Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-sa/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Patient population:
Hospitalized adult non-critically ill (non-ICU) patients in general medicine, surgical, perioperative, short-stay, and OB/GYN areas with Type 1 diabetes (T1DM), Type 2 diabetes (T2DM), stress hyperglycemia, diabetes secondary to medications, prediabetes, and gestational diabetes.
Objectives:
To promote safe, effective glycemic management in hospitalized patients targeting blood glucose (BG) to published goals while preventing hypoglycemia.
Significance:
Approximately 30–35% of admitted patients have diabetes. Hyperglycemia is a well-established risk factor for adverse hospital outcomes. Additionally, hypoglycemia can have deleterious consequences. Safe and effective glucose management is of paramount importance in the hospital. Additionally, a hospital admission is an opportune time to address diabetes control.
Key points:
Principles of Glycemic Management in the Hospital
The following principles are the foundation of treating patients with glycemic management issues:
BG targets in non-critically ill patients. [I-C]
Pre-meal
100–140 mg/dL for typical situations
120–180 mg/dL for significant comorbid conditions, hypoglycemia unawareness
Random
≤180 mg/dL for typical situations
<200 mg/dL for limited life expectancy and terminal illness
Physiologic insulin treatment in the hospital. Insulin is the preferred drug for glycemic control in the hospital. Table 1 lists the currently available formulations of insulin.
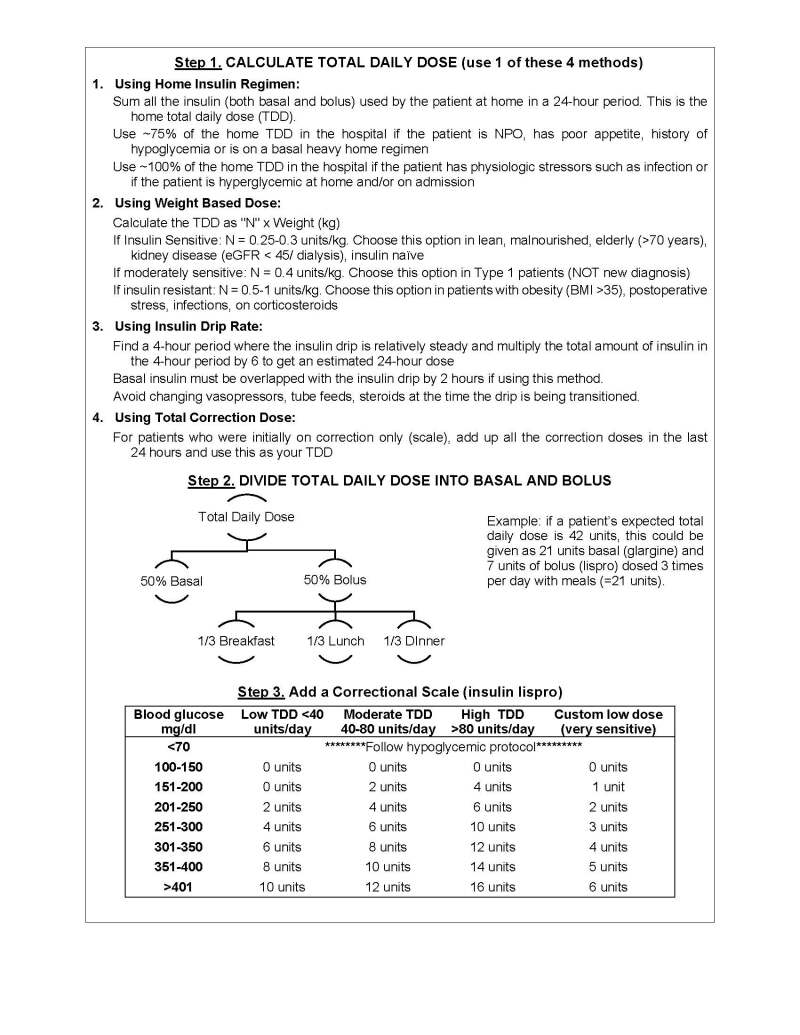
Basal-bolus insulin regimens most closely approximate physiologic insulin release and are the preferred inpatient insulin regimens. A step-wise approach to prescribing a basal-bolus insulin regimen is shown in Figure 1.
Monitoring glucose in the hospital. All patients with T1DM, T2DM, cystic fibrosis-related diabetes, patients on high-dose steroids, or those with stress hyperglycemia must have their BG monitored with point-of-care BG checks before meals (QAC) and at bedtime (QHS), or every 6 hours if NPO. [I-D]
Hemoglobin A1c. A1c should be checked in all hospitalized patients with known diabetes mellitus who have not had a measurement in the last 3 months, [I-B] and when the diagnosis of diabetes is being considered.
Management of Specific Clinical Scenarios
Outlined below are operational steps to achieve glycemic control in specific clinical situations:
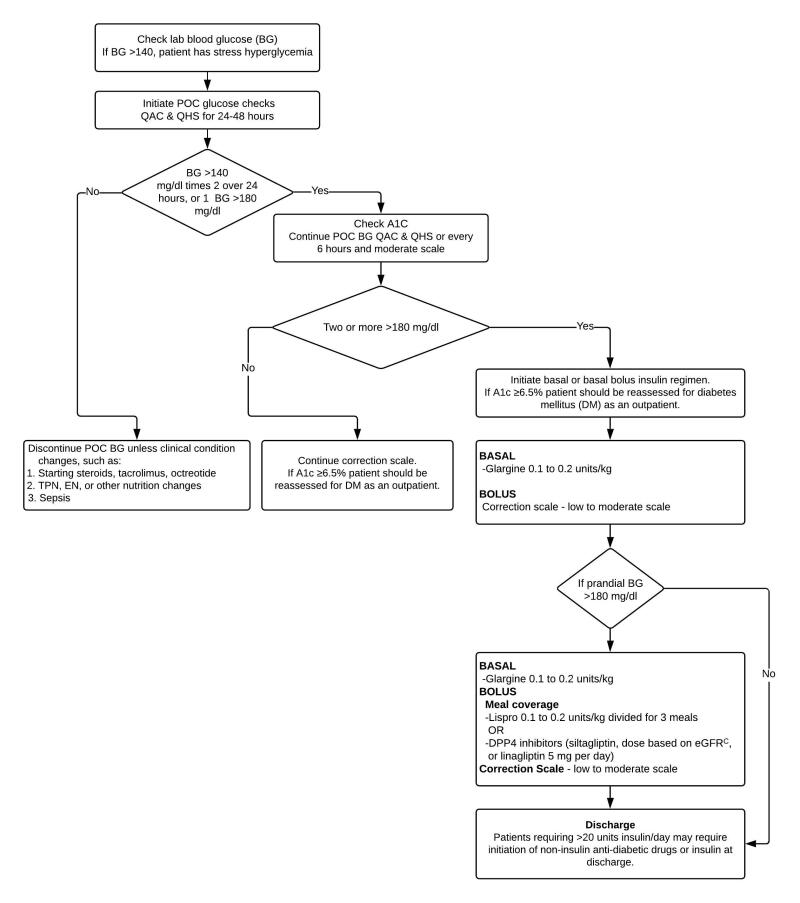
Stress hyperglycemia in patients without diabetes (Figure 2)
T2DM, taking only non-insulin agents prior to admission (Figure 3)
T2DM, taking basal-only insulin, or basal insulin plus non-insulin agents, prior to admission (Figure 5)
Taking basal-bolus insulin prior to admission (Figure 6)
Titrate insulin based on Table 2.
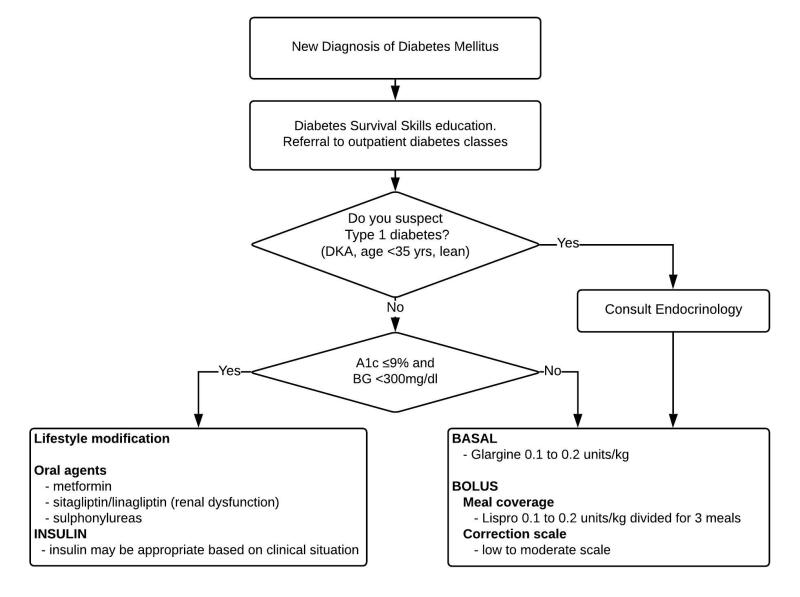
New diagnosis of diabetes made in the hospital (Figure 7)
Before discharge, provide diabetes management education focused on survival skills (Table 3). Provide follow-up in an outpatient comprehensive diabetes program.
For all patients with a new diagnosis of suspected T1DM, obtain Endocrine consultation. [I-D]
T1DM
Never hold basal insulin in a patient with T1DM.
For T1DM patients who count carbohydrates at home, continue meal insulin dosing based on carbohydrate ratios in the hospital (with modification of the ratio if needed). [I-D]
For T1DM patients on a sensitivity factor or custom correction scale at home, use this as the basis for a custom correction scale in the hospital. [II-D]
Patients using an insulin pump (continuous subcutaneous insulin infusion) at home.
Contact the Endocrinology Service for assistance with managing all insulin pump patients in the hospital. A formal consult is not required if patient is educated, insulin pump orders are in place, and BG is well managed.
Patients on insulin-pump therapy at home can continue to use their pumps in the hospital if they meet existing safety criteria. (At University of Michigan Health System (UMHS), see the Insulin Subcutaneous Infusion Pump Therapy Policy.)
Steroid-induced hyperglycemia see Table 5
Pregnancy and Peripartum. For antepartum glycemic management, see Table 6. For postpartum glycemic management, see Table 7.
Perioperative Diabetes Management
Preoperative assessment of glycemic control.
Check a preoperative A1c in all diabetes patients to assess control (if not done within the prior 3 months).
Check a preoperative A1c on patients without known diabetes if they meet the criteria in Figure 8.
Glycemic management in the preoperative period.
Table 8 shows recommendations for preoperative medication management.
Figure 10 shows recommendations for BG monitoring and control in the preoperative/holding area.
Figure 8 discusses when to consider delaying surgery due to hyperglycemia during the preoperative evaluation, and Figure 9 discusses the same for the day of surgery.
Patients who use an insulin pump for diabetes management should be instructed on preoperative management. (At UMHS see the Perioperative Insulin Pump Guideline.)
Intraoperative glycemic management see Figure 11
Glycemic management in the post-anesthesia care unit see Figure 12
Perioperative use of glucocorticoids. If BG is >180 mg/dL in the preoperative area or patient has significant insulin resistance, the anesthesia team should consider not administering dexamethasone.
Anesthesia and surgery teams should adhere to guidelines developed for administration of supplemental steroids in the perioperative period. At UMHS see Prophylaxis for PONV in the Adult Patient.
Postoperative floor management of diabetes and transition of diabetes care see Figure 12
Patients on an insulin drip in PACU who are moving to an ICU bed should continue on an insulin drip in the ICU.
Patients on an insulin drip in PACU who are moving to a floor bed should be transitioned off the insulin drip as soon as it is safe to do so after arrival on the floor using the following parameters:
- -
If no prior history of diabetes (ie, stress hyperglycemia only), insulin drip can be discontinued once it is <2units/hour and the patient should be written for a low or moderate dose correction scale. If correction scale alone does not provide adequate control within 24 hours follow Figure 2 for initiation of scheduled insulin.
- -
If the patient has a prior history of Type 2 diabetes but is not on insulin at home (i.e. diet controlled or non-insulin agents only), insulin drip can be discontinued once it is <2units/hour and the patient should be written for a moderate dose correction scale. If correction scale alone does not provide adequate control within 24 hours follow Figure 3 for initiation of scheduled insulin. Patients with A1c >7.5 or on 2 or more oral agents at home may need scheduled insulin initiated sooner.
