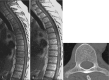
Central nervous system (CNS) infections carry high morbidity and mortality risks. The spectrum of infection includes: meningitis; ventriculitis; cerebritis/brain abscesses; subdural/epidural empyemas; and encephalitis.
The most common emerging inflammatory disorders that may affect the CNS are IgG-related disease, autoimmune encephalitis, neuromyelitis optica spectrum disorders (NMOSD), and Anti-GQ1b IgG antibody syndrome. Sarcoidosis and vasculitis may also affect the brain and spinal cord. Magnetic resonance imaging (MRI) is the method of choice in detection, evaluation, and follow-up of CNS infection and inflammation.
In this chapter, the imaging findings and diagnostic roadmaps of most common clinical challenges in infectious and inflammatory CNS disorders are discussed and illustrated.
Keywords:
Infection, Inflammation, Abscess, Meningitis, Vasculitis, Autoimmune encephalitis, Vasculitis, IgG-related diseases6.1. Meningitis
CT and MR imaging findings in meningitis are nonspecific, with MR being superior to CT in the detection of meningeal pathology. Imaging findings in meningitis include: high signal intensity of subarachnoid spaces on FLAIR, leptomeningeal enhancement, subdural effusions, ventricular debris, and hydrocephalus, as well as high signal changes on diffusion-weighted MR imaging (DWI), and infarcts [1].
6.1.1. MR Imaging
On fluid-attenuated inversion-recovery (FLAIR) sequences, a high signal will be present in the subarachnoid spaces, which reflects the high protein content in the cerebral spinal fluid (CSF). Differential diagnosis includes metastatic disease, subarachnoid hemorrhage, with the administration of oxygen and some sedation agents. Leptomeningeal enhancement can be best depicted with post-contrast 3D T2-FLAIR (Fig. 6.1a–c) [2].
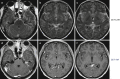
Fig. 6.1
Superiority of post-contrast 3D T2-FLAIR (a–c) to post-contrast T1WI (d–f) in the detection of leptomeningeal enhancement
6.1.2. Enhancement Pattern
Intracranial meningeal enhancement patterns are pachymeningeal (dura-arachnoid) or pia-subarachnoid (leptomeningeal). In a pachymeningeal enhancement pattern, the dura and the outer layer of the arachnoid will enhance. This pattern will be seen in intracranial hypotension, after surgery, granulomatous diseases, meningioma, and metastatic disease. Leptomeningeal enhancement follows the pial surface of the brain and fills the subarachnoid spaces of the sulci and cisterns. This pattern suggests an infectious origin (bacterial, viral, fungal), or, in some cases, carcinomatous meningitis. Thin, linear enhancement will be seen in meningitis, whereas thick, lumpy, nodular enhancement suggests carcinomatous origin (Figs. 6.2 and 6.3). Unilateral, thin, leptomeningeal enhancement may be due to non-infectious diseases, such as neurosarcoidosis, vasculitis, IgG-related pachymeningitis, and Sturge–Weber syndrome (Fig. 6.2).
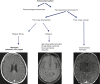
Fig. 6.2
Different enhancement patterns in meningeal diseases
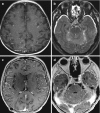
Fig. 6.3
Thin, linear enhancement (a, b) suggests infectious origin, whereas a thick, nodular enhancement will favor neoplastic diseases (c, d)
6.1.3. Diffusion-Weighted MR Imaging (DWI) in Meningitis
Hyperintense DWI lesions may be seen in several locations: (a) in the ventricles representing purulent fluid; (b) in the cortical sulci; and (c) in the Virchow-Robin spaces (VRSs) (Fig. 6.4).
