sakamoto T, Nakatani Y, Mizumaki K, Inoue H. Xanthine oxidase inhibition prevents atrial fibrillation in a canine model of atrial pacing-induced left ventricular dysfunction. Journal of Cardiovascular Electrophysiology. 2012;23(10):1130–1135. [PubMed: 22587612]
- Saku K, Kakino T, Arimura T, Sakamoto T, Nishikawa T, Sakamoto K, Ikeda M, Kishi T, Ide T, Sunagawa K. Total mechanical unloading minimizes metabolic demand of left ventricle and dramatically reduces infarct size in myocardial infarction. PLOS ONE. 2016;11(4):e0152911. [PMC free article: PMC4849631] [PubMed: 27124411]
- Sakurai T. Orexin deficiency and narcolepsy. Current Opinion in Neurobiology. 2013;23(5):760–766. [PubMed: 23663890]
- Sakurai T, Mieda M. Connectomics of orexin-producing neurons: Interface of systems of emotion, energy homeostasis, and arousal. Trends in Pharmacological Sciences. 2011;32(8):451–462. [PubMed: 21565412]
- Salcman M, Scott EW, Schepp RS, Knipp HC, Broadwell RD. Transplantable canine glioma model for use in experimental neuro-oncology. Neurosurgery. 1982;11(3):372–381. [PubMed: 6290929]
- Sanders WE Jr, Richey MW, Malkin RA, Masson SC, Ransbury TJ, Urtz MW, Ideker RE. Novel intravascular defibrillator: Defibrillation thresholds of intravascular cardioverter-defibrillator compared to conventional implantable cardioverter-defibrillator in a canine model. Heart Rhythm. 2011;8(2):288–292. [PubMed: 21034853]
- Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, Jackman WM. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000;102(22):2774–2780. [PubMed: 11094046]
- Schol J, Sakai D. Cell therapy for intervertebral disk herniation and degenerative disc disease: Clinical trials. International Orthopaedics. 2019;43:1011–1025. [PubMed: 30498909]
- Schwartz RS, Holmes DR, Van Tassel RA, Hauser R, Henry TD, Mooney M, Matthews R, Doshi S, Jones RM, Virmani R. Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC: Cardiovascular Interventions. 2010;3(8):870–877. [PubMed: 20723861]
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. International Journal of Molecular Sciences. 2017;18(6):1296. [PMC free article: PMC5486117] [PubMed: 28629171]
- Shah K, Drury T, Roic I, Hansen P, Malin M, Boyd R, Sumer H, Ferguson R. Outcome of allogeneic adult stem cell therapy in dogs suffering from osteoarthritis and other joint defects. Stem Cells International. 2018;2018:7309201. [PMC free article: PMC6046133] [PubMed: 30050578]
- Sharif-Alhoseini M, Khormali M, Rezaei M, Safdarian M, Hajighadery A, Khalatbari MM, Safdarian M, Meknatkhah S, Rezvan M, Chalangari M, Derakhshan P, Rahimi-Movaghar V. Animal models of spinal cord injury: A systematic review. Spinal Cord. 2017;55(8):714–721. [PubMed: 28117332]
- Shorofsky M, Maguy A, Nattel S. Consequences of atrial or ventricular tachypacing on the heat shock proteins (HSP) level of expression and phosphorylation. McGill Journal of Medicine. 2009;12(2):34. [PMC free article: PMC2997257] [PubMed: 21264054]
- Shumway NE, Lower RR, Hurley EJ, Dong E Jr, Stofer RC. Congenital heart disease. Changing concepts in the surgical treatment. California Medicine. 1962;97:148–151. [PMC free article: PMC1575247] [PubMed: 13912411]
- Sigwart U. Living history of medicine: Vascular scaffolding, from dream to reality. European Heart Journal. 2017;38(16):1245–1248. [PubMed: 26792876]
- Simpson S, Edwards J, Ferguson-Mignan TF, Cobb M, Mongan NP, Rutland CS. Genetics of human and canine dilated cardiomyopathy. International Journal of Genomics. 2015;2015:204823. [PMC free article: PMC4525455] [PubMed: 26266250]
- Simpson S, Dunning MD, Brownlie S, Patel J, Godden M, Cobb M, Mongan NP, Rutland CS. Multiple genetic associations with Irish wolfhound dilated cardiomyopathy. Biomedical Research International. 2016;2016:6374082. [PMC free article: PMC5187458] [PubMed: 28070514]
- Smolek T, Madari A, Farbakova J, Kandrac O, Jadhav S, Cente M, Brezovakova V, Novak M, Zilka N. Tau hyperphosphorylation in synaptosomes and neuroinflammation are associated with canine cognitive impairment. Journal of Comparative Neurology. 2016;524(4):874–895. [PubMed: 26239295]
