Continuing Education Activity
Seborrheic dermatitis is a common papulosquamous skin disease that is most prevalent in infancy and middle age, and it usually presents differently in these two age groups. This activity will help to identify the different presentations of seborrheic dermatitis and illustrate how each may influence management decision making that leads to improved treatment outcomes.
Objectives:
- Describe the burden of seborrheic dermatitis from an epidemiological, clinical, and economic perspective and be aware of its potential to impair quality of life.
- Identify the hypotheses concerned with understanding the diverse pathophysiological mechanisms underlying the development of seborrheic dermatitis.
- Explain the reasons for the relevant differential diagnoses for seborrheic dermatitis in the younger and older cohorts.
- Outline an evidence-based formulary for the treatment of seborrheic dermatitis that includes conservative measures, over-the-counter products, and prescription treatments that are age-appropriate and likely to improve compliance, along with facilitating the variation of treatment at home. Good communication, patient education, and involvement of the dermatologist and pharmacist in the identification of suitable treatments are all important in this regard.
Introduction
Seborrheic dermatitis (SD) is a common inflammatory skin disease presenting with a papulosquamous morphology in areas rich in sebaceous glands, particularly the scalp, face, and body folds. The infantile (ISD) and adult (ASD) variants reflect the condition’s bimodal occurrence.
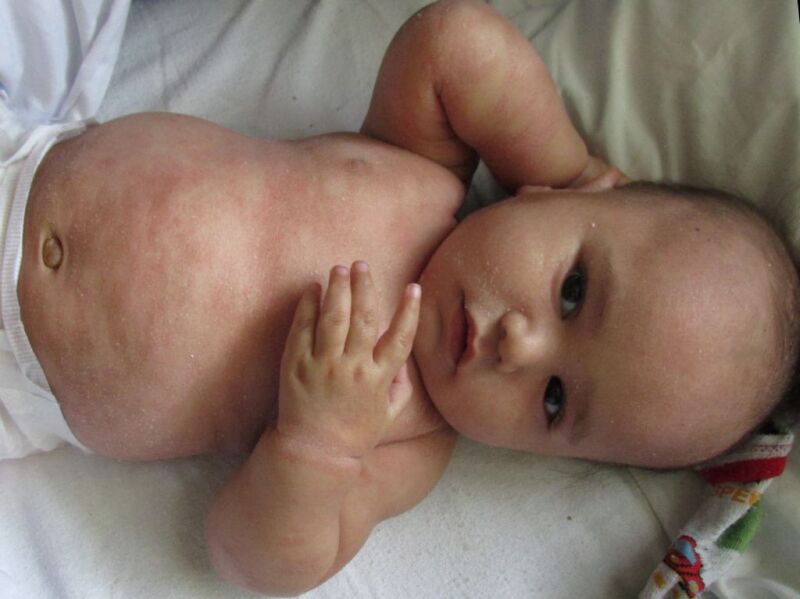
Infants are not usually troubled by seborrheic dermatitis, but it may cause significant parental anxiety, often appearing as firm, greasy scales on the crown and frontal regions of the scalp. It occurs in the first three months of life and is mild,self-limiting, and resolving spontaneously in most cases by the first year of life.ASD, on the other hand, is characterized by a relapsing and remitting pattern of disease and is ranked third behind atopic and contact dermatitis for its potential to impair the quality of life.[1]
Etiology
There are multiple factors associated with the development of seborrheic dermatitis, and their disparate nature has led to many proposals about its cause and pathogenesis, but the onset of SD appears linked to the interplay of normal microscopic skin flora (especially Malassezia spp.), the composition of lipids on the skin surface and individual susceptibility. Neither the level of sebum produced nor the amount of yeast appears to be significant factors.[2]
Epidemiology
The worldwide prevalence of seborrheic dermatitis is around 5%, but the prevalence of its non-inflammatory variant, dandruff, is probably closer to 50%. SD affects all ethnic groups in all regions globally.[3] The prevalence of SD is bimodal with a peak in the first three months of life and then from adrenarche to a second peak after the fourth decade. In Australian preschool children, the prevalence of SD was approximately 72% at three months then fell rapidly with an overall incidence of 10%. Furthermore, analysis of the Rotterdam Study data found that 14% of middle-aged and elderly adults had SD.[4] In HIV-AIDS, however, 35% with early HIV infection have SD, and the prevalence reaches 85% in patients with AIDS.[5]