- -
If the patient has a prior history of Type 2 diabetes on basal or basal-bolus therapy at home and the insulin drip is <2units/hour the insulin drip can be discontinued and the patient can be transitioned to SC insulin using Figure 5 or Figure 6. If they are using >2units/hour they should be continued on the insulin drip until their insulin requirements stabilize then transitioned to a basal-bolus regimen. Use Figure 1 to estimate their 24-hour drip requirements taking into account when they last dose their long-acting insulin prior to surgery and the dose, then follow Step 2 and Step 3 to initiate basal-bolus regimen. The insulin drip is discontinued 2 hours after the first injection of long- or intermediate-acting insulin.
Nutritional Issues
Nutrition education
For patients with newly diagnosed diabetes, consult an inpatient Registered Dietitian Nutritionist (RDN) for diabetes nutrition education.
For long term follow up and reinforcement, refer patients to an outpatient diabetes class, RDN, or Certified Diabetes Educator as needed.
Oral nutrition
Give a consistent carbohydrate diet to hospitalized diabetes patients when possible. At UMHS see Table 9.
Glycemic management of patient receiving enteral nutrition. (Table 10)
Total Parenteral Nutrition (TPN)
At UMHS, see Adult UMHS Hospital Guideline for Addition of Insulin to TPN.
Patients with T1DM or T2DM on insulin and TPN may have basal insulin up to 40% of Total Daily Dose (TDD), in addition to TPN insulin.
Other Issues
U-500 Insulin
Do not prescribe U-500 insulin without both approval and consultation from endocrinology. (At UMHS, U-500 insulin cannot be prescribed without approval and consultation from endocrinology).
Hypoglycemia management
Hypoglycemia in the inpatient setting should prompt an evaluation for cause and, if appropriate, a change in existing insulin regimen (Table 2).
At UMHS, manage hypoglycemia in accordance with the Hypoglycemia Treatment Algorithm: Adult
Diabetes education in the hospital setting
Focus on survival skills (Table 3).
Preparing patients for discharge
Modify diabetes therapy at the time of discharge in accord with Figure 4.
Table 1.
Currently Available Insulin Formulations
| Rapid-Acting | Ultra-Rapid Acting | Short-Acting | ||||
|---|---|---|---|---|---|---|
| (Meal Time and Correction Scale Coverage) | (Meal Time and Correction Scale Coverage) | (Meal Time and Correction Scale Coverage) | ||||
| Brand Name | Apidra | NovoLog | Humalog Admelog | Afrezza | Fiasp | Humulin R, Novolin R |
| Generic name | Glulisine | Aspart | Lispro | Aspart | Regular | |
| Formulations | Vial, Pen | Inhaled | Vial, Pen | Vial | ||
| Color | Clear | N/A | Clear | Clear | ||
| Onset | 5–15 minutes | <15 minutes | 7–13 minutes | 30 to 60 minutes | ||
| Peak | 1 to 2 hours | 50 minutes | 30–90 minutes | 2 to 3 hours | ||
| Duration | 3 to 5 hours | 2–3 hours | 3–5 hours | 6 hours | ||
| Notes | May need to take 5 −15 minutes before meal | Take right at start of meal | Take 30 minutes before meal | |||
| Intermediate-Acting (Basal Coverage) | Long-acting (Basal Coverage) | Ultra-long Acting | ||||
|---|---|---|---|---|---|---|
| Brand Name | Novolin N | Humulin N | Lantus | Levemir | Toujeo | Tresiba |
| Generic name | NPH | Glargine | Detemir | Glargine U-300 | Degludec | |
| Formulations | Vial | Vial, Pen | Vial, Pen | Vial, Pen | Pen | Pen |
| Color | Cloudy | Clear | Clear | Clear | Clear | |
| Onset | 2 to 4 hours | Begins working in 1 hour. Reaches full activity within 4 to 5 hours and continues at a constant level for 24 hours. | 50 to 120 minutes | 6 hours | 30 to 90 minutes | |
| Peak | 6 to 10 hours | 6 to 8 hours | No peak | No peak | ||
| Duration | 10 to 12 hours | 16 hours | 36 hours | 42 hours | ||
| Notes | Do not mix with other insulin. Given once or twice daily. | |||||
| Combination Insulin | Other Insulins | ||||
|---|---|---|---|---|---|
| Brand Name | Humulin 70/30 | NovoLog 70/30 Humalog 75/25 | Novolin 70/30 | Humulin R U-500 | Humalog U-200 |
| Generic name | NPH/Regular | Aspart protamine /Aspart Lispro protamine/Lispro | NPH/Regular | Regular U-500 | Lispro U-200 |
| Formulations | Vial, Pen | Vial, Pen | Vial | Vial | Pen |
| Color | Cloudy | Cloudy | Cloudy | clear | |
| Onset | 30–60 minutes | 10 to 20 minutes | 30 to 60 minutes | 30 minutes | 15 minutes |
| Peak | varies | 1 to 3 hours & 6 to 10 hours | 2 to 3 hours & 6 to 10 hours | 8 hours | 30–90 minutes |
| Duration | 10–16 hours | 10 to 12 hours | 10 to 12 hours | Up to 24 hours | 3–5 hours |
| Notes | Do not mix with other insulin. Mixing could alter insulin action. | ||||
Figure 2.
Clinical Approach to Stress Hyperglycemia with No History of Diabetes
BG = blood glucose; DM = diabetes mellitus; POC = point of care; QAC = before meals; QHS = after meals.
Figure 3.
Management of Type 2 Diabetes on Non-Insulin Agents Only Prior to Admission
BG = blood glucose; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; POC = point of care; QAC = before meals; QHS = after meals; q = every, NIA- non-insulin anti-diabetic agent
a. One notable exception is DPP4-inhibitors such as sitagliptin or linagliptin, which can be safely continued on admission if desired.
b. A1c >7.5 represents glucose control that could be improved. However, in patients with significant comorbidities, A1c <8% may be acceptable.
c Sitagliptin dose is based on eGFR: ≥ 45 cc/min = 100 mg daily, 30–44 cc/min = 50 mg daily, < 30 cc/min = 25 mg daily.
Figure 4.
Modifying the Diabetes Medication Regimen at the Time of Discharge
NIA = non-insulin antidiabetic agent
Figure 5.
Management of Type 2 Diabetes for Patients on Basal Insulin Only or Basal Insulin Plus Non-Insulin Agents Prior to Admission
a. One exception is DPP4-inhibitors such as sitagliptin or linagliptin, which can be safely continued on admission if desired.
b. A1c >7.5 represents glucose control that could be improved. However, in patients with significant comorbidities, A1c <8% may be acceptable.
c. Basal: use closer to 100% in patients poorly controlled on their home doses, use closer to 75% in patients who report hypoglycemia or who are at risk of hypoglycemia (AKI, malnourished, etc.).
d. CORRECTION SCALE: Low dose = <40 units, moderate dose = 40-80 units, high dose = >80 units continue correctional insulin scale.
e. Sitagliptin dose is based on eGFR: ≤45mL/min = 100 mg daily, 30-44mL/min = 50 mg daily, <30mL/min = 25 mg daily.
BG = blood glucose; DM = diabetes mellitus; POC = point of care; QAC = before meals; QHS = after meals; AKI = Acute Kidney Injury, NIA- non-insulin anti-diabetic agent
Table 2.
Insulin Titration
| Blood Glucose (BG) | Fasting Morning BG | Pre-Lunch/Pre-Dinner | Bedtime BG |
|---|---|---|---|
| <54mg/dl | Follow hypoglycemia protocol* Reduce glargine by 50% | Follow hypoglycemia protocol* Reduce prior meal lispro by 50% | Follow hypoglycemia protocol* Reduce dinner lispro by 50% |
| <70 mg/dl | Reduce glargine by 20% and up to 50% if BG <54 mg/dl | Reduce prior meal lispro by 20% And follow hypoglycemia protocol if needed | Reduce dinner lispro by 20% and follow hypoglycemia protocol if needed |
| 70–99 mg/dl | Reduce glargine by 10% | Reduce prior meal lispro by 10% | Reduce dinner lispro by 10% |
| 100–140 mg/dl | No change in glargine (unless patient has had other hypoglycemic episodes) | No change in prior meal lispro dose | No change dinner lispro dose |
| 141–180 mg/dl | Increase glargine by 10% | Increase prior meal lispro by 10% | No change in prior meal lispro |
| >180 mg/dl | Increase glargine by 20% | Increase prior meal lispro by 20% | Increase prior meal lispro by 10% |
These are recommendations but consider all other factors when making dose changes
- *
At UMHS see Hypoglycemia Treatment Algorithm: Adult
Figure 6.
Management of Type 2 Diabetes on Basal Bolus Insulin Prior to Admission
a. A1c >7.5 represents glucose control that could be improved. However, in patients with significant comorbidities, A1c <8% may be acceptable.
b. BASAL: use closer to 100% in patients on a balanced basal-bolus regimen, use closer to 75% in patients who report hypoglycemia or who are at risk of hypoglycemia (AKI, malnourished, etc.).
c. IF NPO – Hold bolus insulin. Reduce basal insulin dose to 70% of home dose. Continue correctional insulin scale.
d. Sitagliptin dose is based on eGFR: ≥45mL/min = 100 mg daily, 30–44mL/min = 50 mg daily, <30mL/min = 25 mg daily.
e. CORRECTION SCALE: The appropriate correction scale can be determined by considering the patient’s known (or estimated) total daily dose (TDD) of insulin. Low dose = TDD <40units, moderate dose = TDD 40–80 units, high dose = TDD >80 units.
BG = blood glucose; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; POC = point of care; QAC = before meals; QHS = after meals; AKI = acute kidney injury.
Table 3.
Inpatient Diabetes Education1
| Basic Survival Skills | Minimum Content to Reinforce |
|---|---|
| Basic understanding of diabetes and the need for treatment | The role of glucose and insulin in the disease process Difference between Type 1 and Type 2 The relationship between glycemic control and complications |
| Role of diet and consistent meal planning | Carbohydrate counting Portion size Spacing of meals |
| Ability to self-test blood glucose and knowledge of glucose targets | How to use home blood glucose monitoring meter When to check BG What to do when results are too high or too low Range for individualized glycemic targets |
| Ability to administer and dispose of medication/insulin | Purpose, timing and dosing of administration of oral medications and insulin in regards to meals Self-administration of insulin Responsible disposal of sharp supplies |
| Ability to recognize and treat hypoglycemia and hyperglycemia | Signs and symptoms, treatment and prevention of hypoglycemia Signs and symptoms, treatment and prevention of Diabetic ketoacidosis (DKA) |
| Knowledge of sick-day guidelines | How to manage blood glucose during illness and prevent severe hypo- and hyperglycemia |
| Knowledge of how and when to call for help | When and who to call for assistance: health care provider or emergency services |
- *
BG: blood glucose
Table 4.
Discharge Checklist
| Discharge Supply Check List |
|---|
| Blood Sugar Test |
| ✓ Glucometer (check insurance for type) ✓ Glucose test strips ✓ Lancets and lancing device |
| Insulin Administration (vials) |
| ✓ Long and short acting insulin as appropriate. Each vial contains 1000 units of insulin ✓ Syringes, which can hold up to 30 units, 50 units, or 100 units ✓ Needles 5/16” or 15/64” 31-gauge |
| Insulin Administration (pens) |
| ✓ Long and short acting insulin pens as appropriate. Each pen contains 300 units of insulin. ✓ Pen needles generally 4mm x 32G |
Table 5.
Approach to the Patient with Steroid-induced Hyperglycemia
| Initial Assessment | |
|---|---|
| When starting glucocorticoid therapy (≥7.5 mg prednisone per day), perform point of care BG monitoring for all patients (with or without diabetes). | |
| BG <180 | POC checks can be discontinued |
| BG <250 (mild) | Increase sliding scale from moderate to high. See regimens below if BG continues to be high. |
| BG >250 (Moderate to severe) | If taking a single daily oral steroid, add a single daily dose of NPH with the prednisone. Give NPH at same time as prednisone and titrate up based on glucose measurements. • For patients without known DM, or with DM well controlled on diet, or ≤2 oral agents, or with ESRD, 5 units of NPH is an appropriate starting point • For all other DM patients 10 units of NPH is an appropriate starting point. If patient is not on basal bolus insulin, use NPH with correction insulin • In patients on basal bolus regimens of glargine and lispro, NPH can be added as a 3rd insulin and timed with the prednisone. • If prednisone is held do NOT give NPH. Patients taking twice daily prednisone (or other long-acting steroids) who are already on a basal bolus regimen, add high dose correction scale and increase and titrate per BG readings. Meal insulin usually needs more adjustment than basal insulin. |
Table 6.