- *Soon-Shiong P, Feldman E, Nelson R, Komtebedde J, Smidsrod O, Skjak-Braek G, Espevik T, Heintz R, Lee M. Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets. Transplantation. 1992;54(5):769–774. VA research was performed at the VA Greater Los Angeles Healthcare System. [PubMed: 1440841]
- *Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surgery, Gynecology, and Obstetrics. 1963;117:385–395. VA research was conducted at the Rocky Mountain Regional VA Medical Center in Aurora, CO and the VA Pittsburgh Healthcare Center in Pittsburgh, PA. [PMC free article: PMC2581919] [PubMed: 14065716]
- *Sun Y, Song G-Q, Yin J, Lei Y, Chen JDZ. Effects and mechanisms of gastrointestinal electrical stimulation on slow waves: A systematic canine study. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2009;297(5):R1392–R1399. VA research was conducted at the VA Medical Center in Oklahoma City, OK. [PMC free article: PMC2777781] [PubMed: 19710395]
- Sunagawa G, Karimov JH, Breitbach M, Robinson NA, Fukamachi K. Impact of a refined advanced design for left atrial appendage exclusion. European Journal of Cardiothoracic Surgery. 2017;52(6):1098–1103. [PubMed: 28633397]
- Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: Implications for atherogenesis. Arteriosclerosis Thrombosis and Vascular Biology. 2007;27(2):346–351. [PubMed: 17122449]
- Tafti M, Nishino S, Aldrich MS, Liao W, Dement WC, Mignot E. Major histocompatibility class II molecules in the CNS: Increased microglial expression at the onset of narcolepsy in a canine model. Journal of Neuroscience. 1996;16(15):4588–4595. [PMC free article: PMC6579009] [PubMed: 8764647]
- Takaseya T, Fumoto H, Saraiva RM, Shiose A, Arakawa Y, Park M, Rao S, Dessoffy R, Kramer LD Jr, Juravic M, Lombardi P, Fukamachi K. Acute feasibility study of a novel device for the treatment of mitral regurgitation in a normal canine model. Innovations (Phila). 2010;5(1):28–32. [PubMed: 22437273]
- Tan AY, Hu YL, Potfay J, Kaszala K, Howren M, Sima AP, Shultz M, Koneru JN, Ellenbogen KA, Huizar JF. Impact of ventricular ectopic burden in a premature ventricular contraction-induced cardiomyopathy animal model. Heart Rhythm. 2016;13(3):755–761. [PMC free article: PMC4762722] [PubMed: 26586453]
- Thannickal TC, John J, Shan L, Swaab DF, Wu M-F, Ramanathan L, McGregor R, Chew K-T, Cornford M, Yamanaka A, Inutsuka A, Fronczek R, Lammers GJ, Worley PF, Siegel JM. Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Science Translational Medicine. 2018;10:eaao4953. [PMC free article: PMC8235614] [PubMed: 29950444]
- Toepp AJ, Bennett C, Scott B, Senesac R, Oleson JJ, Petersen CA. Maternal Leishmania infantum infection status has significant impact on leishmaniasis in offspring. PLOS Neglected Tropical Diseases. 2019;13(2):e0007058. [PMC free article: PMC6391032] [PubMed: 30759078]
- Toledo-Pereyra LH. Heart transplantation. Journal of Investigative Surgery. 2010;23(1):1–5. [PubMed: 20232998]
- Triani F, Tramutola A, Di Domenico F, Sharma N, Butterfield DA, Head E, Perluigi M, Barone E. Biliverdin reductase-A impairment links brain insulin resistance with increased Aβ production in an animal model of aging: Implications for Alzheimer disease. Biochimica et Biophysica Acta. Molecular Basis of Disease. 2018;1864(10):3181–3194. [PubMed: 29981845]
- Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Frontiers in Behavioral Neuroscience. 2013;7:28. [PMC free article: PMC3629303] [PubMed: 23616752]
- Tsutsui H, Spinale FG, Nagatsu M, Schmid PG, Ishihara K, DeFreyte G, Cooper G, Carabello BA. Effects of chronic beta-adrenergic blockade on the left ventricular and cardiocyte abnormalities of chronic canine mitral regurgitation. Journal of Clinical Investigation. 1994;93(6):2639–2648. [PMC free article: PMC294505] [PubMed: 7911128]
- Uminski K, Kadkhoda K, Houston BL, Lopez A, MacKenzie LJ, Lindsay R, Walkty A, Embil J, Zarychanski R. Anaplasmosis: An emerging tick-borne disease of importance in Canada. IDCases. 2018;14:e00472. [PMC free article: PMC6278667] [PubMed: 30524954]