Risk factors for the development of seborrheic dermatitis include:
- Age
- Male sex
- Increased sebaceous gland activity
- Immunodeficiency, including[6]:
- Lymphoma
- Renal transplantation
- HIV-AIDS
- Neurological and psychiatric disease, including[7]:
- Parkinson disease
- Stroke
- Alzheimer dementia
- Major depression
- Autonomic dysfunction
- Exposure to drug treatment, including:
- Dopamine antagonists
- Immunosuppressants
- Psoralen/PUVA
- Lithium
- Low ambient humidity and/or low ambient temperature
Pathophysiology
Proposed mechanisms for the pathogenesis of seborrheic dermatitis include[8][9][10]:
- Disruption of the skin’s microbiota
- An impaired immune reaction to Malassezia spp. associated with a diminished T-cell response and activation of complement
- The increased presence of unsaturated fatty acids on the skin surface
- Disruption of cutaneous neurotransmitters
- Abnormal shedding of keratinocytes
- Epidermal barrier disturbances associated with genetic factors
- The role of Malassezia spp. also includes the degradation of sebum and consumption of saturated fatty acids, disrupting the lipid balance on the skin surface. Further evidence for the involvement of Malassezia spp. includes their isolation from SD lesions and the significant resolution of SD with antifungal treatment.
However, there is insufficient evidence to link ISD with the development of ASD.
Histopathology
The dermatopathology of seborrheic dermatitis is non-specific, but the surface and infundibular epidermis usually show a superficial perivascular infiltrate of lymphocytes, acanthosis, focal spongiosis, and focal parakeratosis.[11][12] “Shoulder parakeratosis” refers to scale-crust accumulation around the infundibular ostia. Malassezia may be present in the stratum corneum.
Histological progression from acute to chronic SD characteristically demonstrates a transition from spongiosis to psoriasiform hyperplasia and the development of a lichenoid lymphocytic infiltrate.Severe SD is often associated with keratinocyte necrosis, focal interface destruction, and leukocytoclasis.
History and Physical
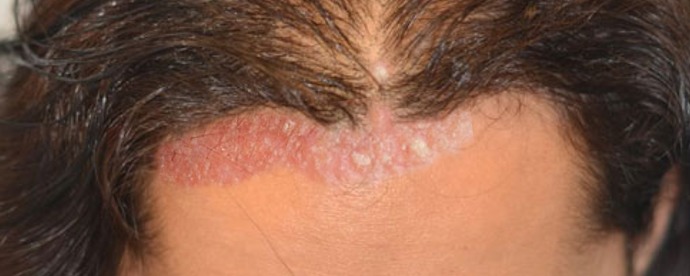
The distribution of lesions is the most important clinical feature of seborrheic dermatitis, with lesions occurring in areas where the skin is rich in sebaceous glands, especially on the scalp and face. ISD is generally asymptomatic, but atopic dermatitis frequently coexists. On the other hand, pruritus is common in ASD, especially with scalp lesions, and patients regularly report burning, but there is usually no history of atopic dermatitis. Seborrheic dermatitis characteristically demonstrates folliculocentric salmon-colored papules and plaques with a fine white scale, and a yellowish crust often described as a greasy scale-crust. It may present in one or more locations, with less scaling on flexural surfaces, and with lesions whose margins tend to be poorly defined.