Antepartum Glycemic Recommendations
| Type of Diabetes | Diet | Glucose Monitoring | Medication |
|---|---|---|---|
| Gestational | Obstetric Diabetes Diet | Fasting and post-meals (4 times daily) | For new treatment regimens, suggest split mixed insulin*, starting at ½ - ¾ of expected needs. Glyburide or metformin for selected patients. |
| Type 2 | Obstetric Diabetes Diet | Pre- and post- meals and bedtime (7 times daily) | For new treatment regimens, suggest split mixed insulin*, starting at ¾ of expected needs. Multiple daily doses of insulin in selected patients. Metformin as an insulin sensitizer for selected patients. |
| Type 1 | Obstetric Diabetes Diet | Pre- and post- meals and bedtime (7 times daily) | For new treatment regimens, suggest multiple daily doses of insulin, starting at ½ of expected needs. |
- *
See Table 1 for a list of mixed insulins
Table 7.
Postpartum Glycemic Recommendations
| Type of Diabetes | Diet | Glucose Monitoring | Medication |
|---|---|---|---|
| Gestational | Regular Diet | Check sugar fasting and after breakfast on postpartum Day 1 | None |
| Type 2 | Obstetric Diabetes Diet | Pre- and post- meals and bedtime (7 times daily) | Evaluate pre-pregnancy control. Metformin (preferred) vs glyburide vs insulin. Start insulin at ½ of pre-pregnancy dose. |
| Type 1 | Obstetric Diabetes Diet | Pre- and post- meals and bedtime (7 times daily) | Insulin. For patients without known pre-pregnancy control consider insulin drip for calculation of needs. May also calculate (as per Figure 1) starting at half of calculated dose. |
| For pump patients, start basal rates at ½ of pre-pregnancy dose. Start bolus ratios at 2/3 of pre-pregnancy. | |||
| For basal coverage use detemir and glargine starting at half of pre-pregnancy doses. For mealtime insulin use rapid-acting insulin starting at 2/3 of pre-pregnancy doses (carb ratio or fixed dose). |
Table 8.
Perioperative Medication Schedule
| Scenario | Day(s) before procedure | Morning of procedure |
|---|---|---|
| Patient takes oral diabetes medications (other than SGLT-2 inhibitors)a | Take usual dose | HOLD dose |
| Patient takes SGLT-2 inhibitors | Hold for 2 days before fasting | HOLD dose |
| Patient takes non-insulin injectablesb Patient takes evening or bedtime insulin | Take usual dose | HOLD dose |
| - NPH | Take usual dose | Take 50% of usual dose |
| - Mixed insulinsc | Take usual dose | Take 50% of usual dose |
| - Glargine/GlargineU-300/Detemir /Reg U-500 (without scheduled meal insulin) | Take 50% of usual dose | Take 50% of usual dose |
| - Degludec (without scheduled meal insulin) | Take 50% of usual dose for 1–2 days prior to procedure | Take 50% of usual dose |
| - Glargine/Glargine U-300/Detemir (as part of a regimen which includes scheduled meal insulin) | Take 70% of usual dose | Take 70% of usual dose |
| - Degludec (as part of a regimen which includes scheduled meal insulin) | Take 70% of usual dose for 1–2 days before procedure | Hold till after procedure |
| - Regular or aspart or lispro or glulisine | Take usual dinner dosee | HOLD dose |
| Patient uses insulin pumpd (UMHS staff see Perioperative Insulin Pump Guideline) | Continue basal rate-unless frequent hypoglycemia
Then reduce to temporary basal rate of 70% | Reduce to temporary basal rate of 70% |
- a
Oral SGLT2 inhibitors - Canagliflozin; Dapagliflozin; Empagliflozin; Ertugliflozin; alone or in combination pills
- b
Noninsulin injectables - Bydureon®, Byetta®, Victoza®, Symlin®, Ozempic®, Xultophy®, Soliqua®, or Trulicity®.
- c
Mixed insulin include (75/25,70/30 or 50/50)
- d
Take 50% of usual dose for sugary beverage diet for bowel prep
- e
Intraoperative and postoperative use of pump should be addressed on an individual basis in consultation with patient’s endocrinologist.
Degludec is extra-long-acting therefore doses need to be reduced for several days prior to NPO status.
SGLT-2 inhibitors are known to cause euglycemic DKA if not reduced for several days prior to procedure.
Figure 9.
Day-of-Surgery Considerations for Delay or Cancellation of Procedure
* Small ketones can be due to overnight fasting
** If the patient is in DKA:
• The patient should be admitted to an observation unit (at UMHS, UH South) for DKA management.
• The surgical team is responsible for the management of the patient until they can be moved to the observation unit.
• If the observation unit is full, the surgical team should find an expedited bed (at UMHS speak to ABCC).
Figure 10.
Glucose Management in the Preop/Holding Area
* Perioperative Insulin Pump Guideline for UMHS only
Note: Patients with Type 1 Diabetes must have some basal insulin at all times. If patient did not take reduced dose of long acting insulin per usual preop instructions, consider starting IV insulin infusion in preop or administering reduced dose of patient’s long-acting insulin).
BG = blood glucose
Figure 11.
Intraoperative Glucose Management
* Perioperative Insulin Pump Guideline for UMHS only
** Patients with Type 1 diabetes must have some basal insulin at all times (long-acting Sub-Q or IV infusion)
BG = Blood glucose; Q = every
Figure 12.
Glucose Management in the Post-Anesthesia Care Unit
* Patients with Type 1 diabetes must have some basal insulin at all times (long-acting Sub-Q or IV infusion).
** Perioperative Insulin Pump Guideline for UMHS only
Table 9.
Consistent Carbohydrate Diets at UMHS
| Carbohydrates per Meala | Diet Order Available in MiChartb |
|---|---|
| 45 g | Consistent Carbohydrate/Diabetes 45 g/meal |
| 60 g | Consistent Carbohydrate/Diabetes 60 g/meal |
| 75 g | Consistent Carbohydrate/Diabetes 75 g/meal |
| 90 g | Consistent Carbohydrate/Diabetes 90 g/meal |
| 105 g | Consistent Carbohydrate/Diabetes 105 g/meal |
- a
For a patient with diabetes, suggested carbohydrate intake is 60 to 75 g of carbs/meal.
- b
Carb-free snacks and food can be added freely. Examples of carb-free snacks include: nuts like macadamia, Brazil nuts, walnuts and almonds (not cashews, chestnuts and pistachios, which are higher in carbs); hard cheese; olives; celery; sliced whole meats like ham or turkey breast (processed meats may have additional carbs added); shrimp; tuna; eggs; and flax crackers (Appendix 3).
Table 10.
Glycemic Management of Patients Treated on Enteral Nutrition (“Tube Feeds”)
| All Enteral Nutrition Therapy | ||
| Standard enteral formula is acceptable for patients with diabetes. No evidence exists that diabetes enteral formulas have a significant impact on clinical outcomes. | ||
| Match insulin dosing in patients on enteral nutrition to the tube feed duration* (continuous tube feeds, bolus tube feeds, or nocturnal tube feeds). | ||
| Continuous Tube Feeds | Bolus Tube Feeds | Nocturnal Tube Feeds (~12 hours) |
| Regular insulin every 6 hours is appropriate. Long-acting basal insulin ALONE is NOT appropriate for patients on continuous tube feeds. Basal insulin should be given for T1DM and T2 patients on insulin at home. Approximately 20% to 40% of the total daily dose of insulin is used as basal to avoid hypoglycemia should tube feeds be interrupted. | Rapid-acting insulin before every bolus is appropriate. Basal insulin should be only 20% to 40% of the total daily dose of insulin | Insulin can be provided with 2 doses of regular insulin, one dose at the start and another dose half-way through the feed. An option, especially for patients with indwelling G or J tubes, is NPH as a single dose prior to tube feeds, If the patient is on ~18-hour tube feeds, three doses of regular insulin during tube feeds can provide adequate coverage |
- *
At UMHS, MiChart order sets should be used when ordering insulin for tube feed coverage. These order set include appropriate precautions in the event of interruption of tube feeds as well as instructions to reduce or hold doses based on blood sugar readings.
Clinical Background
Glycemic management in the hospital setting can be challenging, and has broad implications for patient outcomes. Approximately 25–30% of hospitalized patients have diabetes. In addition, patients without diabetes can have hyperglycemia due to the stress of illness, medications and nutritional changes. Hyperglycemia, hypoglycemia and glucose variability are all common in the hospital setting. The goal of management is to avoid both hyperglycemia (which is independently associated with increased morbidity and mortality) and hypoglycemia (which is also associated with poor outcomes).
General Principles
Insulin and Its Components
Recommendations:
- Insulin is the preferred drug for glycemic control in the hospital.
- BG targets for non-critically ill patients are ≤ 140 mg/dL for pre-meal BGs and ≤ 180 mg/dL for random BGs.
- Basal-bolus insulin regimens most closely approximate physiologic insulin release and are the preferred inpatient insulin regimen.
- Avoid using “sliding scale” regimens as sole therapy for more than 24–48 hours.
A wealth of literature and clinical experience exists for using insulin in the hospital setting. Insulin does not interact with other medications and can safely be used in patients with multisystem disease (especially renal and hepatic dysfunction) in appropriately adjusted doses and with modified glycemic targets.
Basal insulin.
These long or intermediate acting (LA, IA) insulins mimic the background insulin produced by the pancreas when patients are not eating. Available insulins include glargine, glargine-300, detemir, degludec, and NPH (isophane insulin). They are generally given once or twice daily.
Bolus insulin.
These short and rapid acting (SA, RA) insulins mimic the insulin response of the pancreas to food. They are used to cover meals and also to correct high BGs rapidly as correction insulin. Insulin that is intended to correct hyperglycemia is often ordered as a scale, and sometimes referred to as “sliding-scale insulin.” (Lispro is the formulary bolus insulin at UMHS.) Table 1 includes a comprehensive list of SA and RA insulins.
Total Daily Dose (TDD). TDD is the sum of all the insulin doses a patient injects during the day and includes both basal and bolus insulins. The bolus component includes both meal and any correction insulin.
When initiating a basal-bolus insulin regimen, a patient’s basal and bolus insulin needs are often approximately equal (the 50:50 rule). For example, for a patient with a TDD of 40 units of insulin, 20 units of basal insulin might be prescribed, along with 20 units of bolus insulin (divided among the meals).
The 50:50 rule is only an approximation and does not apply to some situations including patients:
- With steroid-induced hyperglycemia and cystic fibrosis, who typically require >50% of their insulin as bolus insulin
- Eating low carbohydrate diets, who typically need <50% of their insulin as bolus insulin.
General Principles of Glycemic Management in the Hospital
Insulin is a high-risk medication and, most significantly, can cause hypoglycemia. Following guidelines reduces the risk of adverse side effects and medication errors. Order sets in the electronic medical record can facilitate basal-bolus insulin prescription. Scheduled insulin is to be administered in a physiologic pattern as basal, bolus, and correctional insulin.
- Basal insulin is given as intermediate or long-acting insulin injected once or twice a day. AM dosing is preferred in patients with end stage renal disease.
- Bolus insulin is given as rapid or short-acting (RA/SA) insulin, which has 2 components:
- Meal insulin: RA insulin is used to cover the carbohydrate content of a meal. This can be given 15 minutes before or immediately after finishing a meal (up to 10 minutes). Hospitalized patients have unpredictable oral intake, therefore should preferably receive their RA insulin immediately after eating.
- Correction insulin: RA insulin is used to lower glucose levels that are above target. Figure 1 shows selection of appropriate correctional scale based on patient’s total daily insulin requirements.
- If a patient is eating, correction insulin can be given with meal dose in one injection.
- If patient is not eating and BG is high, correctional insulin should be given alone.
- For patients on oral agents and/or TDD of insulin of ≤0.5 units/kg/day, sitagliptin or linagliptin can replace bolus meal doses. A correction scale should still be prescribed.
Total daily dose of insulin is calculated based on four options: See Figure 1.
- Home regimen with modifications: For patients on a home regimen, decrease outpatient doses by 25%, especially if a patient is tightly controlled at home or has risk factors for hypoglycemia (such as poor oral intake or basal heavy regimen).
- Weight based: Especially for insulin naïve patients, those with high A1c, or with significant hypo- or hyperglycemia on current regimen. Use the appropriate N factor as described in Figure 1.
- Insulin drip transition: An estimated total daily dose can be calculated to convert IV to subcutaneous insulin therapy.
- Correction scale: For patients initially on correction scale-only insulin, a total daily dose can be estimated based on their total correction in the last 24 hours.
Patient comorbidities must be considered when calculating insulin doses. ESRD, hypoglycemia unawareness, severe CAD, neurological injury and malnutrition predispose a patient to hypoglycemia.