- VA (U.S. Department of Veterans Affairs). Health services research and development: Cancer. 2017. [March 17, 2020]. https://www
.research .va.gov/topics/cancer.cfm. - VA. Appendix 8: Current VA dog projects. Submitted to the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs on November 28, 2018. 2018a. [March 2, 2020]. https://www
.research .va.gov/programs/animal_research /canine_research /active_projects.cfm. - VA. Appendix 6: Sample accomplishments of VA research with dogs, 1960-current decade. Submitted to the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs on November 28, 2018. The list of 30 past research studies is available at. 2018b. [February 11, 2020]. https://www
.research .va.gov/programs/animal_research /canine_research /accomplishments.cfm. - VA. U.S. Department of Veterans Affairs FY 2018-2024 strategic plan. 2019. [February 27, 2020]. https://www
.va.gov/oei /docs/VA20182024strategicPlan.pdf. - VA. Public health: Infectious diseases. 2020. [March 2, 2020]. https://www
.publichealth .va.gov/exposures /infectious-diseases. - VA ORD (Office of Research and Development). VA canine documents submitted to the National Academies of Sciences, Engineering, and Medicine. 2018. [November 19, 2019]. https://www
.research .va.gov/programs/animal_research /canine_research /VA-CanineResearch-NAS.pdf. - VA ORD. VA research on cardiovascular disease. n.d.a. [February 12, 2020]. https://www
.research .va.gov/topics/cardio.cfm. - VA ORD. VA research on spinal cord injury. n.d.b. [October 9, 2019]. https://www
.research .va.gov/topics/sci.cfm. - VA ORD. VA research on diabetes. n.d.c. [November 14, 2019]. https://www
.research .va.gov/topics/diabetes.cfm. - VA ORD. Overview of VA research on Alzheimer's disease. n.d.d. [November 19, 2019]. https://www
.research .va.gov/topics/alzheimers.cfm. - Vainio O. Translational animal models using veterinary patients—An example of canine osteoarthritis (OA). Scandinavian Journal of Pain. 2012;3(2):84–89. [PubMed: 29913782]
- Van Dam D, De Deyn PP. Animal models in the drug discovery pipeline for Alzheimer's disease. British Journal of Pharmacology. 2011;164(4):1285–1300. [PMC free article: PMC3229762] [PubMed: 21371009]
- van der Linde HJ, Van Deuren B, Somers Y, Loenders B, Towart R, Gallacher DJ. The electro-mechanical window: A risk marker for Torsade de Pointes in a canine model of drug induced arrhythmias. British Journal of Pharmacology. 2010;161(7):1444–1454. [PMC free article: PMC3010558] [PubMed: 21054337]
- Vargas HM. A new preclinical biomarker for risk of Torsades de Pointes: Drug-induced reduction of the cardiac electromechanical window. British Journal of Pharmacology. 2010;161(7):1441–1443. [PMC free article: PMC3010557] [PubMed: 20698854]
- Vasilevko V, Head E. Immunotherapy in a natural model of A pathogenesis: The aging beagle. CNS & Neurological Disorders—Drug Targets. 2009;8(2):98–113. [PubMed: 19355931]
- VHA ORD (Veterans Health Administration Office of Research and Development). Guidance document AR2017-001 (rev3): Canine, feline and non-human primate research protocols. 2017. [February 12, 2020]. https://www
.research .va.gov/programs/animal_research /CanineFelineNHP.pdf. - Vilahur G, Padro T, Badimon L. Atherosclerosis and thrombosis: Insights from large animal models. Journal of Biomedicine and Biotechnology. 2011;2011:907575. [PMC free article: PMC3022266] [PubMed: 21274431]
- Vischer AS, Connolly DJ, Coats CJ, Fuentes VL, McKenna WJ, Castelletti S, Pantazis AA. Arrhythmogenic right ventricular cardiomyopathy in boxer dogs: The diagnosis as a link to the human disease. Acta Myologica. 2017;36(3):135–150. [PMC free article: PMC5953225] [PubMed: 29774304]
- Voorhees IEH, Dalziel BD, Glaser A, Dubovi EJ, Murcia PR, Newbury S, Toohey-Kurth K, Su S, Kriti D, Van Bakel H, Goodman LB, Leutenegger C, Holmes EC, Parrish CR. Multiple incursions and recurrent epidemic fade-out of H3N2 canine influenza A virus in the United States. Journal of Virology. 2018;92(16):e00323–18. [PMC free article: PMC6069211] [PubMed: 29875234]
- Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, Rowald A, Seáñez I, Caban M, Pirondini E, Vat M, McCracken LA, Heimgartner R, Fodor I, Watrin A, Seguin P, Paoles E, Van Den Keybus K, Eberle G, Schurch B, Pralong E, Becce F, Prior J, Buse N, Buschman R, Neufeld E, Kuster N, Carda S, von Zitzewitz J, Delattre V, Denison T, Lambert H, Minassian K, Bloch J, Courtine G. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563(7729):65–71. [PubMed: 30382197]
- Wakili R, Yeh YH, Qi XY, Greiser M, Chartier D, Nishida K, Maguy A, Villeneuve LR, Boknik P, Voigt N, Krysiak J, Kaab S, Ravens U, Linke WA, Stienen GJ, Shi Y, Tardif JC, Schotten U, Dobrev D, Nattel S. Multiple potential molecular contributors to atrial hypocontractility caused by atrial tachycardia remodeling in dogs. Circulatoin: Arrhythmia and Electrophysiology. 2010;3(5):530–541. [PubMed: 20660541]
- *Walter JS, Dunn RB, Wurster RD, Laghi F. Microstimulators and intramuscular hook electrodes for the stimulation of respiratory muscles. Journal of Spinal Cord Medicine. 2007;30:338–345. VA research was performed at the Edward J. Hines Jr. VA Hospital in Hines, IL. [PMC free article: PMC2031938] [PubMed: 17853655]
- Walton MB, Cowderoy E, Lascelles D, Innes JF. Evaluation of construct and criterion validity for the Liverpool Osteoarthritis in Dogs (LOAD) clinical metrology instrument and comparison to two other instruments. PLOS ONE. 2013;8(3):e58125. [PMC free article: PMC3591443] [PubMed: 23505459]
- Wang S, Zhou X, Huang B, Wang Z, Liao K, Saren G, Lu Z, Chen M, Yu L, Jiang H. Spinal cord stimulation protects against ventricular arrhythmias by suppressing left stellate ganglion neural activity in an acute myocardial infarction canine model. Heart Rhythm. 2015;12(7):1628–1635. [PubMed: 25778432]
- Wang X, Li G. Angiotensin-(1-7) prevent atrial tachycardia induced sodium channel remodeling. Pacing and Clinical Electrophysiology. 2014;37(10):1349–1356. [PubMed: 24861429]
- Wang Y, Eltit JM, Kaszala K, Tan A, Jiang M, Zhang M, Tseng GN, Huizar JF. Cellular mechanism of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2014;11(11):2064–2072. [PMC free article: PMC4252777] [PubMed: 25046857]
- Warren PM, Alilain WJ. Plasticity induced recovery of breathing occurs at chronic stages after cervical contusion. Journal of Neurotrauma. 2019;36(12):1985–1999. [PMC free article: PMC6599385] [PubMed: 30565484]
- Warren PM, Steiger SC, Dick TE, MacFarlane PM, Alilain WJ, Silver J. Rapid and robust restoration of breathing long after spinal cord injury. Nature Communications. 2018;9(1):4843. [PMC free article: PMC6258702] [PubMed: 30482901]
- Watanabe Y, Miyagawa S, Fukushima S, Daimon T, Shirakawa Y, Kuratani T, Sawa Y. Development of a prostacyclin-agonist-eluting aortic stent graft enhancing biological attachment to the aortic wall. Journal of Thoracic and Cardiovascular Surgery. 2014;148(5):2325–2334. [PubMed: 25224552]
- Watkins L Jr, Burton JA, Haber E, Cant JR, Smith FW, Barger AC. The rennin-angiotensin-aldosterone system in congestive failure in conscious dogs. Journal of Clinical Investigations. 1976;57(6):1606–1617. [PMC free article: PMC436820] [PubMed: 180056]
- Weaver ME, Pantely GA, Bristow JD, Ladley HD. A quantitative study of the anatomy and distribution of coronary arteries in swine in comparison with other animals and man. Cardiovascular Research. 1986;20(12):907–917. [PubMed: 3802126]
- Wei J, Zhang Y, Li Z, Wang X, Chen L, Du J, Liu J, Liu J, Hou Y. GCH1 attenuates cardiac autonomic nervous remodeling in canines with atrial-tachypacing via tetrahydrobiopterin pathway regulated by microRNA-206. Pacing and Clinical Electrophysiology. 2018;41(5):459–471. [PubMed: 29436714]
- Weina PJ, Neafie RC, Wortmann G, Polhemus M, Aronson NE. Old world leishmaniasis: An emerging infection among deployed U.S. military and civilian workers. Clinical Infectious Diseases. 2004;39(11):1674–1680. [PubMed: 15578370]
- White R. Presentation at the Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs Public Workshop on the Uses of Dogs in Biomedical Research, March 27. Washington, DC: 2019.
