NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
OVERVIEW
Introduction
Phentermine is a sympathomimetic amine and anorectic agent used for the short term therapy of obesity. Phentermine which has been in clinical use for more than 50 years has not been linked convincingly to either serum enzyme elevations during therapy or to instances of clinically apparent liver injury.
Background
Phentermine (fen' ter meen) is a structural analogue to amphetamine and has similar activity in suppressing appetite, but has few of the other central nervous system effects of amphetamines and only mild abuse potential. Its effect in increasing weight loss is probably mediated by anorectic activity that is the result of enhancement of norepinephrine release in appetite centers in the brain. Phentermine was approved as a therapy for obesity in the United States in 1959 but was recommended only for short term use (less than 12 weeks) and in combination with behavioral modification, caloric restriction and exercise. Nevertheless, phentermine is widely used with more than 2 million prescriptions filled in the United States yearly. Phentermine is a Schedule IV drug, meaning that it has proven, but low abuse potential and has an accepted medical use. Phentermine is available by prescription as 30 mg tablets of phentermine base and 37.5 mg tablets of phentermine hydrochloride in multiple generic forms and under various trade names including Adipex-P, Obenix and Lonamin. The usual dose is 30 or 37.5 mg once daily. A combination of phentermine with topiramate (an anticonvulsant that has weight loss effects) has recently been approved for use for long term use in patients who are obese or are overweight and have obesity related conditions. This combination consists of fixed lower doses of phentermine and topiramate (3.75/23, 7/46, 11.25/69 and 15/92 mg) and is available in capsules under the brand name Qsymia. Common side effects of phentermine include nervousness, excitability, insomnia, headache, dry mouth, sweating, tachycardia, palpitations, nausea, constipation, and thirst. Rare severe adverse events include atrial fibrillation, acute psychosis and pulmonary hypertension.
Hepatotoxicity
Phentermine has not been linked to an increased rate of serum enzyme elevations during therapy; however, results of ALT monitoring during phentermine therapy have rarely been reported. Despite long term availability and wide use of phentermine, there have been no published reports linking it to clinically apparent acute liver injury.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Drug Class: Weight Loss Agents, see also Phentermine-Topiramate
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Phentermine – Generic, Adipex-P®, Lonamin®
DRUG CLASS
Weight Loss Agents
Product labeling at DailyMed, National Library of Medicine, NIH
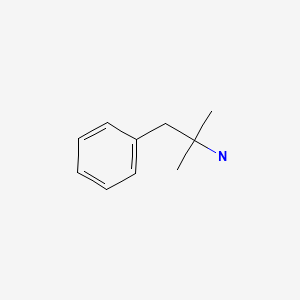
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Phentermine | 122-09-8 | C10-H15-N |
|
ANNOTATED BIBLIOGRAPHY
References updated: 06 June 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 483-91.(Expert review of hepatotoxicity published in 1999; phentermine is not discussed).
- Westfall TC, McArthur H, Westfall DP. Adrenergic agonists and antagonists. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 191-224.(Textbook of pharmacology and therapeutics; phentermine is listed as a sympathomimetic agent used for weight loss).
- Cohen A, De Felice EA, Leb SM, Fuentes JG, Rothwell KG, Truant AP. Double-blind comparison of efficacy, safety, and side effects of Bionamin, phentermine compound, and placebo in the treatment of exogenous obesity. Curr Ther Res Clin Exp. 1968;10:323–34. [PubMed: 4969672](Controlled trial of 12 weeks of phentermine vs a combination of phentermine and amphetamine vs placebo in 120 obese prison inmates; no significant changes in serum bilirubin, ALT or Alk P in any group).
- Langlois KJ, Forbes JA, Bell GW, Grant GF Jr. A double-blind clinical evaluation of the safety and efficacy of phentermine hydrochloride (Fastin) in the treatment of exogenous obesity. Curr Ther Res Clin Exp. 1974;16:289–96. [PubMed: 4208343](Controlled trial of 16 weeks of phentermine vs placebo in 70 obese subjects; weight loss was more with phentermine [16 vs 4 pounds]; there were no differences in changes of any “safety parameters”).
- Bray GA. A concise review on the therapeutics of obesity. Nutrition. 2000;16:953–60. [PubMed: 11054601](Review of drug treatment of obesity; hepatotoxicity is not discussed).
- Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord. 2002;26:262–73. [PubMed: 11850760](Metaanalysis of published studies of antiobesity medications; phentermine has been studied in 9 controlled trials published between 1969-92; no discussion of side effects).
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med. 2005;143:380–5. [PubMed: 16144896](History of the approval of medications for obesity from initial agents approved in 1947 to sibutramine in 1997; phentermine was first approved in 1959, before the Kefauver-Harris amendment requiring proof of efficacy from controlled trials).
- Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–46. [PubMed: 15809465](Systematic review of efficacy and safety of medications for obesity; phentermine therapy is associated with a significant, but modest increase in weight loss; there have been no systematic reports of adverse events, but serious adverse events were not reported in 9 controlled trials of phentermine).
