NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.
Thyroid Stimulating Hormone (TSH) is a heterodimeric glycoprotein hormone that regulates thyroid homeostasis upon interaction with the TSH receptor (TSHR). TSH binds to the TSH receptor, which couples preferentially to the G-alpha (s) (Gs) protein, resulting in activation of adenylate cyclase and increase in cyclic adenosine 3′, 5′ monophosphate (cAMP). In this report, we describe the discovery and SAR studies of a series of dihydroquinazolin-4-ones as TSHR agonists. ML109 (CID-25246343) is the first selective and orally available small-molecule TSHR agonist, and the probe will be a useful pharmacological tool to study TSHR biology in thyroidal and extrathyroidal tissues.
Assigned Assay Grant #: X01 MH080680
Screening Center Name & PI: NIH Chemical Genomics Center, Christopher Austin
Chemistry Center Name & PI: NIH Chemical Genomics Center, Christopher Austin
Assay Submitter & Institution: Marvin Gershengorn, NIDDK
PubChem Summary Bioassay Identifier (AID): 1401
Probe Structure & Characteristics
PubChem CID: 25246343
Probe MLSMR ID: MLS002576689
Internal ID: NCGC00161870
Chemical Formula: C31H29N3O5
Exact Mass: 523.21
Recommendations for scientific use of the probe
This probe is a member of a series of TSHR agonists. The current state of the art is lacking any small molecule TSHR agonists. This probe can be used to study TSHR functions in vitro and in vivo, and could be used as a lead for development of drugs to replace recombinant human TSH in patients with thyroid cancer. It is highly selective for human TSHR over other glycoprotein hormone receptors, such as LHCGR and FSHR, and interacts with the receptor’s serpentine domain. In primary cultures of human thyrocytes, this compound increases mRNA levels for thyroglobulin, thyroperoxidase, sodium iodide symporter, and deiodinase type 2, as well as deiodinase type2 enzyme activity. Moreover, oral administration of the agonist stimulated thyroid function in mice, resulting in increased serum thyroxine and thyroidal radioiodide uptake1. This report supplants the previous report of activity with the [2-(furan-2-ylmethylamino)-2-oxoethyl] adamantane-1-carboxylate series.
1. Introduction
Thyroid Stimulating Hormone (TSH) is an α/β heterodimeric glycoprotein hormone secreted from the anterior pituitary gland. It belongs to the glycoprotein hormone family, including Chorionic Gonadotropin (CG), Luteinizing Hormone (LH), and Follicle Stimulating Hormone (FSH)2,3. TSH binds to the TSH receptor (TSHR), which couples preferentially to the G-alpha (s) (Gs) protein, resulting in activation of adenylate cyclase and increase in cyclic adenosine 3′, 5′ monophosphate (cAMP). THSR is mainly expressed in thyroid follicular cells4 and regulates their growth and function5. Recombinant TSH is currently used for follow-up in patients with thyroid cancer who are receiving thyroid hormone suppression therapy, specifically for screening of residual tumor after surgery6, but it is difficult to produce and must be administered intramuscularly. An orally active small molecule TSHR agonist would serve as a valuable research tool for studying TSHR pharmacology and physiology, especially the extrathyroidal role of TSHR, and would have multiple advantages for therapeutic applications.
There are few literature reports of TSHR agonists (all from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) group), and most are confined to a class of thienopyrimidines with little structural diversity and no selectivity for TSHR over LHR or FSHR. The thienopyrimidine Org 41841 (Fig. 1) was first described in 2002 as an orally active LH agonist with in vivo efficacy in mice following oral administration7. It was later found to have modest TSHR activity, with an EC50 of 7700nM and 220nM for TSHR and LHCGR, respectively8. This finding was followed with a series of synthesized analogs for SAR studies. Some of the analogs achieved LHR selectivity, but none were TSHR selective9. Another TSHR agonist is from a high throughput screen at NCGC. MLS000098157 was identified as a 2μM agonist of hTSHR from the 2-(furan-2-ylmethylamino)-2-oxoethyl] adamantane-1-carboxylate series (Fig. 1). That series is supplanted by this report.

