This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
Continuing Education Activity
Valproic acid (VPA) ia as a highly prevalent medication with multifaceted therapeutic applications in various neurological and psychiatric disorders. The therapeutic uses of VPA include epilepsy treatment across different seizure types, bipolar disorder management, and migraine prophylaxis. The mechanism of action for VPA involves the enhancement of inhibitory neurotransmission and the modulation of voltage-gated ion channels. Coordination among interprofessional healthcare professionals, including neurologists, psychiatrists, pharmacists, and nurses, is crucial for appropriate patient selection, dosage adjustment, and monitoring of VPA therapy. This activity analyzes and assesses the indications, pharmacology, potential drug interactions, and adverse effects of VPA therapy, encompassing its effectiveness across various clinical scenarios. Furthermore, this activity also critically reviews the evidence supporting VPA's application in specific populations, including pediatric patients, pregnant women, and older individuals. The assessment also delves into the effectiveness of communication among healthcare providers, patients, and caregivers, regarding the advantages, potential risks, and adherence to VPA treatment.
Objectives:
- Assess the diverse mechanisms through which valproic acid exerts its therapeutic effects across various medical conditions.
- Analyze the evidence supporting the use of valproic acid in specific patient populations, such as pediatric patients, pregnant women, and older individuals.
- Identify the diverse therapeutic applications of valproic acid in neurological and psychiatric disorders, including epilepsy, bipolar disorder, and migraine prophylaxis.
- Collaborate with interprofessional healthcare teams, including neurologists, psychiatrists, pharmacists, and nurses, to ensure coordinated care, appropriate dosing adjustments, and comprehensive monitoring of patients on valproic acid therapy.
Indications
Valproic acid (VPA) was initially synthesized in 1882 by Burton as a derivative of valeric acid, which is a branched, short-chain, and naturally occurring fatty acid present in both plants and animals. However, the anticonvulsant properties of VPA were discovered by Eymard in 1962 while attempting to synthesize new compounds from VPA. Eymard observed that one of the compounds of VPA, 2-propylpentanoic acid, exhibited a melting point similar to that of phenytoin, the antiepileptic drug. Later, Eymard decided to conduct experiments on mice and established that the compound possessed a potent anticonvulsant effect against maximal electroshock seizures.
In 1966, Meunier and colleagues conducted the first human trial of VPA involving 12 individuals with refractory epilepsy. Their findings indicated that valproic acid notably decreased the occurrence and intensity of seizures in most patients without causing significant adverse effects. The US Food and Drug Administration (FDA) approved VPA in 1978 to treat absence seizures, also known as petit mal seizures, a form of generalized epilepsy characterized by brief episodes of altered consciousness. In 1983, the FDA approved the use of valproic acid for treating complex partial seizures, also termed psychomotor seizures, a type of focal epilepsy characterized by impaired consciousness and automatisms.[1] Since its introduction, VPA has gained extensive utilization as a first-line or adjunctive therapy for various epilepsy types.
Divalproex sodium is the stable, coordinated compound of sodium valproate and VPA. Due to its distinctive extensive spectrum of anticonvulsant effects, divalproex sodium is used to manage a wide range of seizure disorders, such as myoclonic epilepsy syndromes, absence epilepsy, generalized convulsions, partial seizures, and status epilepticus.[2][3] Divalproex sodium also efficiently manages acute depressive episodes of bipolar mood disorder and severe manic or mixed episodes.[4][5]
Over the past decades, the drug's utilization has experienced a substantial upswing, not only as a mood stabilizer but also as a replacement for lithium treatment. However, a retrospective cohort study involving individuals with bipolar disorder revealed that those under lithium treatment exhibited a reduced risk of suicide attempts and suicide-related deaths compared to those receiving divalproex sodium.[6] Nevertheless, the 2002 American Psychiatric Association (APA) guidelines recommend treating severe manic or mixed episodes, suggesting the commencement of either lithium or divalproex sodium in combination with an atypical antipsychotic as the preferred first-line therapeutic approach.
Patients encountering milder episodes can opt for treatment with divalproex, lithium, or an atypical antipsychotic as monotherapy options. Lamotrigine is a modern antiepileptic agent that has received approval to prevent depressive episodes in individuals with bipolar disorder.[7] The pharmacological guidelines for treating bipolar disorder continue to be complex, suggesting the addition of lamotrigine for patients exhibiting partial response to a combined regimen of lithium and divalproex.[8][9]
When using VPA, it is important to exercise caution as the drug can prolong the elimination half-life of lamotrigine.[10] Furthermore, VPA has gained substantial recognition for its effectiveness in preventing migraine headaches since the FDA approved this indication in 1996.[11][12] Within the pediatric population, VPA has exhibited promising efficacy in treating bipolar mood disorder and conduct disorder. This drug specifically addresses irritability, aggression, and impulsivity symptoms, showcasing its potential in this context.[10] Research into the utilization of VPA in cancer therapy is still in its early stages, offering valuable insights into novel realms of its potential application.[13]
Recent research has also unveiled the effectiveness of VPA as an adjuvant therapy in conditions such as HIV, cancer, and neurodegenerative diseases, owing to its histone deacetylase (HDAC) inhibition property.[14][15] VPA is a widely used therapy for pediatric epilepsy due to its diverse targets and favorable safety profile. The significant variability in dose requirements and interactions with a broad spectrum of drugs necessitates consistent patient follow-up and the implementation of therapeutic drug monitoring.
