This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Show detailsIntroduction
The sensory system receives and processes information that generates an individual's awareness of their environment. Various sensory perceptions then influence voluntary and involuntary motor activity to facilitate interaction with the world.[1]
Broadly, sensations fall into two categories: General and special senses. General senses include touch, pain, temperature, proprioception, vibration, and pressure. Special senses include vision, hearing, taste, and smell. Special senses are processed via cranial nerves and differ from the pathway utilized in processing general senses. This article is focused on the discussion of the general senses.
Both innocuous and harmful mechanical and thermal stimuli are processed by different receptors and nerve fiber types in the skin, follow specific ascending pathways through the spinal cord (dorsal column or spinothalamic tract), and eventually relay to the somatosensory cortex.[2] Based on these different pathways, the clinical picture of patients presenting with sensory abnormalities can help elucidate the suspected location of a spinal cord or central lesion based on the anatomical region and the type of sensation affected.[1]
Cellular Level
Different receptor types and free nerve endings, each with their own specific function, generate the overall sense of touch, position in space, and pain. For the purposes of this article, “mechanoreceptor” refers to the mechanosensory end-organ structure and its associated low-threshold mechanoreceptor (LTMR).[3]
Mechanoreceptors (Pressure, Vibration, and Proprioception)
Non-painful mechanical stimuli compose the sense of touch via low-threshold mechanoreceptors. Four main types of receptors are responsible for sensing light pressure, firm pressure, dynamic pressure, vibration, and relative position.
- Merkel Discs
Merkel discs are found in both hairy and non-hairy (glabrous) skin. They are heavily concentrated at the fingertips and basal layer of the epidermis and have large myelinated axons. Merkel’s discs facilitate the detection of pressure, static touch, corners, edges, curvatures, and position sense.[3] Merkel discs are associated with slow-adapting type 1 receptors.[4]
- Ruffini Endings
Ruffini nerve endings (or Ruffini corpuscles) are concentrated at the fingertips but are located throughout the dermis as well as in joints and fascia.[5] Ruffini endings facilitate the detection of stretch, slipping, or sliding of objects across the skin surface and joint angle changes. Ruffini endings are associated with slow-adapting type 2 receptors.[3]
- Meissner Corpuscle
Meissner’s corpuscles are located in the dermal papillae of non-hairy (glabrous) skin. Meissner’s corpuscles have a “coin stack” morphology and facilitate the detection of low-frequency vibrations, fine touch, and fine dynamic skin movement.[6] Meissner corpuscles have large, myelinated axons associated with rapid-adapting type 1 receptors.[3]
- Pacinian Corpuscle
Pacinian corpuscles are located in the deep dermis, especially throughout the palm and fingertips, as well as ligaments and joints.[5] Pacinian corpuscles are large onion-like structures surrounding a nerve ending and facilitate the detection of high-frequency vibration and pressure. Pacinian corpuscles have large myelinated axons associated with rapid-adapting type 2 receptors.[3]
- Muscle Spindle Mechanoreceptors and Golgi Tendon Organs
Muscle spindle mechanoreceptors are located in skeletal muscle bellies, and Golgi tendon organs are located within skeletal muscle-tendon junctions. These receptors facilitate the detection of limb position and movement, referred to as proprioception. These receptors are activated by muscle stretch and contractile forces.[7]
Nociceptors (Pain) and Thermoreceptors (Temperature)
Noxious stimulation, such as intense heat, cold, or chemical exposure, is converted into an electric signal by free nerve endings that terminate in the dermis and epidermis and have a pseudounipolar morphology. These nerve endings are also present on some viscera and can be further classified into A-delta fibers or C-fibers.
A-delta fiber nerve endings branch in clusters to allow for a more precise receptive field; they are mostly myelinated except at the terminals and are considered medium in diameter with fast signal conductors. “Fast” pain is sometimes referred to as “first” pain, as in the immediate pain felt when pricked by a pin.
On the other hand, C-fibers are spread further apart and lack precision, are unmyelinated with smaller diameters and therefore are slower in signal conduction. “Slow” pain is sometimes referred to as “second” pain, as in the slightly delayed ache felt after stubbing a toe.[8][9]
Nerve Fiber Classification
The nerve fiber types along which action potentials propagate are classified according to diameter and conduction velocity, with an Erlanger Gasser classification (A-C) as well as a numerical classification (I-IV). From A (largest, myelinated, fastest) to C (smallest, unmyelinated, slowest), nerve fiber diameter decreases, as does relative conduction velocity.