Figure 1
TSHR agonists.
2. Materials and Methods
All commercially available reagents and solvents were purchased and used without further purification. All microwave reactions were carried out in a sealed microwave vial equipped with a magnetic stir bar and heated in a Biotage Initiator Microwave Synthesizer. All compounds for biological testing were purified using a Waters semi-preparative HPLC equipped with a Phenomenex Luna® C18 reverse phase (5 micron, 30 × 75 mm) column having a flow rate of 45 ml/min. The mobile phase was a mixture of acetonitrile and H2O, each containing 0.1% trifluoroacetic acid. During purification, a gradient of 30% to 80% acetonitrile over 8 minutes was used with fraction collection triggered by UV detection (220 nM). Pure fractions passed through PL-HCO3 MP SPE (Varian) to remove trifluoroacetic acid and concentrated under vacuum on a lyophilizer. 1H spectra were recorded using an Inova 400 (100) MHz spectrometer (Varian).
2.1. Assays
i. qHTS Assay for agonists of the TSH receptor [AID-926; Primary DR]
Assay Description
TSHR is a seven-transmembrane receptor that couples to the Gs protein, resulting in activation of adenylate cyclase and increase in intracellular cAMP level. This cell-based assay utilized a cyclic nucleotide gated ion channel (CNG) as a biosensor for measurement of increase in intracellular cAMP level stimulated by TSHR agonists. The HEK293 cell line expressing human TSHR and a modified CNG was used as the primary screen assay9,10,11. The membrane potential dye was used to detect the membrane depolarization upon the activation of CNG by increased cAMP level. This assay was optimized in a homogenous 1536-well plate format.
TSHR cell line and Parental cell lines used in membrane potential assay
A HEK293 cell line stably expressing the TSHR as well as a modified CNG was purchased from BD biosciences (TSHR ACTOne cell line, Rockville, MD). When the TSHR is stimulated, intracellular cAMP level increases, which activates CNG channels to cause membrane depolarization. A fluorescent membrane potential dye (BD biosciences) was utilized to detect the membrane depolarization in this assay (Fig. 2). The cells were maintained in DMEM medium (Invitrogen) containing 10% FBS (Hyclone), 100 units/ml Penicillin, 100 ug/ml Streptomycin (Invitrogen), and 250 ug/ml Geneticin (Invitrogen) and 1 μg/ml Puromycin (Invitrogen) at 37°C in 5% CO2. A parental HEK293 cell line expressing CNG without TSHR was used in a parallel screen to eliminate compounds that activate targets other than TSHR.
Assay Protocol
Reagents used are listed in Table 1. Cells from 4 confluent T175 flasks were seeded into 25 to 30 T175 flasks at a density of 3 to 4 million cells per flask. After 3 days of growth, the flasks were harvested, and the fresh cells were used for dispensing into 1536-well plates. This process was repeated on a daily basis for screening purposes. The cells were resuspended in 2% FCS DMEM medium at 500,000 cells/ml, and 4μl of resuspended cells were dispensed into each well of black, clear bottom, 1536-well tissue culture–treated plates using a Multidrop Combi dispenser. After overnight culture at 37°C with 5% CO2, the cells were generally 60% to 70% confluent in the 1536-well plates. After overnight incubation, 4μl of membrane potential dye containing 50μM of the phosphodiesterase inhibitor RO 20- 1724 was added to each well, and the plates were incubated for 60 min at room temperature. A total of 23nl of compound or positive control (30nM TSH or 10μM forskolin for TSHR- expressing or parental cells, respectively) in DMSO was added to each well using a pintool (Kalypsys, San Diego, CA), and the plates were further incubated for 30 min at room temperature. Plates were measured with an excitation of 535/20 nm, an emission of 590/20 nm, a gain of 150, and 5 flashes per well on an Envision plate reader (PerkinElmer, Boston, MA) (Table 2).
Table 1
Reagents and resources for the TSH receptor screen.
Table 2
Assay protocol for the TSHR and parental cell lines in 1536 well format.
Center Summary of Results
Both TSHR and parental (lacking TSHR) lines were screened across 463 compound plates (for a total of 926 plates). The average signal-to-basal ratio for the entire screen was 2.01, the CV was 12.1%, and the Ẓ′ value was 0.4. Plates with a Ẓ′ factor less than 0.2 (approximately 5% of the total plates) were rescreened. The relatively low Ẓ′ is likely due to the use of HEK293 cells, which typically do not adhere well after treatment with membrane potential dyes.
ii. Counterscreen in ACTOne parental cell line for TSHR agonists. [AID:398; Primary DR]
Assay Description
All 72,030 compounds were tested in qHTS format in the parental cell line lacking the TSH receptor.
Assay Protocol
The assay protocol and reagents are identical to: qHTS Assay for TSHR agonists Primary screen [AID: 926; Primary] but screened in the parental HEK 293 cell line, which lacks the TSH receptor.
Center Summary of Results
Prioritized compounds had no activity in the parental cell line.
iii. Confirmation Concentration-Response Assay for TSHR agonists in ACTOne TSHR cells [AID:939; Confirmatory DR]
Assay Description
To confirm activity in the original assay, select samples active in the primary screen were obtained in DMSO solution from the MLSMR and/or as powders from compound vendors.
Assay Protocol
The assay protocol and reagents are identical to: qHTS Assay for TSHR agonists Primary screen [AID: 926; Primary]
Center Summary of Results
Prioritized compounds had a high confirmation rate.
iv. Counterscreen in ACTOne parental cell line for TSHR agonists. [AID:953; Confirmatory DR]
Assay Description
To confirm activity in the original assay, select samples active in the primary screen were obtained in DMSO solution from the MLSMR and/or as powders from compound vendors.
Assay Protocol
The assay protocol and reagents are identical to: qHTS Assay for TSHR agonists Primary screen [AID: 926; Primary], but screened in the parental HEK 293 cell line, which lacks the TSH receptor.
Center Summary of Results
Prioritized compounds were inactive in the parental cell line.
v. Counterscreen in TSHR cell line using Homogeneous Time Resolved Fluorescence (HTRF) cAMP assay in TSHR cells [AID:933; Confirmatory DR]
Assay Description
Recently, a time resolved fluorescence resonance energy transfer (TR-FRET) based cAMP assay has been available for screens from Cisbio (12,13). It uses an anti-cAMP antibody labeled with Eu3+, which binds to a d2-dye labeled cAMP tracer, resulting in TR-FRET. The cAMP from the cell lysate can displace the d2 labeled cAMP tracer that interrupts the TR-FRET (Fig. 3). Because this cAMP assay uses a different detection system, it can help to further eliminate the false positive compounds.