FDA-Approved Indications
VPA holds FDA-approved indications for a diverse range of neurological and psychiatric conditions, as listed below.
- As a monotherapy and adjunctive therapy to address complex partial seizures in adult and pediatric patients aged 10 and older.
- As a standalone and adjunctive therapy to treat both simple and complex absence seizures in adults and pediatric patients.
- As an adjunctive therapy for patients with multiple seizure types, encompassing absence seizures.
Off-Label Uses
Beyond its FDA-approved indications, VPA also finds utility in several off-label applications, as listed below.
- Manic episodes associated with bipolar disorder
- Migraine prophylaxis
- Emergency treatment of status epilepticus[16]
- Diabetic peripheral neuropathy
- Postherpetic neuralgia
- Impulsivity, agitation, and aggression
Mechanism of Action
VPA is a medication with diverse mechanisms of action that are not yet fully comprehended. Some of the suggested mechanisms of action for VPA include:
- Inhibition of voltage-gated sodium channels: VPA obstructs the entry of sodium ions into neurons, leading to decreased neuron excitability and firing rate. This prevents the generation and propagation of abnormal electrical impulses responsible for triggering seizures.
- Inhibition of gamma-aminobutyric acid (GABA) transaminase: VPA inhibits the function of the GABA transaminase enzyme, which is responsible for degrading GABA, the predominant inhibitory neurotransmitter in the brain. This process increases the levels and activity of GABA, thereby enhancing the inhibition of neuronal function and ultimately diminishing the vulnerability to seizures. In cases of neuropathic pain, VPA has been demonstrated to impede neurogenic inflammation through GABA-A receptor-mediated inhibition.
- Enhancement of GABA synthesis: VPA stimulates the synthesis of GABA by increasing the expression and activity of glutamic acid decarboxylase (GAD), the enzyme responsible for converting glutamate, which is the primary excitatory neurotransmitter in the brain, into GABA. This process also contributes to the increase in GABA levels and activity. GABA is synthesized from α-ketoglutarate via the tricarboxylic acid (TCA) cycle and metabolized into succinate semialdehyde. This intermediate is further transformed into succinate by GABA transaminase and succinate semialdehyde dehydrogenase, respectively. Prior research has indicated that VPA inhibits GABA transaminase and succinate semialdehyde dehydrogenase, thereby elevating GABA concentration by reducing its degradation (see Image. Mechanism of Action of Valproic Acid).
- Inhibition of HDACs: VPA inhibits the action of HDAC enzymes, notably HDAC1, which are involved in the regulation of gene expression by modifying the acetylation status of histones, which are proteins that wrap around DNA.[17] This process leads to changes in chromatin structure, subsequently influencing the transcription of many genes, including those linked to neuronal plasticity, synaptic transmission, neurogenesis, neuroprotection, and inflammation. This phenomenon could potentially elucidate a portion of the enduring effects of VPA on mood, cognition, neurodevelopment, apoptosis, and its potential antitumor properties.[18]
- Modulation of calcium channels: VPA modulates the activity of various calcium channels, including T-type, L-type, and N-type calcium channels. These channels are essential in neuronal signaling, neurotransmitter release, gene expression, and cellular survival. The exact effects of VPA on calcium channels are complex and depend on several factors, such as channel subtype, location, state, and co-expression with other proteins. Certain studies indicate that VPA effectively inhibits T-type calcium channels, which have been implicated in absence seizures and thalamocortical oscillations. Other studies suggest that VPA enhances L-type calcium channels, which are involved in neuronal plasticity and neuroprotection. VPA may also affect N-type calcium channels associated with neuropathic pain and migraine.[19]
In summary, VPA's antiepileptic effects are manifested through the attenuation of high-frequency neuronal firing, achieved through the blockade of voltage-gated sodium, potassium, and calcium channels. The medication influences the biochemical occurrence of aura and impacts nociception by modulating GABA or glutamate-mediated neurotransmission. Moreover, VPA also influences signaling systems, such as the Wnt/beta-catenin and ERK pathways, which similarly interfere with inositol and arachidonate metabolism.[20] Valproate use is also critical in expressing multiple genes involved in cell survival, transcription regulation, ion homeostasis, signal transduction, and cytoskeletal modifications. Both immediate biochemical effects and long-term genomic influences explain the underlying effect of VPA in treating all the conditions, as mentioned in the table in the "Indications" section of this article.[21][22][18][20]
Pharmacokinetics
Comprehending the pharmacokinetics of VPA is essential in understanding its absorption, distribution, metabolism, and elimination processes within the body. This information is also essential to comprehend how the medication is affected and influenced by dosages, formulations, dietary intake, age, gender, body weight, liver function, genetic polymorphisms, and potential drug interactions.[23]
Absorption: VPA is readily absorbed from the gastrointestinal tract after oral administration. The bioavailability of VPA ranges from 81% to 89%, depending on the specific drug formulation and food intake. The absorption rate of VPA is influenced by the gastric emptying time and the dissolution rate of the particular formulation. The delayed-release and extended-release formulations of VPA are designed to reduce the peak plasma concentration and increase the drug's duration of action. The peak plasma concentration of VPA occurs within 1 to 4 hours after the oral administration of immediate-release formulations and within 4 to 17 hours after the oral administration of delayed-release or extended-release formulations. The absorption of VPA is enhanced by food, especially meals rich in fat. This can elevate the bioavailability by up to 35% and extend the time required to reach peak concentration by up to 4 hours.