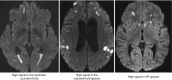
Fig. 6.4
Diffusion-weighted MRI abnormalities in meningitis
6.1.4. Tuberculous Meningitis
In tuberculous meningitis (TBM), FLAIR will show hyperintense subarachnoid spaces and the cisterns at the base of the brain (interpeduncular fossa, suprasellar, perimesencephalic, and pontine cisterns) with intense leptomeningeal enhancement on post-contrast T1WI or post-contrast FLAIR. In addition, surrounding brain edema will be recognized as a high signal on T2WI and FLAIR. In children, communicating or non-communicating hydrocephalus may occur. Spread of the infection to small vessels with arteritis and subsequent thrombosis will lead to arterial infarctions. Bilateral infarctions in the basal ganglia region and in the internal capsule on DWI should raise the suspicion of tuberculous meningitis. Narrowing or occlusion of the arteries may be seen on MR angiography (MRA). Cranial nerve involvement with thickened enhancing nerves is a common finding (Fig. 6.5).
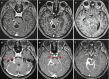
Fig. 6.5
Proven tuberculous meningitis with multiple ring-enhancing tuberculomas in the basal cistern (a–c). With disease progression, there was perineural spread with thickened and enhancing cranial nerves (d, e, red arrows)
6.2. Brain Abscess
A brain abscess is a focal infection of the brain that begins as a localized area of softening of the brain parenchyma (cerebritis), and develops into a collection of pus surrounded by a capsule.
6.2.1. MR Imaging of Brain Abscesses
6.2.1.1. T2-Wi
Bacterial brain abscesses have a T2 high-signal-intensity center, a T2 low-signal-intensity capsule, prominent perifocal edema, and ring-like enhancement on post-contrast T1WI. Fungal abscesses usually demonstrate low T2 signal in the center. Parasitic abscesses, such as toxoplasma abscesses, may show centrally high or low signal.
6.2.1.2. Diffusion-Weighted MR Imaging
A high signal on DWI, with low ADC values in the pyogenic abscess cavity, is consistent with restricted diffusion due to the high viscosity pus. Fungal abscesses may show high, low, or intermediate signals on trace DWI in the abscess cavity. Restricted diffusion may be seen in the walls of the fungal abscess called “intracavitary projections.” High signal on DWI (with low ADC values) is due to fungal organisms located at the periphery of the lesions and mucoid material (Fig. 6.6).
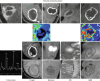
Fig. 6.6
Diagnostic scheme for differentiation of ring-enhancing brain lesions
6.2.1.3. Susceptibility-Weighted MR Imaging (SWI)
On susceptibility-weighted MR imaging (SWI), pyogenic brain abscesses show the “dual rim sign,” (hypointense outer layer and hyperintense inner layer) [3]. Fungal abscesses do not show the dual rim sign, but rather, are recognized by the thick dark rim on SWI. In addition, fungal abscesses may have multiple, punctate, intralesional dark signals indicative of hemorrhage (common in Aspergillus infection) (Fig. 6.6) [4, 5].
In demyelinating lesions, which present as ring-enhancing lesions, SWI will demonstrate linear dark structures that run through the lesion, representing dilated veins. SWI can also be used to differentiate glioblastoma that presents as a peripheral or ring-enhancing mass from an infectious abscess. Glioblastoma shows dark dots and lines on SWI due to hemorrhage and neoangiogenesis.
6.2.1.4. Perfusion MRI
Perfusion MRI demonstrates low regional cerebral blood flow (rCBV) in all infectious abscesses regardless of etiological agents. Very rarely, in bacterial brain abscesses, high rCBV in the enhancing part will be detected due to the vascularization present in the early capsular stage. Neoplastic lesions (glioblastoma, metastases), as well as demyelinating lesions, may present as ring-like enhancing brain lesions, and can mimic a brain abscess. Perfusion will be useful as it will show increased rCBV in the enhancing part of neoplastic lesions, but low rCBV in demyelinating lesions (Fig. 6.6) [1, 5].
6.2.1.5. MR-Spectroscopy
To distinguish between aerobic and anaerobic pyogenic abscesses, MR spectroscopy (MRS) can be used. Succinate and acetate signal resonances suggest an anaerobic agent. In fungal abscesses lipids, lactate, amino acids, and trehalose (3.6–3.8 ppm) are commonly detected.
