This is a work of the US government and distributed under the terms of the Public Domain
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Rx Marketplace Dynamics
The pharmaceutical marketplace is a complex amalgam of competing interests. There is no one specific pharmaceutical marketplace. There is no one price for a specific drug product. There are multiple customers, multiple distribution channels, multiple prescription drug reimbursement systems, multiple purchasing arrangements, multiple pricing methodologies, multiple marketing techniques, and multiple cost control tools. As history has shown, legislation, regulation, and market adjustments in one sector of the market often result in a chain reaction of intended and unintended consequences throughout the entire pharmaceutical marketplace.
Lawmakers considering how best to provide outpatient prescription drugs to Medicare beneficiaries are keenly aware of these consequences. As the debate continues, numerous issues and questions have been raised. The answers, however, are often cloaked in rhetoric and blanketed in politics. Terms meaning different things to different people are tossed around freely. The economics of the pharmaceutical marketplace are extremely complex: the companies themselves range in size from newly merged behemoths to very small one-product start-ups; some of the manufacturers are multinational, spanning the globe, while others are domestic and still others are foreign companies seeking to do business in America. Despite their differences, each company has the identical goal—to be profitable. Herein lies the rub. The prescription drug industry—the most profitable of all U.S. industries, based on revenue, equity and assets, according to Fortune Magazine—represents both the greatest strengths and the greatest weaknesses of American capitalism. “Hardly a day has gone by lately without some new reflection of the tension between the commercial and scientific accomplishments of the drug companies on the one hand, and the disparity in the spread of the benefits from those accomplishments.”1
Much of today’s political dialogue revolves around the notion of profitability and “public good.” How much profit is too much? Who should decide? Profitability and price, patents and promise—all are at the core of today’s congressional debates. Arguments fly around Capitol Hill: on the one hand, many of the elderly upset with the high cost of drugs are delighted when their drug company stock dividends continue to soar; on the other hand, those representing the low-income elderly argue that seniors should not have to choose between necessities to be able to afford life-sustaining medication. Some groups claim that the only way Medicare beneficiaries can afford their drugs is to buy them in Canada or Mexico; others point out that problems are so great in the health care systems in these countries that Canadian and other citizens are coming by busloads into America to purchase care. These arguments hold for the non-elderly as well.
In an effort to get beyond the hype, the Forum has organized this background briefing. The session has been structured as a one-half day institute, a tutorial on pharmaceutical marketplace dynamics. It will explain how the marketplace works by exploring a number of topics, including competition, generics, intellectual property, research and development, pricing, distribution, and current federal programs such as the Department of Veterans’ Affairs federal supply schedule and Medicaid rebates. Following this discussion will be a luncheon briefing highlighting Prescription Drug Coverage, Spending, Utilization, and Prices, the April 2000 report to the president from the Office of the Assistant Secretary for Planning and Evaluation (ASPE), Department of Health and Human Services (DHHS).
Pharmaceutical Industry Profile
According to a new University of Maryland School of Pharmacy study sponsored by the BlueCross BlueShield Association of America and reported in the April 24, 2000, issue of Medicine and Health, “U.S. pharmaceutical spending in 2004 will total $212 billion—more than twice last year’s total of $105 billion.” The study also reports that “prescription drug spending will rise 15-to-18 percent annually over the next five years.”
According to the Pharmaceutical Research and Manufacturers of America (PhRMA), the United States is the largest market for pharmaceuticals, accounting for approximately one-third of global pharmaceutical sales (Figure 1).
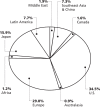
Figure 1
World Pharmaceutical Market –1997. Source: Pharmaceutical Research and Manufacturers of America, 1999 Industry Profile, PhRMA, Washington, D.C., 1999.
Although there are hundreds of U.S. pharmaceutical companies today, including large and small firms producing both branded and generic products, IMS data indicate that the 100 U.S. companies with the greatest dollar volume of sales make up approximately 96 percent of the U.S. market. “PhRMA reported that the domestic U.S. sales of its members (nearly all of the approximately 100 major research-intensive brand name manufacturers) were $81 billion in 1998. In contrast, the generic industry reported sales of $8 billion in 1998.”2
In recent years, numerous mergers and acquisitions (see Table 1) have brought about a major shift in the pharmaceutical landscape—with staggering results. For example,
the combined R&D [research and development] budget of the newly merged Glaxo Wellcome and SmithKline Beecham is on par with that of the entire National Cancer Institute and is approximately 10 times the research budget of the private Howard Hughes Medical Institute.3
Table 1
Mergers and Acquisitions in the Pharmaceutical Industry.
Industry officials argue that, although these newly merged titans are vast, they hold a relatively small share of the total drug market. The flip side, according to critics, is that individual companies dominate specific therapeutic markets. For example, “Schering-Plough controls 40 percent of the market for allergy medicines; and Warner-Lambert controls 48 percent of the market for cholesterol drugs. Glaxo SmithKline, as the new company will be called, will dominate the market for asthma, AIDS, and migraine drugs, as well as for vaccines.”4
Consumer advocates are worried that these mergers could lead to fewer drug products and higher prices. Company spokespersons point out that merger and acquisitions ultimately benefit consumers by strengthening their R&D pipelines.