Figure 3
Schematic of cAMP competitive immunoassay.
Assay Protocol
The 95 selected compounds were tested for TSHR agonism in the TR-FRET based cAMP assay, which measures cAMP concentrations in cell lysates. The readout in the HTRF cAMP assay is distinct from that in the membrane potential dye measurement assay used in the qHTS, allowing us to further eliminate assay-related false positive compounds. Of the 95 compounds, 49 showed TSH agonist activity in the TSHR cell line without the activities in the parental cell line. Taken together, these results indicate that these 49 compounds are true small molecule TSHR agonists.
Assay Protocol
HEK 293 cell lines stably expressing the TSH, FSH, or LH receptors were used as the counter-screens for these compounds in the TR-FRET cAMP assay format. These lines did not contain the CNG and were therefore incapable of being used in the primary assay format. An assay protocol was summarized in Table 2. Cells were plated in white, solid bottom 1536 well tissue culture treated plates and assayed as described in Table 3.
Table 3
HTRF assay in TSH, LH, and FSH cell lines.
Table 4Reagents for HTRF counterscreen
| Reagents | Resources | Catalog Number |
|---|---|---|
| HTRF cAMP dynamic 2 kit | Cisbio | 62AM4PEJ |
| DMEM Media | Invitrogen | 11765 |
| Geneticin | Invitrogen | 10131027 |
| Fetal Bovine Serum | Hyclone | SH30071.03 |
| Penicillin-Streptomycin | Invitrogen | 15140 |
| RO 20-1724 | Sigma | B8279 |
vi. Counterscreen in TSHR cell line using Homogeneous Time Resolved Fluorescence (HTRF) cAMP assay in LH cells [AID:1402; Confirmatory DR]
Assay Description
To confirm activity in the original assay, select samples active in the primary screen were obtained in DMSO solution from the MLSMR and/or as powders from compound vendors.
Assay Protocol
The assay protocol and reagents are identical to the HTRF assay run in TSH cells qHTS Assay [AID: 933; Primary].
Center Summary of Results
Prioritized compounds had a high confirmation rate.
vii. Counterscreen in TSHR cell line using Homogeneous Time Resolved Fluorescence (HTRF) cAMP assay in FSH cells [AID:1403; Confirmatory DR]
Assay Description
To confirm activity in the original assay, select samples active in the primary screen were obtained in DMSO solution from the MLSMR and/or as powders from compound vendors.
Assay Protocol
The assay protocol and reagents are identical to the HTRF assay run in TSH cells qHTS Assay [AID: 933; Primary].
Center Summary of Results
Prioritized compounds had a high confirmation rate.
viii. Secondary Concentration-Response Assay for Agonists of the Thyroid Stimulating Hormone Receptor: ELISA Activity Detection [AID:2104; Confirmatory DR]
Assay Description
To confirm activity in the original assay, select samples active in the confirmatory assays were resynthesized and assayed by the low-throughput ELISA assay format.
Assay Protocol
The assay protocol and reagents are described in detail in reference 11 (1) [AID: 2104; Secondary]. Transiently transfected HEK-EM293 cells or cells stably expressing TSHR, LHCGR, or FSHR were seeded into 96-well plates at a density of 50,000 cells/well in DMEM containing 10% FBS. Cells were cultured for 24 h before incubation for 1 h in serum-free DMEM containing 1mM 3-isobutyl-1-methylxanthine (Sigma) and TSH, LH, FSH, or small-molecule ligands in a humidified 5% CO2 incubator at 37°C. Following aspiration of the media, cells were lysed using lysis buffer of the cAMP-Screen Direct system (Applied Biosystems). The cAMP content of the cell lysate was determined using the method described in the manufacturer’s protocol. Reagents are listed in Table 5.
Table 5
Reagents for ELISA secondary screen.
Center Summary of Results
Compounds were much more potent using ELISA assay than HTRF format assay.
2.2. Probe Chemical Characterization
Synthesis of probe molecule
The synthesis of the probe molecule commenced with amide formation between commercially available acid 1a and benzyl amine to afford 3a and subsequent demethylation to 31a (Scheme 1). The second component of the aminal was synthesized via ether formation between benzyl chloride 4a and p-acetamide phenol to afford 5a. The ytterbium catalyzed condensation between 31a and 5a afforded the probe molecule 6a.

Scheme 1
Synthesis of probe.
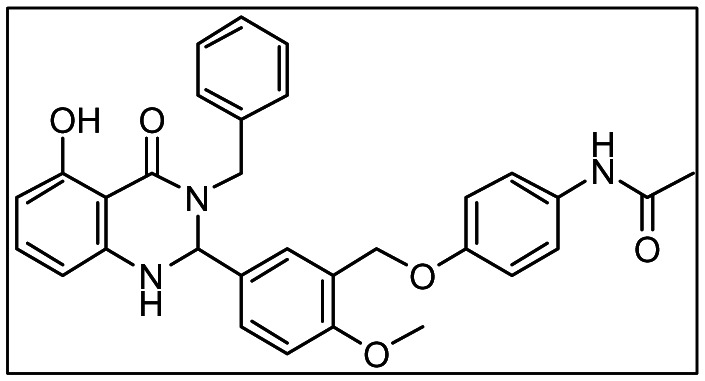
MLS002576689 (CID: 25246343/ML109):
1H NMR (400 MHz, DMSO-d6) δppm 2.00 (s, 3 H), 3.78 (d, J=15.5Hz, 1H), 3.80 (s, 3H), 4.96 (s, 2 H), 5.15 (d, J=15.3 Hz, 1 H), 5.73 (d, J=1.96 Hz, 1 H), 6.05–6.08 (m, 2 H), 6.86–6.90 (m, 2 H), 7.01 – 7.11 (m, 2 H), 7.19 – 7.36 (m, 6 H), 7.41–7.48 (m, 4 H) 9.77 (s, 1 H), 12.26 (s, 1 H); HPLC: tR = 6.19 min, UV254 = 96%; HRMS (ESI): m/z calcd for C31H29N3O5 [M+1]+ 524.2185, found 524.2184. Solubility (PBS, pH = 7.4, 23 °C): 2μM.
Table 6Compounds submitted to the MLSMR
| MLS002576689 | Probe |
| MLS003221407 | Analogue |
| MLS003221408 | Analogue |
| MLS003221409 | Analogue |
| MLS003221410 | Analogue |
| MLS003221411 | Analogue |
2.3. Probe Preparation
Synthesis of 2,3-dihydroquinazolin-4(1H)-one (6) and quinazolin-4(1H)one (7) analogs
As depicted in scheme 2, 2-aminobenzamides 3 were prepared by either amide couplings of 2-aminobenzoic acids 1 with different amines or reactions of isatoic anhydrides 2 with amines. Reactions of benzyl chlorides 4 with different phenols under microwave irradiation generated aldehydes 5. Condensations of aldehydes 5 with 2-aminobenzamides 3 yielded 2,3-dihydroquinazolin-4-ones 6. The 2,3-dihydroquinazolin-4-ones 6 were rapidly oxidized by DDQ at room temperature to produce quinazolin-4-ones 7.