The mildest form of SD is a non-inflammatory variant commonly referred to as pityriasis capitis or sicca.[12] It affects the scalp and “beard region” and is associated with the shedding of small light-colored flakes of skin, often seen on a background of dark clothing as “dandruff.” Sudden onset of severe SD should be a red flag for the presence of HIV-AIDS, with early recognition and diagnosis of HIV-AIDS significantly improving long-term outcomes. Common clinical presentations include reddening of the face, scaling, and dandruff. On darker skin, there may be persistent dyschromia with variable hyper/hypo-pigmentation. Other conditions associated with Malassezia spp may be present, including pityriasis versicolor and (Pityrosporum/Malassezia) folliculitis in adults and neonatal cephalic pustulosis.[13] Lesions on the anterior chest tend to have a psoriasiform morphology but frequently have a petaloid appearance, with such annular lesions commonly observed on the face in darker skin phenotypes. A pityriasiform variation (with a collarette scale mimicking pityriasis rosea) is rare.
Adult SD:
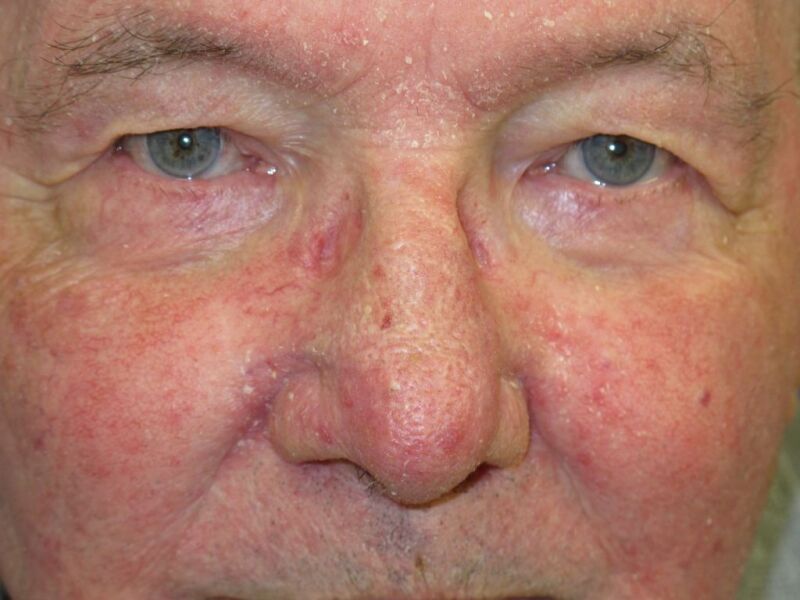
The face, scalp, and chest are the sites most commonly involved in ASD, with around 88%, 70%, and 27% of cases developing lesions in these areas, respectively.[14] On the head and neck, seborrheic dermatitis is characteristically symmetrical and involves the central third of the face, including the malar region, the center of the forehead, the eyebrows (especially their medial aspects), the postauricular area, and the external ear canal. SD characteristically affects the nasolabial and alar folds, and blepharitis is a common finding with the involvement of the anterior (lash) line.
Psoriasis requires differentiating from seborrheic dermatitis in adults. It is characterized by indurated red papules and plaques that have a sharply defined margin and a loose, silvery lamella scale. The nails may show psoriatic changes, and the Auspitz sign is positive. The existence of sebopsoriasis as a separate clinical entity has come into dispute.[7]
Infantile SD:
ISD usually appears in the second week of life and tends to last 4 to 6 months. It can present in the facial distribution of ASD, the diaper region, the skin creases of the neck, and the axillae. The rash is usually not itchy or painful, and the infants look content, but parents may be distressed. It is generally mild and self-limiting. A common presentation known as cradle cap refers to as an adherent yellowish scale-crust that arises on the crown and front of the scalp, developing from a bran-like scale, serous ooze, and a greasy crust, to create a firm mass that may progress to involve the whole scalp.
Pityriasis amiantacea may be present in ISD. It represents a set of clinical findings that may occur in older infants or young children but is not specific to seborrheic dermatitis. Typically, there are thick, silvery or yellow scales enveloping scalp hairs and binding them in tufts and can also be present in scalp psoriasis, atopic dermatitis, and tinea capitis. Atopic dermatitis is important in the differential diagnosis of ISD. It tends to be pruritic and prone to weeping and typically occurs on the face and limb flexures (elbow and knee) and spares the trunk. The child tends to be upset by the rash, and scratch marks are a common finding.