Research and Development
Research and development in the pharmaceutical arena is risky business. According to PhRMA,
- U.S. pharmaceutical companies will spend $26.4 billion this year to discover and develop new medicines.
- It costs an average of $500 million to discover and develop just one new medicine. It takes nearly 15 years from the time a drug is discovered in the laboratory until it gets to the drug store.
- The United States leads the world in developing new medicines. Of the 152 major medicines launched worldwide over the last 20 years, U.S. companies developed nearly half.
- Pharmaceutical companies now put back $1 out of every $5 in revenues for R&D.
Over the years, the relationship between pharmaceutical R&D and profit—18.5 percent return on gross revenues in 1998, among pharmaceutical firms in the Fortune 500—have ignited heated discussions in and around Capitol Hill. Studies have been commissioned, hearings have been held, and countless policy debates have taken place. But, points of contention remain.
On the one hand, says Stephen Schondelmeyer, a pharmacoeconomist and professor in the University of Minnesota’s College of Pharmacy,
the drug companies give the impression that they need those profits to fund R&D. But, no, that’s not true. The 18.5 percent profit is accounted for separately from the 20 percent they say they spend on R&D. … On average, for every $100 spent on a drug at the manufacturer’s level, the actual cost of making it is about $10 to $15. A further $20 goes to R&D. About $15 goes to taxes and administrative costs. About $30 goes to advertising and marketing. And about $20 is profit.5
When asked if the industry could afford to lower its drug prices, given its robust profit margins, the industry answer is “Without reasonable returns on R&D investments, companies will not attract the investment capital needed to fund ongoing research to discover and develop lifesaving, cost-effective medicines, of which about 600 are in the pipeline.”6 The president of PhRMA, Alan F. Holmer, has reiterated in written statements and in testimony that, should market dynamics and legislative action negatively affect prices and profits, R&D activity would not come to a screeching halt; rather, research cutbacks would ensue and priorities would shift, effectively placing some diseases on the research “back burner.”
In many ways, companies are being forced to rethink their R&D portfolios as the very nature of R&D itself is changing. As a result of sophisticated new tools, such as computer modeling, 3-D computer-visualization techniques, combinational chemistry, and X-ray crystallography, the process of discovery, while still somewhat serendipitous, is much less haphazard than it was 20 years ago.
As the biotechnology revolution advances and as the Human Genome Project is completed, the very essence of our understanding of disease and therapy is undergoing a profound shift. Scientists today now realize that many diseases are actually a collection of several different diseases, each with a unique molecular cause.
The idea, therefore, of identifying a magic bullet that works for all cancer or all heart disease seems increasingly naive. Not surprisingly, big drug companies are terrified by a future in which they must develop a different drug for each type of hypertension, rather than a single blockbuster product.7
These scientific advances could prove to be a double-edged sword for some pharmaceutical companies.
For big pharmaceutical companies, the inevitable loss of market share caused by niche drug production will be offset both by the opportunity to intensify collaborations with thoughtful clinical investigators, and the ability to discover, develop and market new drugs more economically. The large R&D budgets afforded by the recent pharmaceutical mergers offer the opportunity to set aside a fraction of these resources to explore such an approach. If big drug companies don’t seize this moment, someone else will.8
Today, innovation and discovery are occurring not only inside but also outside the large pharmaceutical companies.
The market will continue to reconfigure itself as the “race for the cures” continues.Drug mergers could create a crisis in innovation, turning today’s pharmaceutical giants into tomorrow’s distribution centers, reliant on more creative outside research firms. To avoid this, big companies will have to divert some of their research money into new, more effective approaches to drug creation.9
Along these lines, the Wall Street Journal reported the following in a February 9, 2000, article, “After Drug-Firm Mergers, the Prescription Is on the Wall for More”:
Pharmaceutical companies have been scooping up biotech concerns for their drug prospects. … But that was when biotech stocks were out of favor and drug stocks were strong. Due to the recent rise in biotech stocks, many trade at multiples greatly higher than traditional pharmaceutical companies. This makes many targets prohibitively expensive. … Though it seems far-fetched, one investor said he wouldn’t be surprised to see a major biotech company such as Amgen acquire a traditional pharmaceutical concern for its sales and marketing.
It is clear that as the secrets of genomics are demystified, promising new miracle products (drugs may no longer be the correct term) will become available. Financing and discovering these products are only part of the risk for a company. The ability to recoup R&D investments is another.