Scheme 2
Analog synthesis. Reagents and conditions: (i) DMC, DIPEA, r.t. 12 h; (ii) ACN, r.t.-50 °C. (iii) K2CO3, DMA, microwave heating, 150 °C, 10 min; (iv) Yb(OTf)3, DMA, microwave heating, 200 °C, 10 min; (v) DDQ, r.t. 1 h
General procedure for the synthesis of 2-aminobenzamides from isatoic anhydride
Amines (1.05 mmol, 1.05 equiv) at room temperature were added to a solution of isatoic anhydride (0.163 g, 1.0 mmol, 1.0 equiv) in 10 mL of anhydrous acetonitrile. The resulting mixture was stirred at room temperature for 2 hours and heated at 50°C for 4 hours. Then, it was concentrated in vacuo to yield the products as solids in 90–99% yields.
2-Amino-N-benzylbenzamide

1H NMR (400 MHz, CHLOROFORM-d) δ 4.61 (s, 1 H), 4.63 (s, 1 H), 5.58 (br. s., 2 H), 6.33 (br. s., 1 H), 6.62–6.66 (m, 1 H), 6.69–6.71 (m, 1 H), 7.19–7.25 (m, 1 H), 7.28 – 7.43 (m, 6 H); LCMS: (electrospray +ve), m/z 227.1 (MH)+; HPLC: tR = 4.38 min, UV254 = 96%.
2-Amino-N-(furan-2-ylmethyl)benzamide

1H NMR (400 MHz, CHLOROFORM-d) δ 4.60 (s, 1 H), 4.61 (s, 1 H), 5.57 (br. s., 2 H), 6.24 – 6.42 (m, 3 H), 6.59 – 6.74 (m, 2 H), 7.16 – 7.25 (m, 1 H), 7.33–7.39 (m, 2 H); LCMS: (electrospray +ve), m/z 217.1 (MH)+; HPLC: tR = 3.77 min, UV254 = 98%.
2-Amino-N-benzyl-6-methoxybenzamide

2-chloro-1,3-dimethylimidazolinium chloride (1.099g, 6.5mmol, 1.3equiv) at room temperature was added to a solution of 2-amino-6-methoxybenzoic acid (0.841g, 5.0mmol, 1.0equiv), benzylamine (0.643g, 6.0mmol, 1.2equiv), and diisopropylethylamine (1.935g, 15.0mmol, 3.0equiv) in 50ml of dichloromethane. The mixture was stirred at room temperature for 6 hours, poured into water, and extracted with dichloromethane. The organic solution was successively washed with aqueous saturated NaHCO3 and water. The organic layer was dried over MgSO4 and the solvent was removed by rotary evaporator. The residue was purified by column chromatography (silica gel, 2% 2.0 M ammonia MeOH solution in CH2Cl2) to give 2-Amino-N-benzyl-6-methoxybenzamide (0.593g, 46%) as a solid. 1H NMR (400 MHz, CHLOROFORM-d) δ 3.81 (s, 3 H), 4.62 (s, 1 H), 4.63 (s, 1 H), 6.07 (vb.s, 2 H), 6.19 (d, J=8.2 Hz, 1 H), 6.32 (d, J=8.2 Hz, 1 H), 7.07 (t, J=8.2 Hz, 1 H), 7.14 – 7.54 (m, 5 H), 8.05 (br. s., 1 H); HPLC: tR = 4.72 min, UV254 = 99%; HRMS (ESI): m/z calcd for C15H16N2O2 [M+1]+ 257.1296, found 257.1294.
N-(4-(5-(3-(Furan-2-ylmethyl)-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)-2-methoxybenzyloxy)phenyl)acetamide

K2CO3 (207mg, 1.5mmol, 5equiv) was added to a solution of 3-(chloromethyl)-4-methoxybenzaldehyde (55.2mg, 300μmol, 1.0equiv) and 4-acetamidophenol (54.4mg, 360μmol, 1.2equiv) in 1.5ml of anhydrous DMA. The mixture was heated in a microwave for 10 min at 150°C. After filtering off the solid, 2-amino-N-(furan-2-ylmethyl)benzamide (77.8mg, 360μmol, 1.2equiv) and Ytterbium trifluoromethanesulfonate (93mg, 150μmol, 0.5equiv) were added to the clear solution. The resulting mixture was heated in a microwave for 10 min at 200 °C. The product was isolated via preparative HPLC purification and solvent was removed by a GeneVac to give N-(4-(5-(3-(furan-2-ylmethyl)-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)-2-methoxybenzyloxy)phenyl)acetamide (47.8mg, 32%) as a solid. 1H NMR (400 MHz, CDCl3) δ 2.11 (s, 3 H), 3.72 (d, J=15.6 Hz, 1 H), 3.83 (s, 3 H), 4.25 (v.b.s, 1H), 5.01 (s, 2 H), 5.26 (d, J=15.6 Hz, 1 H), 5.71 (s, 1 H), 6.03 – 6.29 (m, 2 H), 6.48 (d, J=7.8 Hz, 1 H), 6.83 (dd, J=18.2, 8.8 Hz, 3 H), 7.10 – 7.61 (m, 7 H), 7.92 (d, J=7.0 Hz, 1 H); HPLC: tR = 5.40 min, UV254 = 91%; HRMS (ESI): m/z calcd for C29H27N3O5 [M+1]+ 498.2029, found 498.2025.
3-(Furan-2-ylmethyl)-2-(4-methoxy-3-(phenoxymethyl)phenyl)-2,3-dihydroquinazolin-4(1H)-one