6.2.2. Tuberculoma and Tuberculous Abscesses
Tuberculous granuloma (tuberculoma) is the most common parenchymal form of CNS TB. The imaging features depend on the stage of infection. Noncaseating tuberculomas have a low T1WI and a high T2WI signal and show nodular enhancement. Caseating tuberculomas demonstrate a low T1- and T2-WI signal with ring-like enhancement. DWI characteristics will also depend on the stage and content; caseating tuberculomas with a T2 high-signal-intensity center show restricted diffusion. Caseating tuberculomas with a T2-low-signal-intensity center have elevated diffusion. MRS can also be used to differentiate pyogenic abscesses from tuberculous lesions (a large lipid peak will be found in T2-hypointense caseating tuberculomas, and large choline and lipid peaks will be detected in T2-hyperintense tuberculomas) (Fig. 6.6).
Tuberculous abscesses are large, multiloculated masses, with thick enhancement and edema with mass effect.
6.3. Autoimmune Encephalitis
The term “autoimmune encephalitis” generally refers to a family of closely related disease processes that share overlapping clinical features and neuroimaging findings, but are ultimately differentiated by the specific antibody subtypes driving the underlying immune-mediated attack on different CNS structures [6–8].
Autoimmune encephalitis can be classified into two major groups: paraneoplastic and non-paraneoplastic.
A subset of patients with autoimmune encephalitis will have no neuroimaging findings despite profound neuropsychiatric dysfunction. Serum antibody testing will ultimately lead to the diagnosis of autoimmune encephalitis.
18FDG-PET could be a helpful alternative with which to narrow the diagnosis when MRI and biological tests are negative. FDG-PET was reportedly more sensitive for AE compared to EEG, MRI, or routine CSF findings. Hypometabolism was found in the cortex, whereas hypermetabolism was seen in the basal ganglia.
N-methyl D-aspartate receptor encephalitis (NMDAr) is one of the most common and best characterized subtypes of autoimmune encephalitis, classically seen in young women and children. It can be paraneoplastic (associated with ovarian cancer) or non-paraneoplastic. Over half of MRIs performed in anti-NMDA receptor encephalitis show NO abnormalities. T2/FLAIR hyperintensity of the medial temporal lobe (Fig. 6.7), frontal lobe, subcortical WM, and periventricular region have been described. Occasionally, leptomeningeal and cortical contrast enhancement will be detected.
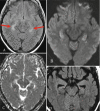
Fig. 6.7
Axial FLAIR (a) shows bilateral high signal in the hippocampus. On trace DWI (b), high signal is detected with a subtle decrease in ADC values (c). On follow-up MR, bilateral hippocampal atrophy is observed (d)
Anti-neuronal nuclear antibody 1(Anti-Hu) encephalitis is associated with lung cancer and is usually associated with a bad prognosis. It will less frequently be recognized as limbic encephalitis, and usually affects the brainstem.
Anti-Yo is associated with ovarian and breast cancer, and it typically presents with paraneoplastic cerebellar degeneration (Fig. 6.8).
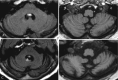
Fig. 6.8
Initial MR exam in a patient with proven anti-Yo encephalitis (a, b). Follow-up MR 2 years later shows marked cerebellar atrophy (c, d)
6.4. Neurosarcoidosis
Neurosarcoidosis is a multisystem granulomatous disease with sarcoid-type granulomas and epithelial cells and its diagnosis is based on the clinical determination of multisystem diseases in combination with histologic confirmation.
Neurosarcoidosis has been described in 5% of patients with sarcoidosis. The disease might present with a variety of clinical presentations, such as cranial neuropathy, encephalopathy, meningitis, hydrocephalus, seizures, as well as spinal cord abnormalities and peripheral neuropathy and myopathy.