Patents
Patent protection is essential for companies investing in pharmaceutical R&D. Unlike many other technological advances, a drug product, once discovered, is relatively easy to reproduce. Without the period of market exclusivity that patents provide, companies would not have the opportunity to recoup their R&D investments. Some argue that patents provide a monopoly, a barrier to market entry for competing products. The other side of that has been articulated by the Congressional Budget Office (CBO):
Patents do not grant complete monopoly power in the pharmaceutical industry. The reason is that companies can frequently discover and patent several different drugs that use the same basic mechanism to treat an illness. The first drug using the new mechanism to treat that illness—the breakthrough drug—usually has between one and six years on the market before a therapeutically similar patented drug (sometimes called a ‘me-too’ drug) is introduced.10
As the future of medical research itself changes, patent policy will face interesting challenges. For example, the area of genetic research has raised significant issues, such as what is patentable (that is, are gene sequences bona fide inventions?).11 As reported in the February 21, 2000, American Medical News article “Gene Patents Raise Concerns for Researchers, Clinicians,” by Vida Foubister: “The proliferation of patents for specific gene sequences may increase costs and decrease quality of diagnostic laboratory testing, limiting access for patients and training opportunities for physicians.”
While the future of patenting and biotechnology is still unfolding, past patent legislation and regulation are still affecting today’s pharmaceutical market. This is true both domestically and abroad, where patent piracy costs the industry hundreds of millions of dollars a year, despite various provisions agreed to in NAFTA (the North American Free Trade Agreement) and GATT (the General Agreement on Tariffs and Trade).
The 1984 Drug Price Competition and Patent Term Restoration Act
Domestically, the prescription drug market was radically altered with the passage of the 1984 Drug Price Competition and Patent Term Restoration Act (commonly referred to as the Hatch-Waxman Act after its authors, Sen. Orrin G. Hatch [R-Utah] and Rep. Henry A. Waxman [D-Calif.]). The act was
intended to strike a balance between promoting innovation (by guaranteeing makers of brand-name drugs a certain number of patent years) and ensuring that consumers have timely access to lower-cost generic medicines (by guaranteeing makers of generic drugs that those patents would eventually end).12
Generics
Since the 1980s, the use of generics has continued to increase (see Table 2). Along with the passage of Hatch-Waxman, the passage of drug-product substitution laws (at the state level) allowing pharmacists to dispense a generic, even in the case of a brand-name prescription, and the active promotion of generic substitution by government health programs and private health plans have all spurred the increase in generic sales.
Table 2
Prescription Drugs Facts and Figures.
Some analysts, such as Elliot Wilbur, a securities analyst with CIBC World Markets in Los Angeles, are of the opinion that
The opportunity for expansion of generic sales is further illustrated in Figure 2.The coming bull market in generics’ stocks will be fueled by a never-before-seen combination of surging supply and powerful demand. On the supply side, Wilbur points out that more than $30 billion worth of brand-name drugs will go off patent over the next five years. That’s more than six times the dollar level that has been available to the generic industry over the past four years. At the same time, says Wilbur, nearly half of all HMOs now include generic compliance stipulations as part of their financial incentive packages offered to physicians. Wilbur anticipates that number will climb to 75 percent over the next five years.13
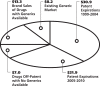
Figure 2
The Generic Opportunity (in billions of dollars). Source: Elliot Wilbur, CIBC World Markets.
With a significant number of top-selling prescription drugs coming off patent in the next few years (Table 3), brand-name research pharmaceutical companies are seeking ways to extend their patent protection. One option has been to revise the 1984 Hatch-Waxman Act. While Waxman agrees that the market is different today and recognizes the changing nature of the drug products, he has expressed concern over the motivations of some of the companies seeking legislative revisions. In a recent speech to an industry group, Waxman articulated
the need to stop—once and for all—the numerous efforts to obtain patent extensions benefitting a few at the expense of many. I am talking about efforts to secure special patent extensions and special extensions of market exclusivity, in direct contravention of the Waxman-Hatch Act and its underlying purpose.14
Table 3
Top 20 Prescription Drugs Coming Off Patent by December 31, 2005.
Seven companies are seeking legislation that would lengthen patent-term extensions from the Patent and Trademark Office. Companies such as Schering-Plough, which produces Claritin, argue that Food and Drug Administration (FDA) approval delays wasted several years of patent protection. However, as noted in the earlier-mentioned CBO study on generics and competition, amending Hatch-Waxman to lengthen patent-term extensions
would not encourage innovation as much as accelerating the FDA approval process by the same amount would. The reason is that lengthening patent terms increases profits today for drugs whose patents are about to expire, but it does not have as great an impact on the incentive to invest in R&D—that is, on the expected average value of the profits from marketing a drug. CBO calculates that increasing the average patent term by one year would raise the expected value of those profits by about $12 million in 1990 dollars. Accelerating the FDA review period by one year would boost returns by much more—about $22 million in 1990 dollars.
Generic Equivalents: Today and Tomorrow
According to the Generic Pharmaceutical Industry Association, the FDA uses three terms to describe generic drug products: pharmaceutical equivalence, bioequivalence, and therapeutic equivalence. Each is discussed below.