Evaluation
It is not necessary to routinely investigate seborrheic dermatitis, but HIV serology should be expedited in cases of severe SD, especially where the onset is sudden. Clinical features of Parkinson disease require recognition in elderly patients. The patient’s medications require a review.
The following tests may be helpful in the differential diagnosis
- KOH examination of skin scrapings
- Swab for microscopy, culture, and sensitivities
- Histology and direct immunofluorescence
- HIV serology; VDRL
- Serum zinc levels
- ANA; ENA; ESR
Treatment / Management
The approach will vary according to the patient’s age and the distribution and severity of the condition. It is essential to discuss good general skincare practices, including the use of a soap-substitute and appropriate moisturizing.[15] Treatments should address the underlying disease process and any secondary features, especially the hyperkeratotic scale, Staphylococcal infection, and associated symptoms, particularly pruritus. A Danish expert group recommended that authorities adopt topical antifungals as first-line treatment and agreed that topical corticosteroids and calcineurin inhibitors should only be used for significant symptoms and to manage moderate to severe flare-ups.[16] In ISD, removing the scale-crust in cradle cap and allaying parental anxiety are important considerations.[17] Sorbolene cream or lotion and a soft-bristled toothbrush can be used to soften and remove the cradle cap scales. On the other hand, it is crucial to relieve itch and discomfort in ASD.
A typical formulary should include antifungals, keratolytics, antipruritics, and anti-inflammatories (topical corticosteroids and calcineurin inhibitors). Moreover, treatment rotation may be more effective and associated with fewer adverse reactions than persisting with monotherapy. For scalp and non-scalp SD treatment, evidence supports the use of topical 1% to 2% ketoconazole, 1% ciclopirox, 1% zinc pyrithione, and 1% hydrocortisone.[18] Intermittent use of a mild topical corticosteroid and imidazole antifungal combination is convenient and can be very effective, but a potent corticosteroid may be necessary for short term treatment of scalp ASD. Shampoos usually contain combinations of agents such as pine or coal tar (antipruritic/keratolytic), salicylic acid (keratolytic), sulfur (antimicrobial/keratolytic), and sulfacetamide (anti-inflammatory/antibacterial). The patient can apply these to the scalp and non-scalp regions and washed-off after 5 to 10 minutes. Care should be taken when using topical salicylic acid, selenium, or zinc for the treatment of ISD, given the lack of safety and efficacy data to inform such treatment, but topical ketoconazole has been shown to be safe in infants with minimal systemic absorption detected.
Side effects associated with topical corticosteroids should be mitigated by intermittent use of site-appropriate potencies or steroid-sparing preparations such as topical 1% pimecrolimus. Another strategy is to employ the inherent anti-inflammatory effect of the topical antifungals, estimated to be equivalent to 1% hydrocortisone.[19] Oral treatment should be a consideration for generalized or refractory disease, and the standard of care utilizes the antifungal and anti-inflammatory properties of ketoconazole (monitor liver function; Black Box warning), itraconazole (check for CYP450 drug interactions; can worsen heart failure), and fluconazole (adjust the dose according to renal function). Itraconazole has the greatest anti-inflammatory effect, while oral terbinafine may be more effective than oral fluconazole in severe SD. Low-dose isotretinoin is non-inferior to topical standard of care but is commonly associated with significant mucocutaneous side effects.[20]
Itraconazole is safe and effective for controlling flares of SD and in preventing relapses.[19] It has also been shown to improve the quality of life in patients with moderate to severe SD.[21] However, given the absence of high-quality safety and efficacy data, a specialist team review is recommended before commencing oral treatment for ISD.In HIV-AIDS, antiretroviral treatment frequently improves SD, and SD may improve with L-dopa therapy in Parkinson’s disease.[22] Future therapies for SD could target improving skin barrier function or restoring the skin’s surface lipid composition.