- White RA, Fogarty TJ, Kopchok GE, Wilson E, Ayres B, Zalewski M, Donayre CE. Evaluation of a modular endovascular bifurcated prosthesis in a canine aortic aneurysm model. Journal of Vascular Surgery. 1996;24:1034–1042. [PubMed: 8976358]
- Wiersma AC, Stabej P, Leegwater PA, Van Oost BA, Ollier WE, Dukes-McEwan J. Evaluation of 15 candidate genes for dilated cardiomyopathy in the Newfoundland dog. Journal of Heredity. 2008;99(1):73–80. [PubMed: 17998275]
- Wiersma M, Meijering RAM, Qi XY, Zhang D, Liu T, Hoogstra-Berends F, Sibon OCM, Henning RH, Nattel S, Brundel B. Endoplasmic reticulum stress is associated with autophagy and cardiomyocyte remodeling in experimental and human atrial fibrillation. Journal of the American Heart Association. 2017;6(10):e006458. [PMC free article: PMC5721854] [PubMed: 29066441]
- Wieschowski S, Silva DS, Strech D. Animal study registries: Results from a stakeholder analysis on potential strengths, weaknesses, facilitators, and barriers. PLOS Biology. 2016;14(11):e2000391. [PMC free article: PMC5104355] [PubMed: 27832101]
- Wiese AJ, Rathbun M, Butt MT, Malkmus SA, Richter PJ, Osborn KG, Xu Q, Veesart SL, Steinauer JJ, Higgins D, Lappi DA, Russell B, Yaksh TL. Intrathecal substance P-saporin in the dog: Distribution, safety, and spinal neurokinin-1 receptor ablation. Anesthesiology. 2013;119(5):1163–1177. [PubMed: 24051388]
- Wilson EP, White RA, Kopchok GE, Donayre CE, deVirgilio C, Geselschap J. Deployment and healing of an e-PTFE encapsulated stent endograft in the canine aorta. Annals of Vascular Surgery. 1997;11:354–358. [PubMed: 9236990]
- Wilson S, Nagel SJ, Frizon LA, Fredericks DC, DeVries-Watson NA, Gillies GT, Howard MA 3rd. The hemisection approach in large animal models of spinal cord injury: Overview of methods and applications. Journal of Investigative Surgery. 2018;31:1–12. [PubMed: 30380340]
- Wilson S, Fredericks DC, Safayi S, DeVries-Watson NA, Holland MT, Nagel SJ, Gillies GT, Howard MA 3rd. Ovine hemisection model of spinal cord injury. Journal of Investigative Surgery. 2019;15:1–13. [PubMed: 31304811]
- Witt CM, Dalton S, O'Neil S, Ritrivi CA, Sanders R, Sharma A, Seifert G, Berhow S, Beinborn D, Witz A, McCaw T, Scott CG, Padmanabhan D, Killu AM, Naksuk N, Asirvatham SJ, Friedman PA. Termination of atrial fibrillation with epicardial cooling in the oblique sinus. JACC: Clinical Electrophysiology. 2018;4(10):1362–1368. [PubMed: 30336883]
- Wittkampf FH, Nakagawa H, Yamanashi WS, Imai S, Jackman WM. Thermal latency in radiofrequency ablation. Circulation. 1996;93(6):1083–1086. [PubMed: 8653827]
- Yaksh TL, Hobo S, Peters C, Osborn KG, Richter PJ Jr, Rossi SS, Grafe MR, Eisenach JC. Preclinical toxicity screening of intrathecal oxytocin in rats and dogs. Anesthesiology. 2014;120(4):951–961. [PMC free article: PMC5392224] [PubMed: 24492326]