- Pharmaceutical equivalence—drug products are considered pharmaceutical equivalents if they have the same active ingredient(s), the same dosage form and are identical in strength as the brand-name product. Even if a generic has a different color, a different taste, or comes in a different shape or package, the FDA considers the product to be equivalent if it meets the same standards for strength, quality, purity and identity as the branded product.
- Bioequivalence—a generic drug is considered bioequivalent if it is absorbed in the bloodstream at the “same rate and extent” as the brand drug.
- Therapeutic equivalence—a generic drug is considered therapeutically equivalent to the comparable brand when the FDA determines the generic is safe and effective, pharmaceutically equivalent, and bioequivalent.
Because biologics (for example, human growth hormone) are difficult to produce and because the FDA currently has no mechanism for measuring the equivalency of generic biotech-based drug products, producing generics in the future will become more complicated. “For the forseeable future, generic manufacturers essentially have to repeat all the development and approval steps that the patent producers do.”15 The overall effect of this on the market, on competition, and on price, sales, and expenditures remains to be seen.
Drug Expenditures
As reported in the March 2000 Kaiser Family Foundation white paper, “Medicare and Prescription Drugs,”
(See Figure 3.)Pharmaceuticals are the fastest-growing component of national health expenditures. In 2000, national drug spending increased by an estimated 11% compared with 7% for physician services and 6% for hospital care. Since 1990, national spending for prescription drugs has tripled. By 2008, that figure is expected to more than double from an estimated $112 billion today to $243 billion.

Figure 3
National Spending for Prescription Drugs (in billions of dollars). Source: Health Care Financing Administration, Office of the Actuary.
Three factors have contributed to the recent increases in pharmaceutical budgets: unit cost inflation, utilization (that is, increases in the absolute number of prescriptions), and intensity (that is, availability of new drug technologies).
Fueling the increase in utilization is the explosion of direct-to-consumer (DTC) advertising by the pharmaceutical companies. The National Institute for Health Care Management’s July 1999 study, “Factors Affecting the Growth of Prescription Drug Expenditures,” reported that “the 10 drugs most heavily advertised directly to consumers in 1998 accounted for $9.3 billion or about 22 percent of the total increase in drug spending between 1993 and 1998.” The study, citing data from the Scott-Levin Source Prescription Audit Data, found that in 1998 pharmaceutical companies spent $8.3 billion promoting their products in the United States, of which approximately $1.3 billion was spent on DTC advertising and $7.0 billion on advertising and detailing to health care professionals.
Other nonprice factors explaining the growth in total drug expenditures include demographic changes (a growing elderly population, changing chronic disease prevalence patterns); the growth in third-party drug coverage, which tends to drive demand; record sales of new products; new product formulations; changing mix of products used; patient noncompliance; and inappropriate prescribing. Another way of looking at total drug expenditures is shown in Figure 4.

Figure 4
Factors Influencing Drug Expenditures. Source: Steven W. Schondelmeyer, Ph.D., PRIME Institute, University of Minnesota, January 2000.
Pharmaceutical Pricing: Multiple Markets, Multiple Prices
As indicated above, pricing alone does not account for the total growth in drug expenditures. But it does, of course, play a role, especially for newer products, many of which are currently working their way through the R&D pipeline.
Defining and comparing pharmaceutical prices is complicated and not always consistent—many terms are used and many methodologies are employed.16 There is no one way to price a product. A variety of factors are considered in drug pricing, among them the relative commercial success of the agent; the prices, product features, and past actions of the competition; specific patient characteristics; the economic and social value of the therapy itself; the decision-making criteria of prescribers and those who influence that decision; company needs in terms of market position, revenue, and other considerations; the current and anticipated insurance reimbursement environment; company abilities, including available budgets and willingness to support the project; and the type of manufacturer supplying the drug.17
Three basic pricing strategies, each chosen to maximize a competitive edge are described by E. M. Kolassa below:The importance of competitive analysis in pricing cannot be overstated. It has been suggested by many researchers that the pricing and presence of competitors, together with the uniqueness or therapeutic value of the new product, are the major determinants of launch prices.18
- Skimming—The product, anticipating little direct competition, is priced above prevailing levels to maximize profits. Prilosec, the first proton pump inhibitor, was priced in this manner, substantially above the price of the H2 antagonists.
- Parity—The product is viewed internally as being little or no different from current competitors and is priced equivalent to the prevailing levels. The nonsedating antihistamine Claritin and the ACE inhibitor Accupril were priced at parity to the market leaders at the times of their launches.
- Penetration—A product is viewed as equal to or slightly inferior to current or anticipated offerings and is priced below prevailing levels in hopes of gaining market share with its low price or of erecting a barrier to entry for anticipated future competitors. Lescol appears to be the only pharmaceutical product to have successfully employed a penetration pricing strategy.19
Manufacturers use these various pricing methodologies depending upon internal strategies, external forces, distribution channels, and specific purchasers. As external market forces change, internal pricing strategies also change (see Figure 5).
