NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
OVERVIEW
Introduction
Elvitegravir is a human immunodeficiency virus (HIV) integrase inhibitor which is used largely in a four drug combination with cobicistat, emtricitabine and tenofovir as therapy of HIV infection. Therapy with this elvitegravir based regimen is often associated with transient serum aminotransferase elevations during therapy, but has not been implicated in cases of clinically apparent acute liver injury.
Background
Elvitegravir (el" vi teg' ra vir) is a 4-quinolone-3-glyoxylic acid and antiretroviral agent that acts by inhibition of viral DNA strand transfer by the HIV integrase, a necessary step in HIV replication. Elvitegravir has been shown to lower serum levels of HIV RNA and to raise CD4 counts. In multiple prospective clinical trials, the combination of elvitegravir with tenofovir and emtricitabine has been found to be as effective as other standard antiretroviral combinations. Elvitegravir is given with cobicistat, a pharmacokinetic enhancer that inhibits CYP 3A4 activity, causing increased levels and more prolonged activity of drugs like elvitegravir that are metabolized by the hepatic cytochrome P450 isomer CYP 3A4. Elvitegravir was approved as a part of a four drug combination including cobicistat, emtricitabine and tenofovir disoproxil fumarate as therapy of HIV infection in 2012 under the brand name Stribild. A similar four drug combination that includes tenofovir alafenamide instead of tenofovir disoproxil fumarate was approved for use in HIV infection in 2016 under the brand name Genvoya. These combinations are available as tablets of 150 mg of elvitegravir with 150 mg of cobicistat, 200 mg of emtricitabine and either 300 mg of tenofovir disoproxil fumarate (Stribild) or 10 mg of tenofovir alafenamide (Genvoya). The recommended dose is one tablet daily, these combination being two of several "single tablet regimens" (STRs) for therapy of HIV infection. Elvitegravir was the second HIV integrase to be approved in the United States and shares structural similarity and resistance patterns with the initial agent, raltegravir. Elvitegravir is also available as a single agent for use with other antiretroviral agents in 85 and 150 mg tablets under the brand name Vitekta. Common side effects of the four drug combination include fatigue, diarrhea, nausea, dizziness, headache, depression, abnormal dreams and skin rashes. Cobicistat has inhibitory activity against several drug metabolizing enzymes besides CYP 3A4, including CYP 2D6 and the P-glycoprotein transporter, making it likely to cause drug-drug interactions and important to avoid when using other agents that are metabolized by the P450 system. Cobicistat also inhibits creatinine secretion, which artificially raises serum creatinine levels without affecting the glomerular filtration rate. With long term use, tenofovir disoproxil fumarate can be associated with decline in kidney function, phosphate wasting and decline in bone mineral density. These adverse effects appear to be less with tenofovir alafenamide which is more potent and is given in a lower dose that the disoproxil fumurate form of tenofovir.
Hepatotoxicity
In premarketing clinical trials, serum ALT elevations greater than 5 times the upper limit of normal occurred in 15% of patients treated with elvitegravir combined with cobicistat, emtricitabine and tenofovir. This rate was somewhat lower than what occurred in comparator arms. Most serum enzyme elevations with elvitegravir based regimens were transient and asymptomatic, and occurred in patients with known underlying chronic liver disease such as hepatitis B or C or alcoholic liver disease. In at least one study, 1% of patients on the elvitegravir based four drug regimen developed "acute hepatitis", but the relatedness of the liver injury to elvitegravir was unclear and clinical details were not provided. No specific reports of clinically apparent liver injury attributed to elvitegravir have appeared in the published literature. The product label for elivitegravir mentions two other forms of acute liver injury that have been linked to potent antiretroviral regimens: the immune reconstitution syndrome when these regimens are started and reactivation of hepatitis B when regimens with anti-HBV acitivity (as with tenofovir) are discontinued. Neither of these effects are specific to elvitegravir or other integrase inhibitors.
