OVERVIEW
Introduction
Pexidartinib is an orally available small molecule multi-kinase inhibitor that is used as an antineoplastic agent in the treatment of tenosynovial giant cell tumors. Pexidartinib is associated with a high rates of serum aminotransferase and alkaline phosphatase elevations during therapy and has been implicated in several cases of clinically apparent liver injury marked by progressive intrahepatic bile duct injury, some of which resulted in liver transplantation or were fatal.
Background
Pexidartinib (pex” i dar’ ti nib) is a potent small molecule inhibitor of several kinase receptors, including colony stimulating factor 1 (CSF1) receptor, FMS-like tyrosine kinase 3 receptor (FLT3) and the proto-oncogene c-kit. These kinase receptors activate intracellular signaling cascades that can promote unregulated cell growth and proliferation. Tenosynovial giant cell tumors (TGCT) are rare, locally aggressive malignancies for which there are no known effective therapies other than surgery. Genetic analyses of the malignant cells in TGCT show a frequent translocation between chromosome 1 (1p13) and 2 (2q35) leading to a mutated CSF1 and its overexpression. In vivo and in vitro studies show that inhibition of the CSF1 receptor leads to inhibition of tumor cell growth and, in clinical trials, pexidartinib was found to induce objective responses in a proportion of patients with symptomatic, advanced TGCTs. Pexidartinib received accelerated approval for this indication in the United States in 2019 and is available in capsules of 200 mg under the brand name Turalio. The recommended dose is 400 mg twice daily, continued until progressive disease or intolerable toxicity occurs. Side effects are common and can include change in hair color, fatigue, nausea, myalgia, arthralgia, diarrhea, abdominal pain, headache and peripheral, facial and periorbital edema. Uncommon, but potentially severe side effects include hepatotoxicity and embryo-fetal toxicity. Largely because of hepatotoxicity, pexidartinib is available only through a restricted FDA Risk Evaluation and Mitigation Strategy (REMS) Program.
Hepatotoxicity
Elevations in serum aminotransferase levels are common during pexidartinib therapy, occurring in 50% to 90% of patients and rising above 5 times the upper limit of the normal range in 12% to 20%. In addition, elevations in alkaline phosphatase levels occur in up to 20% of treated persons. In registration trials, clinically apparent liver injury with jaundice developed in 5% of patients. The time to onset of liver injury was typically between 2 and 6 weeks, and the pattern of liver enzyme elevations was mixed or cholestatic. Autoimmune and immune-allergic features were not prominent. Liver biopsy demonstrated bile duct injury and loss, and at least 3 patients in studies for conditions other than TGCT developed bile duct paucity and features of vanishing bile duct syndrome that ultimately led to liver transplantation in one subject. Pexidartinib has had limited clinical use and the frequency and spectrum of acute liver injury with its use is not yet well defined.
Likelihood score: B (likely cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the cholestatic liver injury due to pexidartinib is not known. Pexidartinib is metabolized in the liver largely by the cytochrome P450 system (largely CYP 3A4) and is susceptible to drug-drug interactions with inhibitors or inducers of the microsomal CYPs.