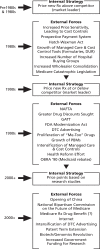
Figure 5
Drug Pricing Methodologies: Internal Strategies & External Forces 1980s, 1990s, & 2000+.
A specific drug product can be priced differently in different markets, at different points in the distribution chain, and for different purchasers. Domestically, the pharmaceutical market can be broken down into various segments essentially falling into two broad categories: private markets and government programs. Markets can be segmented in other ways as well, such as by branded and generic as well as chronic and acute.
The Private Market
The private marketplace includes retail (which comprises traditional chain drug stores, mass merchandisers, independent pharmacies, supermarket pharmacies, and mail order pharmacies); wholesale; hospital; managed care organizations and providers (such as clinics, long-term care facilities—including nursing homes—outpatient facilities, and physician offices); and the Internet.
Retail, Wholesale, Mail Order and the Internet. On its Web site (www.nacds.org), the National Association of Chain Drug Stores (NACDS) reports the following figures:
- Nationwide, there are more than 30,000 pharmacies operated by traditional chain pharmacy companies, supermarkets, and mass merchants. In addition, there are another 20,000 independent pharmacies.
- In recent years, the retail prescription drug industry has grown dramatically. The number of retail prescriptions dispensed each year increased from 2.0 billion in 1992 to 2.6 billion in 1997. This represents a 23 percent increase in just five years. In 1998, this number reached 2.73 billion.
- The chain pharmacy is the leading component of this industry. It dispenses more than 60 percent of these prescriptions, which equals 1.6 billion prescriptions a year or 4 million each day.
According to data from the April 2000 DHHS ASPE report, Prescription Drug Coverage, Spending, Utilization, and Prices:
- Over the past few years, the wholesale drug industry has become quite concentrated. While there are still a number of wholesalers in operation, the top five wholesalers account for 90 percent of the entire wholesale drug market.20 In 1998, the net sales of prescription drugs by wholesalers were $57 billion.
- Mail order pharmacy accounts for about 12 percent of the total retail prescription market. Between 1997 and 1998 mail service pharmacy grew by 19 percent.21 This compares to the total prescription market, which grew by 18.5 percent.22 It is interesting to note that Internet pharmacies use mail order to distribute their products and are members of the mail order pharmacies’ professional organization.
Government Programs
The federal market encompasses such government programs as the Federal Employee Health Benefit program, the Department of Veterans Affairs (VA) and its federal supply schedule (FSS), Medicaid, and various public health service programs. Various government programs have legislated drug pricing and reimbursement methodologies.
The VA Federal Supply Schedule. The Veterans Health Care Act established a mandatory federal ceiling price on a manufacturer’s sales of innovator medicines to four federal agencies: the VA, the Department of Defense, the Public Health Service (including the Indian Health Service), and the Coast Guard. The formula establishes an upper limit on all procurements by any of the four agencies equal to 76 percent of the weighted average nonfederal selling price for the product, limited to annual increases of no more than the increase in the consumer price index-urban (CPI-U). The FSS generally limits annual increases in any pharmaceutical price over the life of a contract (typically five years) to the increase in the CPI-U over the same period.
Medicaid Rebates. The Omnibus Budget Reconciliation Act of 1990 (OBRA) established the Medicaid rebate program. The basic formula requires that, in exchange for having their products reimbursed (that is, on the formulary), pharmaceutical manufacturers rebate to the states the greater of (a) 15.1 percent of the average manufacturer price (AMP)23 paid by wholesalers for brand-name drugs that Medicaid beneficiaries purchase as outpatients or (b) the difference between AMP and the manufacturer’s “best price.” The best price is the lowest price offered to any other customer, excluding FSS prices and prices to state pharmaceutical assistance programs. Similarly, manufacturers pay a rebate equal to 11 percent of the AMP on generic and over-the-counter drugs.
If a brand-name drug’s AMP increases faster than the inflation rate, an additional rebate is imposed so that manufacturers cannot offset the basic rebate by raising their AMP. The additional rebate is equal to the difference between the current AMP and a base-year AMP increased by the inflation rate as measured by the consumer price index.24
OBRA 93 changed the pricing schedule of single-source and innovator multiple-source drugs approved by the FDA after October 1990.
In general, OBRA 93 had an impact on the computation of the unit rebate amount for covered outpatient drugs. The effective date for implementation of OBRA 93 was October 1, 1993. Presently, more than 500 manufacturers have rebate agreements with the Federal Government which, in turn, address approximately 55,000 drug products.25
Medicaid—which accounted for over $2.2 billion worth of pharmaceutical manufacturer rebates in 1997—is not alone in its use of rebates. As the number of HMOs and PBMs increased, manufacturers began to offer rebates in an effort to gain access to managed care patients.26
These programs add another dimension to drug pricing variability. The price a manufacturer sets often changes as it makes its way through the distribution chain and onto the negotiating table. It is not unusual for one specific product to be priced differently in different markets (see Table 4).
