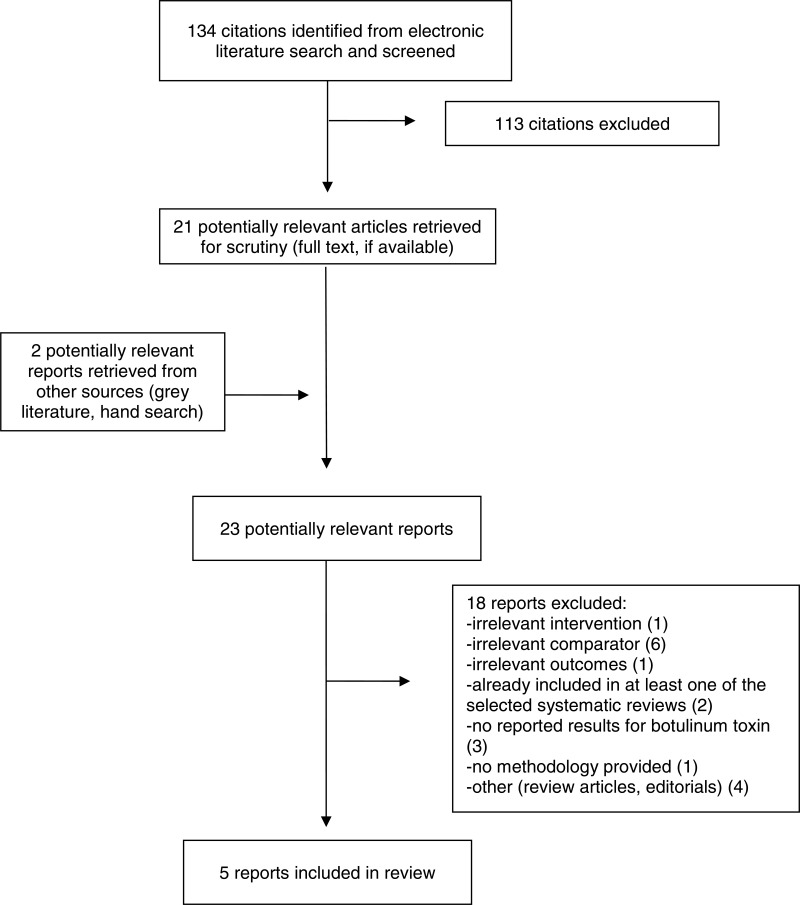
Demographics N = 59 BTA group n = 30 Placebo group n = 29 Age, years, median [IQR] BTA: 43 [30 to 55] Placebo: 40 [31 to 54] BMI, median [IQR] BTA: 23 [22 to 27] Placebo: 27 [24 to 29] Nulliparous, number (%) BTA: 11 (37) Placebo: 17 (59) History of pelvic floor physical therapy, number (%) BTA: 23 (77) Placebo: 20 (69) Sexually active, number (%) BTA: 17 (57) Placebo: 8 (28) Years of pain, median [IQR] BTA: 7 [3 to 10] Placebo: 5 [3 to 10] Other pain disorders, number (%) BTA: 22 (73) Placebo: 23 (79) “The intervention group had more participants rating their pain as severe or moderate at baseline compared with the placebo group.” (p1.e4) BTA vs. Placebo, changes in pain from baseline, median (Wong-Baker FACES Pain Rating Scale) Left muscle group, BTA vs. placebo, median [IQR] Coccygeus 2 weeks: −1 [–2 to −1] vs. −3 [–4 to −1], P = 0.046 4 weeks: −2 [–4 to −1] vs. −2 [–4 to −1], P = 0.72 12 weeks: −3 [–5 to −1] vs. −2 [–5 to −1], P = 0.390 Iliococcygeus 2 weeks: −2 [–3 to −1] vs. −2 [–5 to −1], P = 0.12 4 weeks: −1 [–3 to 0] vs. −1 [–4 to 0], P = 0.92 12 weeks: −3 [–5 to −1] vs. −2 [–4 to −1], P = 0.46 Obturator onternus 2 weeks: −1 [–3 to 0] vs. −1 [–5 to 0], P = 0.84 4 weeks:–2 [–4 to 0] vs. −2 [–4 to −1], P = 0.82 12 weeks:–3 [–5 to −3] vs. −3 [–3 to 0], P = 0.22 Piriformus 2 weeks: −1 [–3 to 0] vs. −2 [–4 to −1], P = 0.12 4 weeks: −2 [–3 to 0] vs. −2 [–4 to 0], P = 0.79 12 weeks: −2 [–4 to 0] vs. −2 [–6 to 0], P = 0.85 Pubcoccygeus 2 weeks: −1 [–3 to 0] vs. −1 [–5 to 0], P = 0.44 4 weeks: −1 [–3 to 1] vs. −2 [–4 to 0], P = 0.29 12 weeks: −3 [–6 to 0] vs. −3 [–5 to 2], P = 0.55 Puborectalis 2 weeks: −1 [–3 to 1] vs. 0 [–1 to 1], P = 0.39 4 weeks: −3 [–4 to −1] vs. −1 [–3 to 0], P = 0.31 12 weeks: −2 [–6 to −1] vs. −1 [–3 to 0], P = 0.21 Right muscle group, BTA vs. placebo, median [IQR] Coccygeus 2 weeks: −2 [–3 to 0] vs. −1 [–3 to 0], P = 0.50 4 weeks: −3 [–4 to −1] vs. −2 [–3 to 0], P = 0.19 12 weeks: −3 [–4 to 0] vs. −2 [–4 to 0], P = 0.54 Iliococcygeus 2 weeks: −1 [–3 to 0] vs. −1 [–3 to 0], P = 0.85 4 weeks: −1 [–5 to 0] vs. −3 [–4 to 0], P = 0.77 12 weeks: −3 [–5 to −1] vs. −1 [–3 to 0], P = 0.28 Obturator