- Yamashita K, Silvernagel J, Kwan E, Kamali R, Ghafoori E, MacLeod R, Dosdall DJ, Ranjan R. Changes in atrial electrophysiological and structural substrate and their relationship to histology in a long-term chronic canine atrial fibrillation model. Pacing and Clinical Electrophysiology. 2019;42(7):930–936. [PubMed: 31127633]
- Yu CH, Song GS, Yhee JY, Kim JH, Im KS, Nho WG, Lee JH, Sur JH. Histopathological and immunohistochemical comparison of the brain of human patients with Alzheimer's disease and the brain of aged dogs with cognitive dysfunction. Journal of Comparative Pathology. 2011;145(1):45–58. [PubMed: 21256508]
- Zeira O, Scaccia S, Pettinari L, Ghezzi E, Asiag N, Martinelli L, Zahirpour D, Dumas MP, Konar M, Lupi DM, Fiette L, Pascucci L, Leonardi L, Cliff A, Alessandri G, Pessina A, Spaziante D, Aralla M. Intra-articular administration of autologous micro-fragmented adipose tissue in dogs with spontaneous osteoarthritis: Safety, feasibility, and clinical outcomes. Stem Cells Translational Medicine. 2018;7(11):819–828. [PMC free article: PMC6216453] [PubMed: 30035380]
- Zeitzer JM, Nishino S, Mignot E. The neurobiology of hypocretins (orexins), narcolepsy, and related therapeutic interventions. Trends in Pharmacological Sciences. 2006;27(7):368–374. [PubMed: 16766052]
- Zhang WH, Zhou QN, Lu YM, Li YD, Zhang L, Zhang JH, Xing Q, Lv WK, Cheng XC, Zhang GG, Wang XS, Gu Q, Lou X, Guli B, Tang BP, Zhou XH. Renal denervation reduced ventricular arrhythmia after myocardial infarction by inhibiting sympathetic activity and remodeling. Journal of the American Heart Association. 2018;7(20):e009938. [PMC free article: PMC6474949] [PubMed: 30371294]
- Zhang X, Chen Z, Sun Y, Xu M, Pan G. Influence of iliac vein stent implantation on the contralateral iliac vein. Vascular and Endovascular Surgery. 2015;49(5-6):119–123. [PubMed: 26335993]
- Zhou M, Liu Y, Xiong L, Quan D, He Y, Tang Y, Huang H, Huang C. Cardiac sympathetic afferent denervation protects against ventricular arrhythmias by modulating cardiac sympathetic nerve activity during acute myocardial infarction. Medical Science Monitor. 2019;25:1984–1993. [PMC free article: PMC6436207] [PubMed: 30877783]
- Zile MR, Tomita M, Nakano K, Mirsky I, Usher B, Lindroth J, Carabello BA. Effects of left ventricular volume overload produced by mitral regurgitation on diastolic function. American Journal of Physiology. 1991;261(5 Pt 2):H1471–H1480. [PubMed: 1951734]
- *Zuleger CL, Kang C, Ranheim EA, Kurzman ID, Macklin MD, Newton MA, Wolchok JD, Vail DM, Eriksson E, Albertini MR. Pilot study of safety and feasibility of DNA microseeding for treatment of spontaneous canine melanoma. Veterinary Medicine and Science. 2017;3(3):134–145. The VA is funding the continuation of this work at the William S. Middleton Memorial VA Medical Center in Madison, WI. [PMC free article: PMC5645840] [PubMed: 29067210]
- Zullig LL, Sims KJ, McNeil R, Williams CD, Jackson GL, Provenzale D, Kelley MJ. Cancer incidence among patients of the U.S. Veterans Affairs health care system: 2010 update. Military Medicine. 2017;182(7/8):e1883–e1891. [PMC free article: PMC5650119] [PubMed: 28810986]