Likelhood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
The possible mechanism of liver injury due to elvitegravir is unknown. Elvitegravir is extensively metabolized in the liver via the cytochrome P450 system (predominantly CYP 3A4), and production of a toxic or immunogenic intermediate may trigger liver injury.
Outcome and Management
The severity of the liver injury linked to elvitegravir therapy has been mild and self-limited, and characterized by serum enzyme elevations without jaundice and few or no symptoms. The enzyme elevations often resolve even with continuation of elvitegravir. In one case report, a patient who developed liver injury while taking dolutegravir was able to tolerate elvitegravir without recurrence. Thus, switching to another integrase inhibitor may be an option in patients with acute liver injury due to an agent in this class, but it shold be done with cautioni.
Drug Class: Antiviral Agents
Other Drugs in the Subclass, Integrase Strand Transfer Inhibitors: Bictegravir, Cabotegravir, Dolutegravir, Raltegravir
See also drugs used in combination, Nucleoside Analogues: Emtricitabine, Tenofovir
Pharmacokinetic Enhancers: Cobicistat
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Elvitegravir – Vitekta®
Elvitegravir, Cobicistat, Emtricitabine, Tenofovir disoproxil fumarate – Stribild®
Elvitegravir, Cobicistat, Emtricitabine, Tenofovir alafenamide – Genvoya®
DRUG CLASS
Antiviral Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Elvitegravir | 697761-98-1 | C23-H23-Cl-F-N-O5 |
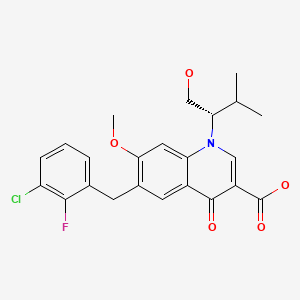
|
| Cobicistat | 1004316-88-4 | C40-H53-N7-O5-S2 |

|
| Emtricitabine | [Refer to Emtricitabine Record.] | ||
| Tenofovir | [Refer to Tenofovir Record.] | ||
ANNOTATED BIBLIOGRAPHY
References updated: 30 January 2018
- Núñez M. Hepatic toxicity of antiviral agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 505-18.(Review of hepatotoxicity of antiviral agents; elvitegravir is not specifically discussed).
- Flexner C. Antiretroviral agents and treatment of HIV infection. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1623-64.(Textbook of pharmacology and therapeutics).
- https://hivinfo
.nih.gov /hiv-source/medical-practice-guidelines /hiv-treatment-guidelines . (Regularly updated guidelines on therapy of HIV infection in adults, adolescents and children). - Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. [PubMed: 10632283](Among 298 patients with HIV infection, ALT elevations above 5 times ULN occurred in 10.4% per year during antiretroviral treatment; factors associated with ALT elevations included ritonavir [27.3%] and coinfection with either HCV or HBV; ALT with bilirubin elevations occurred in 3 patients; all 3 had coinfection and 2 were on indinavir).
- Torti C, Lapadula G, Casari S, Puoti M, Nelson M, Quiros-Roldan E, Bella D, et al. EPOKA-MASTER Study Group. Incidence and risk factors for liver enzyme elevation during highly active antiretroviral therapy in HIV-HCV co-infected patients: results from the Italian EPOKA-MASTER Cohort. BMC Infect Dis. 2005;5:58. [PMC free article: PMC1188059] [PubMed: 16018804](Among 1038 HIV-HCV coinfected patients starting antiretroviral therapy, the risk of ALT elevations above 5 times ULN was 17.1% yearly in treatment naive and 8.2% in treatment experienced patients; risk factors being baseline ALT levels and use of nonnucleoside reverse transcriptase inhibitors).