Typical Formulary May Include:
i. Topical Creams, Ointments, and Lotions
- 2% salicylic acid + 2% sulfur in sorbolene cream or emulsifying ointment
- 2% ketoconazole cream
- 1% clotrimazole + 1% hydrocortisone cream
- 10% sulfacetamide + 5% sulfur lotion
- Betamethasone dipropionate 0.05% lotion
- 0.03% and 0.1% tacrolimus ointment
ii. Shampoos
- 1% zinc pyrithione
- 1% to .5% selenium sulfide
- 2% ketoconazole
- 1% ciclopirox
- 5% coal tar + 2% salicylic acid
- 0.1% and 0.03% tacrolimus
iii. Oral Medication
- Itraconazole
- Fluconazole
- Terbinafine
Differential Diagnosis
Important Considerations in the Differential Diagnosis for Adult SD
Scalp
- Psoriasis - usually nonpruritic and tends to affect the occipital and frontal regions, whereas SD tends to affect the vertex and parietal regions
- Eczema (contact) -due to the use of different shampoo and hair dye
- Darier’s disease - yellowish-brown clusters of rough dome-shaped papules in Seborrheic distribution; acanthosis
Face
- Psoriasis - rarely occurs in isolation; pitted nails
- Lupus erythematosus (LE) – discoid LE is associated with skin atrophy and scarring alopecia
- Rosacea – look for erythema and telangiectasia; it may cause Meibomianitis, along the posterior lid line
- Acne vulgaris – look for comedones, which are its hallmark
- Staphylococcal blepharitis (anterior lash line)
- Eczema (contact) - eyelids commonly involved (irritant - dry, scaly; or allergic - swollen, vesicular)
- Darier’s disease- Nail changes
Trunk
- Psoriasis - sharply-defined red plaques with a loose, silvery lamella scale
- Pityriasis rosea - herald spot; collarette scale; Christmas tree distribution
- Pityriasis versicolor - not symmetrical; hypo/hyperpigmentation
- Subacute lupus erythematosus - photosensitive distribution
- Eczema (nummular) - intense pruritus
- Tinea corporis - raised leading edges and central clearing; uncommon in infants
- Erythema annulare centrifugum - recurrent polycyclic lesions that slowly expand and disappear
- Darier disease - Greasy wart-like papules and plaques
- Grover disease (transient acantholytic dermatosis) - acanthosis
- Drug reaction - drug history (neuroleptic; immunosuppressant; PUVA; lithium)
- Parapsoriasis - elderly; very slow growing; resistant to treatment
- Pemphigus foliaceus - fragile, painful blisters - Nikolsky sign is positive
- Secondary syphilis - lesions on the palms and soles; a history of chancre
Intertriginous Areas
- Psoriasis (inverse) - sharply-defined border
- Dermatitis (Contact) - itchy; vesicular
- Tinea cruris - advancing border; very uncommon in infants
- Erythrasma - coral-red fluorescence under Wood Lamp
- Candidiasis - satellite lesions; obesity; a history of immunodeficiency
- Hailey-Hailey disease (familial benign pemphigus) - acanthosis
Important Considerations in the Differential Diagnosis for ISD
Cradle Cap
- Tinea capitis - (look for broken hairs or “black dots”); very uncommon in adults
- Impetigo - yellow, honey-colored crusting
Diaper Region
- Irritant contact dermatitis – tends to spare the skin folds
- Candidiasis – either secondary or from colonization with fecal yeast; look for satellite lesions
- Infantile psoriasis – sharply-defined red plaques with silver scale
- Histiocytosis X (Langerhans cell histiocytosis) – tends to be confined to the skin folds with a purpuric rash on the body
- Acrodermatitis enteropathica – look for periorificial involvement and check zinc levels
Prognosis
ISD usually affects the scalp and is mild and self-limiting, whereas ASD presents a chronic pattern of skin disease characterized by relapses and remissions. ASD is very controllable but not curable.
Complications
Seborrheic dermatitis usually takes a benign course, and serious complications are very rare. The intertriginous areas and eyelids are prone to secondary bacterial infections, especially during acute flares, and the diaper region is particularly prone to overgrowth with Candida spp.