An appropriate clinic history helps determine the appropriate inpatient glycemic regimen.
Consider the following points:
- Does the patient have a previous diagnosis of diabetes?
- Does the patient have T1DM or T2DM?
- How long has the patient had diabetes?
- What prior agents has the patient been on?
- What is the patient’s current diabetes regimen?
- What is the patient’s adherence to their home regimen?
- Does the patient check his/her BG, if so how often?
- What are the patient’s BGs generally at home?
- What is the patient’s previous A1c?
- Who manages the patient’s diabetes as an outpatient?
- Does the patient have any macrovascular complications (CVD, PVD, stroke)?
- Does the patient have any microvascular complications such as retinopathy, neuropathy, or nephropathy?
- Can interventions in the hospital, including education, supplies, medications, or social interventions, improve DM care at home?
Glycemic monitoring in the hospital.
Recommendations:
- Order at least one laboratory BG measurement for all patients admitted to the hospital (with or without diabetes) to assess for unrecognized diabetes or hyperglycemia. [I-B]
- Monitor all patients with T1DM, T2DM, cystic fibrosis related diabetes, stress hyperglycemia, and those on high dose steroids tube feeds or TPN with point-of-care BG checks before meals (QAC) and at bedtime (QHS), or every 6 hours if NPO. [I-D]
- In patients with T2DM managed with noninsulin agents or in those with stress hyperglycemia, if BGs are < 140 mg/dL for 24 hours with minimal or no correctional insulin administration, then point-of-care BG monitoring can be discontinued. [II-D]
- If medications or nutrition known to increase BG are added, or if the patient’s clinical condition changes, reinstitute point-of-care monitoring. [I-C]
Patients with or without diabetes can experience significant hyperglycemia in the hospital due to physiologic stress associated with illness. Admission glucose measurement can identify those with stress hyperglycemia or with previously undiagnosed diabetes.2–5 Also, if BG is elevated, obtain an A1c to differentiate between stress hyperglycemia and newly diagnosed diabetes.
For patients with known diabetes, BG control and medication requirements can vary significantly between the hospital and the home setting. Therefore, frequent monitoring is essential to prevent both hyper- and hypoglycemia.
Perform point-of-care BG checks using the hospital’s glucose meters (not the patient’s home meter or continuous glucose sensor). Document the checks in the medical record. Patients who use continuous glucose monitoring (CGM) devices at home may continue to use these devices in the hospital for self-monitoring. However, this does not replace QAC & QHS point-of-care checks in the hospital. Insulin should always be dosed based on point-of-care readings and never off the CGM device in the hospital.6
If BGs are <140 mg/dL for 24 hours with no or minimal insulin correction, BG checks may be discontinued in patients with T2DM on non-insulin agents or stress hyperglycemia. Patients who require insulin (even if their BGs are at target) should continue to have regular monitoring QAC & QHS (or q6 hours if NPO).
Patients started on steroids (≥7.5mg prednisone or equivalent per day), tube feeds, or TPN are at high risk of hyperglycemia and require continued monitoring even if they are euglycemic on admission.7–10 See the individual sections on steroids, tube feeds, and TPN for further information on these special circumstances.
Hemoglobin A1c.
Recommendations:
- Check A1c in patients without a previous diagnosis of diabetes who require scheduled insulin during their hospitalization to distinguish between prediabetes and stress hyperglycemia. [I-B]
- Do not check A1c in patients with recent blood transfusion. The value is not reliable. See other confounding factors below. [III-C]
A1c is a measure of glycated hemoglobin and gives an estimate of glycemic control over the last 3 months. A1c ≥6.5% is consistent with the diagnosis of diabetes, and between 5.7–6.4% suggestive of prediabetes.13
A1c levels are useful for patients without and with a diagnosis of diabetes.
Data are mixed regarding the use of A1c as a predictor of poor outcomes in patients with known diabetes in the inpatient setting.22–24 For patients with high A1c, the hospitalization may be an opportune time to work on improving glucose control at home.
A1C can be artificially elevated or lowered by several conditions.25,26
- A1c is elevated by chronic anemia from iron deficiency, B12 or folic acid deficiency, asplenia, uremia, severe hypertriglyceridemia, severe hyperbilirubinemia, chronic alcoholism, chronic aspirin or salicylate use, chronic opioids and lead poisoning.
- A1C is lowered in acute blood loss, hemolytic anemias, splenomegaly, pregnancy, ribavirin, and interferon use.
- Transfusion and hemoglobinopathies have variable effects
Management of Specific Clinical Scenarios
Stress Hyperglycemia
Recommendations:
- The management of stress hyperglycemia in hospitalized patients without diabetes is described in Figure 2.
- If a patient requires more than 20 units of insulin per day, recommend BG monitoring at home and consider either an oral agent or insulin upon discharge.
- Reassess A1c as an outpatient.
In the hospital setting, stress hyperglycemia is defined as any BG >140 mg/dL in patients with an A1c of < 6.5%.27 Patients with hyperglycemia whose A1c is higher than 6.5% likely exhibit hyperglycemia due to undiagnosed diabetes rather than stress hyperglycemia.12 Separately confirm and follow up with another test of glycemia (fasting plasma glucose or A1c) as an outpatient.
Inpatient Management of Type 2 Diabetes Patients Taking Only Non-insulin Agents Prior to Admission
Recommendations:
- Management recommendations for patients on non-insulin agents at admission are shown in Figure 3.
- Discontinue non-insulin diabetes agents on admission to the hospital in acutely ill patients. One notable exception is the DPP4-inhibitors sitagliptin or linagliptin, which are shown to be safe to continue on admission.
- Insulin is appropriate in the hospital for those who have significant doses of non-insulin agents withheld at admission or those with poor glycemic control (A1c >7.5%) at home.
- Resuming non-insulin agents 1–2 days prior to discharge is reasonable if no contraindications.
- Intensify home regimen on discharge if appropriate, as per Figure 4.
Discontinue the use of most noninsulin agents during hospitalization except in select stable patients who are expected to have regular meals.28
- Sulfonylureas – discontinue because of the risk of hypoglycemia, as nutritional intake is unpredictable. This is especially true for glyburide which can cause prolonged hypoglycemia.
- Metformin:
- -
If contrast studies are anticipated, renal function is fluctuating, or estimated glomerular filtration rate (eGFR) < 45, hold on admission.
- -
If no contraindications are present (eg, renal failure, IV contrast), it may be reinstituted close to discharge.
- Thiazolidinediones (TZDs) – very slow acting and can cause fluid retention and liver toxicity.
- Glucagon-like peptide-1 (GLP-1) agonists – cause nausea and can slow gastric emptying.
- Sodium/glucose cotransporter 2 (SGLT2) inhibitors – cause glycosuria and volume changes.
Sitagliptin and linagliptin, the dipeptidyl peptidase-4 (DPP-4) inhibitor, has been evaluated for use in the inpatient setting. Out of the four DPP-4 inhibitors approved for the outpatient treatment of hyperglycemia alone or in combination with other oral agents, sitagliptin and linagliptin have been evaluated in randomized control trials for hospital use and found to be safe and effective therapy for inpatient management of general medicine and surgery patients with T2DM.29–32 The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS), found no adverse cardiovascular outcomes. It needs to be dosed according to the patient’s eGFR.33
Linagliptin has the advantage of not having any adjustment for renal failure.
In general, reinstitute home oral agents prior to discharge from the hospital if no contraindications develop and BG control was reasonable on them prior to admission (Figure 4). Reduce insulin doses (by about 25%) when oral agents are added back for discharge and BG is well controlled on insulin.
In low acuity patients who are being transferred to inpatient psychiatry, inpatient rehab, or a long-term care facility, consider reinstituting home regimen assuming no contraindications.
Inpatient Management of Type 2 Diabetes Patients Taking Basal-only Insulin, or Basal Insulin Plus Non-Insulin Agents, Prior to Admission
Recommendations:
Most patients require about 50% of insulin administered as basal insulin and 50% as bolus insulin. Patients taking high doses of basal-only insulin at home may actually be using their basal insulin to cover some of their meal requirements. Patients on “basal-heavy” regimens are more prone to hypoglycemia when NPO. Switching these patients to a basal-bolus regimen in the hospital is often useful and requires adjustment of basal dose.
Inpatient Management of Type 2 Diabetes Patients Taking Basal-bolus Insulin Prior to Admission
Recommendations:
Patients on basal-bolus insulin therapy can generally continue their insulin regimen in the hospital with adjustment as indicated in Figure 6. One notable exception is for patients who are on mixed insulin (see Table 1 for listing of mixed insulins). Mixed insulins contain both intermediate-acting and short-acting insulin combined in a single injection typically dosed twice a day. Patients on this type of insulin are prone to hypoglycemia unless they maintain a consistent meal schedule. Given that PO intake is variable in hospitalized patients it may to be safer to convert to a traditional basal-bolus regimen. To convert, add up the amount of insulin the patient uses per day to get a total daily dose and give 50% as basal insulin and 50% as bolus insulin. For instance, a patient on Novolin 70/30 who takes 50units with breakfast and 40units with dinner could be converted to 45units glargine and 15units lispro TID with meals. A dose reduction of up to 25% from the home total daily dose can still be considered in patients where there is concern for hypoglycemia as indicated in Figure 6.
New Diagnosis of Diabetes (in the hospital)
Recommendations:
- The algorithm for patients with a new diagnosis of diabetes in the hospital is detailed in Figure 7.
- Consult the Endocrinology Service for patients admitted with a new diagnosis of suspected T1DM. [I-D]
- Ensure basic diabetes education and survival skills prior to discharge for both T1DM and T2DM patients with a new diagnosis of diabetes. See education section below, and Table 3.
A new diagnosis of T1DM should be considered in patients presenting with diabetic ketoacidosis (DKA) or patients with hyperglycemia who do not fit the typical pattern for T2DM (patients <35 years or lean patients). Antibodies can be useful to help distinguish T1DM from T2DM. However, if T1DM is suspected, do not delay the initiation of insulin while awaiting the results of antibodies.
GAD-65 antibody tests are a useful first step in patients with suspected T1DM, and other antibody tests can be used on an outpatient basis.34
Endocrine consultation is appropriate for patients with new T1DM because insulin requirements can be highly variable and tend to trend down around the time of discharge (“honeymoon period”). These patients require close follow-up after discharge.
The A1c can help determine which patients require insulin versus which can be managed with lifestyle changes or oral agents. Although the American Diabetes Association suggests a cut off A1c of 10% to start insulin vs oral agents, this may be too high and 9% is more appropriate in the hospital setting.
Inpatient Management of Patients with Type 1 Diabetes
Recommendations:
- Never hold basal insulin in a patient with T1DM.
- For T1DM patients on carbohydrate ratios at home, continue mealtime insulin dosing based on the carbohydrate ratios in the hospital (with modification of the ratio if needed). [I-D]
- For patients on a sensitivity factor or custom correction scale at home, use this as the basis for a custom correction scale in the hospital. [II-D]
The key difference between T1DM and T2DM patients in the inpatient setting is that basal insulin should NEVER be held in a patient with T1DM as they do not make their own insulin. In the absence of studies specifically addressing inpatient glycemic targets in the T1DM population, for this population adhering to the same BG goals as the T2DM population is reasonable.
The absence of insulin, even for short periods of time (>2 hours), produces a catabolic state and can lead to DKA. DKA can occur at BGs as low as 250 mg/dL. This is especially true in patients both with T1DM and T2DM who are on a SGLT-2 inhibitor (euglycemic DKA). Maintain a high degree of clinical suspicion for DKA in any patient with T1DM or ketosis-prone type 2 who has been without basal insulin for >2–3 hours.
T1DM patients typically count carbohydrates. They can start with their home carbohydrate ratio in the hospital and adjust for high or low BG levels with correction. To help with this calculation, carbohydrate content of meals is on the menu in carbohydrate consistent diets. Insulin for carbohydrates must be prorated to a patient’s actual intake. The patient will need to be able to communicate with nursing regarding their carbohydrate intake and insulin to carbohydrate ratios. The carbohydrate ratio must be documented in medication orders and the amount of insulin given at each meal must be documented in the medication administration record.
Doses for T1DM patients on fixed doses of meal insulin may need to be reduced for decreased appetite or poor oral intake.
If the patient uses a sensitivity factor, this can guide the choice of correction scale. The standard low-dose correction scale at UMHS may be too aggressive for many T1DM patients, especially for those that are insulin sensitive or those with renal insufficiency. In these circumstances, a custom correction scale of 1:50 >150 (ie, 1 unit of insulin for every 50 mg/dL that the BG is greater than 150 mg/dL) may be more appropriate or a custom scale using their home sensitivity factor may be ordered.
