NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Menini A, editor. The Neurobiology of Olfaction. Boca Raton (FL): CRC Press/Taylor & Francis; 2010.
5.1. INTRODUCTION
Several excellent reviews have detailed the conservation of principles between insect, amphibian, and mammalian olfactory systems, and many important contributions to the field have been made by cross-phyla comparisons. However, within the limitations of this reference, in this chapter we will emphasize embryonic development and axon targeting in the rodent olfactory system, focusing on the mouse. To aide comparisons between studies in other rodents, the developmental ages in studies in the rat will be adjusted and referred to as the equivalent developmental age in mice. We apologize for any papers we may have missed or not had the opportunity to discuss in detail due to space limitations.
The olfactory system is one of the most precocious sensory systems to develop in the embryo. The primary olfactory pathway is comprised of two components, the olfactory epithelium (OE) and the olfactory bulb (OB). The secondary olfactory pathway includes multiple cortical regions, all of which are directly innervated by OB projection neurons, and are collectively referred to as the olfactory cortex. Major regions of the olfactory cortex include the anterior olfactory nucleus (AON), the olfactory tubercle, the piriform cortex, and the entorhinal cortex (reviewed in Wilson et al. 2006). These regions are innervated by mitral and/or tufted cell axons via the lateral olfactory tract (LOT).
During the earliest stages of primary olfactory pathway formation, the OE and the OB undergo simultaneous, yet independent, developmental programs (Lopez-Mascaraque and De Castro 2002). However, as development progresses and axons from the OE innervate the nascent OB, their developmental programs become interrelated (Treloar et al. 1999; Matsutani and Yamamoto 2000; Lopez-Mascaraque et al. 2005). The early separation in developmental programs is perhaps not surprising given the spatial segregation of the regions that give rise to these two structures (see below). Despite a common ectodemal origin, the OE (the peripheral component of the olfactory system) is derived from the olfactory placode, while the OB emerges from the germinal zones of the neural tube, like other central nervous system (CNS) structures.
5.2. FORMATION OF THE NASAL CAVITY
The olfactory placodes that give rise to the OE are specialized areas of cranial non-neural ectoderm found in the rostrolateral regions of the head. This specialized epithelial thickening invaginates to form a simple nasal pit. Like the majority of placodes, some mesenchymal cells migrate away from the placodal epithelium and differentiate as either secretory cells or glial cells (Scholsser et al. 2006). In rodents, the formation of the nasal pit occurs soon after the closure of the neural tube; the formation of the OE precedes the formation of the OB. In mice, the olfactory placodes are identified as epithelial thickenings as early as embryonic day 9 (E9), which is equivalent to Theiler Stage 14 (TS14; Theiler 1972). It must be noted that the embryonic days stated here are based on E0 being designated the day a positive vaginal plug is identified on the morning after mating. Due to differences in criteria between researchers in naming embryonic stages by days, we have included the Theiler stages as an unambiguous standard, which can easily be cross-referenced with common resources such as The House Mouse: Atlas of Mouse Development (Thieler 1972), The Atlas of Mouse Development (Kaufman 1992), and the Emap Digital Atlas of Mouse Development (http://genex.hgu.mrc.ac.uk/Atlas/intro.html). It is important for readers to note that careful attention should be paid to the staging and nomenclature strategy adopted by individual researchers, as this is crucial for interpretation and for comparison between studies. Staging embryos using established developmental landmarks is essential; at early stages, individual embryos can vary by as much as two to three stages in their development across the uterine horn, making staging by days postconception (dpc) insufficient for early developmental analyses.
By E10/TS16, the olfactory placode has thickened considerably since its initial appearance and begins to invaginate, appearing much like a simple bowl. This structure is referred to as the olfactory pit and is the beginning of the nasal cavity. Twelve hours later (E10.5/TS17), the nasal pits have invaginated further, forming distinct marginal rims. At E11/TS18, the olfactory pit has deepened considerably and the rims are beginning to unite, forming the nostrils. By E11.5/TS19, the nostrils are narrowed to small slits and the nasal pit has further invaginated into a more complex nasal cavity. In the medial wall of the newly formed nasal cavity, the vomeronasal organ (VNO) has invaginated further into a separate, distinct cavity. Thus, by E11.5/TS19, the main olfactory and vomeronasal cavities/epithelia can be distinguished. A schematic representation of the formation of the nasal cavity from the olfactory placode is shown in Figure 5.1.

FIGURE 5.1
Schematic diagram of the developing nasal cavity from Thieler Stage (TS) 15/embryonic day 9.5 (E9.5) to TS19/E11.5. At TS15, the olfactory placodes (shaded gray) are located very laterally, and are only a few cell layers thick. By TS16, the placodes have (more...)
During this period, there is a marked realignment of the polarity of the olfactory system, from rostrolateral to rostral. While the placodes are initially laterally located on the embryo, as development proceeds and the nasal pits and nostrils form, the nasal cavity shifts orientation from a rostrolateral position to a more rostral location. This is best seen in horizontal sections through the head (Figure 5.1); the placodes are laterally located and as they invaginate and nasal pits form, the orientation of the developing nasal cavity gradually moves rostrally until the nares form at the most rostral tip of the head.
5.2.1. Molecular Basis of Nasal Pit Formation
The signals that control olfactory placodal induction are not as well understood as other sensory placodes. Perhaps the best insights into olfactory placode induction come from a recent study in chick, where several transcription factors were identified whose spatiotemporal expression patterns reflect olfactory fate acquisition (Bhattacharyya et al. 2008). Transcripts for Dlx5 and Pax6 are present quite early at the neurula stage, however four stages later, expression of Dlx5 is upregulated while expression of Pax6 is inversely downregulated. In contrast, Dlx3 is expressed at low levels early on, but is upregulated once cells are committed to an olfactory fate (Bhattacharyya et al. 2008). Furthermore, other work in Xenopus has demonstrated that the induction of the olfactory placode is blocked by hedgehog signaling (Cornesse et al. 2005). Recent studies in the mouse have identified the transcription factors Sox2, Oct-1 (encoded by the Pou2fl gene), and Pax6 as combinatorial components of the molecular pathway used to induce the olfactory placode (Donner et al. 2006). Mice with mutations in both Sox2 and Pou2fl fail to undergo nasal morphogenesis, but mice with just a single mutation in either gene exhibit normal developmental staging (Donner et al. 2006). Similarly, in the Pax6 mutant mouse small eye (Pax6Sey/Sey), the olfactory placodes fail to differentiate (Hill et al. 1991; Grindley et al. 1995).
Following placode induction, the subsequent development of the nasal cavity involves signaling by retinoic acid (RA), fibroblast growth factors (FGFs), and bone morphogenetic proteins (BMPs) from the adjacent frontonasal mesenchyme and olfactory ectoderm (e.g., LaMantia et al. 2000; Kawauchi et al. 2004, 2005; reviewed in Beites et al. 2005; Balmer and Lamantia 2005; Rawson and LaMantia 2006). RA was shown to be involved in mesenchymal/epithelial (M/E) signaling and, together with Fgf8, Bmp4, and Sonic hedgehog (Shh), is involved in defining axes in the developing OE and olfactory nerve (LaMantia et al. 2000; Bhasin et al. 2003; reviewed in Balmer and Lamantia 2005; Rawson and LaMantia 2006). Previous work has demonstrated that RA signaling between the placode and the associated mesenchyme is essential to generate both the molecular and cellular diversity of the OE and to establish the appropriate axon connections within the primary olfactory pathway (LaMantia et al. 2000).
Using in vitro assays, RA, Fgf8, Shh, and Bmp4 were found to provide different axial M/E signals: RA is a lateral signal, Bmp4 is a posterior signal, and Fgf8 and Shh are both medial signals. When their signaling is blocked or augmented in vitro, distinct aspects of olfactory pathway differentiation and patterning are compromised (LaMantia et al. 2000). Complementing these findings is a recent study on the role of Fgf8 in the developing olfactory system using a conditional knockout approach to create mice with Fgf8 inactivated in the OE from the earliest stages of development (Kawauchi et al. 2005). In normal development, Fgf8 mRNA is expressed in the rim of the invaginating nasal pit in a small domain of cells termed the morphogenetic center, which partially overlaps with the domain of putative OE neural stem cells later in gestation. In the Fgf8 null mice, the initial invagination of the nasal pit and the initiation of the OE differentiation occur, but development halts shortly thereafter due to apoptosis of cells in the morphogenetic center and adjacent developing neuroepithelium. Consequently, a definitive OE and VNO fail to develop in these mice. Thus, Fgf8 is crucial for the proper development of the OE, the nasal cavity, and the VNO (Kawauchi et al. 2005).
This process of molecular signaling between the mesenchyme and epithelium (M/E) is not unique to the olfactory system and is common at other sites of nonaxial induction, such as the limb buds, heart, and brachial arches (reviewed in Balmer and Lamantia 2005; Rawson and LaMantia 2006). At these sites (the olfactory placode included), local production of RA and the RA synthetic enzyme Raldh2 in the mesenchyme are autonomous (Bhasin et al. 2003). However, in the placode there are some differences in M/E signaling compared to the other sites, such as unique expression of RALDH3 in the placode (Bhasin et al. 2003; Kawauchi et al. 2004), and different effects of Fgf8 on the RA receptor RARb and Raldh2 (Bhasin et al. 2003). These findings indicate that the olfactory system has apparently modified a general mechanism of M/E regulation to meet its requirements of establishing the primary olfactory pathway.
5.3. PRIMARY OLFACTORY EPITHELIAL GENESIS
The OE is a pseudostratified neuroepithelium that comprises multiple cell types. In addition to mature and immature OSNs, which reside in the middle pseudolayer, there are two subpopulations of basal cells, the horizontal basal cells (HBCs) and the globose basal cells (GBCs), as well as supporting or sustentacular cells that reside in the apical pseudolayer. These cell types all differentiate after placodal induction and are thought to come from a common progenitor (reviewed in Nicolay et al. 2006). The lineage of olfactory sensory neurons (OSNs) has been established by a number of laboratories using a combination of in vitro and in vivo studies (reviewed in Kawauchi et al. 2004; Beites et al. 2005). Briefly, a population of self-renewing stem cells, probably the GBCs (Caggiano et al. 1994; Huard et al. 1998), give rise to a population of transit-amplifying cells that express mammalian achaete-scute homolog 1 (Mash 1+), a basic helix-loop-helix (bHLH) transcription factor essential for OSN development (Guillemot et al. 1993). Mash 1+ cells give rise to a second transit-amplifying progenitor, the intermediate precursor (INP), which expresses another bHLH transcription factor, neurogenin 1 (Ngn 1) (Cau et al. 1997, 2002). Daughter cells from dividing INPs differentiate into OSNs, which are easily identified by a variety of markers, including the neural cell adhesion molecule (NCAM) and the olfactory marker protein (OMP) (reviewed in Nicolay et al. 2006).
Interestingly, at the very earliest stages of epithelial development, neurogenesis follows a slightly different scheme than later in embryogenesis, postnatal and adult stages (see Chapter 10). At early embryonic stages (e.g., E11-E14), the majority of dividing cells are found apically in the developing OE, while later in development proliferation is found predominantly near the base of the OE (Smart 1971; Carson et al. 2006).