Erythroderma has been reported in immunosuppressed neonates with generalized ISD, but it is more frequently a feature in adults with HIV-AIDS. However, research has not firmly established that SD causes erythroderma per se, given its predilection for sebaceous-rich skin.[7][13] The most common problems associated with both ISD and ASD relate to misdiagnosis of the condition.[23]
Deterrence and Patient Education
- Parental education is useful in allaying anxiety associated with ISD, and well-informed adults can learn to be confident at managing their condition
- For ASD, it requires emphasizing to the patient that there is no cure, but it can be well controlled and managed primarily at home.[16]
- Many of the treatments for SD are available without a prescription, over the counter at the pharmacy, or increasingly on supermarket shelves. Directing the patient to the selection of such products may save consultation time and associated costs.
Enhancing Healthcare Team Outcomes
SD is a difficult condition to manage in the adult, and its management is best by an interprofessional team.
- Consider the impact of SD on psychosocial functioning and quality-of-life and remember that SD may accompany neurological or psychiatric disease.[24]
- The dermatologist and pharmacist can help to promote the appropriate use of topical corticosteroids and employ steroid-sparing alternatives.
- New evidence suggests diet may play a role in SD, with a high fruit intake associated with a 25% lower risk of SD, whereas a “Western” diet was associated with a 47% increased risk of SD.[25]
- Atopic dermatitis and ISD frequently coexist, whereas psoriasis often occurs in adults with SD, but there is usually no history of atopic dermatitis
- Review the diagnosis in recalcitrant cases of SD and consider immunodeficiency in sudden, severe cases
- Topical antifungals have an anti-inflammatory effect which contributes significantly to their efficacy and is a convenient steroid-sparing strategy
Even though primary clinicians manage the condition, it is best to refer the complex patient to the dermatologist for guidance. Dermatology specialty-trained nurses can also help by counseling the patient, providing direction on medical management, and monitoring and charting treatment progress. A pharmacist should also be on the case, with assistance in selecting the most appropriate agents, verifying dosing, offering patient education, and performing medication reconciliation, informing the prescriber of any issues encountered. Close communication between interprofessional team members is vital to improving outcomes.

Figure
Infantile Seborrhoeic Dermatitis DermNet New Zealand

Figure
Seborrhoeic Dermatitis DermNet New Zealand

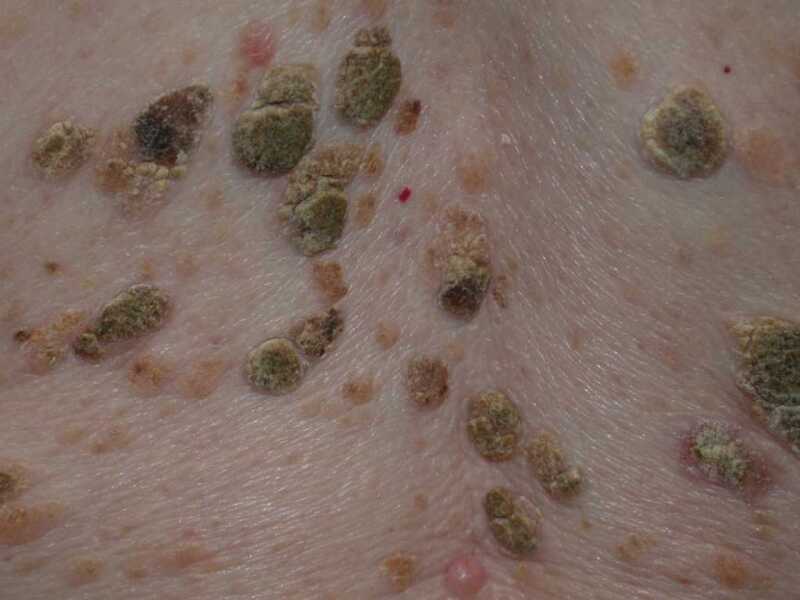
Figure
Seborrhoeic Keratoses DermNet New Zealand

Figure
Seborrheic Dermatitis Contributed by S bhimji, MD
References
- 1.