Inpatient Management of Patients Using an Insulin Pump (continuous subcutaneous insulin infusion) at Home
Recommendations:
- Patients on insulin-pump therapy at home can continue to use their pumps in the hospital if they meet existing safety criteria.
- Contact the Endocrinology Consult Service for approval/direction to continue use of the insulin pump and if needed, assistance with pump management during hospitalization (exception is OB). A formal consultation is not required in all cases.
An insulin pump is a device that delivers rapid-acting insulin as a slow continuous subcutaneous infusion (basal component). The patient gives boluses of rapid acting insulin via the pump when the patient is eating or has high glucoses (mealtime and correctional component). The patient must prompt the pump to give boluses, either by entering the carbohydrates consumed to get a mealtime bolus or by entering the BG reading to get a correction bolus. Since the pump uses rapid acting insulin, the patient should never suspend or remove their pump for >2 hour (unless long-acting is given in its place).
At UMHS, please see the Insulin Subcutaneous Infusion Pump Therapy Policy for assistance in deciding which patients can appropriately continue their pumps in the hospital, and which patients should be changed to a different management strategy. The admitting service must contact Endocrinology for approval/direction to continue use of the insulin pump therapy during hospitalization. The order set for insulin infusion pump settings and use must be completed in the EHR by the admitting service or Endocrinology. Orders for pump therapy must be placed in the patient records (at UMHS, use MiChart; entry is located as part of the END subcutaneous insulin therapy order set). The pump order set documents the basal rates, carbohydrate ratios, and correction factors used by the pump. It also allows nursing to document BG readings and boluses in the medication administration record.
Patients on a pump must have their BGs checked 4–5 times per day on the hospital BG meters. Checking on a home meter linked to the pump does NOT substitute for checking on the hospital meter, as those readings are not documented in the medical record.
Some insulin pumps are capable of incorporating continuous glucose monitoring data in order to self-adjust insulin dosing. These types of automated insulin delivery systems (including hybrid closed loop technology) has not yet been tested in the hospital. In accordance with the current insulin pump policy at UMHS we recommend that patients on these types of devices have automated insulin delivery turned off and instead use “manual mode” while they are in the hospital. Automated insulin delivery technologies are a rapidly changing field and the endocrine consult service should be involved to help clarify which type of device the patient is using and whether or not it is appropriate for the hospital setting.
Steroid Induced Hyperglycemia
Recommendations:
An approach to the patient with steroid-induced hyperglycemia is shown in Table 5.
Steroids can cause hyperglycemia in patients with and without DM. Steroids increase mealtime insulin requirements to a greater extent than fasting (basal) insulin requirements.
Options available for controlling hyperglycemia with steroid regimens depend on the steroid doses and degree of hyperglycemia, summarized in Table 5.
Prednisone once daily in the morning causes a BG peak between lunch and dinner. Hyperglycemia in patients on single dose prednisone in the AM can be treated by adding a single dose of NPH timed with steroid administration. If patient is already on basal bolus regimen, NPH can be added as a third insulin in addition to a basal and bolus insulin. This is particularly beneficial for patients on a rapid steroid taper, as the NPH dose can be decreased in a stepwise fashion with each decrease in the prednisone dose without disturbing the basal bolus regimen.
For patients on long acting steroids, or multiple doses of steroids per day, a basal-bolus regimen with a moderate to high dose correction scale may be more useful. The insulin regimen will need to be adjusted as per BG levels and each time steroids are increased or decreased.35
For very high-dose dexamethasone treatments like CVAD (Cyclophosphamide, Vincristine Sulfate, Doxorubicin Hydrochloride [Adriamycin], Dexamethasone), high doses of basal-bolus and correction insulin are necessary with an emphasis on giving adequate meal insulin. An insulin drip may be necessary for uncontrolled hyperglycemia.36
Pregnancy and Peripartum
Recommendations:
See Table 6 for antepartum BG management recommendations and Table 7 for postpartum recommendations.
Antepartum management.
Diabetes in pregnancy can be pregestational or gestational, and it is associated with increased risk for adverse outcomes including DKA, pregnancy loss, malformation, hypertensive disorders in pregnancy, macrosomia, shoulder dystocia, cesarean delivery, neonatal hypoglycemia, and NICU admission. Excellent BG control in pregnant patients with diabetes is essential to healthy pregnancy outcomes. [I-A]
Insulin needs can decrease in early pregnancy, but rise over the course of the second and third trimesters as insulin resistance increases.
Glycemic goals during pregnancy are to maintain BG <95 mg/dL fasting, <100 mg/dL before meals, and <120 mg/dL two hours postprandially. These goals apply both to patients with pregestational and gestational diabetes.
For patients admitted during pregnancy, continuation of outpatient dosing is usually an appropriate starting point. When calculating new insulin regimens, expected daily insulin needs are approximately 0.7 units/kg in the first trimester, 0.8 units/kg in the second trimester, 0.9 units/kg in the third trimester. Initiating therapy at 1/2 – 3/4 of these expected doses is often reasonable.
Consider the oral hypoglycemic agents glyburide and metformin for use in select patients with gestational diabetes. [II-B] Patients at risk for failure with these medications have fasting BGs ≥110 mg/dL, 50-gram glucose challenge test results of ≥200 mg/dL, or diagnosis prior to 24 weeks’ gestation. [II-C]
Postpartum management.
Breastfeeding is recommended for all patients. While breastfeeding may reduce insulin requirements, this reduction is not a contraindication to breastfeeding. Feeding infants artificial milk (formula) is associated with increased likelihood of diabetes in children. [I-C]
Need for insulin falls dramatically soon after delivery, but little data exists for insulin dosing in postpartum patients. Suggestions for initial therapy and monitoring of BG in postpartum patients are presented in Table 7. [I-E]
Glycemic goals in postpartum patients are similar to those for other hospitalized patients.
For patients who are considered to be at risk for pregestational diabetes (ie, diagnosed early in gestation, or with A1c of 6.5 or more), a protocol of glucose monitoring and diabetes treatment more intensive than shown in Table 7 may be appropriate. [II-E]
Perioperative Diabetes Management
Preoperative assessment of glycemic control.
Recommendations:
- Check a preoperative A1c in all diabetes patients to assess glycemic control if not done within the prior 3 months.
- Check a preoperative A1c on patients without known diabetes if they meet the criteria in Figure 8.
Newly diagnosed diabetes may impact operative, intraoperative and postoperative BG management.37 The United States Preventive Services Task Force (USPSTF) has recommended screening for diabetes in hypertensive adults only, while the ADA recommends screening in all adults age >45 and in younger overweight patients with at least one additional risk factor.38,39
If A1c >8.5% and time permits, after discussing with surgeon and reviewing type of surgery, consider improving glucose control for planned major procedures as shown in Figure 8. Delaying surgery is not prudent in patients requiring urgent elective surgery as they may present with emergent complications while awaiting improved glycemic control.40,41
Current observational data are not conclusive that delaying surgery in non- emergent patients to improve BG control will improve surgical outcomes.
Glycemic management in the preoperative period.
Recommendations:
- Table 8 shows recommendations for preoperative medication management.
- Figure 10 shows recommendations for BG monitoring and control in the preoperative/holding area.
- Patients who use an insulin pump for diabetes management should be instructed on preoperative management. (At UMHS see the Perioperative Insulin Pump Guideline.)
Patients must receive clear instructions on how to adjust their diabetes regimen in anticipation of surgery. Insulins generally need to be reduced or withheld due to NPO status. Additionally, the non-insulin agents are generally not appropriate for the perioperative setting and should be held the morning prior to the procedure. When patients arrive at the preoperative area, confirm the amounts of their last doses of insulin and non-insulin agents. For pump patients, review current pump settings.
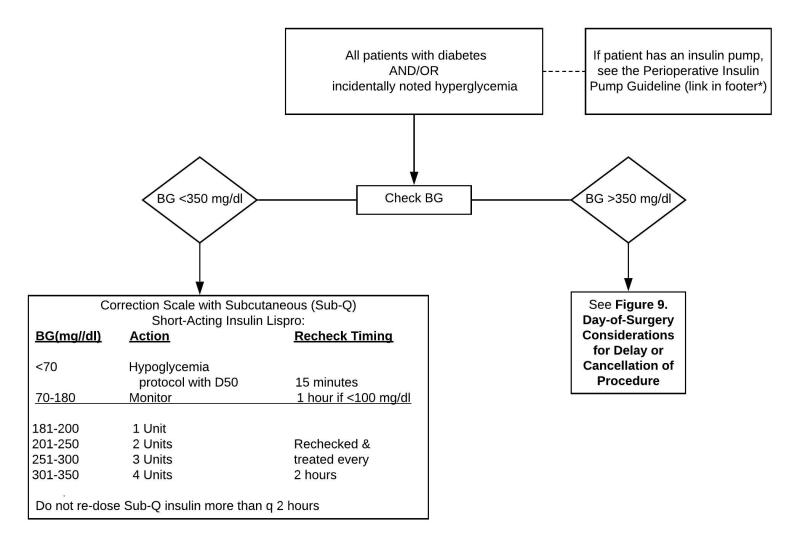
Hyper- or hypoglycemia in the preoperative area needs to be addressed for BG >180 mg/dL with rapid acting insulin injected subcutaneously via correction scale listed in Figure 10 for patients with and without a history of DM undergoing procedures not requiring initiation of an insulin drip.
Use rapid acting insulin for subcutaneous administration due to its quick onset of action. Give subcutaneous insulin no more frequently than every 2 hours, as a greater frequency can lead to insulin stacking and hypoglycemia.
Use regular insulin for IV administration. IV insulin may not be used as bolus alone as it is short-lived and must be followed by an IV insulin infusion. Depending on length and complexity of procedure and level of BG upon arrival, starting an IV insulin drip in the preoperative area may be appropriate.
IV insulin drip may also be appropriate for insulin pump patients undergoing long procedures. Remove or suspend the insulin pump. Do not stop the insulin drip until the patient is either restarted on their pump, or in rare cases, on long-acting basal insulin if admitted to the hospital (UMHS staff see Perioperative Insulin Pump Guideline.).
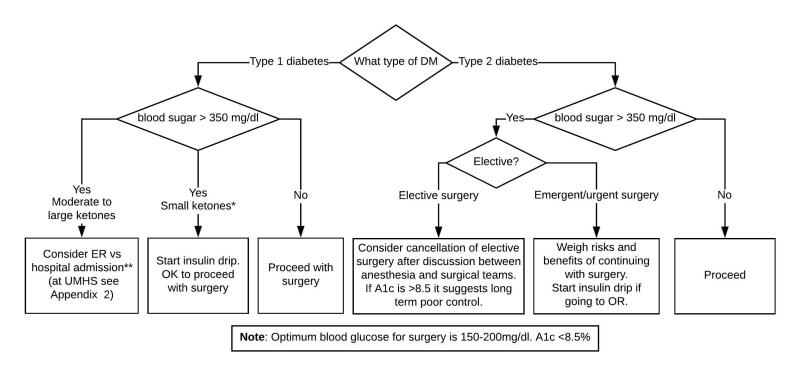
In T1DM with BG >350 mg/dL, check urine ketones. (Ketones may be present alone in patients who have long starvation periods.)
- If BG >350 mg/dL with or without small urine ketones and no anion gap, start T1DM insulin drip and proceed with the procedure. (At UMHS, see the Adult Custom Insulin Infusion Protocol for Type 1 Diabetes Mellitus.)
- In case of moderate to large ketones and anion gap, discuss with surgeon pros and cons of elective surgery and considerations to cancel. Patient will need management of ketotic state (at UMHS please see Appendix 2 to determine if ER or Hospital Admission is appropriate). Ketoacidosis is gradually corrected with insulin infusion and dextrose utilizing the institutional DKA protocol.
In patients with T2DM and BG >350 mg/dL the surgeon and anesthesiologist need to weigh the risk and benefits of the procedure.
At UMHS, hypoglycemia (< 70 mg/dL) in the preoperative area should be treated as per the Hypoglycemia Treatment Algorithm: Adult.
Intraoperative blood glucose (BG) management.
Recommendations:
- Intraoperative glycemic management is summarized in Figure 11.
- Considerations based on type of surgical procedure are summarized in Appendix 1.