Table 4
Illustrative Example of Pricing for Brand Name Prescription Drugs.
Many cross-national drug pricing comparisons have been made over the years. While these findings are significant, they merit caution in how the comparisons are made and in how the conclusions are drawn. Because markets, demographics, and values vary, and because medical practices and economic circumstances also vary, it is difficult simply to transfer one country’s pricing methods to another. Nevertheless, for many reasons, price differentials between products purchased in the U.S. and other countries are oftentimes substantial, leaving many to question why the gap is so wide as well as whether and how it should be narrowed.
The Forum Session
This Forum session, designed as an institute providing background information as well as raising policy issues, will begin with a discussion of the supply side of the market. Glenna M. Crooks, Ph.D., president and chief executive officer, Strategic Health Policy International, will open with a discussion of “The Price of Innovation: Internal R&D Decisions,” answering questions such as the following: How are R&D decisions made? Who makes them? How has the nature of R&D changed over the past decade or two (for example, greater reliance on computers and government/academic funded research, fewer “hit and miss” scenarios, big companies as holding companies for innovation investments, and strategic alliances)? How is risk spread across classes of drugs? How is R&D in big companies different from R&D in small companies?
Judith L. Wagner, Ph.D., principal analyst with the Congressional Budget Office, will provide a briefing on “Pharmaceutical Marketplace Dynamics,” covering a multitude of questions, including the following: How the market interacts with R&D decisions. How does the market work? To whom do drug companies sell (industrial organization)? What is competition in this market? How do companies compete? Is competition compromised by recent mergers? What are the effects of generics, intellectual property, PBMs and formularies, and DTC advertising on the marketplace? At what point(s) do the supply and demand sides of the market come together?
The program will continue with an overview of the demand side of the market. John M. Coster, Ph.D., R.Ph., vice president of federal and state affairs for the National Association of Chain Drug Stores, will present “The Private Market,” describing dynamics in the retail market—pharmacies, hospitals, managed care organizations, mail order; across distribution channels—wholesalers, buying groups, prime vendors; and within the Internet.
Dan Mendelson, managing director of the Health Strategies Consultancy, will present “The Federal Market,” highlighting current government programs (FFHBP, Medicaid Rebates, VA FSS, PHS programs) and potential future government action, including the intended and unintended consequences of a Medicare prescription drug benefit and various price control bills.
Wrapping up the morning session will be Patricia M. Danzon, Ph.D., the Celia Moh professor in the health care department at the University of Pennsylvania’s Wharton School. Danzon will answer questions involving “The International Market,” including the following: Are international price comparisons valid? What can we learn from them? How and why do prices differ between the United States and other countries? For which products are the differences greatest? Could the costs be more equitably distributed? What are the higher U.S. prices actually funding (R&D versus marketing)? What have been the effects of GATT and NAFTA? What does the opening of China mean for pharmaceutical markets?
The morning session will be followed immediately by a luncheon briefing highlighting the findings of the recently released DHHS report to the president, Prescription Drug Coverage, Spending, Utilization, and Prices. John F. Hoadley, Ph.D., director of the division of health financing policy in the office of the assistant secretary for planning and evaluation will provide an overview of the study and its findings. He will be joined by other members of the research team who will be on hand to answer questions.
Pharmaceutical Pricing Terms27
- Actual Acquisition Cost (AAC)
Retail pharmacy reimbursement arrangements are often based on the AWP (see below) plus a fee. Knowing that retailers no longer pay the published AWP for prescription drugs, many payers attempted to reduce the reimbursement by discounting the AWP by 5% to as much as 20%. Because this system penalized the pharmacies that are unable to secure significant discounts from wholesalers, some payers have instituted a payment schedule on the basis of actual acquisition cost plus a fee. Billing complexities and schemes, however, make it difficult to ascertain the actual acquisition cost.
- Average Manufacturer’s Price (AMP)
This term was developed by the drafters of OBRA 90 and is used to describe the average price received by a manufacturer, after discounts, for products sold to the retail class of trade. The AMP is used for computing the rebates that are paid to state Medicaid programs.
- Average Wholesale Price (AWP)
Neither an average price nor a price charged by wholesalers, this figure is a vestige of earlier times. Few, if any, wholesalers even consider AWP today when pricing their prescription products. It is, however, commonly used by retailers and others who dispense medications as the basis for many pricing decisions. Due to its availability from many sources, the AWP is often used as a surrogate for actual prices when studying prescription price trends.
- Cash Discounts
Most pharmaceutical firms offer incentives to their customers for rapid payment of invoices. The most common terms offered are a 2% discount if the full bill is paid within 10 days of receiving the invoice. Thus a wholesaler that pays the regular ex-factory price actually pays only 98% of that price if it pays within 10 days. The wholesaler that sells at cost plus 3%, then, is actually charging a markup of roughly 5%.