Distribution: VPA exhibits a strong affinity for plasma proteins, primarily albumin, with a binding rate of approximately 90%. The binding rate of VPA is concentration-dependent and decreases as the plasma concentration increases. The VPA distribution volume is relatively low, ranging from 0.1 to 0.4 L/kg. This suggests that the drug is mainly distributed in the extracellular fluid and does not readily permeate tissues. However, VPA can cross the blood-brain barrier and enter the cerebrospinal fluid (CSF), where it exerts its anticonvulsant effects. The concentration of VPA within the CSF typically ranges between 10% and 20% of its plasma concentration. In addition, VPA can also cross the placental barrier and enter fetal circulation, potentially causing teratogenic effects. The fetal concentration of VPA generally ranges from 70% to 100% of the maternal concentration. Moreover, VPA can be excreted into breast milk, reaching concentrations similar to those in the plasma.
Metabolism: VPA is extensively metabolized in the liver through various pathways involving oxidation, glucuronidation, and beta-oxidation, as well as conjugation with carnitine or glycine. VPA is primarily metabolized via the glucuronidation pathway, which is mediated by the enzyme UDP-glucuronosyltransferase (UGT), leading to the formation of valproate glucuronide, the major inactive metabolite of VPA. The other major metabolites of VPA include 2-propyl-4-pentenoic acid (4-ene-VPA), 2-propyl-2-pentenoic acid (2-ene-VPA), 3-keto-VPA, 3-hydroxy-VPA, and valproyl-CoA. Among these metabolites, certain ones possess anticonvulsant activity, whereas others exhibit toxicity or inactivity.
Elimination: The primary route of elimination for VPA is through the kidneys, involving the excretion of its metabolites via urine. About 30% to 50% of the administered dose of VPA is excreted unchanged in the urine, whereas the rest is excreted as metabolites. Several factors, including dosages, age, gender, renal functions, genetic polymorphisms, and drug interactions, influence the elimination rate of VPA within the body. The elimination rate of VPA is subject to dosage dependency and saturation effects, signifying that it adheres to zero-order kinetics at higher doses and shifts to first-order kinetics at lower doses.
Administration
Available Dosage Forms
VPA is administered to individuals orally through tablets, sprinkles, capsules, or via the intravenous route.
Strength
The tablet form of the medication comes in 2 variations: a delayed-release formulation with strengths of 125 mg, 250 mg, and 500 mg and an extended-release formulation with strengths of 250 mg and 500 mg.
The capsule form of the medication is available in 125 mg strength. The injectable formulation is offered at a concentration of 100 mg/mL.
Adult Dosage
Treatment for simple or complex absence seizures: VPA can be used as monotherapy or adjunctive therapy in patients with complex partial seizures. The usual starting dosage of VPA for this treatment is 15 mg/kg/d, with an increase of 5 to 10 mg/kg/d every 7 days. The maximum dosage of VPA is 60 mg/kg/d, and if doses exceed 250 mg daily, the medication is administered in divided doses.[24]
Treatment for mania: The initial VPA dosage typically ranges from 250 to 500 mg, which is taken 3 times a day. In the case of the extended-release formulation, the initial dosage starts at 25 mg/kg once daily, with a rapid increase of up to 60 mg/kg/d to achieve the desired clinical effect.
Treatment for migraines: The initial VPA dosage ranges from 250 to 500 mg for migraine prophylaxis, taken twice daily for 1 week. The extended-release formulation of the drug can be initiated at 500 mg and administered once daily for 1 week. Depending on the circumstances, the dosage can be escalated to a maximum of 1000 mg daily if deemed necessary.[25]
Therapeutic ranges of valproic acid: The therapeutic range for total valproate in epilepsy is 50 to 100 mcg/mL, and in mania, it is 50 to 125 mcg/mL. VPA generally takes around 14 days to achieve maximum concentration within the body.
Specific Patient Population
Renal impairment: Dosage adjustments for VPA are not required for patients with renal impairment. However, it is recommended that patients be monitored closely for possible adverse effects.