- Group I or A-alpha fibers: Ia fibers transmit proprioceptive input from primary endings of muscle spindles. Ib fibers transmit proprioceptive input from Golgi tendon organs.
- Group II or A-beta fibers: Transmit proprioceptive input from secondary endings of muscle spindles and touch and pressure input from specialized receptors in the skin and deep tissues (Merkel discs, Ruffini endings, Meissner corpuscles, and Pacinian corpuscles).
- Group III or A-delta fibers: Transmit “fast” pain, cold, pressure, and touch from the skin and visceral afferents.
- Group IV or C fibers: Transmit “slow” pain, heat, and pressure from the skin, muscle, and visceral afferents.[10]
Neurotransmitters
Sensory stimuli initiate action potentials and are considered excitatory. Glutamate is the primary excitatory neurotransmitter and, therefore, pivotal to the sensory system. Substance P and calcitonin-gene-related peptide (CGRP) also serve an important role in transmitting painful stimuli via A-delta and C nerve fibers.[9][11]
Development
The embryological ectoderm gives rise to the sensory system. Surface ectoderm generates the epidermis, neural ectoderm generates the brain and spinal cord, and neural crest generates the dorsal root ganglia and peripheral nervous system.[12]
The cortex derives from the telencephalon of the embryologic brain vesicles, the thalamus derives from the diencephalon, and the midbrain derives from the mesencephalon.[13]
Organ Systems Involved
As discussed in this article, the sensory system involves the integumentary, peripheral, and central nervous systems.
Mechanism
Ascending Pathways
The sensory system follows two ascending pathways from the periphery to the central nervous system. Primary somatosensory neurons in both pathways are pseudounipolar. Sensory cell bodies are located in the dorsal root ganglion, with one peripheral axon that receives sensory input and one central axon that travels to the spinal cord.[8]
1) Spinothalamic Pathway
The spinothalamic tract (STT) is further divided into the lateral spinothalamic tract, which transmits pain and temperature, and the anterior spinothalamic tract, which transmits touch and pressure. Primary neurons of the STT have a peripheral axon with either A-delta or C-fibers, a cell body in the dorsal root ganglion, and a central axon that enters the ipsilateral spinal cord. The primary synapse in the gray matter of the ipsilateral spinal cord transmits the action potential to a second-order neuron that crosses (decussates) at the anterior white commissure approximately two vertebral levels above the level of the sensory input. The STT ascends along the contralateral spinal cord until synapsing in the thalamus in the ventral posterolateral nucleus (VPL) to a third-order neuron in the sensory cortex.[9]
2) Dorsal Column Pathway
The dorsal column medial lemniscus (DCML) pathway transmits pressure, vibration, fine touch, and proprioception. Primary neurons have a peripheral axon with specialized sensory endings such as mechanoreceptors discussed previously, a cell body in the dorsal root ganglion, and a central axon that enters the ipsilateral dorsal columns of the spinal cord and ascends to the brainstem where the action potential is transmitted to a second order neuron in the nucleus cuneatus (for the upper body) or nucleus gracilis (for the lower body) of the medulla. The second-order neuron crosses (decussates) in the medulla and continues to ascend along the contralateral medial lemniscus until synapsing in the thalamus in the VPL to a third-order neuron in the sensory cortex.[14]
Thalamus
Although the functions of thalamic nuclei may not be as discrete as previously thought, the general consensus regarding sensory processing holds that sensations other than olfaction are primarily relayed through particular thalamic nuclei. The ventral posterolateral nucleus (VPL) and ventral posteromedial nucleus (VPM) relay bodily sensation, facial sensation, and taste to the parietal lobe's somatosensory cortex. The lateral geniculate nucleus (LGN) relays visual input to the calcarine sulcus. The medial geniculate nucleus (MGN) relays auditory input to the auditory cortex of the temporal lobe.[15]
Sensory Cortex
The second-order neurons of both the STT and DCML synapse at the VPL of the thalamus. From the thalamus, third-order neurons from the internal capsule travel to the somatosensory cortex. The somatosensory cortex is in the post-central gyrus of the parietal lobe of the brain and processes sensory input from the contralateral body with a somatotopic organization in which the foot, leg, trunk, forelimbs, and face are sequentially represented from medial to lateral.[16][17]
The somatosensory cortex is subdivided into Brodmann areas 1, 2, 3a, and 3b with seemingly reciprocal connections. Area 3b is considered the primary somatosensory cortex in which cutaneous tactile stimuli are processed. Area 1 also responds to cutaneous tactile stimuli but is thought to be more specific to texture perception. Area 3a mostly processes proprioception from joint and muscle stretch and potentially heat-induced pain. Area 2 processes deep cutaneous stimuli and proprioception.[18]
Additional Processing of Pain
While the somatosensory cortex processes the type, location, and intensity of painful stimuli, painful stimuli also have an affective component (fear and aversion). Pain is also processed through the brainstem parabrachial nucleus and amygdala and transmitted to the cingulate gyrus and insular cortex.[9][19]
Related Testing
The most important and ubiquitous test of the sensory system is the physical and neurological exam. A detailed neurologic exam helps physicians determine when further testing or diagnostic modalities are indicated, such as skin biopsy to assess nerve fiber density or radiographic studies of the spine or brain.