Typical imaging findings, although nonspecific, include dural thickening or dural masses, leptomeningeal involvement (Fig. 6.9), enhancing and non-enhancing parenchymal lesions (Figs. 6.10 and 6.11), and cranial nerve involvement (Fig. 6.12). The latter is the most common finding. Spine involvement may exist, even less frequently, such as lesions in the vertebral body, spinal cord lesions, and spinal and nerve root enhancement (Fig. 6.13).
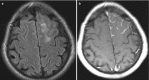
Fig. 6.9
Axial FLAIR (a) and T1-weighted post-contrast administration (b) demonstrate diffuse, focal, increased FLAIR signal in the underlying brain parenchyma and focal left-sided leptomeningeal enhancement in a patient with neurosarcoidosis
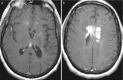
Fig. 6.10
Axial post-contrast-enhancing T1-weighted images (a, b) demonstrating focal periventricular granulomatous contrast-enhancing masses
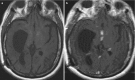
Fig. 6.11
Axial pre- (a) and post-contrast T1-weighted (b) images demonstrating basal granulomatous contrast-enhancing mass lesions obstructing the CSF circulation and causing secondary hydrocephalus
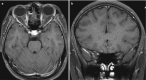
Fig. 6.12
Axial (a) and coronal (b) post-contrast T1-weighted images showing thickening and pathological contrast enhancement in the posterior pre-chiasmatic portion of the right optic nerve
Common radiological imaging methods include CT and MRI. Imaging findings on CT are, in general, nonspecific and might include hydrocephalus, periventricular hypoattenuation and contrast enhancement, calcification, meningeal contrast enhancement, focal white matter lesions, and lesions in the optic nerves or chiasm. The imaging modality of choice for the evaluation of suspected neurosarcoidosis is MRI. The protocol should include FLAIR/T2-weighted, T1-weighted pre- and post-contrast administration, DWI and T2∗, or SWI. MRI can better depict leptomeningeal abnormalities and lesions in the brain parenchyma, as well as demonstrate the cranial nerve involvement, which often presents as cranial nerve thickening and enhancement. The most common nerves involved are the optic nerve and the facial nerves. There may also be involvement of the pituitary gland. Spinal involvement, both of the vertebra and the spinal cord, is better seen with MRI. It should be noted that MRI can be normal in patients with neurosarcoidosis, especially in patients on corticosteroids or if the only presenting symptom is cranial neuropathy.
6.4.1. Leptomeningeal Involvement
Leptomeningeal involvement occurs in up to 40% of patients with neurosarcoidosis. It might be focal, multifocal, or diffuse, or even mass-like, and present as linear or nodular enhancement along the brain surface and basal regions. It is best depicted after contrast enhancement (Fig. 6.9). Differential diagnosis for leptomeningeal enhancement should include meningeal carcinomatosis, lymphoma, leukemia, and fungal and bacterial infections.
6.4.2. Dural or Focal Mass Lesions
The granulomatous lesions might, if located in the basal regions or adjacent to the ventricles, cause obstruction of the CSF (cerebral spinal fluid) circulation and lead to hydrocephalus (Fig. 6.10). The dural lesions are usually isointense, appearing as a gray mass on T1 sequences of MRI and hypointense on T2 sequences of MRI. Potential differential diagnosis includes meningiomas, metastases, plasmacytoma, and granulomatous infections.
6.4.3. Cranial Nerve Involvement
The involvement of the cranial nerves, most commonly the optic nerve and the fascial nerves, might be demonstrated as thickening and enhancement of the involved nerves, best seen on T1-weighted post-contrast administration and on T2-weighted images (Fig. 6.12a, b). Here, the 3D-CISS sequences might be of value for better depiction of the nerves. Note that several nerves might be involved.