Hepatic impairment: The use of VPA is contraindicated in patients with hepatic impairment and known or suspected mitochondrial disorders caused by mutations in the polymerase-gamma (POLG) gene, such as Alpers-Huttenlocher syndrome, which is due to the increased risk of acute liver failure. The use of VPA in patients with other types of hepatic impairment requires caution and close monitoring of liver function tests (LFTs). In patients with mild-to-moderate hepatic impairment (Child-Pugh class A or B), the dose of VPA should be decreased by 50%. For those with severe hepatic impairment (Child-Pugh class C), the dose of VPA should be reduced by 75%. The plasma concentration of VPA should be maintained within the therapeutic range of 50 to 100 mcg/mL.
Pregnancy considerations: VPA can traverse the placental barrier and enter fetal circulation, potentially leading to teratogenic effects. VPA increases the risk of major congenital malformations, particularly neural tube defects (NTDs) such as spina bifida and anencephaly. The risk of NTDs is estimated to be about 10 times higher than the general population, with estimated rates ranging from 1% to 2% compared to the usual 0.1% to 0.2%. Other malformations reported with VPA exposure include craniofacial, cardiovascular, hypospadias, and limb defects. VPA can also affect fetal neurodevelopment and cause cognitive impairment, behavioral problems, and autism spectrum disorders. The use of VPA in pregnancy is contraindicated for migraine prophylaxis, as the potential benefits do not outweigh the potential risks involved with the medication.[26][27]
Breastfeeding considerations: VPA can be excreted into breast milk, reaching concentrations similar to those in the plasma. The adverse effects caused by VPA in breastfed infants include sedation, irritability, poor feeding, weight loss, thrombocytopenia, or liver dysfunction. The risk of adverse effects is higher in infants younger than 2 months, especially those who are premature, possess low birth weight, or have underlying medical conditions.
Pediatric patients: The use of VPA in pediatric patients requires caution and close monitoring of clinical and laboratory parameters. The dosage of VPA should be personalized, considering factors such as age, weight, seizure type, concurrent medications, and plasma concentration. The initial dosage of VPA for pediatric patients usually ranges from 10 to 15 mg/kg/d, divided into 2 or 3 doses. The dosage can be increased by 5 to 10 mg/kg per week until seizure control is achieved or adverse effects occur. The usual maintenance dose of VPA for pediatric patients is 20 to 60 mg/kg/d. There is a risk of fatal hepatotoxicity associated with the drug, especially in children younger than 2.[28]
Older patients: Older patients might experience heightened vulnerability to the adverse effects of VPA due to age-related changes in pharmacokinetics and increased susceptibility to hepatotoxicity. In this population, it is often advisable to commence with lower initial doses and implement more gradual titration schedules. Regularly monitoring liver function, renal function, and potential drug interactions is paramount in ensuring VPA's safe and effective use in older patients.
Adverse Effects
Common Adverse Effects of Valproic Acid
The common adverse effects of VPA include gastrointestinal, neurological, and hematological symptoms. These adverse effects usually occur at the beginning of VPA treatment or after dosage modifications. However, these adverse effects usually diminish or resolve over time or with appropriate dosage adjustments. The adverse effects associated with the medication include headache, abdominal pain, somnolence, dizziness, thrombocytopenia, asthenia, nausea and vomiting, diarrhea, tremor, weight changes, alopecia, constipation, emotional lability, insomnia, petechiae and ecchymosis, depression, rash, nervousness, appetite changes, alanine transaminase (ALT) and aspartate aminotransferase (AST) elevation, tinnitus, blurred vision, nystagmus, photosensitivity, myalgia, and dyspnea.
Severe Adverse Effects of Valproic Acid
VPA can lead to several severe adverse reactions, including hepatotoxicity, hallucinations, suicidality, psychosis, toxic epidermal necrolysis, Stevens-Johnson syndrome, anaphylaxis, hyponatremia, SIADH, pancreatitis, thrombocytopenia, pancytopenia, hyperammonemia, myelosuppression, hypothermia, aplastic anemia, bleeding, erythema multiforme, polycystic ovarian syndrome, cerebral pseudoatrophy, encephalopathy, and coma.[29] Although these adverse effects are infrequent, they can potentially be life-threatening. Therefore, immediate medical intervention and discontinuation of VPA are imperative in such cases. The abrupt discontinuation of the drug can trigger withdrawal seizures.
Drug-Drug Interactions
VPA interacts with an array of drugs, some of which can be beneficial and enhance the therapeutic effect of VPA or reduce its adverse effects. Conversely, certain interactions can be detrimental, potentially diminishing the therapeutic efficacy of VPA or increasing its adverse effects. Some interactions are bidirectional, impacting both VPA and the concurrently administered medication. VPA's interactions can be categorized into pharmacokinetic or pharmacodynamic interactions, further divided into enzyme-based or transporter-based interactions.
- Enzyme-inducing drug interactions: VPA is a weak inhibitor of various cytochrome P450 (CYP) enzymes, including CYP2C9 and CYP2C19. Coadministration of drugs that induce these enzymes, such as carbamazepine, phenytoin, and rifampin, can increase the metabolism of VPA. This may result in decreased plasma concentration levels and potentially compromise the efficacy of the medication. Dosage adjustments or alternative treatment options may be necessary in these cases.