- Bray GA. Drug Insight: appetite suppressants. Nat Clin Pract Gastroenterol Hepatol. 2005;2:89–95. [PubMed: 16265126](Review of the mechanism of action and clinical efficacy of drugs that suppress appetite including sympathomimetic agents [amphetamine, phentermine, sibutramine], serotonin reuptake inhibitors [bupropion, fenfluramine, fluoxetine], GABAergic agents [topiramate, zonisamide], cannabinoid antagonists [rimonabant] and various peptides [leptin, neuropeptide Y, melanocortin-4]).
- Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf. 2006;29:277–302. [PubMed: 16569079](Review of safety of drug therapy of obesity; the only mention of liver adverse events was “a case of reversible hepatotoxicity associated with sibutramine”).
- Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab. 2010;12:876–82. [PubMed: 20920040](Randomized controlled trial of 12 weeks of phentermine vs placebo in 74 patients with obesity found no severe adverse events and no change in blood chemistry results from baseline).
- Diet, drugs and surgery for weight loss. Treat Guidel Med Lett. 2011;9(104):17–22. [PubMed: 21436767](Concise review of approved and unapproved medical and surgical approaches to obesity; the sympathomimetic amines are the oldest weight loss drugs, but are approved for short term use only; no mention of hepatotoxicity in discussion of side effects of phentermine).
- Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–52. [PubMed: 21481449](Randomized controlled trial of 56 weeks of two doses of phentermine/ topiramate vs placebo in 2487 overweight or obese patients; no mention of ALT levels or hepatotoxicity).
- Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. [PMC free article: PMC3260065] [PubMed: 22158731](Controlled extension to two years of a randomized controlled trial of phentermine/topiramate vs placebo in 676 obese patients; weight loss was sustained and common side effects were similar with longer therapy: “No dose related changes were observed in shift summaries of selected laboratory values”).
- Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330–42. [PMC free article: PMC3270297] [PubMed: 22051941](Randomized controlled trial of two dose regimens of phentermine/topiramate vs placebo in 1267 obese subjects; common side effects were paresthesias, dry mouth, constipation, headache, change in taste, insomnia and depression; no mention of ALT levels or hepatotoxicity).
- Kang JG, Park CY. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J. 2012;36:13–25. [PMC free article: PMC3283822] [PubMed: 22363917](Review of the safety and efficacy of current and potentially future medications for obesity; mentions that phentermine has been available for 50 years, but there is little data on its long term efficacy and safety).
- 2 new drugs for weight loss. Med Lett Drugs Ther. 2012;54(1398):69–71. [PubMed: 22992487](Concise review of phentermine/topiramate and lorcaserin for weight loss shortly after their approval for use in the US; in discussion of side effects, no mention of hepatotoxicity).
- Kim HO, Lee JA, Suh HW, Kim YS, Kim BS, Ahn ES, Roh YJ, et al. Postmarketing surveillance study of the efficacy and safety of phentermine in patients with obesity. Korean J Fam Med. 2013;34:298–306. [PMC free article: PMC3791337] [PubMed: 24106582](Among 795 obese Korean patients enrolled in a prospective surveillance program and treated with phentermine [37.5 mg daily], the mean weight loss at 12 weeks was 3.8 kg and 30% of patients reported adverse events, but none were serious and there was no mention of ALT elevations or hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996-2012 identified 176 cases, none of which were attributed to phentermine or other weight loss agents).
- Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. [PMC free article: PMC3928674] [PubMed: 24231879](Systematic review of the literature on the efficacy of long term use of drugs for obesity that were FDA approved [at the time of the analysis] mentions that phentermine, diethylpropion and phendimetrazine are approved for short term use only, but that orlistat, lorcaserin and phentermine/topiramate are approved for long term use although their efficacy is modest; no discussion of hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one unlikely fatal case was attributed initially to phentermine).
- Aagaard L, Hallgreen CE, Hansen EH. Serious adverse events reported for antiobesity medicines: postmarketing experiences from the EU adverse event reporting system EudraVigilance. Int J Obes (Lond). 2016;40:1742–7. [PubMed: 27478924](Analysis of adverse event reports from antiobesity medications to the European pharmacovigilance database [EudraVigilance] between 2007 and 2013 identified 4941 reports detailing 13,957 individual adverse events, 90% serious, only 15 attributed to phentermine with 2 deaths, but none were classified as hepatobiliary).
- Diet, drugs, devices, and surgery for weight management. Med Lett Drugs Ther. 2018;60(1548):91–8. [PubMed: 29913463](Concise review of the medical and surgical therapies for obesity mentions that orlistat is modestly effective in inducing weight loss and that “severe liver injury has been reported rarely, but no cause-and-effect relationship has been established; but discussion of other weight loss agents [phentermine/topiramate, naltrexone/bupropion, lorcaserin and liraglutide] does not mention ALT elevations or hepatotoxicity).
- Lewis KH, Fischer H, Ard J, Barton L, Bessesen DH, Daley MF, Desai J, et al. Safety and effectiveness of longer-term phentermine use: clinical outcomes from an Electronic Health Record Cohort. Obesity (Silver Spring). 2019;27:591–602. [PubMed: 30900410](Among 13,972 adults who initiated therapy with phentermine between 2010-15 identified by electronic health records, those who remained on long term therapy [n=144] lost an average of 7.5% of weight vs none in those on short term therapy and there were no differences in composite adverse cardiovascular outcomes with long term continuous or intermittent therapy; no mention of hepatotoxicity or ALT elevations).