Clinical Significance
Understanding the sensory system and the ascending pathways to the brain provides the foundation for understanding certain clinical conditions. The conditions listed below are not described extensively; their relation to the sensory system is highlighted.
Lateral hemisection of the spinal cord (Brown-Sequard injury) results in the ipsilateral loss of all sensations at the dermatome corresponding to the level of the injury. Damage to the ascending sensory tracts causes the loss of ipsilateral proprioception, tactical sensation, and vibration below the level of the injury (dorsal columns) and loss of contralateral nociception and temperature sensation below the level of the injury (spinothalamic tract).[20]
Demyelination of the dorsal columns due to vitamin B12 deficiency or traumatic injury to the posterior spinal cord, thereby affecting the dorsal columns, results in bilateral impairment of proprioception and vibration below the level of the injury. Because the spinothalamic tract is not involved, pain and temperature sensations are intact.[20]
Trauma to the central cord or compression of the central cord from an expanding syrinx impacts the anterior white commissure where the second-order neurons of the spinothalamic tract cross. Clinically, this can include bilateral loss of pain and temperature sensation.[21]
Tabes dorsals is a manifestation of tertiary syphilis in which demyelination of the dorsal columns and dorsal root ganglion lead to impaired proprioception and sensation, resulting in ataxia.[22]
The symptoms of a parietal brain lesion (due to trauma, neoplasm, infarct, infection, etc.) within the somatosensory cortex can provide clues to its location due to the somatotopic organization of the somatosensory cortex.[16]
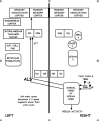
Figure
The diagram illustrates the spinothalamic tract pathway as part of the anteriolateral system Contributed by Campbell University School Of Osteopathic Medicine
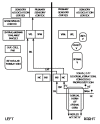
Figure
This diagram illustrates the pathway of the dorsal column medial lemniscus (posterior column pathway) in a schematic fashion Contributed and Used with Permission from Campbell University School Of Osteopathic Medicine

Figure
Sensory Homunculus. The illustration is a sensory homunculus of the primary somatosensory cortex. OpenStax, Public Domain, via Wikimedia Commons

Figure
Diagram of the different mechanoreceptors located throughout the layers of the skin. Contributed by Rian Kabir, MD
References
- 1.
- Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013 Aug 21;79(4):618-39. [PMC free article: PMC3811145] [PubMed: 23972592]
- 2.
- Bishop B. Pain: its physiology and rationale for management. Part I. Neuroanatomical substrate of pain. Phys Ther. 1980 Jan;60(1):13-20. [PubMed: 6243183]
- 3.
- Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science. 2014 Nov 21;346(6212):950-4. [PMC free article: PMC4450345] [PubMed: 25414303]
- 4.
- Feng J, Hu H. A novel player in the field: Merkel disc in touch, itch and pain. Exp Dermatol. 2019 Dec;28(12):1412-1415. [PMC free article: PMC6800577] [PubMed: 31001848]
- 5.
- Suarez-Rodriguez V, Fede C, Pirri C, Petrelli L, Loro-Ferrer JF, Rodriguez-Ruiz D, De Caro R, Stecco C. Fascial Innervation: A Systematic Review of the Literature. Int J Mol Sci. 2022 May 18;23(10) [PMC free article: PMC9143136] [PubMed: 35628484]
- 6.
- Suazo I, Vega JA, García-Mesa Y, García-Piqueras J, García-Suárez O, Cobo T. The Lamellar Cells of Vertebrate Meissner and Pacinian Corpuscles: Development, Characterization, and Functions. Front Neurosci. 2022;16:790130. [PMC free article: PMC8959428] [PubMed: 35356056]
- 7.