- Enzyme-inhibiting drug interactions: VPA is primarily metabolized via the glucuronidation pathway, mediated by UGT enzymes. Drugs that inhibit UGT enzymes, such as aspirin, felbamate, and some nonsteroidal anti-inflammatory drugs (NSAIDs), can interfere with glucuronidation, leading to increased plasma concentrations of VPA. In such scenarios, monitoring VPA levels closely and considering potential dosage adjustments in patients is advisable.
- Protein-binding interactions: VPA exhibits substantial binding to proteins, predominantly to albumin. When VPA is administered concurrently with other highly protein-bound medications, such as salicylates and sulfonamides, the potential exists for competition over albumin-binding sites. This could potentially elevate the free fraction of VPA and increase the risk of adverse effects of the drug. In such cases, monitoring for signs of VPA toxicity and adjusting the dosages as needed is advisable.
- Other interactions (antiepileptic drugs): VPA can interact with other antiepileptic drugs, including lamotrigine and phenobarbital. This can result in modifications to plasma concentrations, potentially impacting both efficacy and toxicity. In such situations, monitoring drug levels and adjusting the dosages as needed is advisable.
Contraindications
VPA is contraindicated in patients with significant hepatic impairment, hypersensitivity to components of the drug or its class, urea cycle disorders, mitochondrial disorders, suspected disorders in patients younger than 2, and during pregnancy, especially for migraine prophylaxis with VPA.
Furthermore, exercising caution with VPA usage is necessary for individuals younger than 2, pediatric and geriatric populations, and patients with renal impairment, organic brain disorders, head injury, mental retardation accompanied by seizure disorders, congenital metabolic disorders, hereditary mitochondrial disorders, decreased gastrointestinal transit time, hepatic disease, active or historical depression, undergoing multiple anticonvulsant treatments, experiencing myelosuppression, and individuals at risk of bleeding.
Box Warnings
- Severe or fatal hepatic failure incidents have been reported within the first 6 months of VPA treatment. Patients younger than 2 are at elevated risk for hepatic toxicity. Anticonvulsant polytherapy is associated with an elevated risk of fatality. In older patients, hepatotoxicity may present with symptoms of weakness, lethargy, anorexia, facial edema, vomiting, and loss of seizure control. Therefore, monitoring symptoms and LFTs at baseline and frequent intervals is recommended, especially during the initial 6 months of VPA treatment.
- Administering VPA to patients with mitochondrial disease, specifically POLG-related mitochondrial disorders, has been shown to amplify the risk of hepatotoxicity and mortality. VPA should only be used in patients older than 2 with suspected mitochondrial disorders who have not responded to alternative anticonvulsant therapies. Close monitoring of LFTs and screening for POLG mutations are recommended in such cases.
- VPA use can cause life-threatening pancreatitis. Cases of hemorrhagic pancreatitis with rapid progression to fatality have been reported across all age groups, irrespective of the treatment duration. If patients exhibit symptoms suggestive of pancreatitis, such as nausea, vomiting, abdominal pain, or anorexia, it is recommended to discontinue the medication and initiate alternative treatment options based on clinical indication.[30][31]
- VPA can cause severe congenital malformations, including NTDs, which result in lower IQ scores when exposed in utero. Furthermore, in utero, exposure to VPA is associated with an elevated risk of autism spectrum disorders in children.[32][33] The administration of VPA to pregnant women for migraine headaches is contraindicated unless no other alternative anticonvulsant therapy is available. According to APA guidelines, women with bipolar disorder who opt to continue VPA treatment during pregnancy should undergo additional screening as their healthcare providers recommend.[34]
Monitoring
LFTs should be monitored at baseline and then at regular intervals, particularly within the first 6 months of VPA treatment or if hereditary mitochondrial disease is present. Complete blood count with differential, coagulation tests, and ammonia levels should be assessed at baseline, periodically, before planned surgical procedures, and throughout pregnancy.
Screening procedures should be conducted for patients to detect signs of depression, alterations in behavior, and suicidality.
Serum drug levels should be monitored, with therapeutic ranges set at 50 to 100 mcg/mL for epilepsy and 50 to 125 mcg/mL for mania. The toxic levels are indicated as >175 mcg/mL before the morning dose of VPA. When hypoalbuminemia is present, assessing valproate's free levels is essential due to its protein-binding nature, as the total concentration measurements may be inaccurate.[35]
Toxicity
Acute VPA toxicity can lead to central nervous system (CNS) depression, metabolic acidosis, hypernatremia, hypoglycemia, hyperammonemia, hepatotoxicity, pancreatitis, or multiorgan failure.[36]
Signs and Symptoms of Overdose
CNS depression might manifest as drowsiness, confusion, ataxia, nystagmus, diplopia, dysarthria, tremor, or coma. Metabolic acidosis can present with tachypnea, hypotension, arrhythmias, or shock. Hypernatremia can manifest as thirst, dry mucous membranes, agitation, seizures, or coma. Hyperammonemia can manifest as lethargy, vomiting, ataxia, or encephalopathy. Hepatotoxicity can be evident through elevated liver enzymes.