Treat intraoperative BG >180 mg/dL with insulin (Figure 11). Intraoperative hyperglycemia (BG >180 mg/dL) has been associated with poorer postoperative morbidity and mortality outcomes including increased risk of cardiovascular complications, infections, and poor graft survival. The use of perioperative glycemic control in the range of 140–180 mg/dL significantly reduces perioperative infections in patients with diabetes undergoing open heart surgery. Evidence is insufficient to support tight intraoperative and postoperative glycemic (80–110 mg/dL) control for the prevention of surgical site infections in all surgical patients.41–43
Patients without a history of DM exhibiting stress related hyperglycemia experience more complications than those with DM. Diligently monitor and treat stress hyperglycemia in non-diabetes surgical patients.
An important goal of BG management in the perioperative period is avoiding wide fluctuations in BG levels. While hyperglycemia is to be avoided, hypoglycemia unrecognized in patients under anesthesia can be life-threatening. Aggressively monitor for and treat hypoglycemia.
Use of glucocorticoids.
Recommendations:
- If BG is >180 mg/dL in the preoperative area, or the patient has significant insulin resistance, the anesthesia team should not administer dexamethasone.
- At UMHS anesthesia and surgery teams should adhere to UMHS guidelines developed for administration of supplemental steroids in the perioperative period.
As described previously, dexamethasone can lead to increased BG levels (which can be difficult to treat) in patients with diabetes undergoing surgery.44–46 High quality data are limited regarding the effect of dexamethasone on BG levels. The decision to administer operative glucocorticoids should be made on a case-by-case basis, using clinical judgment along with interdisciplinary communication.
Glycemic management in the post-anesthetic care unit
Recommendations:
- Glycemic management in the post-anesthesia care unit is summarized in Figure 12.
- If a patient is started on an insulin drip intra-operatively
- -
If the patient does NOT have type 1 diabetes and is requiring less than 2 units/hour at the conclusion of the case the insulin infusion can be discontinued prior to arrival in PACU.
- -
If the patient has type 1 diabetes OR is requiring more than 2 units/hour at the conclusion of the case the insulin drip should be continued in PACU.
- Patients on an insulin drip in PACU should have their BG assessed every hour and insulin drip titrated to maintain a BG level of 120–180mg/dl
- Patients with known Type 2 diabetes who are not on an insulin drip in PACU or anyone treated with SQ insulin in pre-op should have their BG checked every 2 hours and should be given correctional insulin SQ for BG >180mg/dl
- Patient with type 1 diabetes should have their BG checked q1 hour in PACU. If they are on an insulin drip this will be titrate every hour. If they are not on an insulin drip, they can receive SQ correction insulin for blood sugar >180mg/dl not more often than every 2 hours.
To facilitate optimal glycemic control in the postoperative setting and throughout the hospital stay, communication regarding intraoperative glycemic control must be clear between anesthesia, Post-Anesthesia Care Unit (PACU), and the first contact for the surgical or admitting team.
- When transferring out of PACU, if there is a need to change the glycemic management orders based on events or needs while in PACU, the PACU nurse must communicate with the first contact for the surgical or admitting team regarding the need to change orders.
- If a patient will transition to the floor or ICU with an insulin infusion, the PACU nurse must inform the first contact for the surgical or admitting team.
Postoperative floor care.
Recommendations:
- Patients on an insulin drip in PACU who are moving to an ICU bed should continue on an insulin drip in the ICU.
- Patients on an insulin drip in PACU who are moving to a floor bed should be transitioned off the insulin drip as soon as it is safe to do so after arrival on the floor using the following parameters:
- -
If no prior history of diabetes (i.e. stress hyperglycemia only), insulin drip can be discontinued once it is <2 units/hour and the patient should be written for a low or moderate dose correction scale. If correction scale alone does not provide adequate control within 24 hours follow Figure 2 for initiation of scheduled insulin.
- -
If the patient has a prior history of Type 2 diabetes but is not on insulin at home (ie, diet controlled or non-insulin agents only), insulin drip can be discontinued once it is <2units/hour and the patient should be written for a moderate dose correction scale. If correction scale alone does not provide adequate control within 24 hours follow Figure 3 for initiation of scheduled insulin. Patients with A1c >7.5 or on 2 or more oral agents at home may need scheduled insulin initiated sooner.
- -
If the patient has a prior history of Type 2 diabetes on basal or basal-bolus therapy at home and the insulin drip is <2units/hour the insulin drip can be discontinued and the patient can be transitioned to SC insulin using Figure 5 or Figure 6. If they are using >2units/hour they should be continued on the insulin drip until their insulin requirements stabilize then transitioned to a basal-bolus regimen. Use Figure 1 to estimate their 24-hour drip requirements taking into account when they last dose their long-acting insulin prior to surgery and the dose, then follow Step 2 and Step 3 to initiate basal-bolus regimen. The insulin drip is discontinued 2 hours after the first injection of long- or intermediate-acting insulin.
BG treatment in the postoperative setting may require more intensive management due to a combination of stress, medication and nutritional factors. BG goal of 110–180 mg/dL improves postsurgical outcomes, especially infections.
Escalation of insulin sliding scales from low to moderate to high to basal-bolus insulin or insulin drip is sometimes necessary for adequate glycemic control. If BG is >250 mg/dL in the first 6 hours after surgery, recheck within 2 hours. If it continues to stay above 250 mg/dL, consider an insulin infusion. Alternatively, initiate a basal bolus regimen immediately.
Insulin correction scales should not be used as sole therapy for managing hyperglycemia for prolonged periods of time (>24 hours). Scheduled insulin should be added if correction scale is unable to keep BG in range. Beyond 24–48 hours after surgery, glycemic management should follow routine inpatient glycemic guidelines.
Nutritional Issues
Nutrition Education
Recommendations:
- Consult an inpatient Registered Dietitian Nutritionist (RDN) for diabetes diet education for patients with newly diagnosed diabetes.
- Refer patients to an outpatient diabetes class, RDN, or Certified Diabetes Educator for long term follow up or reinforcement as needed.
Oral Nutrition
Recommendations:
- Order a Consistent Carbohydrate diet based on carbohydrate requirements, to coordinate insulin dosage to carbohydrate consumption.
- A 60–75 g carbohydrate/meal diet is typically appropriate for a patient with diabetes and no other special nutritional needs. If the patient requests, a higher carbohydrate amount per meal it may be ordered.
Table 9 shows consistent carbohydrate diets available at UMHS. If patients request additional food to feel satisfied, protein and fats can be added freely to the diet.
Obstetric patients have different nutritional needs than the typical patient with diabetes. See Table 6 and Table 7 for dietary recommendations based on clinical scenario.
Enteral Nutrition (“Tube Feeds”)
Recommendations:
- Glycemic management of patient treated with enteral nutrition is shown in Table 10.
- Standard enteral formula is acceptable for patients with diabetes. (No evidence shows that diabetes enteral formulas have a significant impact on clinical outcomes.)
- Basal insulin dosing in patients on enteral nutrition depends on the tube feed duration and type of tube feed: continuous tube feeds, bolus tube feeds, or nocturnal tube feeds.
- Basal insulin alone is not appropriate to cover tube feeds. Long-acting basal insulin should be 20–40% of the TDD. The rest of insulin coverage in tube feeds is by appropriate short-acting or rapid-acting insulins.
- For continuous tube feeds, regular insulin every 6 hours is appropriate.
- For bolus tube feeds, rapid-acting insulin before every bolus is appropriate
- At UMHS, use MiChart order sets when ordering insulin for tube feed coverage. These order sets include appropriate precautions in the event of interruption of tube feeds as well as instructions to reduce or hold doses based on BG readings.
Continuous tube feeds.
The preferred insulin regimen for patients on continuous tube feeds is scheduled regular insulin given subcutaneously every 6 as hours. Regular insulin is of shorter duration, which is useful if tube feed is suddenly discontinued. This regimen also allows for rapid titration of insulin doses up and down. At UMHS, appropriate precautions are added in the tube feed order set in MiChart and its use encouraged. In addition to scheduled regular insulin, a regular insulin sliding scale every 6 hours may be ordered. A sliding scale alone is likely to be inadequate and should not be used for >24 hours.
In patients with diabetes, do not use long-acting insulin alone to cover tube feeds due to the risk of hypoglycemia if tube feeds are decreased or discontinued. Long-acting insulin can be added at 20–40% of TDD. If using home basal insulin doses, reduce dose 50% and add regular insulin every 6 hours for tube feed coverage.
Nocturnal tube feeds.
If the patient is on nocturnal tube feeds (ie, feeding given continuously for a period of 10–12 hours, overnight), use 2 doses of regular insulin, one at the start and one halfway through the feed. An alternative approach is to give NPH as a single dose prior to tube feeds, especially for patients with indwelling G or J tubes.
If the patient is on ~18-hour tube feeds, three doses of regular insulin during tube feeds can provide adequate coverage.
Bolus tube feeds.
BG management for bolus tube feeds is appropriate with rapid-acting insulin scheduled with each feed along with a rapid-acting insulin sliding scale.
Tube feed precautions.
If tube feeds are inadvertently discontinued or interrupted, scheduled regular insulin should be withheld. In cases where insulin has been given and tube feeds discontinued after the injection, increase BG monitoring frequency to every 1–2 hours; if BG is trending down or <100 mg/dL, intravenous D5 should be started at the same rate as the tube feed. Continue until BG has stabilized or patient is 6 hours out from the last regular insulin dose.
Total Parenteral Nutrition (TPN)
Recommendations:
- Patients with T1DM or T2DM on insulin may have basal insulin up to 40% of TDD, in addition to TPN insulin.
- At UMHS please see Adult UMHS Hospital Guideline for Addition of Insulin to TPN.
Add regular insulin directly to the TPN solution rather than using subcutaneous insulin to cover TPN. This provides a safety mechanism in that if the TPN is stopped for any reason, the patient will not have excess insulin on board. Adding insulin to the TPN solution also provides maximal flexibility once TPN is cycled to be given over a period of < 24 hours. The ratio of insulin to carbohydrates remains the same, even if the TPN rate changes (ie, the carbs are administered at a faster rate over shorter time and the insulin is also administered at a faster rate over shorter time, so the ratio stays fixed).
T1DM patients are an exception and should ALWAYS have subcutaneous basal insulin at all times in addition to TPN insulin. Basal insulin is also added for patients with T2DM on insulin at home or requiring very high doses of insulin with TPN. [UMHS staff see the UMHS Ambulatory Care Diabetes guideline.]
Other Issues
U-500 insulin
Recommendations:
Do not prescribe U-500 insulin without consulting the Endocrinology Service.
U-500 insulin is five times more concentrated than regular U-100 insulin. It is given 2–3 times per day with meals and takes the place of both basal and bolus insulin. Its use is generally restricted to patients on >200 units of insulin per day. It is highly error prone due to discrepancies in how doses are reported (dosed in milliliters and units).
As part of the history, inquire about what type of syringe the patient uses (TB syringe, U-500 syringe, U-100 syringe, or pen) and how the patient draws up the insulin (eg, “I draw up my insulin using an insulin syringe to the ‘20’ mark” means that the patient is taking 0.2ml or 100 units).
Patients on U-500 often need significant dose reductions in the hospital. They sometimes need to be switched to basal glargine and bolus lispro with meals.
Providers MUST contact endocrinology for consultation prior to use of U-500 in the hospital because of the high risk of medication errors and need for significant dose adjustment in hospital setting. At UMHS, the pharmacy will not dispense without an approving endocrinologist. Staff at UMHS should refer to the Utilization of Insulin Concentrate U-500 Policy for further details.
Hypoglycemia Management
Recommendations:
- In the inpatient setting, evaluate for the cause of hypoglycemia and, if appropriate, change existing insulin regimen (Table 2).
- UMHS staff are to manage hypoglycemia in accordance with the Hypoglycemia Treatment Algorithm: Adult.
Diabetes Education in the Hospital Setting
Recommendations:
Focus diabetes education for inpatients on the basic survival skills as detailed in Table 3.
Early intervention with ample opportunities for patients to practice skills using teach-back methods reduces readmissions and emergency room visits.47
Bedside nurses often have optimal teaching moments when they are administering medications and checking BG levels. The inpatient setting poses a challenge in that patients are acutely ill, under stress and may also be in pain. Inpatient diabetes education should focus on the basic survival skills necessary to safely prepare patients for discharge (Table 3). Keep sessions brief and focused, and minimize interruptions for a more productive learning experience.
Preparing Patients for Discharge
Recommendations:
- Figure 4 summarizes the management of diabetes at the time of discharge.
- Patients with A1c >8% may benefit from intensification of their diabetes regimen at discharge. See Figure 4 for recommendations based on preadmission home regimen.
- For patients on non-insulin agents before admission, consider prescribing insulin at discharge for the following patients:
- -
Patients needing significantly more than 20 units of insulin per day during their hospital stay – consider basal insulin. (Each of the non-insulin agents is roughly equivalent to 10-units of insulin.)