5.4. DEVELOPMENT OF THE OLFACTORY NERVE
Soon after OSNs differentiate, they extend an axon that pierces the basement membrane of the OE, enters the underlying frontonasal mesenchyme, and begins the process(es) of navigating from the developing OE to the rostral portion of the telencephalon where the OB will develop. OSN axons do not migrate independently, a population of migratory cells also exits the olfactory placode and migrates with the OSN axons, forming an accumulation of axons and cells that have been termed the “migratory mass” (Valverde et al. 1992). The exact nature of the migratory cells is still uncertain, but probably includes precursors of the olfactory ensheathing glial cells (Valverde et al. 1992), some cells that migrate to other brain regions, including the (gonadotropin-releasing hormone (GnRH) or LHRH) expressing cells (Schwanzel-Fukuda and Pfaff 1989; Wray et al. 1989a, 1989b; Valverde et al. 1993; De Carlos et al. 1995), as well as some migratory cells that are OMP+, express OR proteins and may be putative “guidepost” cells for growing OSN axons (Conzelmann et al. 2002). It is not yet established whether OSN axons or migratory cells exit the placode first, but both axons and cells exit very early in embryonic development and migrate through the mesenchyme medially, toward the telencephalon (Whitesides and LaMantia 1996). OSN axons exit the OE and make an immediate turn toward the telencephalon (Treloar et al. 1996; Whitesides and LaMantia 1996). To date, it is unclear which cues underlie this highly stereotypic turn, but differentially expressed ECM molecules in the frontonasal mesenchyme may form a permissive pathway for growing OSN axons (Whitesides and LaMantia 1996).
The formation of the olfactory pathway is unique among sensory systems in that OSNs are the only sensory transduction cells that do not follow established migratory pathways (reviewed in Balmer and LaMantia 2005). The initial establishment of the olfactory nerve involves OSN axons and migratory cells pioneering a pathway, using guidance cues present in the mesenchyme as well as chemotrophic cues released from the telencephalon. The migratory mass grows/projects along the mediorostral surface of the telencephalon, not coming in contact with its surface until E11.5 in mice, when it establishes contact with the rostral-most tip of the telencephalon (Figure 5.2). By E12, the migratory mass has formed a presumptive olfactory nerve layer (pONL; Figure 5.2), with OSN axons entering the CNS though small fenestrations in the basement membrane of the telencephalon (Marin-Padilla and Amieva 1989; Treloar et al. 1996; Gong and Shipley 1996; Balmer and LaMantia 2004; Figure 5.2). A small subpopulation of OSN axons do not remain restricted to the pONL, instead they extend into the ventricular zone of the telencephalon (Pellier et al. 1994; Figure 5.2), where they are hypothesized to stimulate OB formation (see below) (Gong and Shipley 1995). By E12.5, these deep penetrating axons are no longer detected in the ventricular zone, although the fate of these axons is unknown. OSN axons remain restricted to the pONL until glomerulogenesis begins (see below), around E15 in mice (Treloar et al. 1999). During this four-day window, from E11.5 to E15, from when the axons first contact the telencephalon and form a pONL to when the axons grow deeper into the OB and establish synaptic connections, a continued growth of OSN axons into the pONL occurs as more OSNs are generated. It has been hypothesized that this waiting period in the pONL is crucial for OSN axons to sort prior to forming appropriate topographic connections in the OB (see below) (Treloar et al. 1999).
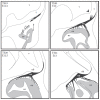
FIGURE 5.2
Schematic diagram of olfactory nerve formation from TS19/E11.5 to TS21/E13. At TS19, OSN axons have first contacted the telencephalon, though they have not penetrated the basement membrane. Twelve hours later, at E12 (early TS20), OSN axons penetrate (more...)
5.4.1. Molecular Basis of Olfactory Nerve Formation
While relatively little is known about the molecular cues that underlie the earliest stages of olfactory nerve formation, studies from transgenic mice have provided insight into some of the molecules that are involved in this process. Perturbation in a number of transcription factors is associated with anomalies in the formation of the olfactory nerve. These include Fez, Klf7, Arx, Emx2, Gli3, Six1, Dlx5, and the hypomorphic Fgf8; all have resulted in a similar phenotype in the olfactory nerve pathway. In most of these transgenics, the OSNs differentiated normally and extended axons, but deficits were seen in the formation of the olfactory nerve (Yoshida et al. 1997; Mallamaci et al. 1998; Yoshihara et al. 2004; Balmer and LaMantia 2004; Laub et al. 2005; Merlo et al. 2007; Ikeda et al. 2007; Chung 2008). OSN axons defasciculate and project aberrantly near the forebrain, rarely entering the CNS. Despite the common phenotype in these knockouts, a common mechanism has not been identified. Furthermore, while many of these molecules are expressed in the OSNs (Dlx5, Six1, Fez, Emx2, Fgf8, Klf7), some are not (Arx, Gli3). This raises the question of whether the projection of OSN axons to the telencephalon is dependent on axon guidance mechanisms or if connectivity is controlled via non-cell-autonomous mechanisms. For example, these genes could affect the formation of the olfactory nerve pathway by affecting the migration and differentiation of migratory cells located along the olfactory nerve pathway, or by influencing axon-mesenchyme interactions via a cell nonautonomous mechanism (Merlo et al. 2007).
Also necessary for OSN axons targeting the OB is expression of a 7-transmembrane (7TM) receptor, like an odor receptor. Feinstein and Mombaerts (2004) demonstrate that when certain mutations are made in ORs, such as chimeric ORs or early stop codons, which they term neomorphic mutations, OSN axons expressing the mutated OR display poor outgrowth and fail to reach the OB. Thus, ORs appear necessary for OSN axon targeting to the OB. However, in mice that have had the beta-adrenergic 7TM receptor substituted into the M71 OR locus, the OSN axons can target or coalesce into glomeruli, indicating that 7TM receptor expression is not a unique axon guidance mechanism specific to ORs (Feinstein and Mombaerts 2004; cf. Chesler et al. 2007). Given the widespread expression of 7TM receptors within the nervous system, it is interesting to consider that 7TM receptor expression may be a ubiquitous axon guidance mechanism.
5.5. DEVELOPMENT OF THE OLFACTORY BULB (OB)
The OB develops from a predetermined region of the rostral telencephalon (Lopez-Mascaraque et al. 1996). During early embryonic development, the telencephalon is prespecified into different areas that develop into distinct adult brain regions. The initial model of area specification, first proposed by Rakic (1988), has received widespread support from numerous studies. The restricted expression patterns of transcription factors and signaling molecules underlie the patterning of the telencephalon (reviewed by Sur and Rubenstein 2005; Rash and Grove 2006; Mallamaci and Stoykova 2006). Graded cues have been identified that control the spatial formation of the axes of the developing telencephalon: FGF proteins set up the rostral-caudal (R-C) axis (Fukuchi-Shimogori and Grove 2001; Garel et al. 2003); Wnt and BMP proteins regulate dorsoventral (D-V) patterning (Furuta et al. 1997; Rubenstein et al. 1999; Hébert at al. 2002; Gunhaga et al. 2003); and medial-lateral (M-L) patterning is thought to be regulated by the transcription factors Emx2, Pax6, and COUP-TF1 (Bishop et al. 2000; Mallamaci et al. 2000; Zhou et al. 2001).
FGF signaling has been implicated in OB specification (Meyers et al. 1998), but it is likely that other cues are also involved in the initial OB formation (Hébert et al. 2003). Macroscopically, the OB is first morphologically distinct at E12.5 as an evagination of the rostral telencephalon (Figure 5.3; Hinds 1968b; Sugisaki et al. 1996; Inaki et al. 2004), but, microscopically, changes can be observed in the rostral telencephalon prior to El2. After pioneer OSN axons have extended into the ventricular zone of the telencephalon (see above), a distinct flexure is observed in the rostral telencephalon (see Figure 5.3). Gong and Shipley (1995) examined the cell-cycle kinetics of cells in the presumptive OB (pOB) and the adjacent neocortex after the arrival of the pioneer axons. Twenty-four hours after the arrival of the axons, the duration of the cell cycle in the pOB was significantly prolonged compared to the cortex, suggesting that these axons influence the formation of the OB. However, the arrival of OSN axons postdates the differentiation of mitral cells, which begins on E10.5–11 (Hinds 1968a). Moreover, studies examining the Pax-6 mutant mouse SeyNeu/SeyNeu (Small eye), which lack an OE, revealed that an OB-like structure (OBLS) develops within the rostral telencephalon without inductive signals from OSN axons (Jimenez et al. 2000). Similarly, olfactory bulbs (OBs) develop in Dlx5-deficient mice in which OSN axons extend from the OE, but fail to reach and innervate the OB (Long et al. 2003; Levi et al. 2003). These more recent studies suggest that OB formation occurs in the absence of any of the deep-penetrating OSN axons described by Gong and Shipley (1995). The exact nature of the signal that induces OB formation warrants further investigation (i.e., Shay et al. 2008). But, regardless of the molecules and mechanisms that underlie these processes, the olfactory system develops initially on two independent timeframes in the olfactory placode and the pOB; they become linked only as development continues (reviewed in Lopez-Mascaraque and De Castro 2002).

FIGURE 5.3
Schematic diagram comparing embryonic (A) and postnatal (B) olfactory connectivity. During the postnatal period, OSNs express a single OR from a single allele (monoallelic expression, represented by different colors), and extend a single unbranched axon (more...)
5.5.1. Projection Neurons
Mitral/tufted (M/T) cell development has been categorized into three distinct phases: (1) a postneurogenesis phase (from E11 to E13), when newly generated cells migrate radially toward the pOB border and then undergo a tangential reorientation; (2) a sensitive period (from E14 to E16), during which cells orient radially upon arrival of OSN axons; and (3) a cell refinement phase (from E17 though adulthood), when cells extend their dendrites and undergo extensive pruning to acquire the mature morphology (Blanchart et al. 2006). During the sensitive period, developing M/T cells extend numerous dendrites apically, which form a presumptive external plexiform layer (pEPL), which is directly apposed to the pONL (Treloar at al. 1999; Bailey et al. 1999; Blanchart et al. 2006). The immature M/T cells have multiple broadly spread apical dendrites and they do not become associated with glomerularlike structures (protoglomeruli) until later in postnatal development. OSN axons remain restricted to the pONL until approximately E15, when they begin to grow in among the M/T dendrites in the pONL and begin to coalesce into protoglomerular structures at E17 (Treloar et al. 1999; Blanchart et al. 2006). M/T cell dendrites do not become associated with these protoglomerular structures until immediately prior to birth, when the uniform dendritic meshwork of the EPL begins to segregate into protoglomerular structures (Treloar et al. 1999).
M/T cells do not begin to refine their immature and broadly spread dendritic arbors (Figure 5.3A) into the mature morphology (Figure 5.3B) until the postnatal period. They undergo extensive pruning/remodeling until a single apical dendrite remains, which projects to and ramifies as an apical tuft within only one glomerulus and numerous lateral dendrites extend horizontally in the EPL (Malun and Brunjes 1996; Lin et al. 2000; Blanchart et al. 2006). This developmental process is believed to be at least partially dependent on the presence of neuronal activity and/or OSN axons (Lin et al. 2000; Matusnami and Yamamoto 2000). A recent report by Tran et al. (2008) examining mitral cell dendritic development in vitro suggests that TGF-beta, released by the OE, may influence dendritic growth.
Some morphologically distinct glomeruli can first be distinguished at birth, although glomerulogenesis appears to continue during the first postnatal days, while the exact timeline remains to be established (see Figure 5.4). Glomeruli do not form uniformly around the circumference of the OB, rather a gradient is seen with glomeruli forming closer to the points where OSNs enter the EPL and later in regions where OSNs take longer to enter the EPL (Blanchart et al. 2006). It will be interesting in future studies to compare the sites of early glomerulogenesis to the entry zones of OSN axons into the OB, as it seems likely that the first glomeruli to form will be from the earliest generated OSN axons and presumably the first axons to reach the OB.