onternus 2 weeks: −1 [–3 to 0] vs. −1 [–4 to 0], P = 0.54 4 weeks: −2 [–3 to 0] vs. −2 [–4 to 0], P = 0.77 12 weeks: −3 [–6 to 0] vs. −2 [–5 to 1], P = 0.45 Piriformus 2 weeks: −2 [–3 to −1] vs. 0 [–3 to 1], P = 0.10 4 weeks: −2 [–4 to −1] vs. −1P = 0.0 [–3 to 0], P = 0.20 12 weeks: −3 [–4 to 0] vs. −1 [–4 to 0], P = 0.42 Pubcoccygeus 2 weeks: −1 [–3 to 1] vs. −2 [–3 to 0], P = 0.44 4 weeks: −2 [–3 to 0] vs. −1 [–4 to 0], P = 0.80 12 weeks: −3 [–5 to 0] vs. −1 [–3 to 2], P = 0.18 Puborectalis 2 weeks: −1 [–3 to 1] vs. 0 [–2 to 1], P = 0.37 4 weeks: −2 [–4 to 0] vs. −1 [–3 to 1], P = 0.54 12 weeks: −2 [–5 to −1] vs. −1 [–4 to 0], P = 0.22 Change in pain from baseline, median [IQR] (VAS), BTA vs. placebo 2 weeks: −0.3 [–3 to 1] vs. −0.3 [–2 to 0.1], P = 0.65 4 weeks: −1 [–4 to 0] vs. −0.2 [–1 to 0.8], P = 0.16 12 weeks: −1 [–4 to 0] vs. 0 [–4 to 1], P = 0.16 PFDI-20 change from baseline, median [IQR], BTA vs. placebo 2 weeks: 3 [–14 to 22] vs. −10 [–27 to −4], P = 0.01 4 weeks: −3 [–22 to 6] vs. −18 [–38 to −2], P = 0.19 12 weeks: −7 [–21 to 11] vs. −21 [–64 to −2], P = 0.11 BTA vs. Placebo, median pain score (VAS) Baseline: P = 0.07 2 weeks: P = 0.045* 4 weeks: P = 0.99 12 weeks: P = 0.94 *In favour of intervention (BTA) BTA vs. Placebo, median PFDIa
score Baseline: P = 0.89 2 weeks: P = 0.12 4 weeks: P = 0.34 12 weeks: P = 0.23 BTA vs. Placebo, median pelvic organ prolapse distress inventory score Baseline: P = 0.15 2 weeks: P = 0.06 4 weeks: P = 0.19 12 weeks: P = 0.18 BTA vs. Placebo, median colorectal-anal distress inventory score Baseline: P = 0.63 2 weeks: P = 0.19 4 weeks: P = 0.23 12 weeks: P = 0.81 BTA vs. Placebo, median pain urgency frequency score Baseline: P = 0.33 2 weeks: P = 0.22 4 weeks: P = 0.61 12 weeks: P = 0.23 Patient Global Impression of Improvement index 48.1% in intervention group rated severity as normal or mild at 12 weeks 63.0% in placebo group rated severity as normal or mild at 12 weeks P = 0.59 At 4 weeks, intervention group more likely to state improvement in symptoms (P = 0.03); at 12 weeks, intervention numerically more likely to state improvement in symptoms but this was not statistically significant (P = 0.10). |