- -
Patients whose oral agents become contraindicated during the hospitalization (eg, changes in renal function).
- Any diabetes medication adjustment must be accompanied with a close follow-up plan and provider contact information for any issues with BG management, as patients’ BG can change rapidly within weeks of discharge.
Diabetes and hyperglycemia are commonly not the principle reason for hospitalization. Hospitalization is an opportunity to improve glycemic control in patients with poor control at baseline. Intensification of inadequate regimens during hospitalization can improve long-term glycemic control and reduce hospital readmissions.1–4
The optimal discharge medication regimen may differ from either the inpatient regimen or the admission regimen. The prehospital regimen is a useful baseline. Inpatients are often treated with insulin, with doses titrated to effect. The inpatient regimen can inform the selection of the discharge regimen as well.
Several factors may affect BG levels after discharge.
- The stress of acute illness or surgery can raise BG. This effect will wane over time as the patient recovers. Either consider this change when developing discharge regimen or arrange for close BG follow-up.
- Significant hyperglycemia can be caused by medications (eg, corticosteroids, thiazide diuretics, calcineurin inhibitors like cyclosporine or tacrolimus, atypical antipsychotics like clozapine or olanzapine). Use of these medications may necessitate adjustment of the diabetes medication regimen.
- Patients with deteriorating renal function can experience prolonged effects of insulin, and might require dose reductions.
- Dietary changes might impact glycemic control after discharge. For example, patients who start on tube feeds often require treatment for glycemic control of tube-feed related hyperglycemia.
Discuss adjustments in the discharge regimen with the patient or caregiver to ensure that they are willing and able to intensify their regimen. The discussion is particularly important for inpatients new to insulin, or who are increasing from a once daily injection to multiple daily injections. All patients need to have basic diabetes knowledge (survival skills) at discharge (Table 3). For new onset DM or new insulin starts, patients will need additional, formal diabetes education after discharge. Patients should be supplied the appropriate supplies at discharge as well (Table 4). For patients with chronic, very poor glycemic control, glycemic targets should be attained gradually to avoid exacerbation of neuropathy or retinopathy.48
Provide anticipatory guidance about what to do in the event of high or low BGs after discharge. The guidance can be provided either as titration instructions or as call parameters with contact information for the appropriate physician.
In the discharge summary, providers should document any changes that were made to the diabetes medical regimen, and identify the medical provider who will be responsible for outpatient diabetes management. Close follow-up should be arranged.
Guideline Creation Process and Considerations
Related National Guidelines
The University of Michigan Health System (UMHS) Clinical Guideline on Inpatient Glycemic Management is generally consistent with the guidelines of the American Diabetes Association (ADA). There are some minor differences:
ADA recommends an A1c of 10% as a cutoff for patients diagnosed with new diabetes to transition from lifestyle changes and oral agents to injected agents. For hospitalized patients we recommend a cutoff at A1c of 9% Table 5 due to the expected increased acuity of these patients.
Related National Performance Measures
Relevant national measures for inpatient glycemic management are:
Centers for Medicare and Medicaid Services: Glycemic Control – Hyperglycemia. Average percentage of hyperglycemic hospital days for individuals with a diagnosis of diabetes mellitus, anti-diabetes drugs (except metformin) administered, or at least one elevated glucose level during the hospital stay.
Centers for Medicare and Medicaid Services: Glycemic Control – Hypoglycemia. The rate of hypoglycemic events following the administration of an anti-diabetic agent.
Funding
The development of this guideline was funded by the University of Michigan Health System.
Guideline Development Team and Disclosures
The multidisciplinary guideline development team represented the following units of University of Michigan Health System:
Anesthesiology: Subramanian Sathishkumar, MBBS
General Surgery: Barbra S Miller, MD
Hospital Medicine: David H. Wesorick, MD
Learning Health Sciences (guideline development methodologist): F. Jacob Seagull, PhD
Literature search services: informationists at the Taubman Health Sciences Library
Metabolism, Endocrinology, and Diabetes: Roma Y Gianchandani, MBBS; Jennifer J Iyengar, MD
Nursing Services: Jennifer L Pesenecker, RN
Nutrition Services: Jackie Kosanka, RD
Obstetrics & Gynecology: Mark C Chames, MD
Pharmacy Services: Simona O. Butler, PharmD
University of Michigan Health System endorses the Guidelines of the Association of American Medical Colleges and the Standards of the Accreditation Council for Continuing Medical Education that the individuals who present educational activities disclose significant relationships with commercial companies whose products or services are discussed. Disclosure of a relationship is not intended to suggest bias in the information presented, but is made to provide readers with information that might be of potential importance to their evaluation of the information.
No relevant personal financial relationships with commercial entities: Simona O Butler, PharmD; Mark C Chames, MD; Roma Y Gianchandani, MBBS; Jennifer L Iyengar, MD; Jackie Kosanka, RD; Barbra S Miller, MD; Jennifer L Pesenecker, RN; Subramanian Sathishkumar, MBBS; F. Jacob Seagull, PhD; David Wesorick, MD.
Relevant personal financial relationships with commercial entities: None.
Strategy for Literature Search
Two different Main searches were used to search this topic, and the strategies are contained in the Search Strategies document. The second strategy was used in searching type 1 diabetes, and stress hyperglycemia. The comprehensive main search on all glycemic management in diabetes and hyperglycemia retrieved 2,486 references. When the search hedges for Guidelines, Clinical Trials, and Cohort Studies were added, the base results are as follows:
Glycemic Management -Guidelines, total results were 111
Glycemic Management -Clinical Trials, total results were 403
Glycemic Management -Cohort Studies, total results were 834
A search on all glycemic management articles that weren’t covered in the rest of the searches was added. The search retrieved 408 articles.
When the search hedges for Guidelines, Clinical Trials, and Cohort Studies were added, the base results are as follow:
Other Glycemic Management -Guidelines, total results were 33
Other Glycemic Management -Clinical Trials, total results were 72
Other Glycemic Management -Cohort Studies, total results were 107
The MEDLINE In-Process database was also searched using the strategy in the search strategies document. The search retrieved 154 documents. The results with the hedges applied are:
Guidelines, total results were 1
Clinical Trials, total results were 11
Cohort Studies, total results were 43
Within the Cochrane Database of Systematic Reviews, 64 reviews were found using the strategy in the search strategies document. Many of the Cochrane results are not relevant, but were retrieved using a strategy similar to what was used in Medline.
Level of evidence supporting a diagnostic method or an intervention:
A = systematic reviews of randomized controlled trials
B = randomized controlled trials
C =systematic review of non-randomized controlled trials or observational studies, non-randomized controlled trials, group observation studies (eg, cohort, cross-sectional, case control)
D = individual observation studies (case or case series)
E = opinion of expert panel.
Search details and evidence tables available at http://www.uofmhealth.org/provider/clinical-care-guidelines.
Recommendations
Guideline recommendations were based on prospective randomized controlled trials if available, to the exclusion of other data; if RCTs were not available, observational studies were admitted to consideration. If no such data were available for a given link in the problem formulation, expert opinion was used to estimate effect size. The “strength of recommendation” for key aspects of care was determined by expert opinion.
The strength of recommendations regarding care were categorized as:
I = Generally should be performed
II = May be reasonable to perform
III = Generally should not be performed
Data Availability
Internal UMHS Guidelines contain proprietary information so are only accessible to appropriate Michigan Medicine staff. For more information, contact the authors or publisher.
Review and Endorsement
Unit review: Drafts of this guideline were reviewed in clinical conferences and by distribution for comment within departments and divisions of the University of Michigan Health System to which the content is most relevant: Anesthesiology; Family Medicine; General Surgery; Hospital Medicine; Metabolism, Endocrinology, and Diabetes; Nursing Services; Nutrition Services; Obstetrics & Gynecology; and Pharmacy Services.
Institutional review: The final document was distributed for institutional review by the Clinical Practice Committee of the University of Michigan Medical Group and by the Executive Committee for Clinical Affairs of the University of Michigan Hospitals. These committees represent a broad spectrum of medical specialties and other health care professionals.
Footnotes
These guidelines should not be construed as including all proper methods of care or excluding other acceptable methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific clinical procedure or treatment must be made by the physician in light of the circumstances presented by the patient.
References
- 1.
- Draznin B. Managing Diabetes and Hyperglycemia in the Hospital Setting: A Clinician’s Guide.
- 2.
- Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. The Journal of clinical endocrinology and metabolism. 2002;87(3):978–982. [PubMed: 11889147]
- 3.
- Okosieme OE, Peter R, Usman M, et al. Can admission and fasting glucose reliably identify undiagnosed diabetes in patients with acute coronary syndrome? Diabetes care. 2008;31(10):1955–1959. [PMC free article: PMC2551634] [PubMed: 18591399]
- 4.
- Gray CS, Scott JF, French JM, Alberti KG, O’Connell JE. Prevalence and prediction of unrecognised diabetes mellitus and impaired glucose tolerance following acute stroke. Age and Ageing. 2004;33(1):71–77. [PubMed: 14695867]
- 5.
- Krebs JD, Robinson GM, Smith RB, Toomath RJ. Follow up testing of hyperglycaemia during hospital admission: combined use of fasting plasma glucose and HbA1c. The New Zealand medical journal. 2000;113(1117):379–381. [PubMed: 11050904]
- 6.
- Klonoff DC, Buckingham B, Christiansen JS, et al. Continuous glucose monitoring: an Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology & Metabolism. 2011;96(10):2968–2979. [PubMed: 21976745]
- 7.
- Cheung NW, Napier B, Zaccaria C, Fletcher JP. Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition. Diabetes care. 2005;28(10):2367–2371. [PubMed: 16186264]
- 8.
- Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2009;15(5):469–474. [PubMed: 19454391]
- 9.
- Korytkowski MT, Salata RJ, Koerbel GL, et al. Insulin therapy and glycemic control in hospitalized patients with diabetes during enteral nutrition therapy: a randomized controlled clinical trial. Diabetes care. 2009;32(4):594–596. [PMC free article: PMC2660455] [PubMed: 19336639]
- 10.
- Montori VM, Bistrian BR, McMahon MM. Hyperglycemia in acutely ill patients. Jama. 2002;288(17):2167–2169. [PubMed: 12413377]
- 11.
- American Diabetes A. Standard of Care 2017. 2017.
- 12.
- Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2009;15(4):353–369. [PubMed: 19454396]
- 13.
- American Diabetes A. Standard of Care 2016.
- 14.
- Mazurek JA, Hailpern SM, Goring T, Nordin C. Prevalence of hemoglobin A1c greater than 6.5% and 7.0% among hospitalized patients without known diagnosis of diabetes at an urban inner city hospital. Journal of Clinical Endocrinology & Metabolism. 2010;95(3):1344–1348. [PubMed: 20080838]
- 15.
- Ochoa PS, Terrell BT, Vega JA, et al. Identification of previously undiagnosed diabetes and prediabetes in the inpatient setting using risk factor and hemoglobin A1C screening. Annals of Pharmacotherapy. 2014;48(11):1434–1439. [PubMed: 25124691]
- 16.
- Oswald GA, Yudkin JS. Hyperglycaemia following acute myocardial infarction: the contribution of undiagnosed diabetes. Diabetic medicine : a journal of the British Diabetic Association. 1987;4(1):68–70. [PubMed: 2951225]
- 17.
- Wexler DJ, Nathan DM, Grant RW, Regan S, Van Leuvan AL, Cagliero E. Prevalence of elevated hemoglobin A1c among patients admitted to the hospital without a diagnosis of diabetes. The Journal of clinical endocrinology and metabolism. 2008;93(11):4238–4244. [PMC free article: PMC2582564] [PubMed: 18697862]
- 18.
- Lee PH, Franks AS, Barlow PB, Farland MZ. Hospital readmission and emergency department use based on prescribing patterns in patients with severely uncontrolled type 2 diabetes mellitus. Diabetes Technology & Therapeutics. 2014;16(3):150–155. [PubMed: 24224752]
- 19.
- Stolker JM, Sun D, Conaway DG, et al. Importance of measuring glycosylated hemoglobin in patients with myocardial infarction and known diabetes mellitus. American Journal of Cardiology. 2010;105(8):1090–1094. [PMC free article: PMC2856846] [PubMed: 20381658]
- 20.
- Umpierrez GE, Reyes D, Smiley D, et al. Hospital discharge algorithm based on admission HbA1c for the management of patients with type 2 diabetes. Diabetes care. 2014;37(11):2934–2939. [PMC free article: PMC4207201] [PubMed: 25168125]
- 21.