- Oliver KM, Florez-Paz DM, Badea TC, Mentis GZ, Menon V, de Nooij JC. Molecular correlates of muscle spindle and Golgi tendon organ afferents. Nat Commun. 2021 Mar 01;12(1):1451. [PMC free article: PMC7977083] [PubMed: 33649316]
- 8.
- Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest. 2010 Nov;120(11):3760-72. [PMC free article: PMC2964977] [PubMed: 21041958]
- 9.
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009 Oct 16;139(2):267-84. [PMC free article: PMC2852643] [PubMed: 19837031]
- 10.
- Catala M, Kubis N. Gross anatomy and development of the peripheral nervous system. Handb Clin Neurol. 2013;115:29-41. [PubMed: 23931773]
- 11.
- Hübel N, Hosseini-Zare MS, Žiburkus J, Ullah G. The role of glutamate in neuronal ion homeostasis: A case study of spreading depolarization. PLoS Comput Biol. 2017 Oct;13(10):e1005804. [PMC free article: PMC5655358] [PubMed: 29023523]
- 12.
- Mazet F. The evolution of sensory placodes. ScientificWorldJournal. 2006 Apr 04;6:1841-50. [PMC free article: PMC5917201] [PubMed: 17205191]
- 13.
- Guy B, Zhang JS, Duncan LH, Johnston RJ. Human neural organoids: Models for developmental neurobiology and disease. Dev Biol. 2021 Oct;478:102-121. [PMC free article: PMC8364509] [PubMed: 34181916]
- 14.
- Attwell CL, van Zwieten M, Verhaagen J, Mason MRJ. The Dorsal Column Lesion Model of Spinal Cord Injury and Its Use in Deciphering the Neuron-Intrinsic Injury Response. Dev Neurobiol. 2018 Oct;78(10):926-951. [PMC free article: PMC6221129] [PubMed: 29717546]
- 15.
- Roy DS, Zhang Y, Halassa MM, Feng G. Thalamic subnetworks as units of function. Nat Neurosci. 2022 Feb;25(2):140-153. [PMC free article: PMC9400132] [PubMed: 35102334]
- 16.
- Sun F, Zhang G, Ren L, Yu T, Ren Z, Gao R, Zhang X. Functional organization of the human primary somatosensory cortex: A stereo-electroencephalography study. Clin Neurophysiol. 2021 Feb;132(2):487-497. [PubMed: 33465535]
- 17.
- Martuzzi R, van der Zwaag W, Dieguez S, Serino A, Gruetter R, Blanke O. Distinct contributions of Brodmann areas 1 and 2 to body ownership. Soc Cogn Affect Neurosci. 2015 Nov;10(11):1449-59. [PMC free article: PMC4631141] [PubMed: 25809404]
- 18.
- Delhaye BP, Long KH, Bensmaia SJ. Neural Basis of Touch and Proprioception in Primate Cortex. Compr Physiol. 2018 Sep 14;8(4):1575-1602. [PMC free article: PMC6330897] [PubMed: 30215864]
- 19.
- Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM. Parabrachial Complex: A Hub for Pain and Aversion. J Neurosci. 2019 Oct 16;39(42):8225-8230. [PMC free article: PMC6794922] [PubMed: 31619491]
- 20.
- McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30(3):215-24. [PMC free article: PMC2031952] [PubMed: 17684887]
- 21.
- Divi SN, Schroeder GD, Mangan JJ, Tadley M, Ramey WL, Badhiwala JH, Fehlings MG, Oner FC, Kandziora F, Benneker LM, Vialle EN, Rajasekaran S, Chapman JR, Vaccaro AR. Management of Acute Traumatic Central Cord Syndrome: A Narrative Review. Global Spine J. 2019 May;9(1 Suppl):89S-97S. [PMC free article: PMC6512200] [PubMed: 31157150]
- 22.
- Hobbs E, Vera JH, Marks M, Barritt AW, Ridha BH, Lawrence D. Neurosyphilis in patients with HIV. Pract Neurol. 2018 Jun;18(3):211-218. [PubMed: 29478035]
Disclosure: Mahesh Gadhvi declares no relevant financial relationships with ineligible companies.
Disclosure: Marlyn Moore declares no relevant financial relationships with ineligible companies.
Disclosure: Muhammad Waseem declares no relevant financial relationships with ineligible companies.
- Physiology, Sensory System - StatPearlsPhysiology, Sensory System - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
See more...