K2CO3 (319mg, 2.3mmol, 5equiv) was added to a solution of 3-(chloromethyl)-4-methoxybenzaldehyde (85mg, 462μmol, 1.0equiv) and phenol (52mg, 554μmol, 1.2equiv) in 2.0ml of anhydrous DMA. The mixture was heated in a microwave for 10 min at 150°C. After filtering off the solid, 2-amino-N-(furan-2-ylmethyl)benzamide (100mg, 462μmol, 1.0equiv) and Ytterbium trifluoromethanesulfonate (286 mg, 231μmol, 0.5equiv) were added to the clear solution. The resulting mixture was heated in a microwave for 10 min at 200°C. The product was isolated via preparative HPLC purification and solvent was removed via reduced pressure lyophilization to give 3-(furan-2-ylmethyl)-2-(4-methoxy-3-(phenoxymethyl)phenyl)-2,3-dihydroquinazolin-4(1H)-one (41.5mg, 20%) as a white solid after triturating with diethyl ether. 1H NMR (400 MHz, CDCl3) δ 3.74 (d, J=15.6 Hz, 1 H), 3.86 (s, 3 H), 4.40 (v.b.s, 1H), 5.08 (s, 2 H), 5.31 (d, J=16.0 Hz, 1 H), 5.74 (s, 1 H), 6.13 (dd, J=3.3, 0.6 Hz, 1 H), 6.25 (dd, J=3.1, 1.9 Hz, 1 H), 6.48 (dd, J=8.0, 0.4 Hz, 1 H), 6.81–6.85 (m, 2H), 6.94–6.98 (m, 3H), 7.21 – 7.32 (m, 5 H), 7.48 (d, J=2.4 Hz, 1 H), 7.98 (dd, J=7.8, 1.4Hz, 1H); HPLC: tR = 6.42 min, UV254 = 92%; HRMS (ESI): m/z calcd for C27H24N2O4 [M+1]+ 441.1820, found 441.1821.
N-(4-(5-(3-(Furan-2-ylmethyl)-4-oxo-3,4-dihydroquinazolin-2-yl)-2-methoxybenzyloxy)phenyl)acetamide

1H NMR (400 MHz, CHLOROFORM-d) δ 2.15 (s, 3 H), 3.95 (s, 3 H), 5.12 (s, 2 H), 5.19 (s, 2 H), 6.12 (d, J=2.7 Hz, 1 H), 6.22 – 6.28 (m, 1 H), 6.93 (d, J=9.0 Hz, 2 H), 7.00 (d, J=8.6 Hz, 1 H), 7.12 (br. s., 1 H), 7.24 (s, 1 H), 7.39 (d, J=9.0 Hz, 2 H), 7.46 – 7.56 (m, 2 H), 7.64 – 7.82 (m, 3 H), 8.33 (d, J=8.2 Hz, 1 H); HPLC: tR = 5.49 min, UV254 = 95%; HRMS (ESI): m/z calcd for C29H25N3O5 [M+1]+ 496.1872, found 496.1873.
N-(4-(5-(3-Benzyl-4-oxo-3,4-dihydroquinazolin-2-yl)-2-methoxybenzyloxy)phenyl)acetamide

1H NMR (400 MHz, CHLOROFORM-d) δ 2.13 (s, 3 H), 3.91 (s, 3 H), 5.04 (s, 2 H), 5.26 (s, 2 H), 6.81 – 7.04 (m, 5 H), 7.11 – 7.26 (m, 4 H), 7.38 (d, J=9.0 Hz, 2 H), 7.46 – 7.58 (m, 2 H), 7.71 – 7.83 (m, 2 H), 8.36 (d, J=7.8 Hz, 1 H); HPLC: tR = 5.73 min, UV254 = 98%; HRMS (ESI): m/z calcd for C31H27N3O4 [M+1]+ 506.2086, found 506.2082.
Synthesis of the probe
2-Amino-N-benzyl-6-hydroxybenzamide (3a)

1-dodecanethiol (0.360g, 1.78 mmol, 2.0equiv) was added to a solution of 2-amino-N-benzyl-6-methoxybenzamide (0.228g, 0.89mmol, 1.0equiv) in 3 mL of anhydrous DMF, followed by adding NaOMe (0.385g of 25% solution in MeOH, 1.78, 2.0equiv). Five copies of this reaction were prepared, and the reaction mixtures were heated in a microwave at 150 °C for 10 min. The reaction mixtures were combined and passed through a silica gel plug, which was washed with an ethyl acetate-methanol mixture (1:1). The solvents were removed and the residue was purified by column chromatography (silica gel, eluent gradient 2:98 ethyl acetate-CH2Cl2 to 1:1 ethyl acetate-CH2Cl2). The desired 2-Amino-N-benzyl-6-hydroxybenzamide (0.780 g, 72%) was isolated as an off-white solid after triturating with diethyl ether/Hexanes. 1H NMR (400 MHz, CHLOROFORM-d) δ 3.55 – 4.20 (vb.s., 2 H), 4.60 (s, 1 H), 4.61 (s, 1 H), 6.25 (d, J=7.8 Hz, 1 H), 6.50 (d, J=8.2 Hz, 1 H), 7.09 (t, J=8.0 Hz, 1 H), 7.16 – 7.52 (m, 5 H), 8.58 (br. s., 1 H),12.05 (br. s., 1 H); HPLC: tR = 3.97 min, UV254 = 98%; HRMS (ESI): m/z calcd for C14H14N2O2 [M+1]+ 243.1140, found 243.1134.
N-(4-(5-(3-Benzyl-5-hydroxy-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)-2-methoxybenzyloxy)phenyl)acetamide (6a)