Management of Overdose
Discontinuation of VPA: The initial step to managing VPA toxicity is discontinuing the medication. This will allow for the removal of the drug and decrease the risk of additional toxicity.
Supportive care: Supportive care is crucial in managing VPA toxicity. This includes monitoring vital signs, maintaining airway patency, administering respiratory support when needed, and addressing fluid and electrolyte imbalances.
Enhanced elimination: In cases of severe toxicity or significantly elevated VPA levels, procedures such as hemodialysis or hemoperfusion may be considered. These methods aid in expediting the removal of VPA from the bloodstream.
Treatment: The patient might require transfer to the medical intensive care unit for ongoing monitoring and care. Stabilizing any life-threatening issues is the top priority.
Initiate early intravenous (IV) access to administer fluids to patients experiencing hypotension. Administer isotonic crystalloid boluses intravenously to those with low blood pressure. In severe cases, vasopressors might be required. Those experiencing significant respiratory depression could need endotracheal intubation and mechanical support for breathing. In cases of seizures resulting from valproate toxicity, benzodiazepines should be given.[37] It has been reported naloxone administration, in doses between 0.8 mg and 2 mg, can counteract central nervous system depression in certain instances of valproate poisoning.[38][39]
If the patient arrives within 2 hours following a valproate overdose, a single dose of activated charcoal is used for gastrointestinal decontamination. The typical dosage for activated charcoal is 1 g/kg of patient weight. Do not administer to sedated patients when the airway can not be safeguarded. Since valproate comes in enteric-coated and extended-release forms that are absorbed slowly, activated charcoal may be administered after the 2-hour mark post-ingestion.[40]
Consider administering L-carnitine to patients who arrive with an acute valproic acid overdose accompanied by changes in mental status. The initial dose of L-carnitine should be 100 mg/kg, administered intravenously. The treatment regimen for L-carnitine then proceeds with doses of 50 mg/kg every 8 hours. It is also essential to concurrently monitor serum ammonia levels; L-carnitine treatment may be discontinued once these levels begin to decline.[41] In cases of acute valproate overdose where patients are asymptomatic, a preventive oral dose of carnitine is recommended at 100 mg/kg/day, divided into four daily doses.
Enhancing Healthcare Team Outcomes
The use of VPA necessitates a collaborative interprofessional healthcare team approach, encompassing a range of practitioners, including physicians, advanced practice practitioners, specialists, pharmacists, clinical pharmacologists, nurses, laboratory technicians, and counselors. The interprofessional team should collaborate to enhance the therapeutic outcomes and mitigate the adverse effects of VPA for every patient.
Interprofessional care includes collaborative decision-making, open communication, and shared responsibility while monitoring patients by involving all team members. Furthermore, accurate patient record-keeping contributes to this process, ensuring that all healthcare team members involved in providing optimum patient care and making therapeutic decisions possess the most updated and precise clinical information to inform their actions. The patient and their family members or caregivers should be involved in therapeutic decisions by the healthcare team whenever possible.
The interprofessional team of healthcare providers should communicate effectively and share relevant information concerning the patient's status, medication history, potential drug interactions, laboratory findings, and treatment objectives. The team should also educate the patient and their caregivers about the benefits and potential risks of VPA, the significance of adhering to the treatment plan and undergoing monitoring, recognizing signs and symptoms of toxicity, and the suitable steps to follow in an emergency.
Through collaborative efforts and open communication, the dosing and management of VPA can be optimized to enhance patient therapy across its diverse indications.
References
- 1.
- Koch-Weser J, Browne TR. Drug therapy: Valproic acid. N Engl J Med. 1980 Mar 20;302(12):661-6. [PubMed: 6766529]
- 2.
- Willmore LJ. Divalproex and epilepsy. Psychopharmacol Bull. 2003;37 Suppl 2:43-53. [PubMed: 15021860]
- 3.
- Olsen KB, Taubøll E, Gjerstad L. Valproate is an effective, well-tolerated drug for treatment of status epilepticus/serial attacks in adults. Acta Neurol Scand Suppl. 2007;187:51-4. [PubMed: 17419829]
- 4.
- Bond DJ, Lam RW, Yatham LN. Divalproex sodium versus placebo in the treatment of acute bipolar depression: a systematic review and meta-analysis. J Affect Disord. 2010 Aug;124(3):228-34. [PubMed: 20044142]
- 5.
- Cipriani A, Reid K, Young AH, Macritchie K, Geddes J. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013 Oct 17;2013(10):CD003196. [PMC free article: PMC6599863] [PubMed: 24132760]
- 6.
- Goodwin FK, Fireman B, Simon GE, Hunkeler EM, Lee J, Revicki D. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003 Sep 17;290(11):1467-73. [PubMed: 13129986]
- 7.