- Chargeback
This is the difference between the price a wholesaler pays a manufacturer (see WAC) and a lower contract price that has been negotiated by a hospital or managed care organization. Because of complexities of tracking products and some legal limitations, the chargeback system was developed as a means for discounted products to be sold through wholesalers. The wholesaler purchases the product at the normal list price and sells the product to hospitals or other contract customers at the discount price. The difference is then paid as a rebate to the wholesaler by the manufacturer. This rebate is called the chargeback.
- Class of Trade
Under federal law, all businesses that sell to the same customer type must be eligible to receive equal pricing consideration, such as discounts and special offers. To assure compliance with this law, most pharmaceutical companies have developed lists of similar customers and grouped them into different classes of trade. Pricing schedules and tactics are then developed for each class of trade.
- Direct Price
The price paid by retailers, before discounts, for products from those manufacturers who sell directly to nonwholesale accounts such as retailers, hospitals, private practice physicians, and public health clinics is called the direct price.
- Earned Margin
Earned margin is a term used by some retail pharmacists to describe the difference between the AWP and the actual product cost, as paid to the wholesaler or manufacturer.
- Ex-Factory Price
This is the actual selling price, before discounts, charged by the manufacturer. (see WAC).
- Gross Profit (Margin)
The difference between acquisition or production cost of a product and its selling price is known as the gross profit margin. The gross profit margin does not include other costs of doing business.
- Loss Leader
A loss leader is a retail promotional pricing tactic in which the retailer charges a price that is below cost to entice customers into the store, hoping that the customers will make additional purchases while there. In retail pharmacy, a loss leader is not always priced below actual costs, but below AWP. It can, however, be argued that the transaction is indeed a loss when factoring in the professional time and services required to fill a prescription. Still, a pharmacy loss leader does not imply selling the product below acquisition cost.
- MAC
The MAC is the maximum allowable cost, which is the federally set reimbursement rate for generic drugs used in the Medicaid system. Many other payers use MAC systems as well. The federal MAC is also called the FFP, which stands for federal financial participation. It is set at 150% of the lowest generally available price for generics.
- Manufacturer’s List Price
As the name implies, the list price is a price that has been published by a manufacturer. Many manufacturers make actual list prices available only to wholesalers, providing a catalog that contains AWPs to the nonwholesale trade (see Ex-Factory Price).
- Net Price
Also known as “landed price,” this is the price, or revenue, realized by a manufacturer after all discounts have been granted.
- Net Profit (Margin)
Net profit margin is the difference in selling price and all costs associated with doing business, allocated on a per-unit basis.
- OBRA 90
The Omnibus Budget Reconciliation Act of 1990, a law drafted by the Senate Committee on Aging, requires manufactures to pay rebates to state and federal governments for products used by Medicaid recipients.
- Rebate
A rebate is a retroactive discount that is paid to a customer after that customer has purchased the product from a wholesaler or retailer. The rebate allows the manufacturer to offer a lower price to some customers without taking on the burden of special distribution mechanisms.
- Standard Cost
The product costing system used by most pharmaceutical firms is called “standard costing” or “fully absorbed cost.” With this system, in addition to the variable costs such as ingredients, packaging, and direct labor, a portion of fixed cost (overhead) is allocated to each product and package. This cost is allocated on the basis of forecasts made at the beginning of the fiscal year. Such a system assures that, when unit volume increases, the incremental cost of a unit will decline, while the incremental cost of a product with a declining sales trend will increase significantly. It is not uncommon for half or more of a product’s standard costs to consist of this fixed cost allocation.
- Wholesale Acquisition Price (WAC)
This term is used by some publishers of pricing data to denote the ex-factory charge, before discounts to the wholesaler.
Endnotes
- 1
David E. Rosenbaum, “The Gathering Storm over Prescription Drugs,” The Nation: Pill Box, New York Times, November 14, 1999.
- 2
Office of the Assistant Secretary for Planning and Evaluation, Prescription Drug Coverage, Spending, Utilization, and Prices, Report to the President, Department of Health and Human Services, Washington D.C., April 2000, 149.
- 3
David A. Shaywitz and Dennis A. Ausiello, “Can Drug Giants Survive the Biomedical Revolution?” Wall Street Journal, February 8, 2000.
- 4
“Drug Mergers Change Health Landscape,” American Medical News, January 31, 2000, 4.
- 5
Patricia Barry, “What’s Behind High Drug Prices in U.S.?” AARP Bulletin, 41, no. 4 (April 2000): 6–7.
- 6
Barry, “High Drug Prices,” 7.
- 7
Shaywitz and Ausiello, “Drug Giants.”
- 8
Shaywitz and Ausiello, “Drug Giants.”
- 9
Shaywitz and Ausiello, “Drug Giants.”
- 10
Congressional Budget Office, “How Increased Competition from Generic Drugs Has Affected Prices and Returns in the Pharmaceutical Industry,” Excerpts, Washington, D.C., July 1998, xi.