FIGURE 5.4
Schematic diagram of the developing olfactory bulb, detailing the emergence of glomeruli. At E13, OSN axons (illustrated in gray) reside in the ONL, directly apposed to a dense meshwork of dendrites from developing projections neurons. No intermingling (more...)
Axogenesis of M/T cells starts shortly after final differentiation, around E11.5 (Lopez-Mascaraque et al. 1996; Walz et al. 2006). Using mice with genetically labeled M/T cells, the first axons extend into the telencephalon at E11.5, and between E12 and E14 these axons elongate, forming an arch along the path of the future LOT (Walz et al. 2006). Prior to the extension of M/T axons, the position of the LOT can be identified by the presence of a subset of early generated neurons that are recognized by a monoclonal antibody (Lot1) (Sato et al. 1998). Lot1+ neurons appear necessary for LOT formation, as the LOT fails to form in organotypic cultures if the Lotl cells are ablated. Between E1 5 and E16, the LOT extends fully, reaching the most caudal extent of the telencephalon, and the first axon collaterals are formed in the region of the AON and posterior piriform cortex (pPC). While the LOT appears formed by E16, from E17 until birth new axons are added to the LOT and growth of collaterals and innervation of higher cortical regions continues. By birth, most of the overall connectivity appears established, although refinement continues at least until the end of the second postnatal week (Walz et al. 2006).
5.5.2. Interneurons
The two remaining largest populations of neurons within the OB are interneurons, the granule cells and periglomerular cells. These cells are largely generated during early postnatal life (Hinds 1968a; Altman 1969; Rosselli-Austin and Altman 1979; Bayer 1983). They are born in the subventricular zone lining the lateral ventricles and migrate into the OB along the rostral migratory stream (RMS) (Luskin 1993). However, while most of the interneurons are generated between E18 and P5 (Hinds 1968a), recent studies have demonstrated that a pioneer population of OB interneurons are generated from precursors in the lateral ganglionic eminence (LGE) between E12.5 and E14.5, which migrate selectively into the pOB in a pathway that presages the RMS (Tucker et al. 2006). These early generated cells differentiate into a wide variety of mature interneurons and a significant number can still be detected 60 days postnatal, indicating that they are not a transient population. Tucker et al. (2006) point out that they probably underestimate the size of early generated interneurons because cells were only pulse-chased with BrdU once at E14.5; an examination of earlier time points will be necessary for understanding OB formation. How these early generated interneurons impact the formation of OB circuitry and synaptogenesis remains to be determined.
5.5.3. Synaptogenesis
The first synapses in the OB can be detected at E14, but are not found in appreciable numbers until E15 (Hind and Hind 1976; Hwang and Cohen 1985), which is coincident with OSN ingrowth into the pEPL and the initiation of glomerulogenesis (Treloar et al. 1999). Synaptogenesis in the three main neuropil regions of the OB (the glomeruli, the EPL, and the internal granule cell layer [GL]) are not uniform during prenatal development (Hinds and Hinds 1976). Synapse formation in the glomerular layer>EPL>internal GL. At E18, the most obvious differences are observed; synaptic density of the glomerular layer is higher than EPL, which is approximately tenfold that of the internal GL. By birth, virtually all synaptic types detected in adults have been found, but the number of synapses continues to increase. In the glomerular layer, the density of synapses reaches a peak around P15-P20, after which it slowly declines, while in the EPL and internal GL, synaptic density continues to increase slightly even up to P44, probably reflecting the addition of new interneurons (Whitman and Greer 2007a, 2007b).
5.5.4. Molecular Basis of Olfactory Bulb (OB) Development
Much of the understanding of the molecular basis underlying the formation of the OB has come from studies of transgenic mice with defects in their OBs. As discussed above, many transcription and growth factors underlie specification of the rostral telencephalon and the pOB. Members of the fibroblast growth factor (FGF) family have been found to be important in patterning the telencephalon. In particular, several lines of evidence suggest that Fgf8 plays an important role in specifying the rostral-caudal axis of the rostral telencephalon (Crossley and Martin 1995; Shimamura and Rubenstein 1997; Meyers et al. 1998; Crossley et al. 2001; Fukuchi-Shimogori and Grove 2001). However, other FGFs, including Fgf15, Fgf17, and Fgf18, are also expressed in rostral telencephalon (McWhirter et al. 1997; Maruoka et al. 1998) and may play important roles in patterning this region. Although the FGF family is very large, with 22 genes identified in mice, they all mediate their responses through a family of four cell surface tyrosine kinase Fgf receptors (FGFRS; reviewed in Itoh and Ornitz 2004). Thus, approaching Fgf function during development is most feasible by targeting/deleting the receptor(s). In mice with a specific disruption of Fgfr1 in the telencephalon (generated using Foxg1-Cre mice), OBs do not develop normally (Hébert et al. 2003). At E12.5, when the OB first becomes macroscopically distinct in wildtype animals (see above), through E16.5 OBs do not form in Foxg1-Cre; Fgfr1flox/flox mice. Between E18.5 and P0, a small OB protrusion develops, however it does not exhibit the characteristic lamination of wildtype OBs (Hébert et al. 2003). OSN axons have formed connections with this OB-like structure and the developing M/T cells have extended axons to higher cortical areas (see below). In wildtype animals, the reduced rate of proliferation in the rostral telencephalon (seen after the arrival of OSN axons) and the associated increase in differentiation relative to surrounding telencephalon (which continues to proliferate at a higher rate) is believed to trigger the evagination of the OB (Gong and Shipley 1995). In these telencephalon-specific Fgfr1 null mice, no change in proliferation is observed in the rostral telencephalon relative to the adjacent cortex after the arrival of OSN axons (Hébert et al. 2003), suggesting the Fgf signaling is playing a role in OB morphogenesis. Since an OB-like structure does form late in development, it appears that other Fgfrs are probably partially compensating for the loss of FGF signaling through Fgfr1.
5.5.4.1. Projection Neurons
To date, only a few genes that are exclusively expressed in M/T cells have been identified. These include the transcription factors Tbr1 (the mammalian brachyury homolog T-brain 1; Bulfone et al. 1995); Tbx21, another member of the Tbr1 subfamily of T-box genes (Faedo et al. 2002; Yoshihara et al. 2005); Idl, a DNA-binding inhibitory helix-loop-helix Id protein (Neuman et al. 1993; Bulfone et al. 1998); neurotensin, a neurotransmitter transiently expressed by developing M/T cells (Kiyama et al. 1991; Walz et al. 2006), and reelin, a gene which encodes a secreted glycoprotein that was identified as having an autosomal mutation in the reeler trait (Schiffmann et al. 1997). Of these four genes, mice with null mutations have only indicated severe olfactory phenotypes with the Tbr1 gene. Mice that lack Tbr1 do form OBs, but they are small and do not have well defined layers (Bulfone et al. 1998). M/T cells fail to form, and the mice die within the first 2 days postnatal, as they do not suckle. Glomerular-like structures form within the mutant OBs, but this may reflect an inherent ability of OSN axons to coalesce rather than the formation of any synapses. Thus, Tbr1 appears necessary for M/T cell, and ultimately OB, development.
5.5.4.2. Interneurons
The interneuron populations also express many transcription factors, which when knocked out result in olfactory phenotypes. Arx is a vertebrate X-linked prd-type homeobox gene expressed by GABAergic interneurons in the OB (Poirier et al. 2004; Yoshihara et al. 2005). Arx-deficient mice die soon after birth and the neonatal mice have smaller OBs (Kitamura et al. 2002), primarily due to deficits in the entry of interneuron progenitors into the OB as well as disruptions in OB lamination (Yoshihara et al. 2005). Given the disorganized lamination and reduced size of the OB, as well as the neonatal lethality, Arx expression by interneuron precursors appears to be important for the development of the OB.
Another transcription factor, Sp8, which is a member of the Spl zinc finger gene family, is expressed by specific subpopulations of OB interneurons including the calretinin-expressing and GABAergic/TH-negative periglomerular cells (Waclaw et al. 2006). Sp8-deficient mice display severe exencephaly, making the analysis of OB formation difficult (Bell et al. 2003; Treichel et al. 2003). However, conditional mutations in Sp8 lead to a severe reduction in embryonic OB interneurons (Waclaw et al. 2006). During postnatal development, more interneurons reach the OB, however lamination defects are distinct, and deficits in specific subpopulations of interneurons are observed (i.e., they misexpress Pax6 and display abnormal migratory behavior) (Waclaw et al. 2006). Thus, Sp8 appears to play an important role in the regulation of interneuron development.
Distal-less/Dlx homeodomain transcription factors regulate the development of multiple cell types derived from the subcortical telencephalon, including the interneurons of the OB (Qiu et al. 1995; Anderson et al. 1997; Bulfone et al. 1998). Dlx1, Dlx2, Dlx5, and Dlx6 are expressed in precursors of OB interneurons (Liu et al. 1997; Stuhmer et al. 2002), with Dlx1 and Dlx1 expression usually preceding that of Dlx5 and Dlx6 (reviewed in Pangnaiban and Rubenstein 2002). In mice deficient for either Dlx1 or Dlx1, the population of TH-expressing periglomerular cells is reduced (Qiu et al. 1995; Long et al. 2003), but mice deficient for both Dlxl and Dlxl have a severe loss (>95%) of GABAergic interneurons (Anderson et al. 1997; Bulfone et al. 1998). As discussed above, mice deficient in Dlx5 OSNs develop, but fail to form connections with the OB (Long et al. 2003; Levi et al. 2003). In addition to this peripheral phenotype, these mice also have a marked decrease in interneuron populations, particularly in the GAD65+/+, GAD67+/+, and TH+/+ populations of granule and periglomerular cells, as well as non-cell-autonomous effects in the mitral cell population, probably due to the absence of input from the OE (Long et al. 2003).
5.6. OLFACTORY SENSORY NEURON TARGETING OLFACTORY EPITHELIUM (OE)—BULB TOPOGRAPHY
Like all sensory systems, the axonal connections in the olfactory system, from the periphery to the CNS, are not random. But unlike other sensory systems, the strategy employed by the olfactory system is unique. In contrast to visual and somatosensory stimuli, olfactory stimuli are not spatially fixed, therefore a spatial representation of the sensory field in the CNS is not a prerequisite when coding olfactory information. Odors are volatile aromatics with distinct chemical properties, varying in their ability to diffuse through the air as well as the mucous bathing the OE, thus the relatively crude spatial patterning of ORs in the OE may reflect the chemical properties of the OR ligands.
5.6.1. Topographic Organization of Olfactory Sensory Neuron Projections
Each OSN expresses a single odorant receptor protein from a single allele (allelic exclusion) (Buck and Axel 1991; Chess et al. 1994; Malnic et al. 1999; Serizawa et al. 2000; see also Chapter 7). Negative feedback from the expressed OR molecules may maintain this one neuron-one receptor rule, although the mechanism remains unclear (Serizawa et al. 2003; Lewcock and Reed 2004; Shykind et al. 2004). Allelic exclusion is a key feature of olfactory biology, ensuring that each neuron is receptive to a unique, defined repertoire of ligands. OSNs expressing the same OR genes are expressed in restricted longitudinal bands of OE that vary in their dorsoventral position (Vassar et al. 1993; Ressler et al. 1993; Strotmann et al. 1994; Iwema et al. 2004; Miyamichi et al. 2005). The initial studies describing these restricted zones of expression identified only four zones (Vassar et al. 1993; Ressler et al. 1993; Strotmann et al. 1994). However, more recent studies recognize that rather than zones with distinct boundaries, there are instead continuous and overlapping expression domains that are unique for each OR (Iwema et al. 2004; Miyamichi et al. 2005).