- Manji HK, Etcheberrigaray R, Chen G, Olds JL. Lithium decreases membrane-associated protein kinase C in hippocampus: selectivity for the alpha isozyme. J Neurochem. 1993 Dec;61(6):2303-10. [PubMed: 8245981]
- 8.
- Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003 Aug;13 Suppl 2:S57-66. [PubMed: 12957721]
- 9.
- López-Muñoz F, Shen WW, D'Ocon P, Romero A, Álamo C. A History of the Pharmacological Treatment of Bipolar Disorder. Int J Mol Sci. 2018 Jul 23;19(7) [PMC free article: PMC6073684] [PubMed: 30041458]
- 10.
- Rana M, Khanzode L, Karnik N, Saxena K, Chang K, Steiner H. Divalproex sodium in the treatment of pediatric psychiatric disorders. Expert Rev Neurother. 2005 Mar;5(2):165-76. [PubMed: 15853487]
- 11.
- Linde M, Mulleners WM, Chronicle EP, McCrory DC. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013 Jun 24;2013(6):CD010611. [PMC free article: PMC10373438] [PubMed: 23797677]
- 12.
- Freitag FG. Divalproex sodium extended-release for the prophylaxis of migraine headache. Expert Opin Pharmacother. 2003 Sep;4(9):1573-8. [PubMed: 12943487]
- 13.
- Činčárová L, Zdráhal Z, Fajkus J. New perspectives of valproic acid in clinical practice. Expert Opin Investig Drugs. 2013 Dec;22(12):1535-47. [PubMed: 24160174]
- 14.
- Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, Coffin JM, Bosch RJ, Margolis DM. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005 Aug 13-19;366(9485):549-55. [PMC free article: PMC1894952] [PubMed: 16099290]
- 15.
- Routy JP, Tremblay CL, Angel JB, Trottier B, Rouleau D, Baril JG, Harris M, Trottier S, Singer J, Chomont N, Sékaly RP, Boulassel MR. Valproic acid in association with highly active antiretroviral therapy for reducing systemic HIV-1 reservoirs: results from a multicentre randomized clinical study. HIV Med. 2012 May;13(5):291-6. [PubMed: 22276680]
- 16.
- Prasad M, Krishnan PR, Sequeira R, Al-Roomi K. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev. 2014 Sep 10;2014(9):CD003723. [PMC free article: PMC7154380] [PubMed: 25207925]
- 17.
- Schwartz C, Palissot V, Aouali N, Wack S, Brons NH, Leners B, Bosseler M, Berchem G. Valproic acid induces non-apoptotic cell death mechanisms in multiple myeloma cell lines. Int J Oncol. 2007 Mar;30(3):573-82. [PubMed: 17273758]
- 18.
- Ghodke-Puranik Y, Thorn CF, Lamba JK, Leeder JS, Song W, Birnbaum AK, Altman RB, Klein TE. Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2013 Apr;23(4):236-41. [PMC free article: PMC3696515] [PubMed: 23407051]
- 19.
- Bolaños JP, Medina JM. Effect of valproate on the metabolism of the central nervous system. Life Sci. 1997;60(22):1933-42. [PubMed: 9180347]
- 20.
- Rosenberg G. The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cell Mol Life Sci. 2007 Aug;64(16):2090-103. [PubMed: 17514356]
- 21.
- Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37 Suppl 2:17-24. [PubMed: 14624230]
- 22.
- Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16(10):669-94. [PubMed: 12269861]
- 23.
- Zaccara G, Messori A, Moroni F. Clinical pharmacokinetics of valproic acid--1988. Clin Pharmacokinet. 1988 Dec;15(6):367-89. [PubMed: 3149565]
- 24.
- Mattson RH, Cramer JA, Williamson PD, Novelly RA. Valproic acid in epilepsy: clinical and pharmacological effects. Ann Neurol. 1978 Jan;3(1):20-5. [PubMed: 350128]
- 25.
- Kinze S, Clauss M, Reuter U, Wolf T, Dreier JP, Einhäupl KM, Arnold G. Valproic acid is effective in migraine prophylaxis at low serum levels: a prospective open-label study. Headache. 2001 Sep;41(8):774-8. [PubMed: 11576201]
- 26.
- Nau H, Hauck RS, Ehlers K. Valproic acid-induced neural tube defects in mouse and human: aspects of chirality, alternative drug development, pharmacokinetics and possible mechanisms. Pharmacol Toxicol. 1991 Nov;69(5):310-21. [PubMed: 1803343]
- 27.
- Hiilesmaa VK, Bardy AH, Granström ML, Teramo KA. Valproic acid during pregnancy. Lancet. 1980 Apr 19;1(8173):883. [PubMed: 6103246]
- 28.
- Cotariu D, Zaidman JL. Developmental toxicity of valproic acid. Life Sci. 1991;48(14):1341-50. [PubMed: 2008152]
- 29.
- Evans RJ, Miranda RN, Jordan J, Krolikowski FJ. Fatal acute pancreatitis caused by valproic acid. Am J Forensic Med Pathol. 1995 Mar;16(1):62-5. [PubMed: 7771387]
- 30.