K2CO3 (690 mg, 5.0mmol, 5equiv) was added to a solution of 3-(chloromethyl)-4-methoxybenzaldehyde (184.6 mg, 1.00 mmol, 1.0equiv) and N-(4-hydroxyphenyl)acetamide (181mg, 1.20mmol, 1.2equiv) in 5.0ml of anhydrous DMA. The mixture was heated in a microwave at 150°C for 10 min. The solid was filtered and the clear solution was added to a microwave tube containing 2-amino-N-benzyl-6-hydroxybenzamide (264mg, 1.09mmol, 1.1equiv). After adding Ytterbium trifluoromethanesulfonate (310mg, 0.5mmol, 0.5equiv), the mixture was heated in a microwave at 200°C for 10 min. The reaction mixture was purified by HPLC. Pure fractions were dried down under vacuum on a lyophilizer. The desired product (132.3mg, 28%) was obtained as a solid after triturating with diethyl ether. 1H NMR (400 MHz, DMSO-d6) δppm 2.00 (s, 3 H), 3.78 (d, J=15.5Hz, 1H), 3.80 (s, 3H), 4.96 (s, 2 H), 5.15 (d, J=15.3 Hz, 1 H), 5.73 (d, J=1.96 Hz, 1 H), 6.05–6.08 (m, 2 H), 6.86–6.90 (m, 2 H), 7.01 – 7.11 (m, 2 H), 7.19 – 7.36 (m, 6 H), 7.41–7.48 (m, 4 H) 9.77 (s, 1 H), 12.26 (s, 1 H); HPLC: tR = 6.19 min, UV254 = 96%; HRMS (ESI): m/z calcd for C31H29N3O5 [M+1]+ 524.2185, found 524.2184.
3. Results
3.1. Summary of Screening Results
72,030 compounds were screened in the primary assay14. Some active compounds were chosen for confirmation according to the scheme shown in Fig. 4.