Initially using a distinct property of OSNs (they contain mRNA in their axons) and later using molecular genetic tracing techniques, the axonal projection patterns of OSNs expressing the same OR were traced to their synaptic target glomeruli in the OB (Vassar et al. 1994; Ressler et al. 1994; Mombaerts et al. 1996; Wang et al. 1998). OSNs expressing the same OR converge on a few glomeruli in the OB, typically a pair, with one glomerulus on the medial surface and one on the lateral surface of each OB. This mosaic topographic pattern of divergent sensory neurons in the OE projecting and converging their axons onto a pair, or small number of glomeruli in the OB has been observed for all ORs examined to date. It is quite different from the point-to-point maps seen in the visual and somatosensory systems that maintain the relationships between sensory neurons in the receptive field in the target fields. The topographic map in the olfactory pathway is also not invariant; while identified glomeruli (i.e., containing axons expressing the same OR) maintain the same general locale between animals, the “neighbor” relationships between glomeruli can vary by a few glomerular diameters and can show variability between OBs within the same animal (Strotmann et al. 2000; Serizawa et al. 2006). This variability is not yet well understood, but may reflect developmental mechanisms underlying the formation of glomeruli. The zonal, or regional, organization seen in the OE appears to be maintained in the OB (Saucier and Astic 1986; Ressler et al. 1994; Vasser et al. 1994; Schoenfeld et al. 1994; reviewed in Mori et al. 1999). OSNs located in the dorsal nasal cavity converge and form glomeruli in the dorsal OB, and ventrally located OSNs project their axons to the ventral OB. Thus, each of the four broad zones/regions is represented in the OB. It should be noted, however, that as it becomes recognized that distinct zonal boundaries do not exist in the OE, and instead there are gradients of each OR along the dorsomedial/ventrolateral axes of the OE, similar gradients are observed in the OB, recapitulating the patterns seen in the OE (Miyamichi et al. 2005).
The topography described here, of glomerular convergence and zonal projections, underlies coding in the olfactory system. As all OSN axons expressing the same OR receptor converge on a stereotypic pair of glomeruli within the OB, decoding sensory input becomes a problem of recognizing which patterns of glomeruli have been activated by specific stimuli. Moreover, through a process of lateral inhibition, the system is extremely sensitive: the convergence of axons on a small number of projection neurons amplifies the signal, while lateral inhibition of surrounding projection neurons via local interneurons further amplifies the signal to noise ratio (reviewed in Mori et al. 1999). Thus, the olfactory system is capable of detecting and discriminating odorants in the parts-per-billion range.
5.6.2. Development of an Olfactory Topographic Map
How does this mosaic topography develop? How do all the OSN axons expressing a single OR from a family of over 1200 genes, converge and form glomeruli in stereotypic positions? Perhaps the easiest model to imagine is that synaptic connections form between the OE and the OB, and OR gene choice occurs later, after connections have been made. This is an attractive model because the precision of targeting would not be active, but rather a retrograde event that could be imposed on the OE by the OB. However, onset of OR expression has been reported as early as E11.25 in mice (Conzelmann et al. 2001), which predates synaptogenesis by 3–4 days (Hinds and Hinds 1976). Even at the earliest embryonic ages, OSNs expressing the same OR have restricted zonal/regional expression patterns, suggesting that retrograde signals from the OB do not influence OR gene choice in the OE.
The question then becomes how can the identity of the OR expressed by OSNs be encoded or represented at growth cones during the formation of the olfactory pathway? Perhaps the most obvious way is by using the OR protein itself. For OR proteins to play an active role in the targeting of OSN axons, they also need to be expressed on axons and growth cones (in addition to their more classical site of expression in olfactory cilia). In transgenic mice that have had the coding region of an OR replaced with a reporter gene, OSN axons fail to converge and form glomeruli, instead they appear to wander within the ONL (Mombaerts et al. 1996; Wang et al. 1998; Feinstein and Mombaerts 2004, Feinstein et al. 2004). Perhaps more convincing evidence of the OR receptor having a role in targeting OSN axons comes from mice that have had the coding region of one OR substituted with the coding region of another OR (Mombaerts et al. 1996; Wang et al. 1998). In these mice, OSN axons change their targeting to a glomerulus in an intermediate position, between that of the host and donor glomeruli. Antibody localization studies have demonstrated expression of OR proteins in OSN axons (Barnea et al. 2004; Strotmann et al. 2004), as has a transgenic line of mice with an OR protein directly fused to a GFP reporter (Feinstein and Mombaerts 2004).
Consistent with the observation that OR proteins are determinants in targeting, even single amino acid changes in key residues of OR protein can alter the targeting of OSN axons, causing them to converge in intermediate or new glomeruli (Feinstein and Mombaerts 2004). These studies collectively demonstrate the requirement of the OR protein in OSN axon targeting. Yet, they also demonstrate that ORs alone are not sufficient for correct targeting. If they were sufficient, the receptor substitution experiments would have revealed complete switching of glomerular position from host to donor instead of the intermediate positions observed. Thus, it seems likely that other guidance cues and mechanisms must be acting in the olfactory pathway, together with the OR proteins, to determine the final points of axon convergence.
A generalized scheme of the development of sensory maps involves sequential activity-independent and activity-dependent mechanisms: axon guidance molecules are utilized to generate the course pattern of innervation of targets, while subsequent refinement of projections is achieved through activity-dependent processes (reviewed in Goodman and Shatz 1993; Katz and Shatz 1996; Tessier Lavigne and Goodman 1996). Not surprisingly, both activity-independent and activity-dependent mechanisms appear to be acting in the formation of the topographic olfactory projection.
5.6.1.1. Role of Molecular Guidance Cues in the Development of a Topographic Map
While OR genes have been identified as the primary candidates for mediating OSN axon targeting, other guidance cues have been identified in establishing the topography in this pathway. Within the developing nervous system, major families of axon guidance cues have been identified, including the netrins, slits, semaphorins, and ephrins (reviewed in Dickson 2002). While these are not the only axon guidance cues, they are the best understood, and members of each of these families have been identified in the olfactory system.
Semaphorins are a family of secreted and transmembrane proteins that have been identified as axon guidance cues in many regions of the developing nervous system (reviewed in Fiore and Puschel 2003; Yazdani and Terman 2006). In mice lacking Sema3A, OSN axons expressing the Sema3A receptor neuropilin 1 (npn1) fail to target glomeruli in the lateral and medial OB like their wild-type (WT) counterparts, instead aberrantly targeting glomeruli in the rostral and ventral OB (Taniguchi et al. 2003; Schwarting et al. 2004). However, differences were observed between the two lines of null mice reported. In one line, OSN axons expressing the OR P2 failed to target their stereotypic lateral and medial glomeruli, instead targeting multiple glomeruli in the ventral OB (Schwarting et al. 2004). While in the other line, P2-expressing OSN axons targeted appropriately (Taniguchi et al. 2003). These mice did differ in their genetic background, which may account for some of the variation observed. While these differences require further investigation, both studies agreed that zonal/regional projections were disrupted based on the expression of cell surface markers. Examination of the glomerular activity patterns in Sema3A null mice via intrinsic optical imaging also revealed distorted glomerular maps (Taniguchi et al. 2003). Thus, the loss of the inhibitory Sema3A signal during development appears to disrupt the zonal/regional specification of the OB.
Another large family of axon guidance cues implicated in the formation of topographic maps in other sensory systems is the Eph receptor tyrosine kinases and their ligands, the ephrins (reviewed in Wilkinson 2001). During development, OSN axons transiently express members of the ephrin family, while cognate Eph receptors are expressed by target cells in the OB (Zhang et al. 1996, 1997; St. John et al. 2000, 2002; St. John and Key 2001; Cutforth et al. 2003). However, expression patterns are not like the gradients found in the retinotectal system. Rather, there is a mosaic-type pattern of expression with neighboring glomeruli expressing very different levels of the ligands (Cutforth et al. 2003). Although the ligands and receptors exhibit highly regulated spatiotemporal patterns of expression in both OSN axons and bulbar targets (St. John et al. 2002), the promiscuous nature of the ligands, which bind to multiple receptors, make it difficult to predict the interactions that may occur. To date, only one study has looked at the functional role that ephrins and Ephs play in establishing olfactory topography. In mice lacking ephrin-A3 and ephrin-A5, OSN axons expressing the ORs P2 and SR1 have glomeruli that are shifted caudally (Cutforth et al. 2003). In mice that overexpress ephrin-A5 only in the P2-expressing OSN axons, glomeruli are shifted rostral relative to wildtype P2 glomeruli (Cutforth et al. 2003). However, it has been suggested that the genetic techniques used in generating these mice (tricistronic constructs) may reduce levels of the P2 protein, which could have effects on targeting independent of the coexpressed protein (Mombaerts 2006). Thus, while it appears that Ephs and ephrins have a role in establishing the olfactory pathway, further studies are needed to fully elucidate the nature of that role.
Recently, insulin-like growth factors (IGF) have been implicated in mediolateral OB targeting (Scolnick et al. 2008). The IGF family members (IGF1 and IGF2) initiate signaling by activating their receptor IGF1R. During early development (E14.5), IGF1 is expressed in a gradient manner along the mediolateral axis (Scolnick et al. 2008). Expression is restricted to the mitral cell layer and glomerular layers by E18.5. IGF2 is expressed around the OB; IGF1R is expressed in OSNs and in axon fascicles. IGF mutagenesis causes axon mistargeting. Instead of innervating the lateral OB, sensory axons innervate ectopic ventromedial glomeruli. This suggests that IGF signaling may play a role in establishing the topography of the olfactory system.
The expression of cell surface sugars of proteoglycans, glycolipids, and glycoproteins has been proposed to provide a “sugar code” or “glycode” for growing axons (St. John et al. 2002; Holt and Dickson 2005). In the olfactory system, a large body of literature describes a number of lectins (carbohydrate-binding proteins) that bind subsets of OSNs in distinct patterns (reviewed in Plendl and Sinowatz 1998). The diversity in the expression of cell surface sugars has been proposed to underlie the development of the olfactory pathway. Most of these studies, however, do not identify which proteoglycan, protein, or lipid the carbohydrate moiety is attached to, making functional studies difficult. Two approaches that have been used to look at the functional relevance of sugars in the developing olfactory system are: (1) assess the role of endogenous lectins; and (2) assess the role of various glycosytransferases, synthetic enzymes in glycan production.
Galectin-1 is an endogenous lactose-binding lectin that has established roles in cell-cell and cell-matrix interactions (reviewed in Camby et al. 2006). In the olfactory system, galectin-1 is expressed by OSNs, ensheathing cells and M/T cells (Mahanthappa et al. 1994; Puche and Key 1996). In vitro, galectin-1 is a potent promoter of neurite outgrowth i (Puche et al. 1996). In mice lacking galectin-1, a subset of OSN axons that can be labeled with the plant lectin Dolichos biflorus agglutinin (DBA) fail to project to their correct targets in the dorsocaudal OB (Puche et al. 1996). Thus, galectin-1-mediated carbohydrate interactions appear to play a role in pathfinding during development of the olfactory projection.