- Cofini M, Quadrozzi F, Favoriti P, Favoriti M, Cofini G. Valproic acid-induced acute pancreatitis in pediatric age: case series and review of literature. G Chir. 2015 Jul-Aug;36(4):158-60. [PMC free article: PMC4732585] [PubMed: 26712070]
- 31.
- Jones MR, Hall OM, Kaye AM, Kaye AD. Drug-induced acute pancreatitis: a review. Ochsner J. 2015 Spring;15(1):45-51. [PMC free article: PMC4365846] [PubMed: 25829880]
- 32.
- Chomiak T, Turner N, Hu B. What We Have Learned about Autism Spectrum Disorder from Valproic Acid. Patholog Res Int. 2013;2013:712758. [PMC free article: PMC3871912] [PubMed: 24381784]
- 33.
- Nicolini C, Fahnestock M. The valproic acid-induced rodent model of autism. Exp Neurol. 2018 Jan;299(Pt A):217-227. [PubMed: 28472621]
- 34.
- Gotlib D, Ramaswamy R, Kurlander JE, DeRiggi A, Riba M. Valproic Acid in Women and Girls of Childbearing Age. Curr Psychiatry Rep. 2017 Sep;19(9):58. [PubMed: 28726062]
- 35.
- Collins-Yoder A, Lowell J. Valproic Acid: Special Considerations and Targeted Monitoring. J Neurosci Nurs. 2017 Feb;49(1):56-61. [PubMed: 28060221]
- 36.
- Schobben F, van der Kleijn E, Vree TB. Therapeutic monitoring of valproic acid. Ther Drug Monit. 1980;2(1):61-71. [PubMed: 6820201]
- 37.
- Sztajnkrycer MD. Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol. 2002;40(6):789-801. [PubMed: 12475192]
- 38.
- Alberto G, Erickson T, Popiel R, Narayanan M, Hryhorczuk D. Central nervous system manifestations of a valproic acid overdose responsive to naloxone. Ann Emerg Med. 1989 Aug;18(8):889-91. [PubMed: 2502939]
- 39.
- Montero FJ. Naloxone in the reversal of coma induced by sodium valproate. Ann Emerg Med. 1999 Mar;33(3):357-8. [PubMed: 10036355]
- 40.
- Graudins A, Aaron CK. Delayed peak serum valproic acid in massive divalproex overdose--treatment with charcoal hemoperfusion. J Toxicol Clin Toxicol. 1996;34(3):335-41. [PubMed: 8667473]
- 41.
- Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010 Jul-Aug;44(7-8):1287-93. [PubMed: 20587742]
Disclosure: Masum Rahman declares no relevant financial relationships with ineligible companies.
Disclosure: Ayoola Awosika declares no relevant financial relationships with ineligible companies.
Disclosure: Hoang Nguyen declares no relevant financial relationships with ineligible companies.
Figures
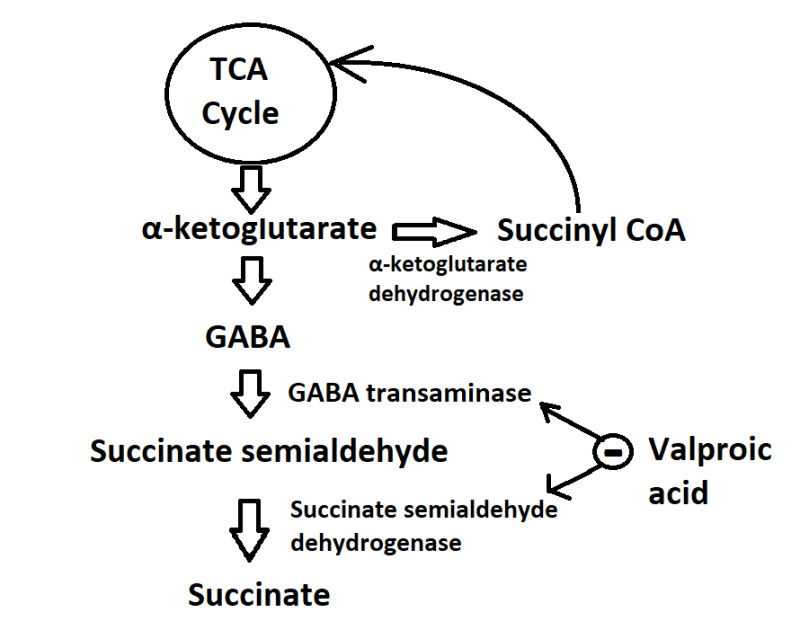
Mechanism of Action of Valproic Acid. The figure illustrates the metabolic pathway of GABA synthesis and its metabolism involving α-ketoglutarate dehydrogenase, GABA transaminase, and succinate dehydrogenase. Valproic acid effectively inhibits the activity of the 2 catabolic enzymes involved in GABA metabolism, leading to the elevation in GABA levels within the central nervous system. Contributed by Masum Rahman