6.4.4. Spine, Spinal Cord, and Spinal Nerve Involvement
Spine and spinal cord involvement are generally uncommon in neurosarcoidosis, but do occur both in combination with other CNS involvement or as an isolated finding. Such involvement may present as leptomeningeal enhancement, pachymeningeal, or intramedullary lesions and rarely involves the vertebral bodies. Typical vertebra lesions can present as focal or diffuse lesions in the vertebral bodies and be misdiagnosed as myeloma, lymphoma, or metastasis. Involvement of the spinal cord and spinal nerve roots might present as focal lesions with low signal intensity on T1, high signal intensity on T2, and nodular enhancement on contrast administration. The increased signal on T2-weighted images compared to that of the gray matter is suspected to be related to inflammation and edema. In addition, leptomeningeal or focal contrast enhancement can be seen along or in the spinal cord or along the nerve roots on T1-weighted images after contrast administration. The intramedullary lesions will normally demonstrate slightly increased diffusivity with correspondingly increased values at the ADC map image, reflecting vasogenic edema and inflammation, even if iso- to high signal intensity with normal to slightly increased diffusion on DWI have been reported in a limited number of patients. Cervical or thoracic involvement is more common than lumbosacral. There is a female predominance for spinal involvement. Potential differential diagnosis for vertebral body involvement in neurosarcoidosis includes, for example, myeloma, lymphoma, and metastasis.
6.5. IgG-Related Diseases
IgG-related diseases encompass a spectrum of fibroinflammatory disorders characterized by IgG4-positive plasma cell infiltration that can affect almost every organ system. They respond well to corticosteroid therapy.
CNS involvement is rare.
IgG-related pachymeningitis is a rare manifestation of the IgG4-related fibroinflammatory disease spectrum. Inflammation leads to a localized or diffuse thickening of the meninges overlying the supratentorial hemispheres, skull base, or spinal cord. The symptoms are related to mass effect, or focal deficits caused by the compression of blood vessels or nerves. This was previously described as idiopathic, hypertrophic, cranial pachymeningitis (IHCPM). Tissue biopsy is the gold standard for diagnosis in most settings (Fig. 6.14).
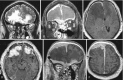
Fig. 6.14
Thick, dural enhancement is observed bilaterally frontally and along the falx (b–f). T2-high signal intensity is also seen in the parenchyma of both frontal lobes, reflecting compression of the brain parenchyma (a)
6.6. Vasculitis
Vasculitis is defined as an inflammation of the vessel wall. The classification of CNS vasculitis can be divided into primary systemic vasculitides and CNS vasculitis associated with other underlying disorders. The inflammation of the vessel wall can affect vessels of various sizes, i.e., small, medium, and large vessels or can affect all the vessels, regardless of size.
Giant cell arteritis, Takayasu’s arteritis, granulomatosis with polyangiitis (GPA), Churg–Strauss syndrome, Kawasaki disease, and primary angiitis of the CNS (PACNS) are considered primary vasculitides. Nervous system vasculitis can also be associated with connective tissue disorders, like systemic lupus erythematosus (SLE), Sjögren’s disease, rheumatoid arthritis (RA), lymphoproliferative diseases, malignancies, drug and substance abuse, or infections. The diagnosis of vasculitis is a combination of symptoms, as well as clinical and laboratory findings, and imaging. Still, today, brain biopsy is considered the gold standard for the final diagnosis of vasculitis.
6.6.1. Imaging in Vasculitis
The MR imaging protocol shall include T1-weighted images pre- and post-contrast, FLAIR/T2-weighted, DWI, and, for the evaluation of microbleeds, SWI or T2∗-w, MRA–3D TOF, and, if possible, vessel wall imaging (VWI) [9–11].
If MRI is inconclusive, digital subtraction angiography can be added to the radiological work-up.