The glycosyltransferase, β1,3-N-acetylglucosaminyltransferase 1 (β3-GnT1), is a key enzyme in lactosamine glycan synthesis and is expressed by a subset of OSNs (Henion et al. 2005). In mice lacking (β3-GnT1, OB innervation and glomerular formation is perturbed in neonatal mice; OSN axons expressing the P2,17, and M72 ORs fail to form glomeruli (Henion et al. 2005). By two weeks postnatal, lactosamine is re-expressed in these mice via a secondary pathway and a regrowth of axons into the glomerular layer occurs. Thus, the carbohydrate lactosamine also appears to have an important role in the formation of the olfactory projection.
Another glycosyltransferase, alpha(l,2)fucosyltransferase (FUT1), synthesizes the blood group H (BGH) carbohydrate alphaFuc(l,2)Gal, which is expressed by all mouse OSNs (e.g., Lipscomb et al. 2002). BGH is the acceptor substrate for a glycosyltransferase that synthesizes blood group A (BGA), which is expressed by a subset of vomeronasal sensory neurons (St. John et al. 2006). In mice that lack FUT1, a delay in the development of the ONL and glomerular layer is observed, but no deficits were seen on OSN targeting (St. John et al. 2006). However, when blood group A transferase (BGAT) was overexpressed on all OSNs using the OMP promoter, VNO axons overshot their targets in the accessory OB and OSNs were observed to make targeting errors (St. John et al. 2006). These studies lend further support to the notion that cell surface carbohydrates are important determinants of OSN axon extension, coalescence, and targeting.
Various cell adhesion molecules (CAMs) have also been proposed to be involved in establishing the topographic olfactory pathway, although the evidence for their involvement is less clear. OCAM, the olfactory CAM, is expressed in a zonal topographic pattern highly suggestive of a role in establishing the topographic projections (Yoshihara et al. 1997; Treloar et al. 2003). However, no disruptions in topography are observed in OCAM null mice (Walz et al. 2006). All OSNs, both immature and mature, express the NCAM (Terkelsen et al. 1989). Mice that lack the NCAM-180 isoform have delays in formation of the olfactory pathway, with OSN axons taking longer to target and form glomeruli, with many axons failing to exit the ONL (Treloar et al. 1997). However, OR-expressing OSN axons have not been assessed in these mice, which would aide the characterization of the delayed phenotype.
Recent evidence also implicates members of the Wnt/Fz family in the extension and perhaps coalesence/targeting of OSN axons (Rodriguez-Gil and Greer 2008). Members of the Wnt family are expressed along the olfactory pathway, the OSN axons express several Fz receptors, and moreover, Wnt5a induces accelerated growth of OSN axons in vitro. While further work remains to be done, the evidence thus far is provocative in suggesting a role for this family of axon guidance molecules in the olfactory system.
5.6.1.2. Role of Functional Activity in the Development of a Topographic Map
When assessing the role of functional activity in the olfactory system, different components of activity must be considered. Activity includes both odor-evoked responses as well as spontaneous responses. While there has not been a great deal of investigation, several key studies have looked at the effects of activity-dependent mechanisms of topographic map formation. One strategy for assessing the role of odor-evoked activity is to block odor stimulation by surgically closing a naris at birth. Although much of the coalesence of OSN axons has occurred by birth, naris closure can still have a profound effect on the specificity of glomeruli. For example, Zou et al. (2004) found that during initial development, OSN axons expressing the same OR can coalesce in supernumerary glomeruli, some of which are heterogeneous for OR expression. Within a few postnatal days, the hypertrophy is corrected and the number of glomeruli stabilizes at approximately two per. However, in mice with unilateral naris closure, the development refinement, the loss of the supernumerary glomeruli, was significantly retarded and many glomeruli received heterogeneous axonal input from OSNs expressing different ORs. Thus, naris closure suggests that beyond any role in primary axon coalesence/targeting, functional activity is important for refining the specificity of glomerular innervation. Genetic approaches have proven to be more tractable to looking at the role of activity in topographic map formation.
Alternative strategies for addressing the role of functional activity in the development of the primary olfactory pathway have used genetic approaches to delete, downregulate, or upregulate downstream members of the OR transduction cascade. Among the first were mice lacking the alpha subunit of the olfactory cyclic nucleotide-gated channel, OCNC1 (now called CNGA2). The mice are anosmic (Brunet et al. 1996) and although the olfactory pathway appears largely intact, the effects of the loss of odor-evoked activity on axonal wiring are somewhat controversial (Lin et al. 2000; Zheng et al. 2000). In a conventional null mutation of OCNC1, axons expressing the P2 odor receptor converge appropriately, while those expressing the M72 odor receptor do not (Lin et al. 2000; Zheng et al. 2000). However, when OCNC1 is selectively mutated in some M72-expressing OSNs, using a “monoallelic deprivation” paradigm, M72 axons expressing OCNC1 segregate into distinct glomeruli from those not expressing OCNC1 (Zheng et al. 2000). These data suggest that postnatal odor-evoked activity does play a role in OSN axon targeting or coalesence, at least for some subsets of OSNs, but it also raises the question of when the odor-induced activity occurs. While there is evidence for in utero functional activity in the olfactory system (i.e., Pedersen et al. 1983), there are also data showing that the temporal onset of OR expression varies significantly among ORs and that some, perhaps, may not appear until perinatal periods (Sullivan et al. 1995; Greer lab unpublished observations). Also, there has been some question regarding the retention of odor transduction among subpopulations of OSNs in the CNGA2 mice (Lin et al. 2004), as well as the possibility that downstream signaling from the G-protein-coupled receptor could influence axons independent of functional CNG channels (Chesler et al. 2007).
Imai et al. (2006) and Chesler et al. (2007) have shown the importance of cAMP signaling in OSN axon extension/coalescence. In both cases, increased levels of cAMP led to the coalescence of OSN axons, independent of odor-induced activity via an OR. Although additional mechanisms may also be involved, Serizawa et al. (2006) further suggested that activity-dependent regulation of cell surface adhesion molecules, such as Kirrel 2 and Kirrel 3, perhaps mediated via cAMP, may contribute to the regulation OSN axon adhesion and coalescence into glomeruli. This suggestion is further supported by data showing that in AC3 knockout mice, OSN axon behavior is aberrant and that glomerulogenesis is perturbed (Zou et al. 2007). Kirrel2/Kirrel3 and their counterparts, ephrin-A5/EphA5, are expressed in a correlated manner with subsets of OR-expressing OSN axons. Therefore, Serizawa et al. (2006) proposed that axon targeting is achieved by expression of a combination of recognition/guidance cues whose expression levels are determined by activity. Coined the “neural identify code,” it will be interesting to see if OR-correlated activity-dependent expression of axon guidance cues does mediate OSN axon fasciculation during development.
Spontaneous, odor-independent activity may also influence OSN axons. Yu et al. (2004) conditionally expressed the tetanus toxin light chain in OSNs, inhibiting synaptic vesicle release and blocking both spontaneous and odor-evoked activity. However, blocking synaptic function did not have a significant effect on the development or topography of the primary olfactory pathway. However, when OSN activity was blocked by the overexpression of the inward rectifying K+ channel, Kir2.1, a delay in the ingrowth of OSN axons into the OB resulted, as well as a gross disorganization of dorsal glomeruli and targeting errors of subpopulations of OSN axons. Thus, while synaptic activity may not be a prerequisite for growth, coalescence, and sorting of OSN axons, electrical transients are required.
5.7. SUMMARY
In this chapter, we have reviewed the basic principles of development and differentiation in the primary olfactory pathway. In particular, we focused on the mechanisms influencing the emergence of the OE and the innervation of the OB by OSN axons. The story is clearly complicated and tight spatiotemporal regulation of molecular expression is required in order for the pathway to develop correctly. Many challenges remain. While myriad molecular mechanisms have been identified in the placode, the developing olfactory pathway, and the OB, how these are integrated to form the highly complex topography between the OE and OB remains to be determined.
ACKNOWLEDGMENTS
The authors express their appreciation to members of their laboratories for helpful discussions and critical readings. Work in the laboratories of the authors has been generously supported by NIH-NIDCD and NIH-NIA.
REFERENCES
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–58. [PubMed: 5361244]
- Anderson S.A., Qiu M., Bulfone A., Eisenstat D.D., Meneses J., Pedersen R., Rubenstein J.L. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. [PubMed: 9247261]
- Bailey M.S., Puche A.C., Shipley M.T. Development of the olfactory bulb: Evidence for glia-neuron interactions in glomerular formation. J Comp Neurol. 1999;415:423–48. [PubMed: 10570454]
- Balmer C.W., LaMantia A.S. Loss of Gli3 and Shh function disrupts olfactory axon trajectories. J Comp Neurol. 2004;472:292–307. [PubMed: 15065125]
- Balmer C.W., LaMantia A.S. Noses and neurons: Induction, morphogenesis, and neuronal differentiation in the peripheral olfactory pathway. Dev Dyn. 2005;234:464–81. [PubMed: 16193510]
- Barnea G., O’Donnell S., Mancia F., Sun X., Nemes A., Mendelsohn M., Axel R. Odorant receptors on axon termini in the brain. Science. 2004;304:1468. [PubMed: 15178793]
- Bayer S. 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res. 1983;50:329–40. [PubMed: 6641865]
- Beites C.L., Kawauchi S., Crocker C.E., Calof A.L. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–16. [PubMed: 15925585]
- Bell S.M., Schreiner C.M., Waclaw R.R., Campbell K., Potter S.S., Scott W.J. Sp8 is crucial for limb outgrowth and neuropore closure. Proc Natl Acad Sci USA. 2003;100:12195–200. [PMC free article: PMC218735] [PubMed: 14526104]
- Bhasin N., Maynard T.M., Gallagher P.A., LaMantia A.S. Mesenchymal/epithelial regulation of retinoic acid signaling in the olfactory placode. Dev Biol. 2003;261:82–98. [PubMed: 12941622]
- Bhattacharyya S., Bronner-Fraser M. Competence, specification and commitment to an olfactory placode fate. Development. 2008;135:4165–77. [PubMed: 19029046]
- Bishop K.M., Garel S., Nakagawa Y., Rubenstein J.L., O’Leary D.D. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–60. [PubMed: 12561075]
- Bishop K.M., Goudreau G., O’Leary D.D. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–49. [PubMed: 10764649]
- Blanchart A., De C., rlos J.A., Lopez-Mascaraque L. Time frame of mitral cell development in the mice olfactory bulb. J Comp Neurol. 2006;496:529–43. [PubMed: 16572431]