- 11
The U.S. Patent and Trademark Office has deemed a gene sequence can be patented if it meets a three-pronged test for utility: its utility must be specific to the gene sequence in question, not to genes in general; it must have a real world substantial utility; and it must be credible to a person of ordinary skill in the art of sequencing.
- 12
Marilyn Werber Serafini, “No Easy Prescription on No-Name Drugs,” National Journal, February 19, 2000, 548.
- 13
Bonar Menninger, “Can Generics Turn It Around?” Healthcare Business, January/February 2000, 20.
- 14
Serafini, “No Easy Prescription,” 548.
- 15
Menninger, “Generics,” 51.
- 16
See “Pharmaceutical Pricing Terms” at the end of this issue brief.
- 17
E. M. Kolassa, Elements of Pharmaceutical Pricing (New York: The Haworth Press, Inc., The Pharmaceutical Products Press, 1997), 33, 46.
- 18
Kolassa, Pharmaceutical Pricing, 49.
- 19
Kolassa, Pharmaceutical Pricing, 50.
- 20
National Wholesale Druggists’ Association, Industry Profile and Health Care Fact Book, Addendum, 1999.
- 21
Anonymous, “Mail Order Drug Sales Leap 10 Percent,” American Druggist, July 1, 1999.
- 22
M. Glaser, “Boom Year,” Drug Topics, April 5, 1999, 47–53.
- 23
Average manufacturer price is the weighted average price to wholesalers for product distributed to the retail pharmacy class of trade, where wholesaler is defined as any entity to whom the manufacturer sells (except relabelers) and where retail pharmacy class of trade excludes hospitals and HMOs.
- 24
Congressional Budget Office, “How the Medicaid Rebate on Prescription Drugs Affects Pricing in the Pharmaceutical Industry,” CBO Papers, January 1996, xi.
- 25
David K. Baugh, Penelope L. Pine, and Steven Blackwell, “Trends in Medicaid Prescription Drug Utilization and Payments, 1990–97,” Health Care Financing Review, 20, no. 3 (Spring 1999), 80 [PMC free article: PMC4194628] [PubMed: 10558022]. (The figures in this quotation that concern the number of manufacturers with rebate agreements with the federal government are derived from a personal communication to the authors from S. Gaston, Baltimore, March 11, 1999.)
- 26
For a more thorough explanation of PBMs, see Robin J. Strongin, The ABCs of PBMs, National Health Policy Forum Issue Brief No. 749, October 27, 1999. [PubMed: 10848111]
- 27
Kolassa, Pharmaceutical Pricing, 30–33.
A background briefing featuring
- Glenna M. Crooks, Ph.D.President and Chief Executive OfficerStrategic Health Policy InternationalFort Washington, Pennsylvania
- Judith L. Wagner, Ph.D.Principal AnalystCongressional Budget Office
- John M. Coster, Ph.D., R.Ph.Vice PresidentFederal and State ProgramsNational Association of Chain Drug Stores
- Dan MendelsonManaging DirectorHealth Strategies ConsultancyWashington, D.C.
- Patricia M. Danzon, Ph.D.Celia Moh ProfessorWharton SchoolUniversity of Pennsylvania
- John F. Hoadley, Ph.D.DirectorDivision of Health Financing PolicyOffice of the Assistant Secretary for Planning and EvaluationDepartment of Health and Human Services
NHPF is a nonpartisan education and information exchange for federal health policymakers.
- NLM CatalogRelated NLM Catalog Entries
- Review The economics of prescription drug prices, government intervention, and the importation of drugs from Canada.[Nurs Econ. 2005]Review The economics of prescription drug prices, government intervention, and the importation of drugs from Canada.Openshaw MS. Nurs Econ. 2005 Nov-Dec; 23(6):307-11, 279.
- Medicine and the pharmaceutical industry: what's right, what's wrong, and what's to come.[Singapore Med J. 1998]Medicine and the pharmaceutical industry: what's right, what's wrong, and what's to come.Tan SY. Singapore Med J. 1998 Mar; 39(3):91-5.
- How to Reduce Prescription Drug Prices: First, Do No Harm.[Mo Med. 2020]How to Reduce Prescription Drug Prices: First, Do No Harm.Atlas S. Mo Med. 2020 Jan-Feb; 117(1):14-15.
- Lowering the cost of prescription drugs.[J La State Med Soc. 2003]Lowering the cost of prescription drugs.Jindal B. J La State Med Soc. 2003 Jan-Feb; 155(1):51.
- Review The High Cost of Prescription Drugs in the United States: Origins and Prospects for Reform.[JAMA. 2016]Review The High Cost of Prescription Drugs in the United States: Origins and Prospects for Reform.Kesselheim AS, Avorn J, Sarpatwari A. JAMA. 2016 Aug 23-30; 316(8):858-71.
- Pharmaceutical Marketplace DynamicsPharmaceutical Marketplace Dynamics
- Decreased renal parenchymal thicknessDecreased renal parenchymal thicknessMedGen
Your browsing activity is empty.
Activity recording is turned off.
See more...