Outcome and Management
Pexidartinib therapy has been associated with transient serum aminotransferase elevations during therapy in at least half of subjects and with several instances of acute liver injury with jaundice and symptoms. Severe progressive cholestasis leading to liver transplantation or death has been described in several patients receiving pexidartinib for which reason it is available only through a restricted REMS Program, which mandates monitoring of liver tests before and during therapy and avoidance of taking other hepatotoxic agents or drugs that might lead to drug-drug interactions. Specific instructions on dose modification as well as temporary and permanent discontinuation for adverse reactions are available from the product label for pexidartinib, a link to which is provided below.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Pexidartinib – Turalio®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
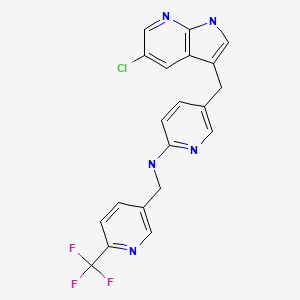
CHEMICAL FORMULA AND STRUCTURE
ANNOTATED BIBLIOGRAPHY
References updated: 01 October 2019
Abbreviations: CSF-1, colony stimulating factor-1; FLT3, FMS-like tyrosine kinase-3; TGCT, tenosynovial giant cell tumor.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of kinase inhibitors).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents published in 2013 before the availability of pexidartinib).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Pathway-targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1203-36.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/scripts/cder/daf/index .cfm?event=overview .process&varApplNo =211810 . (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA scientific review of the new drug application for safety and efficacy; in the “Other Reviews” section on pexidartinib, liver injury is discussed in detail. In the controlled trial of pexidartinib, 66% patients developed ALT elevations on treatment and 5 subjects had severe hepatotoxicity which was typically cholestatic or mixed in pattern and was associated with loss of bile ducts and prolonged jaundice; other trials of pexidartinib noted a similar rate and pattern of hepatotoxicity including 2 further cases of vanishing bile duct syndrome, yielding total of 12 probable cases of clinically apparent hepatotoxicity). - Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, Chmielowski B, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med. 2015;373(5):428–37. [PubMed: 26222558](Among 43 patients with advanced TGCT treated with pexidartinib in a dose-finding and extension study, 52% had an objective response, but side effects were common including ALT or AST elevations; in the extension study 61% had dose reductions and 30% temporary discontinuations).
- Giustini N, Bernthal NM, Bukata SV, Singh AS. Tenosynovial giant cell tumor: case report of a patient effectively treated with pexidartinib (PLX3397) and review of the literature. Clin Sarcoma Res. 2018;8:14. [PMC free article: PMC6038319] [PubMed: 30002809](47 year old woman with symptomatic TGCT had an objective response within months of starting pexidartinib and had stable disease on therapy for 55 months; no mention of abnormal liver test results).
- Piawah S, Hyland C, Umetsu SE, Esserman LJ, Rugo HS, Chien AJ. A case report of vanishing bile duct syndrome after exposure to pexidartinib (PLX3397) and paclitaxel. NPJ Breast Cancer. 2019;5:17. [PMC free article: PMC6570645] [PubMed: 31240240](62 year old woman with ER-positive, HER2-negative breast cancer developed fever 3 weeks after starting therapy with paclitaxel and pexidartinib who developed liver injury after admission; antibiotic therapy was started, followed by cholecystectomy [bilirubin 10.9 mg/dL, ALT 194 U/L, Alk P 377 U/L], liver biopsy showing a cholestatic hepatitis with bile duct loss and subsequent course marked by persistent pruritus, jaundice and cholestasis, ultimately undergoing successful liver transplant, the explant showing cirrhosis and ductopenia).
- Tap WD, Gelderblom H, Palmerini E, Desai J, Bauer S, Blay JY, Alcindor T, et al. ENLIVEN investigators. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet. 2019;394:478–87. [PMC free article: PMC6860022] [PubMed: 31229240](Among 120 adults with symptomatic, advanced TGCT enrolled in a randomized controlled trial, response rates were seen in 24 of 61 pexidartinib [39%] but none of 59 placebo recipients, while side effects were common including change in hair color [67% vs 3%], fatigue [54% vs 36%], nausea [38% vs 41%], peripheral edema [13% vs 2%], facial edema [13% vs 2%] and ALT elevations [28% vs 1%] which were above 5 times ULN in 10% vs none, while 3 patients developed liver injury with jaundice which was often prolonged and led to apparent bile duct paucity in one subject).
- Lee JH, Chen TW, Hsu CH, Yen YH, Yang JC, Cheng AL, Sasaki SI, et al. A phase I study of pexidartinib, a colony-stimulating factor 1 receptor inhibitor, in Asian patients with advanced solid tumors. Invest New Drugs. 2019 Mar 2; [Epub ahead of print] [PMC free article: PMC6985061] [PubMed: 30825104](Among 11 Asian patients with various symptomatic, advanced solid tumors treated with pexidartinib, the only patient with an objective response had a TGCT; side effects included ALT or AST elevations above 3 times ULN in 36% and bilirubin elevations in 18%).
Publication Details
Publication History
Last Update: October 10, 2019.
Copyright
Publisher
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda (MD)
NLM Citation
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Pexidartinib. [Updated 2019 Oct 10].