- Briata P., Di Blas E., Gulisano M., Mallamaci A., Iannone R., Boncinelli E., Corte G. EMX1 homeoprotein is expressed in cell nuclei of the developing cerebral cortex and in the axons of the olfactory sensory neurons. Mech Dev. 1996;57:169–80. [PubMed: 8843394]
- Brunet L.J., Gold G.H., Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–93. [PubMed: 8893025]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175–87. [PubMed: 1840504]
- Bulfone A., Smiga S.M., Shimamura K., Peterson A., Puelles L., Rubenstein J.L. T-brain-1: A homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. [PubMed: 7619531]
- Bulfone A., Wang F., Hevner R., Anderson S., Cutforth T., Chen S., Meneses J., Pedersen R., Axel R., Rubenstein J.L. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 1998;21:1273–82. [PubMed: 9883721]
- Caggiano M., Kauer J.S., Hunter D.D. Globose basal cells are neuronal progenitors in the olfactory epithelium: A lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–52. [PubMed: 8060615]
- Camby I., Le Mercier M., Lefranc F., Kiss R. Galectin-1: A small protein with major functions. Glycobiology. 2006;16:137R–57R. [PubMed: 16840800]
- Carson C., Murdoch B., Roskams A.J. Notch 2 and Notch 1/3 segregate to neuronal and glial lineages of the developing olfactory epithelium. Dev Dyn. 2006;235:1678–88. [PubMed: 16518823]
- Cau E., Casarosa S., Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–80. [PubMed: 11934853]
- Cau E., Gradwohl G., Fode C., Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–21. [PubMed: 9108377]
- Chesler A., Zou D.-J., Le Pichon C., Peterlin Z., Matthews G., Pei X., Miller M., Firestein S. A G protein/cAMP signal cascade is required for axonal convergence into olfactory glomeruli. PNAS. 2007;104:1039–44. [PMC free article: PMC1783360] [PubMed: 17215378]
- Chess A., Simon I., Cedar H., Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78:823–34. [PubMed: 8087849]
- Chung W.C., Moyle S.S., Tsai P.S. Fibroblast growth factor 8 signaling through fibroblast growth factor receptor 1 is required for the emergence of gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:4997–5003. [PMC free article: PMC2582917] [PubMed: 18566132]
- Conzelmann S., Levai O., Breer H., Strotmann J. Extraepithelial cells expressing distinct olfactory receptors are associated with axons of sensory cells with the same receptor type. Cell Tissue Res. 2002;307:293–301. [PubMed: 11904765]
- Conzelmann S., Malun D., Breer H., Strotmann J. Brain targeting and glomerulus formation of two olfactory neuron populations expressing related receptor types. Eur J Neurosci. 2001;14:1623–32. [PubMed: 11860457]
- Cornesse Y., Pieler T., Hollemann T. Olfactory and lens placode formation is controlled by the hedgehog-interacting protein (Xhip) in Xenopus. Dev Biol. 2005;277:296–315. [PubMed: 15617676]
- Crossley P.H., Martin G.R. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. [PubMed: 7768185]
- Crossley P.H., Martinez S., Ohkubo Y., Rubenstein J.L. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. [PubMed: 11734354]
- Cutforth T., Moring L., Mendelsohn M., Nemes A., Shah N.M., Kim M.M., Frisen J., Axel R. Axonal ephrin-As and odorant receptors: Coordinate determination of the olfactory sensory map. Cell. 2003;114:311–22. [PubMed: 12914696]
- De Carlos J.A., L’pez-Mascaraque L., Valverde F. The telencephalic vesicles are innervated by olfactory placode-derived cells: A possible mechanism to induce neocortical development. Neuroscience. 1995;68:1167–78. [PubMed: 8544990]
- Donner A.L., Episkopou V., Maas R.L. Sox2 and Pou2f1 interact to control lens and olfactory placode development. Dev Biol. 2006 Nov 6; [Epub ahead of print] [PMC free article: PMC3276313] [PubMed: 17140559]
- Faedo A., Ficara F., Ghiani M., Aiuti A., Rubenstein J.L., Bulfone A. Developmental expression of the T-box transcription factor T-bet/Tbx21 during mouse embryogenesis. Mech Dev. 2002;116:157–60. [PubMed: 12128215]
- Feinstein P., Bozza T., Rodriguez I., Vassalli A., Mombaerts P. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117:833–46. [PubMed: 15186782]
- Feinstein P., Mombaerts P. A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell. 2004;117:817–31. [PubMed: 15186781]
- Fiore R., Puschel A.W. The function of semaphorins during nervous system development. Front Biosci. 2003;8:s484–99. [PubMed: 12700098]
- Fukuchi-Shimogori T., Grove E.A. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–74. [PubMed: 11567107]
- Furuta Y., Piston D.W., Hogan B.L. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–12. [PubMed: 9187146]
- Garel S., Huffman K.J., Rubenstein J.L.R. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–14. [PubMed: 12642494]
- Gong Q., Shipley M.T. Evidence that pioneer olfactory axons regulate telencephalon cell cycle kinetics to induce the formation of the olfactory bulb. Neuron. 1995;14:91–101. [PubMed: 7826645]
- Gong Q., Shipley M.T. Expression of extracellular matrix molecules and cell surface molecules in the olfactory nerve pathway during early development. J Comp Neurol. 1996;366:1–14. [PubMed: 8866842]
- Goodman C.S., Shatz C.J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell. 1993 72 Suppl:77–98. [PubMed: 8428376]
- Guillemot F., Lo L.C., Johnson J.E., Auerbach A., Anderson D.J., Joyner A.L. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–76. [PubMed: 8221886]
- Gunhaga L., Marklund M., Sjodal M., Hsieh J.C., Jessell T.M., Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–7. [PubMed: 12766771]
- Hébert J.M., Lin M., Partanen J., Rossant J., McConnell S.K. FGF signaling through FGFR1 is required for olfactory bulb morphogenesis. Development. 2003;130:1101–11. [PubMed: 12571102]
- Hébert J.M., Mishina Y., McConnell S.K. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron. 2002;35:1029–41. [PubMed: 12354394]
- Henion T.R., Raitcheva D., Grosholz R., Biellmann F., Skarnes W.C., Hennet T., Schwarting G.A. b1,3-N-acetylglucosaminyltransferase 1 glycosylation is required for axon pathfinding by olfactory sensory neurons. J Neurosci. 2005;25:1894–1903. [PMC free article: PMC6726059] [PubMed: 15728829]
- Hinds J.W. Autoradiographic study of histogenesis in the mouse olfactory bulb. I. Time of origin of neurons and neuroglia. J Comp Neurol. 1968a;134:287–304. [PubMed: 5721256]
- Hinds J.W. Autoradiographic study of histogenesis in the mouse olfactory bulb. II. Cell proliferation and migration. J Comp Neurol. 1968b;134:305–22. [PubMed: 5721257]
- Hinds J.W., Hinds P.L. Synapse formation in the mouse olfactory bulb. I. Quantitative studies. J Comp Neurol. 1976;169:15–40. [PubMed: 956463]
- Hirata T., Nakazawa M., Yoshihara S., Miyachi H., Kitamura K., Yoshihara Y., Hibi M. Zincfinger gene Fez in the olfactory sensory neurons regulates development of the olfactory bulb non-cellautonomously. Development. 2006;133:1433–43. [PubMed: 16540508]
- Holt C.E., Dickson B.J. Sugar codes for axons? Neuron. 2005;46:169–72. [PMC free article: PMC3687205] [PubMed: 15848796]
- Huard J.M., Youngentob S.L., Goldstein B.J., Luskin M.B., Schwob J.E. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–586. [PubMed: 9786409]
- Hwang H.M., Cohen R.S. Freeze-fracture analysis of synaptogenesis in glomeruli of mouse olfactory bulb. J Neurocytol. 1985;14:997–1018. [PubMed: 3831249]
- Ikeda K., Ookawara S., Sato S., Ando Z., Kageyama R., Kawakami K. Six1 is essential for early neurogenesis in the development of olfactory epithelium. Dev Biol. 2007;311:53–68. [PubMed: 17880938]
- Imai T., Sakano H. Odorant receptor-mediated signaling in the mouse. Curr Opin Neurobiol. 2008;18:251–60. [PubMed: 18721880]
- Imai T., Suzuki M., Sakano H. Odorant receptor-derived cAMP signals direct axonal targeting. Science. 2006;314:657–61. [PubMed: 16990513]
- Inaki K., Nishimura S., Nakashiba T., Itohara S., Yoshihara Y. Laminar organization of the developing lateral olfactory tract revealed by differential expression of cell recognition molecules. J Comp Neurol. 2004;479:243–56. [PubMed: 15457507]
- Itoh N., Ornitz D.M. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–69. [PubMed: 15475116]
- Iwema C.L., Fang H., Kurtz D.B., Youngentob S.L., Schwob J.E. Odorant receptor expression patterns are restored in lesion-recovered rat olfactory epithelium. J Neurosci. 2004;24:356–69. [PMC free article: PMC6729985] [PubMed: 14724234]
- Jimenez D., Garcia C., de Castro F., Chedotal A., Sotelo C., de Carlos J.A., Valverde F., Lopez Mascaraque L. Evidence for intrinsic development of olfactory structures in Pax-6 mutant mice. J Comp Neurol. 2000;428:511–26. [PubMed: 11074448]
- Katz L.C., Shatz C.J. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–38. [PubMed: 8895456]
- Kawauchi S., Beites C.L., Crocker C.E., Wu H.H., Bonnin A., Murray R., Calof A.L. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci. 2004;26:166–80. [PubMed: 15711058]
- Kawauchi S., Shou J., Santos R., Hébert J.M., McConnell S.K., Mason I., Calof A.L. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–23. Epub Nov 2. [PubMed: 16267092]
- Kitamura K., Yanazawa M., Sugiyama N., Miura H., Iizuka-Kogo A., Kusaka M., Omichi K., Suzuki R., Kato-Fukui Y., Kamiirisa K., Matsuo M., Kamijo S., Kasahara M., Yoshioka H., Ogata T., Fukuda T., Kondo I., Kato M., Dobyns W.B., Yokoyama M., Morohashi K. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–69. [PubMed: 12379852]
- Kiyama H., Sato M., Emson P.C., Tohyama M. Transient expression of neurotensin mRNA in the mitral cells of rat olfactory bulb during development. Neurosci Lett. 1991;128:85–89. [PubMed: 1922952]
- Klenoff J., Greer C. Postnatal development of olfactory receptor cell axonal arbors. J Comp Neurol. 1998;390:256–67. [PubMed: 9453669]
- LaMantia A.S., Bhasin N., Rhodes K., Heemskerk J. Mesenchymal/epithelial induction mediates olfactory pathway formation. Neuron. 2000;28:411–25. [PubMed: 11144352]
- Laub F., Lei L., Sumiyoshi H., Kajimura D., Dragomir C., Smaldone S., Puche A.C., Petros T.J., Mason C., Parada L.F., Ramirez F. Transcription factor KLF7 is important for neuronal morphogenesis in selected regions of the nervous system. Mol Cell Biol. 2005;25:5699–5711. [PMC free article: PMC1157008] [PubMed: 15964824]
- Levi G., Puche A.C., Mantero S., Barbieri O., Trombino S., Paleari L., Egeo A., Merlo G.R. The Dlx5 homeodomain gene is essential for olfactory development and connectivity in the mouse. Mol Cell Neurosci. 2003;22:530–43. [PubMed: 12727448]
- Lewcock J.W., Reed R.R. A feedback mechanism regulates monoallelic odorant receptor expression. Proc Natl Acad Sci USA. 2004;101:1069–74. [PMC free article: PMC327152] [PubMed: 14732684]
- Lin D.M., Wang F., Lowe G., Gold G.H., Axel R., Ngai J., Brunet L. Formation of precise connections in the olfactory bulb occurs in the absence of odorant-evoked neuronal activity. Neuron. 2000;26:69–80. [PubMed: 10798393]
- Lipscomb B.W., Treloar H.B., Greer C.A. Novel microglomerular structures in the olfactory bulb of mice. J Neurosci. 2002;22(3):766–74. [PMC free article: PMC6758479] [PubMed: 11826106]
- Liu J.K., Ghattas I., Liu S., Chen S., Rubenstein J.L. Dlx genes encode DNA-binding proteins that are expressed in an overlapping and sequential pattern during basal ganglia differentiation. Dev Dyn. 1997;210:498–512. [PubMed: 9415433]
- Long J.E., Garel S., Depew M.J., Tobet S., Rubenstein J.L. DLX5 regulates development of peripheral and central components of the olfactory system. J Neurosci. 2003;23:568–78. [PMC free article: PMC6741869] [PubMed: 12533617]
- López-Mascaraque L., De Carlos J.A., Valverde F. Early onset of the rat olfactory bulb projections. Neuroscience. 1996;70:255–66. [PubMed: 8848129]