- Sampogna F, Linder D, Piaserico S, Altomare G, Bortune M, Calzavara-Pinton P, Vedove CD, Girolomoni G, Peserico A, Sala R, Abeni D. Quality of life assessment of patients with scalp dermatitis using the Italian version of the Scalpdex. Acta Derm Venereol. 2014 Jul;94(4):411-4. [PubMed: 24287710]
- 2.
- Zani MB, Soares RC, Arruda AC, de Arruda LH, Paulino LC. Ketoconazole does not decrease fungal amount in patients with seborrhoeic dermatitis. Br J Dermatol. 2016 Aug;175(2):417-21. [PubMed: 26920094]
- 3.
- Palamaras I, Kyriakis KP, Stavrianeas NG. Seborrheic dermatitis: lifetime detection rates. J Eur Acad Dermatol Venereol. 2012 Apr;26(4):524-6. [PubMed: 21521374]
- 4.
- Sanders MGH, Pardo LM, Franco OH, Ginger RS, Nijsten T. Prevalence and determinants of seborrhoeic dermatitis in a middle-aged and elderly population: the Rotterdam Study. Br J Dermatol. 2018 Jan;178(1):148-153. [PubMed: 28856679]
- 5.
- Scognamiglio P, Chiaradia G, De Carli G, Giuliani M, Mastroianni CM, Aviani Barbacci S, Buonomini AR, Grisetti S, Sampaolesi A, Corpolongo A, Orchi N, Puro V, Ippolito G, Girardi E., SENDIH Study Group. The potential impact of routine testing of individuals with HIV indicator diseases in order to prevent late HIV diagnosis. BMC Infect Dis. 2013 Oct 10;13:473. [PMC free article: PMC3852490] [PubMed: 24112129]
- 6.
- Lally A, Casabonne D, Imko-Walczuk B, Newton R, Wojnarowska F. Prevalence of benign cutaneous disease among Oxford renal transplant recipients. J Eur Acad Dermatol Venereol. 2011 Apr;25(4):462-70. [PubMed: 20738465]
- 7.
- Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013 Jul-Aug;31(4):343-351. [PubMed: 23806151]
- 8.
- Paulino LC. New perspectives on dandruff and seborrheic dermatitis: lessons we learned from bacterial and fungal skin microbiota. Eur J Dermatol. 2017 Jun 01;27(S1):4-7. [PubMed: 28690211]
- 9.
- Honnavar P, Chakrabarti A, Prasad GS, Singh P, Dogra S, Rudramurthy SM. β-Endorphin enhances the phospholipase activity of the dandruff causing fungi Malassezia globosa and Malassezia restricta. Med Mycol. 2017 Feb 01;55(2):150-154. [PubMed: 27497434]
- 10.
- Turner GA, Hoptroff M, Harding CR. Stratum corneum dysfunction in dandruff. Int J Cosmet Sci. 2012 Aug;34(4):298-306. [PMC free article: PMC3494381] [PubMed: 22515370]
- 11.
- Sampaio AL, Mameri AC, Vargas TJ, Ramos-e-Silva M, Nunes AP, Carneiro SC. Seborrheic dermatitis. An Bras Dermatol. 2011 Nov-Dec;86(6):1061-71; quiz 1072-4. [PubMed: 22281892]
- 12.
- Schwartz JR, Messenger AG, Tosti A, Todd G, Hordinsky M, Hay RJ, Wang X, Zachariae C, Kerr KM, Henry JP, Rust RC, Robinson MK. A comprehensive pathophysiology of dandruff and seborrheic dermatitis - towards a more precise definition of scalp health. Acta Derm Venereol. 2013 Mar 27;93(2):131-7. [PubMed: 22875203]
- 13.
- Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012 Jan;25(1):106-41. [PMC free article: PMC3255962] [PubMed: 22232373]
- 14.
- Peyrí J, Lleonart M., Grupo español del Estudio SEBDERM. [Clinical and therapeutic profile and quality of life of patients with seborrheic dermatitis]. Actas Dermosifiliogr. 2007 Sep;98(7):476-82. [PubMed: 17669302]
- 15.