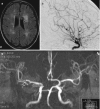
The MR imaging findings in patients with vasculitis may vary depending on the vessels involved. The examination can be normal and include different findings, such as acute ischemic changes, old infarcts, nonspecific focal or multiple hyperintense white matter lesions, confluent white matter lesions, patchy or linear contrast enhancement, leptomeningeal contrast enhancement, and brain atrophy (Fig. 6.15). Microbleeds are rare in contrast to, for example, amyloid angiopathy, a common differential diagnosis. On MR angiography (3D TOF) shows multiple, focal, irregular stenosis of more than one of the medium or large vessels (small vessels are not visualized on MRA) (Figs. 6.16 and 6.19b). The digital subtraction angiography might demonstrate focal stenosis and the string-of-bead sign in various intracranial vessels (Figs. 6.17, 6.18, and 6.19c).

Fig. 6.15
Different imaging findings in patients with known vasculitis; (a) hyperintense FLAIR lesions, (b) confluent white matter signal abnormalities, (c) leptomeningeal contrast enhancement, (d) cortical infracts, (e) multiple acute lacunar infarcts
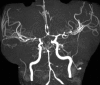
Fig. 6.16
MRA 3D TOF demonstrating bilateral multiple focal stenosis of the anterior and middle cerebral arteries
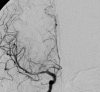
Fig. 6.17
Coronal view after right, selective, internal carotid artery injection, demonstrating focal stenosis in the right pericallosal artery
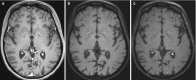
Fig. 6.18
On post-contrast T1wi, enhancement of the branches of the left posterior cerebral artery is noted (a). Intense enhancement of the vessel wall on black-blood sequence (b, c) suggests vasculitis
6.6.2. Primary Angiitis of the CNS (PACNS)
Primary angiitis of the CNS (PACNS) is a rare disease of unknown etiology with a predominance in males compared to females (7:3). Imaging will demonstrate irregularities in the peripheral vessels that results in acute ischemic changes that are secondary to the inflammation of the vessel wall, stenosis, or obstruction of the vessel lumen, with increased local coagulation and alterations in vasomotor tone. A differential diagnosis for PACNS includes the reversible cerebral vasoconstriction syndrome (RCVS). RCVS more commonly affects women and is characterized by severe headaches (thunderclap headache) with or without focal neurologic deficits and/or seizures. In RCVS, the segmental constriction of cerebral arteries resolves within 3 months.
6.6.3. Granulomatosis with Polyangiitis (GPA)
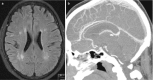
Granulomatosis with polyangiitis (GPA) (previously called Wegener’s granulomatosis) is a systemic disease of small and medium vessels with an unclear pathogenesis. The disease primarily affects the upper and lower respiratory airways, joints, eyes, skin, heart, liver, glands, and, in 25–50%, the peripheral nervous system in the form of necrotizing vasculitis, peripheral neuropathies, and cranial nerve palsy. The involvement of the CNS is far less frequent, with only 2–8% in the form of CNS vasculitis with intracranial granulomas directly or due to spread from the sinus and orbits. CNS involvement in this form is associated with a very high mortality rate (>90%) if untreated. This may present with focal granulomas that have spread from the frontal sinus (Fig. 6.20), or focal white matter hyperintesies and/or leptomeningeal contrast henhancement (Fig. 6.21).
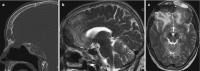
Fig. 6.20
(a) Sagittal CT (bone window) demonstrates opacification of the frontal sinus. (b, c) Granuloma extension into the frontal lobes bilaterally with surrounding edema and gliosis
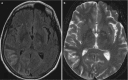
Fig. 6.21
Axial (a) FLAIR, (b) T2-weighted, and (c, d) T1-weighted image after contrast administration demonstrating focal brain swelling and leptomeningeal contrast enhancment
6.6.4. Neurosyphilis
Neurosyphilis (CNS lues) is a classic infectious cause of cerebral vasculitis. The patient often presents with an ischemic event predominantly related to the middle cerebral arteries or branches of the basilar artery. Typical imaging findings are multiple lacunar infarcts, arteritis changes with focal stenosis, ectasia of the basilar artery, focal areas of increased T2 signal in the white matter, and the presence of patchy enhancement (Fig. 6.22).