- Lopez-Mascaraque L., de Castro F. The olfactory bulb as an independent developmental domain. Cell Death Differ. 2002;9:1279–86. [PubMed: 12478464]
- Mahanthappa N.K., Cooper D.N., Barondes S.H., Schwarting G.A. Rat olfactory neurons can utilize the endogenous lectin, L-14, in a novel adhesion mechanism. Development. 1994;120:1373–84. [PubMed: 8050350]
- Mallamaci A., Muzio L., Chan C.H., Parnavelas J., Boncinelli E. Area identity shifts in the early cerebral cortex of Emx2S/S mutant mice. Nat Neurosci. 2000;3:679–86. [PubMed: 10862700]
- Mallamaci A., Stoykova A. Gene networks controlling early cerebral cortex arealization. Eur J Neurosci. 2006;23:847–56. [PubMed: 16519650]
- Malnic B., Hirono J., Sato T., Buck L.B. Combinatorial receptor codes for odors. Cell. 1999;96:713–23. [PubMed: 10089886]
- Malun D., Brunjes P.C. Development of olfactory glomeruli: Temporal and spatial interactions between olfactory receptor axons and mitral cells in opossum and rats. J Comp Neurol. 1996;368:1–16. [PubMed: 8725290]
- Marin-Padilla M., Amieva M.R. Early neurogenesis of the mouse olfactory nerve: Golgi and electron microscopic studies. J Comp Neurol. 1989;288:339–52. [PubMed: 2794142]
- Maruoka Y., Ohbayashi N., Hoshikawa M., Itoh N., Hogan B.M., Furuta Y. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech Dev. 1998;74:175–77. [PubMed: 9651520]
- Matsutani S., Yamamoto N. Differentiation of mitral cell dendrites in the developing main olfactory bulbs of normal and naris-occluded rats. J Comp Neurol. 2000;418:402–10. [PubMed: 10713569]
- McWhirter J.R., Goulding M., Weiner J., Chun J., Murre C. A novel fibroblast growth factor gene expressed in the developing nervous system is a downstream target of the chimeric homeodomain oncoprotein E2A-Pbx1. Development. 1997;124:3221–32. [PubMed: 9310317]
- Merlo G.R., Mantero S., Zaghetto A.A., Peretto P., Paina S., Gozzo M. The role of Dlx homeogenes in early development of the olfactory pathway. J Mol Histol. 2007;38:347–58. [PubMed: 17588208]
- Meyers E.N., Lewandoski M., Martin G.R. An Fgf8 mutant allelic series generated by Creand Flp-mediated recombination. Nat Genet. 1998;18:136–41. [PubMed: 9462741]
- Miyamichi K., Serizawa S., Kimura H.M., Sakano H. Continuous and overlapping expression domains of odorant receptor genes in the olfactory epithelium determine the dorsal/ventral positioning of glomeruli in the olfactory bulb. J Neurosci. 2005;25:3586–92. [PMC free article: PMC6725380] [PubMed: 15814789]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol. 2006;22:713–37. [PubMed: 17029582]
- Mombaerts P., Wang F., Dulac C., Chao S.K., Nemes A., Mendelsohn M., Edmondson J., Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–86. [PubMed: 8929536]
- Nedelec S., Foucher I., Brunet I., Bouillot C., Prochiantz A., Trembleau A. Emx2 homeodomain transcription factor interacts with eukaryotic translation initiation factor 4E (eIF4E) in the axons of olfactory sensory neurons. Proc Natl Acad Sci USA. 2004;101:10815–20. [PMC free article: PMC490017] [PubMed: 15247416]
- Neuman T., Keen A., Zuber M.X., Kristjansson G.I., Gruss P., Nornes H.O. Neuronal expression of regulatory helix-loop-helix factor Id2 gene in mouse. Dev Biol. 1993;160:186–95. [PubMed: 8224536]
- Nicolay D.J., Doucette J.R., Nazarali A.J. Transcriptional regulation of neurogenesis in the olfactory epithelium. Cell Mol Neurobiol. 2006;26:803–21. [PubMed: 16708285]
- Panganiban G., Rubenstein J.L. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371–86. [PubMed: 12223397]
- Pedersen P., Stewart W., Greer C., Shepherd G. Evidence for olfactory function in utero. Science. 1983;221:478–80. [PubMed: 6867725]
- Pellier V., Astic L., Oestreicher A.B., Saucier D. B-50/GAP-43 expression by the olfactory receptor cells and the neurons migrating from the olfactory placode in embryonic rats. Dev Brain Res. 1994;80:63–72. [PubMed: 7955361]
- Plendl J., Sinowatz F. Glycobiology of the olfactory system. Acta Anat (Basel). 1998;161:234–53. [PubMed: 9780362]
- Poirier K., Van Esch H., Friocourt G., Saillour Y., Bahi N., Backer S., Souil E., Castelnau-Ptakhine L., Beldjord C., Francis F., Bienvenu T., Chelly J. Neuroanatomical distribution of ARX in brain and its localisation in GABAergic neurons. Mol Brain Res. 2004;122:35–46. [PubMed: 14992814]
- Puche A.C., Key B. N-acetyl-lactosamine in the rat olfactory system: Expression and potential role in neurite growth. J Comp Neurol. 1996;364:267–78. [PubMed: 8788249]
- Puche A.C., Poirier F., Hair M., Bartlett P.F., Key B. Role of galectin-1 in the developing mouse olfactory system. Dev Biol. 1996;179:274–87. [PubMed: 8873770]
- Qiu M., Bulfone A., Martinez S., Meneses J.J., Shimamura K., Pedersen R.A., Rubenstein J.L. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995;9:2523–38. [PubMed: 7590232]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–76. [PubMed: 3291116]
- Ramon-Cueto A., Avila J. Olfactory ensheathing glia: Properties and function. Brain Res Bull. 1998;46:175–87. [PubMed: 9667810]
- Rash B.G., Grove E.A. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006;16:25–34. [PubMed: 16426837]
- Rawson N.E., LaMantia A.S. Once and again: Retinoic acid signaling in the developing and regenerating olfactory pathway. J Neurobiol. 2006;66:653–76. [PubMed: 16688760]
- Ressler K.J., Sullivan S.L., Buck L.B. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. [PubMed: 7683976]
- Ressler K.J., Sullivan S.L., Buck L.B. Information coding in the olfactory system: Evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1995;79:1245–55. [PubMed: 7528109]
- Rodriguez-Gil D., Greer C. Wnt/Frizzled family members mediate olfactory sensory neuron axon extension. J Comp Neurol. 2008;511:301–17. [PMC free article: PMC2570497] [PubMed: 18803244]
- Rosselli-Austin L., Altman J. The postnatal development of the main olfactory bulb of the rat. J Dev Physiol. 1979;1:295–313. [PubMed: 551115]
- Rubenstein J.L., Anderson S., Shi L., Miyashita-Lin E., Bulfone A., Hevner R. Genetic control of cortical regionalization and connectivity. Cereb Cortex. 1999;9:524–32. [PubMed: 10498270]
- Sato Y., Hirata T., Ogawa M., Fujisawa H. Requirement for early-generated neurons recognized by monoclonal antibody lot1 in the formation of lateral olfactory tract. J Neurosci. 1998;18:7800–10. [PMC free article: PMC6793018] [PubMed: 9742149]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–51. [PubMed: 16677629]
- Schoenfeld T.A., Clancy A.N., Forbes W.B., Macrides F. The spatial organization of the peripheral olfactory system of the hamster. Part I: Receptor neuron projections to the main olfactory bulb. Brain Res Bull. 1994;34:183–210. [PubMed: 8055347]
- Schwanzel-Fukuda M., Pfaff D.W. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–64. [PubMed: 2645530]
- Schwarting G.A., Raitcheva D., Crandall J.E., Burkhardt C., Puschel A.W. Semaphorin 3A-mediated axon guidance regulates convergence and targeting of P2 odorant receptor axons. Eur J Neurosci. 2004;19:1800–10. [PubMed: 15078553]
- Scolnick J.A., Cui K., Duggan C.D., Xuan S., Yuan X.B., Efstratiadis A., Ngai J. Role of IGF signaling in olfactory sensory map formation and axon guidance. Neuron. 2008;57:847–57. [PMC free article: PMC2364597] [PubMed: 18367086]
- Serizawa S., Ishii T., Nakatani H., Tsuboi A., Nagawa F., Asano M., Sudo K., Sakagami J., Sakano H., Ijiri T., Matsuda Y., Suzuki M., Yamamori T., Iwakura Y., Sakano H. Mutually exclusive expression of odorant receptor transgenes. Nat Neurosci. 2000;3:687–93. [PubMed: 10862701]
- Serizawa S., Miyamichi K., Nakatani H., Suzuki M., Saito M., Yoshihara Y., Sakano H. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science. 2003;302:2088–94. [PubMed: 14593185]
- Serizawa S., Miyamichi K., Takeuchi H., Yamagishi Y., Suzuki M., Sakano H. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127:1057–69. [PubMed: 17129788]
- Shay E., Greer C., Treloar H. Dynamic expression patterns of ECM molecules in the developing mouse olfactory pathway. Dev Dyn. 2008;237:1837–50. [PMC free article: PMC2787191] [PubMed: 18570250]
- Shimamura K., Rubenstein J.L. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–18. [PubMed: 9226442]
- Shykind B.M., Rohani S.C., O’Donnell S., Nemes A., Mendelsohn M., Sun Y., Axel R., Barnea G. Gene switching and the stability of odorant receptor gene choice. Cell. 2004;117:801–15. [PubMed: 15186780]
- Smart I.H. Location and orientation of mitotic figures in the developing mouse olfactory epithelium. J Anat. 1971;109:243–51. [PMC free article: PMC1271004] [PubMed: 5558232]
- St John J.A., Clarris H.J., Key B. Multiple axon guidance cues establish the olfactory topographic map: How do these cues interact? Int J Dev Biol. 2002;46:639–47. [PubMed: 12141452]
- St John J.A., Claxton C., Robinson M.W., Yamamoto F., Domino S.E., Key B. Genetic manipulation of blood group carbohydrates alters development and pathfinding of primary sensory axons of the olfactory systems. Dev Biol. 2006;298:470–84. [PubMed: 16884711]
- St John J.A., Key B. EphB2 and two of its ligands have dynamic protein expression patterns in the developing olfactory system. Dev Brain Res. 2001;126:43–56. [PubMed: 11172885]
- St John J.A., Pasquale E.B., Key B. EphA receptors and ephrin-A ligands exhibit highly regulated spatial and temporal expression patterns in the developing olfactory system. Dev Brain Res. 2002;138:1–14. [PubMed: 12234653]
- St John J.A., Tisay K.T., Caras I.W., Key B. Expression of EphA5 during development of the olfactory nerve pathway in rat. J Comp Neurol. 2000;416:540–50. [PubMed: 10660883]
- Strotmann J., Conzelmann S., Beck A., Feinstein P., Breer H., Mombaerts P. Local permutations in the glomerular array of the mouse olfactory bulb. J Neurosci. 2000;20:6927–38. [PMC free article: PMC6772838] [PubMed: 10995837]
- Strotmann J., Levai O., Fleischer J., Schwarzenbacher K., Breer H. Olfactory receptor proteins in axonal processes of chemosensory neurons. J Neurosci. 2004;24:7754–61. [PMC free article: PMC6729612] [PubMed: 15342743]
- Strotmann J., Wanner I., Helfrich T., Beck A., Meinken C., Kubick S., Breer H. Olfactory neurones expressing distinct odorant receptor subtypes are spatially segregated in the nasal neuroepithelium. Cell Tissue Res. 1994;276:429–38. [PubMed: 8062338]
- Stuhmer T., Anderson S.A., Ekker M., Rubenstein J.L. Ectopic expression of the Dlx genes induces glutamic acid decarboxylase and Dlx expression. Development. 2002;129:245–52. [PubMed: 11782417]
- Sugisaki N., Hirata T., Naruse I., Kawakami A., Kitsukawa T., Fujisawa H. Positional cues that are strictly localized in the telencephalon induce preferential growth of mitral cell axons. J Neurobiol. 1996;29:127–37. [PubMed: 8821172]
- Sullivan S.L., Bohm S., Ressler K.J., Horowitz L.F., Buck L.B. Target-independent pattern specification in the olfactory epithelium. Neuron. 1995;15:779–89. [PubMed: 7576628]
- Sur M., Rubenstein J.L. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–10. [PubMed: 16272112]
- Taniguchi M., Nagao H., Takahashi Y.K., Yamaguchi M., Mitsui S., Yagi T., Mori K., Shimizu T. Distorted odor maps in the olfactory bulb of semaphorin 3A-deficient mice. J Neurosci. 2003;23:1390–97. [PMC free article: PMC6742284] [PubMed: 12598627]
- Terkelsen O.B., Bock E., Mollgard K. NCAM and Thy-1 in special sense organs of the developing mouse. Anat Embryol (Berl). 1989;179:31131–38. [PubMed: 2567582]
- Tessier-Lavigne Goodman C.S. The molecular biology of axon guidance. Science. 1996;274:1123–33. [PubMed: 8895455]
- Theiler K. The House Mouse. Development and Normal Stages from Fertilization to 4 Weeks of Age. Berlin-Heidelberg-New York; Springer: 1972.