- Augustin M, Kirsten N, Körber A, Wilsmann-Theis D, Itschert G, Staubach-Renz P, Maul JT, Zander N. Prevalence, predictors and comorbidity of dry skin in the general population. J Eur Acad Dermatol Venereol. 2019 Jan;33(1):147-150. [PubMed: 29953684]
- 16.
- Hald M, Arendrup MC, Svejgaard EL, Lindskov R, Foged EK, Saunte DM., Danish Society of Dermatology. Evidence-based Danish guidelines for the treatment of Malassezia-related skin diseases. Acta Derm Venereol. 2015 Jan;95(1):12-9. [PubMed: 24556907]
- 17.
- Victoire A, Magin P, Coughlan J, van Driel ML. Interventions for infantile seborrhoeic dermatitis (including cradle cap). Cochrane Database Syst Rev. 2019 Mar 04;3(3):CD011380. [PMC free article: PMC6397947] [PubMed: 30828791]
- 18.
- Cheong WK, Yeung CK, Torsekar RG, Suh DH, Ungpakorn R, Widaty S, Azizan NZ, Gabriel MT, Tran HK, Chong WS, Shih IH, Dall'Oglio F, Micali G. Treatment of Seborrhoeic Dermatitis in Asia: A Consensus Guide. Skin Appendage Disord. 2016 May;1(4):187-96. [PMC free article: PMC4908450] [PubMed: 27386464]
- 19.
- Das A, Panda S. Use of Topical Corticosteroids in Dermatology: An Evidence-based Approach. Indian J Dermatol. 2017 May-Jun;62(3):237-250. [PMC free article: PMC5448257] [PubMed: 28584365]
- 20.
- Kamamoto CSL, Nishikaku AS, Gompertz OF, Melo AS, Hassun KM, Bagatin E. Cutaneous fungal microbiome: Malassezia yeasts in seborrheic dermatitis scalp in a randomized, comparative and therapeutic trial. Dermatoendocrinol. 2017;9(1):e1361573. [PMC free article: PMC5821162] [PubMed: 29484095]
- 21.
- Abbas Z, Ghodsi SZ, Abedeni R. Effect of itraconazole on the quality of life in patients with moderate to severe seborrheic dermatitis: a randomized, placebo-controlled trial. Dermatol Pract Concept. 2016 Jul;6(3):11-6. [PMC free article: PMC5006547] [PubMed: 27648378]
- 22.
- Skorvanek M, Bhatia KP. The Skin and Parkinson's Disease: Review of Clinical, Diagnostic, and Therapeutic Issues. Mov Disord Clin Pract. 2017 Jan-Feb;4(1):21-31. [PMC free article: PMC6174479] [PubMed: 30363435]
- 23.
- Schwartz JL, Clinton TS. Darier's disease misdiagnosed as severe seborrheic dermatitis. Mil Med. 2011 Dec;176(12):1457-9. [PubMed: 22338367]
- 24.
- Pärna E, Aluoja A, Kingo K. Quality of life and emotional state in chronic skin disease. Acta Derm Venereol. 2015 Mar;95(3):312-6. [PubMed: 24978135]
- 25.
- Sanders MGH, Pardo LM, Ginger RS, Kiefte-de Jong JC, Nijsten T. Association between Diet and Seborrheic Dermatitis: A Cross-Sectional Study. J Invest Dermatol. 2019 Jan;139(1):108-114. [PubMed: 30130619]
Disclosure: Dan Tucker declares no relevant financial relationships with ineligible companies.
Disclosure: Sadia Masood declares no relevant financial relationships with ineligible companies.
Publication Details
Author Information and Affiliations
Authors
Dan Tucker; Sadia Masood1.Affiliations
Publication History
Last Update: February 16, 2023.
Copyright
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Publisher
StatPearls Publishing, Treasure Island (FL)
NLM Citation
Tucker D, Masood S. Seborrheic Dermatitis. [Updated 2023 Feb 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.