- Jain MK. Drug-induced liver injury associated with HIV medications. Clin Liver Dis. 2007;11:615–39. vii-viii. [PubMed: 17723923](Review of hepatotoxicity of antiretroviral medications; ALT elevations occur in 2-18% of patients during treatment, but often resolve spontaneously even without dose modification; classes of injury include hypersensitivity [nevirapine, efavirenz, abacavir], mitochondrial injury [stavudine, didanosine, zidovudine], flares of hepatitis B [lamivudine, emtricitabine, tenofovir], flares of hepatitis C [any potent regimen], idiosyncratic injury [ritonavir, nevirapine, efavirenz], cholestatic hepatitis [many agents]).
- Soriano V, Puoti M, Garcia-Gascó P, Rockstroh JK, Benhamou Y, Barreiro P, McGovern B. Antiretroviral drugs and liver injury. AIDS. 2008;22:1–13. [PubMed: 18090386](Review of hepatotoxicity of antiretroviral drugs with recommendations on management; therapy should be stopped if symptoms arise or with overt jaundice [direct bilirubin] or there is evidence of mitochondrial toxicity or ALT is above 10 times ULN; therapy should be stopped at lower ALT levels if a newly marketed agent is being used; important to rule out other causes; problematic agents include didanosine, stavudine and zidovudine; nevirapine and efavirenz, full dose ritonavir and tipranavir).
- Cohen C, Elion R, Ruane P, Shamblaw D, DeJesus E, Rashbaum B, Chuck SL, et al. Randomized, phase 2 evaluation of two single-tablet regimens elvitegravir/cobicistat/ emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for the initial treatment of HIV infection. AIDS. 2011;25:F7–12. [PubMed: 21412057](Controlled trial of at least 48 weeks of elvitegravir with cobicistat versus efavirenz, both combined with emtricitabine and tenofovir in 71 treatment naive adults with HIV infection; found similar efficacy and safety; no mention of ALT elevations or hepatotoxicity).
- Molina JM, Lamarca A, Andrade-Villanueva J, Clotet B, Clumeck N, Liu YP, Zhong L, et al. Study 145 Team. Efficacy and safety of once daily elvitegravir versus twice daily raltegravir in treatment-experienced patients with HIV-1 receiving a ritonavir-boosted protease inhibitor: randomised, double-blind, phase 3, non-inferiority study. Lancet Infect Dis. 2012;12:27–35. [PubMed: 22015077](Controlled trial of elvitegravir vs raltegravir, both combined with a ritonavir boosted protease inhibitor and a second antiretroviral agent in 702 treatment experienced patients with HIV infection; found similar efficacy and safety; 2 patients on elvitegravir and 5 on raltegravir stopped therapy because of acute hepatitis, but details were not given; ALT elevations above 5 times the ULN occurred in 2% of patients on elvitegravir versus 5% on raltegravir).
- Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, Gallant JE, et al. GS-US-236-0102 study team. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–48. [PubMed: 22748591](Controlled trial of at least 48 weeks of elvitegravir/cobicistat vs efavirenz, combined with emtricitabine and tenofovir in 700 treatment naive patients with HIV infection; found similar efficacy and safety; side effects included diarrhea, nausea, fatigue, dizziness, headache and rashes leading to discontinuation in 4% of patients; ALT elevations occurred in 15% of elvitegravir treated subjects; no mention of clinically apparent liver injury).
- DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, Ramanathan S, Wei X, et al. GS-236-0103 Study Team. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–38. [PubMed: 22748590](Controlled trial of at least 48 weeks of elvitegravir/cobicistat versus atazanavir/ritonavir, combined with emtricitabine and tenofovir in 708 treatment naive patients with HIV infection; found similar efficacy and safety; ALT elevations occurred in 15% of elvitegravir treated patients, but "patients with clinically significant liver function test abnormalities generally had concurrent underlying hepatic disease").