Figure 4
High-throughput screen.
3.2. Dose Response Curves for Probe

Figure 5Activity of MLS002576689 in the ELISA cAMP assay
3.3. Scaffold/Moiety Chemical Liabilities
A potential site for undesired chemical reactivity in the probe would be the aminal, which could undergo oxidation or hydrolysis to the amine and aldehyde. We found experimentally that the rate of oxidation is dependent on the aryl ring substitution. The 5-hydroxy substituted probe molecule is particularly resistant to oxidation. It was dissolved in acetonitrile and oxygen was bubbled through the solution, but no oxidation was witnessed, even after several hours. In fact, the probe compound only underwent oxidation when treated with MnO2. The other chemical concern for this scaffold would be hydrolysis. The hydrolytic stability of this scaffold was monitored at neutral and basic pH and the t1/2 was 16 h. The stability, not surprisingly, suffered at low pH and the t1/2 was 3 h1.
3.4. SAR Tables
Table 7Structure-Activity relationship study*
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | SID | CID | R1 | R2 | R3 | R4 | R5 | R6 | X | Y | EC50 | Emax |
| 1 | 26755506 | 661788 | H | H |
 | OMe | H | NHAc | NH | O | 1.16 | 101 |
| 2 | 29216393 | 16759579 | H | H | Bn | OMe | H | NHAc | NH | O | 0.29 | 99.8 |
| 3 | 29216998 | 17757110 | H | H | n-Bu | OMe | H | NHAc | NH | O | 0.83 | 112 |
| 4 | 29218134 | 17757294 | H | H |
 | OMe | H | NHAc | NH | O | 3.98 | 70.3 |
| 5 | 29216997 | 17757109 | H | H | H | OMe | H | NHAc | NH | O | n.d. | 39.5 |
| 6 | 29218154 | 17757314 | H | H | Bn | OMe | H | NHAc | N | O | 0.96 | 97.4 |
| 7 | 57655046 | 25246367 | H | OMe | Bn | OMe | H | NHAc | NH | O | 4.48 | 49.1 |
| 8 | 26755506 | 25246343 | H | OH | Bn | OMe | H | NHAc | NH | O | 0.09 | 100 |
| 9 | 29218135 | 17757295 | H | H |
 | OMe | H | NHAc | NH | O | n.d. | 10.1 |
| 10 | 29216999 | 17757111 | H | H |
 | OMe | H | NHAc | NH | O | n.d. | 19.7 |
| 11 | 29218136 | 17757296 | H | H |
 | OMe | H | NHAc | NH | O | 0.43 | 102.5 |
| 12 | 103073331 | 49789101 | OH | H | Bn | OMe | H | NHAc | NH | O | 0.33 | 93.3 |
| 13 | 103073332 | 49789102 | H | H | Bn | H | H | NHAc | NH | O | 1.86 | 36.8 |
| 14 | 103073333 | 49789103 | H | F | Bn | OMe | H | NHAc | NH | O | 0.28 | 94.7 |
| 15 | 29216969 | 2887852 | H | H |
 | OMe | H | NHAc | NH | O | 5.09 | 75.1 |
| 16 | 29218141 | 17757301 | H | H | Bn | OMe | H | OMe | NH | O | 2.16 | 46.3 |
| 17 | 103073337 | 49789106 | H | H | Bn | OMe | H | H | NH | O | 2.57 | 25.6 |
| 18 | 103073338 | 49789107 | H | H | Bn | OMe | NHAc | H | NH | O | 4.34 | 24.8 |
| 19 | 103073339 | 46190126 | H | H | Bn | OMe | H | NHAc | NH | S | 0.08 | 95.7 |
| 20 | 29216989 | 17757102 | H | H |
 | OMe | H | NHAc | N | O | 0.93 | 59 |
| 21 | 103073340 | 49789108 | H | OH | Bn | OMe | _ | _ | NH | _ | n.d. | 17.7 |
| 22 | 103073341 | 49789109 | H | NH2 | Bn | OMe | H | NHAc | NH | O | 3.78 | 72.6 |
| 23 | 103073342 | 49789110 | H | NHMs | Bn | OMe | H | NHAc | NH | O | 0.98 | 90.8 |
| 24 | 29218140 | 17757300 | H | H | Bn | OMe | H | CN | NH | O | n.d. | 11.7 |
| 25 | 103073343 | 49789111 | H | H | Bn | OMe | F | H | NH | O | n.d. | 7.4 |
| 26 | 103073344 | 49789112 | H | OH | Bn | OMe | H | NHAc | NH | S | 0.018 | 69.6 |
| 27 | 103073345 | 49789113 | H | OH | Bn | OMe | H | NHAc | N | O | 0.55 | 98.6 |
3.5. Cellular Activity
Both primary and secondary assays are cell-based assays.
3.6. Profiling Assays
In vitro ADME studies were performed on the probe molecule (Table 8). This data indicates that the probe precipitates out of solution at 2 μM, and exhibits a high rate of clearance and good absorption. Additionally, the probe does not inhibit CYP, is modestly affected by the Pgp inhibitor.
Table 8
Probe (CID-25246343/ML109) ADME properties.
Table 9Summary of data in PubChem
| PubChem AID | Type | Target | Conc. Range | Samples Tested |
|---|---|---|---|---|
| 926 | Primary DR | TSHR cell line | 0.5nM-46μM | 72030 |
| 938 | Primary DR counterscreen | Parental cell line | 0.5nM-46μM | 72030 |
| 939 | Counterscreen | TSHR cell line | 0.5nM-46μM | 151 |
| 933 | Counterscreen | TSHR cell line HTRF | 0.5nM-46μM | 346 |
| 953 | Counterscreen | Parental cell line | 0.5nM-46μM | 151 |
| 1403 | Counterscreen | FSHR cell line HTRF | 0.5nM-46μM | 29 |
| 1402 | Counterscreen | LHR cell line HTRF | 0.5nM-46μM | 31 |
| 2104 | Secondary | TSHR cell line | 0.5nM-46μM | 35 |
| 1401 | Summary | N/A | N/A | N/A |
4. Discussion
Among the confirmed hits, NCGC00168126 (CID-661788) was the most selective for TSH receptor, with no detectable agonist activity at the closely related LHCGR or FSHR, and was therefore selected for chemistry optimization. We explored substitution patterns throughout the scaffold. The analogs were first evaluated using the HTRF cAMP assay (AID-933). However, due to increased satisfaction with the consistency of results from the ELISA assay (AID-2104), the ELISA assay was used to guide our SAR study. As shown in Table 7, when R1 was either an OH or H (entries 12 and 2), there was no substantial change in activity. However, the R2 substitution influences activity. OH improved activity (entry 8), but NH2, NHMs and OMe (entries, 22, 23, and 7) all resulted in a loss of activity. Furyl, benzyl, butyl and phenethyl groups were tolerated at R3 (entries 1, 2, 3 and 11), but pyridyl, tertiary amine, or an alcohol (entries 4, 9 and 10) resulted in a loss of activity. This indicates that lipophilic groups are well tolerated at R3, but polar groups that could behave as either hydrogen bond donors or acceptors are detrimental to activity. When R3 was an H (entry 5), activity was completely lost. The removal of the OMe moiety at R4 resulted in an inactive compound (entry13). Activity was lost when R5 was a functionality other than H (entries 18 and 25), or when R6 was a functionality other than NHAc (entries 16, 17, 18, 24 and 25). The R5 and R6 containing aryl ring is crucial for activity and very sensitive to substitution patterns. This was further demonstrated in the inactive analog where this entire ring was removed (entry 21). Y was oxygen in most analogs tested, but the activity improved when Y was sulfur (entries 19 and 26). Most analogs tested were with X as NH, but several oxidized compounds (X = N) were tested (entries 6, 20, and 27). The oxidized compounds were less active than the un-oxidized counterparts, but remain interesting analogs due to the improved air and acid stability. In vivo studies (Figure 6) showed that CID-25246343/ML109 could increase secretion of T4 and thyroidal iodide uptake in mice after administration by esophageal gavage, suggesting CID-25246343/ML109 is an orally available small molecule that can stimulate thyroid gland function1.
4.1. Comparison to existing art and how the new probe is an improvement
The probe is the first orally active TSHR agonist. The probe series represents the most potent and selective small molecule TSHR agonists reported to date.