Footnotes
- 1
L. B. Kinter, personal communication, November 6, 2019.
- 2
The Animal Welfare Act of 1966 (U.S. Code 7 Chapter 54: Transportation, sale, and handling of certain animals) mandated that USDA collect annual reports listing the numbers of vertebrate animals (excepting mice, rats, and birds) used by all USDA registered academic and industrial research facilities as well as federal research facilities, including the VA. Reports from individual facilities are publicly available and organized by state at https://acis
.aphis.edc .usda.gov/ords/f?p=118:205:0 (accessed December 10, 2019). - 3
L. B. Kinter, personal communication, November 6, 2019.
- 4
Text was modified after the release of the prepublication report to the sponsor to clarify that some of the criteria in Recommendation 1 are not new to the VA. The committee intends for the criteria, old and new, to be applied as a complete set.
- 5
W. Ron DeHaven (Vice Chair), Joan Hendricks, Jonathan Kimmelman, Lewis Kinter, Nancy Marks, Christian Newcomer, William Potter, David Powell, Margaret Riley, and Rodney White.
- 6
Rhonda Cornum (Chair), Donna Arnett, Warren Casey, Chris Green, and Sarah Lathrop.
- 7
Consolidated Appropriations Act of 2018, Sec. 254, p. 825 (U.S. Congress, 2018).
- 8
Id.
- 9
H.R. 1865—Further Consolidated Appropriations Act of 2020. Division F Title II Section 249. Available at https://www
.congress .gov/bill/116th-congress/house-bill/1865 (accessed March 5, 2020). - 10
Further Consolidated Appropriations Act of 2020, Sec. 249(a)(b), pp. 665–666 (U.S. Congress, 2019).
- 11
Id., p. 666.
- 12
Id.
- 13
Id.
- 14
Five committee members (Rhonda Cornum [Chair], Donna Arnett, Warren Casey, Chris Green, and Sarah Lathrop) dissent to Recommendation 2. The dissenters acknowledged the English dictionary definition of “necessary” (“required to be achieved, or essential”) as outlined in the Statement of Task to recommend to the VA when the laboratory dog was necessary in biomedical research (i.e., the dog is the only model that will yield scientific results directly related to veterans' health). This is exactly what federal law currently directs. As the Statement of Task did not request an evaluation of other animal models, the dissenters conclude the majority's Recommendation 2 strays beyond the Statement of Task. Additionally, the five committee members argue that a broader ethical framework that is responsive to the public's perception of animal research be considered, especially given that research conducted by the VA is publicly funded.
- 15
Text was modified after the release of the prepublication report to the sponsor to clarify that some of the criteria in Recommendation 2 are not new to the VA. The committee intends for the criteria, old and new, to be applied as a complete set.
- 16
Non-human primates were excluded from the committee's consideration according to the Statement of Task provided by the VA.
- 17
7 U.S. Code § 2132(g). The section goes on to clarify the Animal Welfare Act's (AWA's) protection of dogs by specifically stating that, “With respect to a dog, the term means all dogs including those used for hunting, security, or breeding purposes.” And when first proposed in the mid-1960s, the working title of the bill that eventually became the AWA was “The Dog Protection Act.”
- 18
This minority approach would closely mirror the framework proposed to the committee by animal ethicist Dr. David DeGrazia, Senior Research Fellow, Department of Bioethics, National Institutes of Health; Elton Professor of Philosophy, The George Washington University.
- TRENDS IN DOG USE IN U.S. RESEARCH FACILITIES
- LABORATORY DOG USE IN BIOMEDICAL RESEARCH AT THE VA
- NEXT STEPS FOR THE USE OF LABORATORY DOGS IN BIOMEDICAL RESEARCH RELATED TO THE VA'S MISSION
- THE VA'S BIOMEDICAL RESEARCH REVIEW PROCESS
- OPERATIONALIZING “NECESSARY” AREAS OF AGREEMENT AND DISAGREEMENT WITHIN THE COMMITTEE
- OPPORTUNITIES TO IMPROVE BIOMEDICAL RESEARCH PROTOCOLS AND REVIEW PROCESSES AT THE VA
- REFERENCES
- Determining the Necessity of Laboratory Dogs in Biomedical Research Funded by or...Determining the Necessity of Laboratory Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs - Necessity, Use, and Care of Laboratory Dogs at the U.S. Department of Veterans Affairs
Your browsing activity is empty.
Activity recording is turned off.
See more...