- Olin JL, Spooner LM, Klibanov OM. Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil Fumarate Single Tablet for HIV-1 Infection Treatment. Ann Pharmacother. 2012;46:1671–7. [PubMed: 23136357](Systematic review of pharmacology, safety and efficacy of the elvitegravir based 4 drug regimen for HIV infection; the most common side effects were diarrhea, nausea, abnormal dreams, depression, ALT elevations [15%] and renal abnormalities [1%]).
- Wills T, Vega V. Elvitegravir: a once-daily inhibitor of HIV-1 integrase. Expert Opin Investig Drugs. 2012;21:395–401. [PubMed: 22321026](Review of the mechanism of action, pharmacokinetics, efficacy and safety of elvitegravir; no discussion of ALT elevations or hepatotoxicity).
- Lee FJ, Carr A. Tolerability of HIV integrase inhibitors. Curr Opin HIV AIDS. 2012;7:422–8. [PubMed: 22886031](Review of safety and adverse events associated with HIV integrase inhibitors – raltegravir, elvitegravir and dolutegravir – focusing largely on myopathy, renal dysfunction and serum lipid abnormalities; no discussion of ALT elevations or hepatotoxicity).
- A 4-drug combination (Stribild) for HIV. Med Lett Drugs Ther. 2012;54(1404):95–6. [PubMed: 23183388](Concise review of the efficacy and safety of Stribild, shortly after its approval in the US; does not mention ALT elevations or hepatotoxicity).
- Zolopa A, Sax PE, DeJesus E, Mills A, Cohen C, Wohl D, Gallant JE, et al. GS-US-236-0102 Study Team. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/ emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;63:96–100. [PubMed: 23392460](Analysis of the second year of study described by Sax et al. [2012] found no new safety concerns; no mention of ALT elevations or hepatotoxicity).
- Rockstroh JK, DeJesus E, Henry K, Molina JM, Gathe J, Ramanathan S, Wei X, et al. GS-236-0103 Study Team. A randomized, double-blind comparison of coformulated Evitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;62:483–6. [PubMed: 23337366](Analysis of the second year of study described by DeJesus et al. [2012] found no new safety concerns; no mention of ALT elevations or hepatotoxicity).
- Surgers L, Lacombe K. Hepatoxicity of new antiretrovirals: a systematic review. Clin Res Hepatol Gastroenterol. 2013;37:126–33. [PubMed: 23522569](Systematic review of hepatotoxicity of new antiretroviral agents including etravirine, rilpivirine, maraviroc, raltegravir, elvitegravir, dolutegravir and darunavir; in 3 large clinical trials of elvitegravir vs raltegravir or efavirenz-based regimens, ALT elevations were less frequent with elvitegravir than raltegravir [15% vs 22%] or efavirenz [18% vs 31%]).
- Genvoya--a new 4-drug combination for HIV. Med Lett Drugs Ther. 2016;58(1488):19–21. [PubMed: 26859659](Concise review of the mechanism of action, clinical efficacy, safety and costs of Genvoya, a single tablet regimen of elvitegravir, tenofovir alafenamide, emtricitabine and cobicistat as therapy of HIV infection, mentions the advantage of tenofovir alafenamide in having less renal and bone toxicity than the disoproxil fumarate and gives the standard warnings about immune reconstitution syndrome with potential liver injury on starting, and reactivation of hepatitis B on stopping antiretroviral reigimens).
- Squillace N, Ricci E, Quirino T, Gori A, Bandera A, Carenzi L, De Socio GV, et al. CISAI Study Group. Safety and tolerability of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate in a real life setting: data from surveillance cohort long-term toxicity antiretrovirals/antivirals (SCOLTA) project. PLoS One. 2017;12(6):e0179254. [PMC free article: PMC5478131] [PubMed: 28632758](Among 280 patients with HIV infection started on therapy with elvitegravir/cobicistat, emtricitabine and tenofovir and monitored for a median of 11 months, serum enzyme elevations occurred in 23 subjects [8%], but most were less than 5 times the upper limit or normal and only 3 led to treatment interruptions).