- Wei NJ, Wexler DJ, Nathan DM, Grant RW. Intensification of diabetes medication and risk for 30-day readmission. Diabetic Medicine. 2013;30(2):e56–62. [PMC free article: PMC3552066] [PubMed: 23126686]
- 22.
- Chan CY, Li R, Chan JYS, et al. The value of admission HbA(1c) level in diabetic patients with acute coronary syndrome. Clinical cardiology. 2011;34(8):507–512. [PMC free article: PMC6652704] [PubMed: 21717470]
- 23.
- Gornik I, Gornik O, Gasparovic V. HbA1c is outcome predictor in diabetic patients with sepsis. Diabetes Research & Clinical Practice. 2007;77(1):120–125. [PubMed: 17141350]
- 24.
- Liberty IF, Abu Freha N, Baumfeld Y, Codish S, Schlaeffer F, Novack V. Prognostic Value of Glycated Hemoglobin for One Year Mortality Following Hospitalization in the Internal Medicine Ward. Israel Medical Association Journal: Imaj. 2015;17(5):277–281. [PubMed: 26137652]
- 25.
- Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. Journal of general internal medicine. 2014;29(2):388–394. [PMC free article: PMC3912281] [PubMed: 24002631]
- 26.
- Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. Jama. 2006;295(14):1688–1697. [PubMed: 16609091]
- 27.
- Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes care. 2013;36(11):3430–3435. [PMC free article: PMC3816910] [PubMed: 23877988]
- 28.
- Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2012;97(1):16–38. [PubMed: 22223765]
- 29.
- Pasquel FJ, Gianchandani R, Rubin DJ, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. The lancetDiabetes & endocrinology. 2017;5(2):125–133. [PubMed: 27964837]
- 30.
- Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a Basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes care. 2013;36(8):2169–2174. [PMC free article: PMC3714500] [PubMed: 23435159]
- 31.
- Vellanki P, Rasouli N, Baldwin D, et al. Glycaemic Efficacy and Safety of Linagliptin compared to Basal-Bolus Insulin Regimen in Patients with Type 2 Diabetes Undergoing Non-Cardiac Surgery: A Multicenter Randomized Clinical Trial. Diabetes Obes Metab. 2018. [PMC free article: PMC7231260] [PubMed: 30456796]
- 32.
- Perez-Belmonte LM, Gomez-Doblas JJ, Millan-Gomez M, et al. Use of Linagliptin for the Management of Medicine Department Inpatients with Type 2 Diabetes in Real-World Clinical Practice (Lina-Real-World Study). J Clin Med. 2018;7(9). [PMC free article: PMC6162816] [PubMed: 30208631]
- 33.
- Green JB, Bethel MA, Paul SK, et al. Rationale, design, and organization of a randomized, controlled Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) in patients with type 2 diabetes and established cardiovascular disease. American Heart Journal. 2013;166(6):983–989.e987. [PubMed: 24268212]
- 34.
- Winter WE, Schatz DA. Autoimmune markers in diabetes. Clinical chemistry. 2011;57(2):168–175. [PubMed: 21127152]
- 35.
- Brady V, Thosani S, Zhou S, Bassett R, Busaidy NL, Lavis V. Safe and effective dosing of basal-bolus insulin in patients receiving high-dose steroids for hyper-cyclophosphamide, doxorubicin, vincristine, and dexamethasone chemotherapy. Diabetes Technology & Therapeutics. 2014;16(12):874–879. [PMC free article: PMC4241952] [PubMed: 25321387]
- 36.
- Grommesh B. HOSPITAL INSULIN PROTOCOL AIMS FOR GLUCOSE CONTROL IN GLUCOCORTICOID-INDUCED HYPERGLYCEMIA. Endocrine Practice. 2015. [PubMed: 26492541]
- 37.
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):e418–499. [PubMed: 17901357]
- 38.
- American Diabetes A. Standards of medical care in diabetes−-2009. Diabetes care. 2009;32 Suppl 1:S13–61. [PMC free article: PMC2613589] [PubMed: 19118286]
- 39.
- Force USPST. Screening for type 2 diabetes mellitus in adults: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2008;148(11):846–854. [PubMed: 18519930]
- 40.
- Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. The New England journal of medicine. 2008;358(24):2545–2559. [PMC free article: PMC4551392] [PubMed: 18539917]
- 41.
- Sheehy AM, Gabbay RA. An overview of preoperative glucose evaluation, management, and perioperative impact. Journal of diabetes science and technology. 2009;3(6):1261–1269. [PMC free article: PMC2787025] [PubMed: 20144379]
- 42.
- Hanazaki K, Maeda H, Okabayashi T. Relationship between perioperative glycemic control and postoperative infections. World J Gastroenterol. 2009;15(33):4122–4125. [PMC free article: PMC2738806] [PubMed: 19725144]
- 43.
- Kao Lillian S, Meeks D, Moyer Virginia A, Lally Kevin P. Peri-operative glycaemic control regimens for preventing surgical site infections in adults. John Wiley & Sons, Ltd;2009. [PMC free article: PMC2893384] [PubMed: 19588404]
- 44.
- Axelrod L. Perioperative management of patients treated with glucocorticoids. Endocrinology and metabolism clinics of North America. 2003;32(2):367–383. [PubMed: 12800537]
- 45.
- Reddy P. Clinical approach to adrenal insufficiency in hospitalised patients. International journal of clinical practice. 2011;65(10):1059–1066. [PubMed: 21762316]
- 46.
- Marik PE, Varon J. Requirement of perioperative stress doses of corticosteroids: a systematic review of the literature. Archives of surgery (Chicago, Ill: 1960). 2008;143(12):1222–1226. [PubMed: 19075176]
- 47.
- Nettles AT. Patient education in the hospital. Diabetes Spectrum. 2005;18(1):44–48.
- 48.
- Leow MK, Wyckoff J. Under-recognised paradox of neuropathy from rapid glycaemic control. Postgrad Med J. 2005;81(952):103–107. [PMC free article: PMC1743196] [PubMed: 15701742]
Appendix 1. BG Considerations Based on Type of Surgical Procedure
BG levels may be impacted by the specific type of operation being performed in addition to consideration of baseline islet cell function, insulin receptor status, comorbidities, and general stress hyperglycemia from anesthesia and surgery.
Liver & Pancreas Operations
Recommendations:
- For operations affecting the liver, monitor glucose levels carefully and more frequently during liver resection, liver transplantation, or cases requiring application of a Pringle maneuver (clamping of hepatic inflow), clamping of the vena cava for >10 minutes, or post ligation of adrenal vein during adrenalectomies for pheochromocytomas
- For pancreatic transplantation, monitor glucose levels carefully and frequently. Anticipate the need for treatment beyond that associated with other types of surgical cases. The threshold to treat hyperglycemia during pancreatic transplants must be discussed with the surgical team. At times, high BG is not treated in order to assess the response to reperfusion of the transplanted pancreas. Careful discussion should happen to avoid the consequences of untreated hyperglycemia including DKA, hyperkalemia, etc.
- For pancreatic resection (Whipple pancreaticoduodenectomy, distal pancreatectomy, or the very rare glucagonoma), monitor glucose levels more frequently. Anticipate the need for treatment with increased amounts of insulin to loss of islet cell mass leading to rebound hyperglycemia
- Special considerations should be given for insulinomas as detailed below
Operations expected to alter intraoperative BG levels include procedures affecting hepatic vascular inflow and outflow, pancreatic procedures, adrenalectomies for pheochromocytomas (risk of hypoglycemia after ligation of adrenal vein), and certain types of benign and malignant tumors.
The anesthesia and surgical teams must anticipate the increased likelihood of change in BG levels. Communication between the anesthesia and surgical teams must occur in multiple settings – preoperatively, intraoperatively, and postoperatively. Expectations for timing of significant shifts in BG should be thoroughly discussed with a plan in place to mitigate significant BG fluctuations as best as possible.
Other types of cases, such as those involving the central nervous system may require additional discussion and planning to ensure optimal outcomes.
Insulinomas are associated with rapid changes in BG. For patients undergoing enucleation or formal pancreatic resection of an insulinoma, BG should be checked every 30 minutes during the case with communication to the surgeon of the BS level.
- Prior to enucleation or formal pancreatic resection of the tumor, maintain patients on a D10 infusion to prevent hypoglycemia.
- Prior to removal of the tumor, the surgeon must communicate with the anesthesia team to discontinue the dextrose infusion and prepare to monitor the BG with an expected upward trend.
Continued assessment of BS should occur every 30 minutes to ensure stability, with communication of BS levels to the surgeon from the anesthesia team.
The BG may climb to a level warranting administration of insulin. After removal of an insulinoma, the BG should be kept between 100 and 180, or 140–180 if the patient is in the intensive care unit.
Craniotomy
Recommendations:
Monitor BG levels closely and treat hyperglycemia aggressively, particularly in patients at risk for glucose-mediated exacerbation of brain injury
Monitor glucose levels with great attention in patients undergoing craniotomy. Steroids are used to treat cerebral edema, but steroids can increase BG and elevations in BG should be avoided in the setting of central nervous system ischemia.
Appendix 3. UH/CVC Room Service Consistent Carbohydrate Snack Ideas
The grams (g) of carbohydrate listed by each item may vary. The nutrition analyses provided here are approximations only.
Snack Ideas that Combine More Than One Item Above:
- Fresh Cut Vegetable and Hummus (17g)
- Apple Slices and Peanut Butter (13g)
- Tuna Salad Packet with Mayonnaise and Saltine Crackers (5g)
- Tuna Salad Mix and Saltine Crackers (6g)
- Egg Salad Mix and Saltine Crackers (8g)
- Sliced Deli Meat (roast beef, turkey or ham), Sliced Cheese and Saltine Crackers (5–7g)
- Tossed Salad and Ranch Dressing (7g)
- Side Caesar Salad with Croutons and Light Caesar dressing (7g)
- Scramble Egg and Salsa (4g)
- Patient population:
- Objectives:
- Significance:
- Key points:
- Clinical Background
- General Principles
- Nutritional Issues
- Other Issues
- Guideline Creation Process and Considerations
- References
- BG Considerations Based on Type of Surgical Procedure
- UMHS ER vs Hospital Admissions
- UH/CVC Room Service Consistent Carbohydrate Snack Ideas
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Moderate-Intensity Insulin Therapy Is Associated With Reduced Length of Stay in Critically Ill Patients With Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic State.[Crit Care Med. 2019]Moderate-Intensity Insulin Therapy Is Associated With Reduced Length of Stay in Critically Ill Patients With Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic State.Firestone RL, Parker PL, Pandya KA, Wilson MD, Duby JJ. Crit Care Med. 2019 May; 47(5):700-705.
- Review Addressing hyperglycemia from hospital admission to discharge.[Curr Med Res Opin. 2010]Review Addressing hyperglycemia from hospital admission to discharge.Moghissi ES. Curr Med Res Opin. 2010 Mar; 26(3):589-98.
- Review Care of Children and Adolescents with Diabetes Mellitus and Hyperglycemia in the Inpatient Setting.[Curr Diab Rep. 2019]Review Care of Children and Adolescents with Diabetes Mellitus and Hyperglycemia in the Inpatient Setting.Kharode I, Coppedge E, Antal Z. Curr Diab Rep. 2019 Aug 23; 19(10):85. Epub 2019 Aug 23.
- Insulin and glucagon ratio in the patho-physiology of diabetic ketoacidosis and hyperosmolar hyperglycemic non-ketotic diabetes.[J Coll Physicians Surg Pak. 2006]Insulin and glucagon ratio in the patho-physiology of diabetic ketoacidosis and hyperosmolar hyperglycemic non-ketotic diabetes.Wahid M, Naveed AK, Hussain I. J Coll Physicians Surg Pak. 2006 Jan; 16(1):11-4.
- Review Perioperative diabetic and hyperglycemic management issues.[Crit Care Med. 2004]Review Perioperative diabetic and hyperglycemic management issues.Coursin DB, Connery LE, Ketzler JT. Crit Care Med. 2004 Apr; 32(4 Suppl):S116-25.
- Inpatient Diabetes Guideline for Adult Non-Critically Ill PatientsInpatient Diabetes Guideline for Adult Non-Critically Ill Patients
- Homologene neighbors for GEO Profiles (Select 98524623) (0)GEO Profiles
- Homologene neighbors for GEO Profiles (Select 97710303) (0)GEO Profiles
- Homologene neighbors for GEO Profiles (Select 87621915) (0)GEO Profiles
- Homologene neighbors for GEO Profiles (Select 6124051) (0)GEO Profiles
Your browsing activity is empty.
Activity recording is turned off.
See more...