4.2. Mechanism of Action Studies
There have been several studies to validate the probe’s exact binding site on TSHR. It was predicted to bind to the serpentine domain of TSHR, which is different from the amino terminal ectodomain binding site of endogenous TSH. This was validated by testing its binding to a TSHR mutant with the amino-terminal ectodomain deleted (KFLR). KFLR is not activated by TSH, but the probe molecule did activate KFLR, albeit with 12% lower efficacy and lower potency (EC50 = 1.7 μM). The binding was then modeled and N5.47 was predicted to be critical for activity, and a site specific mutant validated this prediction1 (Figure 7).
4.3. Planned Future Studies
The oxidized compounds present an attractive scaffold for further optimization due to the improved air and acid stability over the probe molecule.
5. References
- 1.
- Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM, Gershengorn MC. Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci U S A. 2009 Jul 28;106(30):12471–6. [PMC free article: PMC2708975] [PubMed: 19592511]
- 2.
- Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends in Biochemical Sciences. 2004;29(3):119–126. [PubMed: 15003269]
- 3.
- Demeure MJ, Doffek KM, Wilson SD. Defective thyrotropin receptor G-protein cyclic adenosine monophosphate signaling mechanism in the FTC human follicular thyroid cancer cell line. Surgery. 1997;122(6):1195–1201. discussion 1201–1192. [PubMed: 9426438]
- 4.
- Latif R, Ando T, Daniel S, Davies TF. Localization and regulation of thyrotropin receptors within lipid rafts. Endocrinology. 2003;144(11):4725–4728. [PubMed: 12960014]
- 5.
- Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115(8):1972–1983. [PMC free article: PMC1180562] [PubMed: 16075037]
- 6.
- Fernandes JK, Day TA, Richardson MS, Sharma AK. Overview of the management of differentiated thyroid cancer. Current Treatment Options in Oncology. 2005;6(1):47–57. [PubMed: 15610714]
- 7.
- van Straten NC, Schoonus-Gerritsma GG, van Someren RG, Draaijer J, Adang AE, Timmers CM, Hanssen RG, van Boeckel CA. The first orally active low molecular weight agonists for the LH receptor: thienopyr(im)idines with therapeutic potential for ovulation induction. Chembiochem. 2002;3(10):1023–1026. [PubMed: 12362369]
- 8.
- Jäschke H, Neumann S, Moore S, Thomas CJ, Colson A-O, Costanzi S, Kleinau G, Jiang J-K, Paschke Raaka BM, Krause G, Gershengorn MC. A low molecular weight agonist signals by binding to the transmembrane domain of Thyroid-Stimulating Hormone Receptor (TSHR) and Luteinizing Hormone/Chorionic Gonadotropin Receptor (LHCGR). J. Biol. Chem. 2006;281:9841–9844. [PubMed: 16488885]
- 9.
- Moore S, Jaeschke H, Kleinau G, Neumann S, Costanzi S, Jiang J, Childress J, Raaka B, Colson A, Paschke R, Krause G, Thomas C, Gershengorn M. Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: structure-activity relationships and selective binding patterns. J. Med. Chem. 2006;49(13):3888–3896. [PMC free article: PMC2543117] [PubMed: 16789744]
- 10.
- Titus S, Neumann S, Zheng W, et al. Quantitative high-throughput screening using a live-cell cAMP assay identifies small-molecule agonists of the TSH receptor. J Biomol Screen. 2008;13(2):120–127. [PMC free article: PMC2653065] [PubMed: 18216391]
- 11.
- Reinscheid RK, Kim J, Zeng J, Civelli O. High-throughput real-time monitoring of Gs-coupled receptor activation in intact cells using cyclic nucleotide-gated channels. Eur J Pharmacol. 2003;478(1):27–34. [PubMed: 14555181]
- 12.
- Yao YLJ, Llorente I, DeBernardi M, Cao L. ACT: One technology: a live cAMP assay. Poster presentation in the 10th Anniversary Conference of Society of Biomolecular Sciences; 2004.
- 13.
- Gabriel D, Vernier M, Pfeifer MJ, Dasen B, Tenaillon L, Bouhelal R. High throughput screening technologies for direct cyclic AMP measurement. Assay Drug Dev Technol. 2003;1(2):291–303. [PubMed: 15090194]
- 14.
- Prystay L, Gagne A, Kasila P, Yeh LA, Banks P. Homogeneous cell-based fluorescence polarization assay for the direct detection of cAMP. J Biomol Screen. 2001;6(2):75–82. [PubMed: 11689101]
- 15.
- Inglese J, Auld DS, Jadhav A, et al. Quantitative high-throughput screening: A titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103(31):11473–11478. [PMC free article: PMC1518803] [PubMed: 16864780]
- PMCPubMed Central citations
- PubChem BioAssay for Chemical ProbePubChem BioAssay records reporting screening data for the development of the chemical probe(s) described in this book chapter
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Identification of Thyroid Stimulating Hormone Receptor Inverse Agonists.[Probe Reports from the NIH Mol...]Review Identification of Thyroid Stimulating Hormone Receptor Inverse Agonists.Huang W, Englund E, Titus S, Southall N, Zheng W, Ferrer M, Marugan J, Neumann S, Gershengorn M. Probe Reports from the NIH Molecular Libraries Program. 2010
- Human TSH receptor ligands as pharmacological probes with potential clinical application.[Expert Rev Endocrinol Metab. 2...]Human TSH receptor ligands as pharmacological probes with potential clinical application.Neumann S, Raaka BM, Gershengorn MC. Expert Rev Endocrinol Metab. 2009 Nov 1; 4(6):669.
- Quantitative high-throughput screening using a live-cell cAMP assay identifies small-molecule agonists of the TSH receptor.[J Biomol Screen. 2008]Quantitative high-throughput screening using a live-cell cAMP assay identifies small-molecule agonists of the TSH receptor.Titus S, Neumann S, Zheng W, Southall N, Michael S, Klumpp C, Yasgar A, Shinn P, Thomas CJ, Inglese J, et al. J Biomol Screen. 2008 Feb; 13(2):120-7. Epub 2008 Jan 23.
- Substitutions of different regions of the third cytoplasmic loop of the thyrotropin (TSH) receptor have selective effects on constitutive, TSH-, and TSH receptor autoantibody-stimulated phosphoinositide and 3',5'-cyclic adenosine monophosphate signal generation.[Mol Endocrinol. 1993]Substitutions of different regions of the third cytoplasmic loop of the thyrotropin (TSH) receptor have selective effects on constitutive, TSH-, and TSH receptor autoantibody-stimulated phosphoinositide and 3',5'-cyclic adenosine monophosphate signal generation.Kosugi S, Okajima F, Ban T, Hidaka A, Shenker A, Kohn LD. Mol Endocrinol. 1993 Aug; 7(8):1009-20.
- Review Structural-Functional Features of the Thyrotropin Receptor: A Class A G-Protein-Coupled Receptor at Work.[Front Endocrinol (Lausanne). 2...]Review Structural-Functional Features of the Thyrotropin Receptor: A Class A G-Protein-Coupled Receptor at Work.Kleinau G, Worth CL, Kreuchwig A, Biebermann H, Marcinkowski P, Scheerer P, Krause G. Front Endocrinol (Lausanne). 2017; 8:86. Epub 2017 Apr 24.
- Identification of Potent and Selective Thyroid Stimulating Hormone Receptor Agon...Identification of Potent and Selective Thyroid Stimulating Hormone Receptor Agonists - Probe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...