- Tran H., Chen H., Walz A., Posthumus J.C., Gong Q. Influence of olfactory epithelium on mitral/tufted cell dendritic outgrowth. Plos ONE. 2008;3:e3816. [PMC free article: PMC2583930] [PubMed: 19043569]
- Treichel D., Schock F., Jackle H., Gruss P., Mansouri A. mBtd is required to maintain signaling during murine limb development. Genes Dev. 2003;17:2630–35. [PMC free article: PMC280612] [PubMed: 14597661]
- Treloar H., Tomasiewicz H., Magnuson T., Key B. The central pathway of primary olfactory axons is abnormal in mice lacking the N-CAM-180 isoform. J Neurobiol. 1997;32:643–58. [PubMed: 9183743]
- Treloar H.B., Gabeau D., Yoshihara Y., Mori K., Greer C.A. Inverse expression of olfactory cell adhesion molecule in a subset of olfactory axons and a subset of mitral/tufted cells in the developing rat main olfactory bulb. J Comp Neurol. 2003;458:389–403. [PubMed: 12619073]
- Treloar H.B., Nurcombe V., Key B. Expression of extracellular matrix molecules in the embryonic rat olfactory pathway. J Neurobiol. 1996;31:41–55. [PubMed: 9120435]
- Treloar H.B., Purcell A.L., Greer C.A. Glomerular formation in the developing rat olfactory bulb. J Comp Neurol. 1999;413:289–304. [PubMed: 10524340]
- Tucker E.S., Polleux F., LaMantia A.S. Position and time specify the migration of a pioneering population of olfactory bulb interneurons. Dev Biol. 2006;297:387–401. [PubMed: 16790240]
- Valverde F., Heredia M., Santacana M. Characterization of neuronal cell varieties migrating from the olfactory epithelium during prenatal development in the rat. Immunocytochemical study using antibodies against olfactory marker protein (OMP) and luteinizing hormone-releasing hormone (LH-RH). Dev Brain Res. 1993;71:209–20. [PubMed: 8491043]
- Valverde F., Santacana M., Heredia M. Formation of an olfactory glomerulus: Morphological aspects of development and organization. Neuroscience. 1992;49:255–75. [PubMed: 1436469]
- Vassar R., Chao S.K., Sitcheran R., Nunez J.M., Vosshall L.B., Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–91. [PubMed: 8001145]
- Vassar R., Ngai J., Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74:309–18. [PubMed: 8343958]
- Waclaw R.R., Allen Z.J. 2nd, Bell S.M., Erdelyi F., Szabo G., Potter S.S., Campbell K. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–16. [PubMed: 16476661]
- Walz A., Mombaerts P., Greer C.A., Treloar H.B. Disrupted compartmental organization of axons and dendrites within olfactory glomeruli of mice deficient in the olfactory cell adhesion molecule, O.CAM. Mol Cell Neurosci. 2006;32:1–14. [PubMed: 16531066]
- Walz A., Omura M., Mombaerts P. Development and topography of the lateral olfactory tract in the mouse: Imaging by genetically encoded and injected fluorescent markers. J Neurobiol. 2006;66:835–46. [PubMed: 16673392]
- Wang F., Nemes A., Mendelsohn M., Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. [PubMed: 9546391]
- Whitesides J.G., LaMantia A.S. Differential adhesion and the initial assembly of the mammalian olfactory nerve. J Comp Neurol. 1996;373:240–54. [PubMed: 8889925]
- Whitman M., Greer C. Adult-generated neurons exhibit diverse developmental fates. Dev Neurobiol. 2007a;67:1079–93. [PubMed: 17565001]
- Whitman M., Greer C. Synaptic integration of adult-generated olfactory bulb granule cells: Basal axodendritic centrifugal input precedes apical dendrodendritic local circuits. J Neurosci. 2007b;27:9951–61. [PMC free article: PMC6672649] [PubMed: 17855609]
- Wilkinson D.G. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–64. [PubMed: 11256076]
- Wilson D.A., Kadohisa M., Fletcher M.L. Cortical contributions to olfaction: Plasticity and perception. Semin Cell Dev Biol. 2006;17:462–70. [PubMed: 16750923]
- Wray S., Grant P., Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA. 1989a;86:8132–36. [PMC free article: PMC298229] [PubMed: 2682637]
- Wray S., Nieburgs A., Elkabes S. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: Evidence for an embryonic origin in the olfactory placode. Dev Brain Res. 1989b;46:309–18. [PubMed: 2655994]
- Yazdani U., Terman J.R. The semaphorins. Genome Biol. 2006;7:211. doi:10.1186/gb–2006–7–3–211. [PMC free article: PMC1557745] [PubMed: 16584533]
- Yoshida M., Suda Y., Matsuo I., Miyamoto N., Takeda N., Kuratani S., Aizawa S. Emx1 and Emx2 functions in development of dorsal telencephalon. Development. 1997;124:101–11. [PubMed: 9006071]
- Yoshihara S., Omichi K., Yanazawa M., Kitamura K., Yoshihara Y. Arx homeobox gene is essential for development of mouse olfactory system. Development. 2005;132:751–62. [PubMed: 15677725]
- Yoshihara Y., Kawasaki M., Tamada A., Fujita H., Hayashi H., Kagamiyama H., Mori K. OCAM: A new member of the neural cell adhesion molecule family related to zone-to-zone projection of olfactory and vomeronasal axons. J Neurosci. 1997;17:5830–42. [PMC free article: PMC6573213] [PubMed: 9221781]
- Yu C.R., Power J., Barnea G., O’Donnell S., Brown H.E., Osborne J., Axel R., Gogos J.A. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–66. [PubMed: 15157418]
- Zhang J.H., Cerretti D.P., Yu T., Flanagan J.G., Zhou R. Detection of ligands in regions anatomically connected to neurons expressing the Eph receptor Bsk: Potential roles in neuron-target interaction. J Neurosci. 1996;16:7182–92. [PMC free article: PMC6578951] [PubMed: 8929427]
- Zhang J.H., Pimenta A.F., Levitt P., Zhou R. Dynamic expression suggests multiple roles of the eph family receptor brain-specific kinase (Bsk) during mouse neurogenesis. Mol Brain Res. 1997;47:202–14. [PubMed: 9221918]
- Zheng C., Feinstein P., Bozza T., Rodriguez I., Mombaerts P. Peripheral olfactory projections are differentially affected in mice deficient in a cyclic nucleotide-gated channel subunit. Neuron. 2000;26:81–91. [PubMed: 10798394]
- Zhou C., Tsai S.Y., Tsai M.J. COUP-TFI: An intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001;15:2054–59. [PMC free article: PMC312763] [PubMed: 11511537]
- Zou D.-J., Chesler A., Le P., chon C., Kuznetsov A., Pei X., Hwang E., Firstein S. Absence of adenylyl cyclase 3 perturbs peripheral olfactory projections in mice. J Neurosci. 2007;27:6675–83. [PMC free article: PMC6672705] [PubMed: 17581954]
- Zou D.-J., Feinstein P., Rivers A.L., Mathews G.A., Kim A., Greer C.A., Mombaerts P., Firestein S. Postnatal refinement of peripheral olfactory projections. Science. 2004;304:1976–79. [PubMed: 15178749]
- Inverse expression of olfactory cell adhesion molecule in a subset of olfactory axons and a subset of mitral/tufted cells in the developing rat main olfactory bulb.[J Comp Neurol. 2003]Inverse expression of olfactory cell adhesion molecule in a subset of olfactory axons and a subset of mitral/tufted cells in the developing rat main olfactory bulb.Treloar HB, Gabeau D, Yoshihara Y, Mori K, Greer CA. J Comp Neurol. 2003 Apr 14; 458(4):389-403.
- Review Performance of a Computational Model of the Mammalian Olfactory System.[Neuromorphic Olfaction. 2013]Review Performance of a Computational Model of the Mammalian Olfactory System.Benjaminsson S, Herman P, Lansner A. Neuromorphic Olfaction. 2013
- Review Neural crest and placode contributions to olfactory development.[Curr Top Dev Biol. 2015]Review Neural crest and placode contributions to olfactory development.Suzuki J, Osumi N. Curr Top Dev Biol. 2015; 111:351-74. Epub 2015 Jan 20.
- The development of axonal connections in the central olfactory system of rats.[J Comp Neurol. 1984]The development of axonal connections in the central olfactory system of rats.Schwob JE, Price JL. J Comp Neurol. 1984 Feb 20; 223(2):177-202.
- Pattern of rat olfactory bulb mitral and tufted cell connections to the anterior olfactory nucleus pars externa.[J Comp Neurol. 1985]Pattern of rat olfactory bulb mitral and tufted cell connections to the anterior olfactory nucleus pars externa.Scott JW, Ranier EC, Pemberton JL, Orona E, Mouradian LE. J Comp Neurol. 1985 Dec 15; 242(3):415-24.
- Development of the Olfactory System - The Neurobiology of OlfactionDevelopment of the Olfactory System - The Neurobiology of Olfaction
- exonuclease GOR [Homo sapiens]exonuclease GOR [Homo sapiens]gi|26665875|ref|NP_758439.1|Protein
- SLC27A6 AND (alive[prop]) (621)Gene
- TMEM254-AS1 TMEM254 antisense RNA 1 [Homo sapiens]TMEM254-AS1 TMEM254 antisense RNA 1 [Homo sapiens]Gene ID:219347Gene
- 219347[uid] AND (alive[prop]) (1)Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...