6.7. Concluding Remarks
Infectious and inflammatory CNS disorders are difficult clinical diagnoses due to the similarities in clinical symptoms and laboratory findings. The role of imaging remains critical and the use of advanced techniques and state-of-the-art MRI of the brain and spine is mandatory for early detection, differentiation, and follow-up.
References
- 1.
- Thurnher MM. Infections in immunocompromised patients. In: Barkhof F, Jäger R, Thurnher M, Rovira A, editors. Clinical neuroradiology. Berlin: Springer; 2019.
- 2.
- Fukuoka H, Hirai T, Okuda T, et al. Comparison of the added value of contrast-enhanced 3D fluid-attenuated inversion recovery and magnetization-prepared rapid acquisition of gradient echo sequences in relation to conventional postcontrast T1-weighted images for the evaluation of leptomeningeal diseases at 3T. AJNR Am J Neuroradiol. 2010;31:868–73. [PMC free article: PMC7964192] [PubMed: 20037130] [CrossRef]
- 3.
- Toh CH, Wei KC, Chang CN, et al. Differentiation of pyogenic brain abscesses from necrotic glioblastomas with use of susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33:1534–8. [PMC free article: PMC7966558] [PubMed: 22422181] [CrossRef]
- 4.
- Antulov R, Dolic K, Fruehwald-Pallamar J, Miletic D, Thurnher MM. Differentiation of pyogenic and fungal brain abscesses with susceptibility-weighted MR sequences. Neuroradiology. 2014;56(11):937–45. [PubMed: 25085012] [CrossRef]
- 5.
- Thurnher MM. Bacterial infections. In: Barkhof F, Jäger R, Thurnher M, Rovira A, editors. Clinical neuroradiology. Berlin: Springer; 2019.
- 6.
- Rey C, Koric L, Guedj E, et al. Striatal hypermetabolism in limbic encephalitis. J Neurol. 2012;259(6):1106–10. [PubMed: 22081102] [CrossRef]
- 7.
- Kelley BP, Patel SC, Marin HL, Corrigan JJ, Mitsias PD, Griffith B. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol. 2017;38(6):1070–8. [PMC free article: PMC7960083] [PubMed: 28183838] [CrossRef]
- 8.
- Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. [PMC free article: PMC5066574] [PubMed: 26906964] [CrossRef]
- 9.
- Razek AAKA, Alvarez H, Bagg S, Refaat S, Castillo M. Imaging spectrum of CNS vasculitis. Radiographics. 2014;34:873–94. [PubMed: 25019429] [CrossRef]
- 10.
- Mandell DM, Mossa-Basha M, Qjao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2017;38(2):218–29. [PMC free article: PMC7963837] [PubMed: 27469212] [CrossRef]
- 11.
- Cannerfelt B, Nystedt J, Jönsen A, Lätt J, van Westen D, Lilja A, Bengtsson A, Nilsson P, Mårtensson J, Sundgren PC. White matter lesions and brain atrophy in systemic lupus erythematosus patients: correlation to cognitive dysfunction in a cohort of systemic lupus erythematosus patients using different definition models for neuropsychiatric systemic lupus erythematosus. Lupus. 2018;27(7):1140–9. [PubMed: 29523054] [CrossRef]
Publication Details
Author Information and Affiliations
Authors
Majda M. Thurnher 1 and Pia C. Sundgren2.
1 and Pia C. Sundgren2.Affiliations
 Corresponding author.
Corresponding author.Publication History
Published online: February 15, 2020.
Copyright
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Publisher
Springer, Cham (CH)
NLM Citation
Thurnher MM, Sundgren PC. Intracranial Infection and Inflammation. 2020 Feb 15. In: Hodler J, Kubik-Huch RA, von Schulthess GK, editors. Diseases of the Brain, Head and Neck, Spine 2020–2023: Diagnostic Imaging [Internet]. Cham (CH): Springer; 2020. Chapter 6. doi: 10.1007/978-3-030-38490-6_6