Abbreviations
- AGREE
Appraisal of Guidelines for Research & Evaluation
- AMSTAR
Assessing the Methodological Quality of Systematic Reviews
- CAUTI
Catheter-associated urinary tract infection
- CI
Confidence interval
- CLABSI
Central line-associated bloodstream infection
- C. difficile
Clostridium difficile
- CPA
Carbapenemase-producing Acinobacter
- CPE
Carbapenemase-producing Enterobacteriaceae
- CT
Clinical trial
- HAI
Healthcare-acquired infection
- HTA
Health technology assessment
- ICU
Intensive care unit
- IRR
Incidence rate ratio
- JBI
Joanna Briggs Institute
- MA
Meta-analysis
- MDRO
Multidrug-resistant organisms
- MRSA
Methicillin-resistant Staphylococcus aureus
- NA
Not applicable
- NR
Not reported
- OR
Odds Ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PX
Pulsed xenon
- RCT
Randomized controlled trial
- RR
Relative risk
- SD
Standard deviation
- SR
Systematic reviews
- UV
Ultraviolet
- VAP
Ventilator-associated pneumonia
- VRE
Vancomycin-resistant enterococci
Context and Policy Issues
Healthcare-acquired infections (HAIs), also known as nosocomial infections, are infections that patients acquire during their presence in a healthcare setting such as hospitals, long-term care facilities, clinics or home care services.1 At any given time in Canada, about 10% of adults and 8% of children have nosocomial infections.2 According to a 2013 Public Health Agency of Canada report, over 200,000 Canadians acquire HAIs each year, and about 8,000 of these patients die as a result of infection.3 HAIs can be caused by all types of microorganisms, including bacteria, viruses, or fungi that are present in the environment of hospitals and healthcare facilities. Common nosocomial infection microorganisms that are currently monitored by the Canadian Nosocomial Infection Surveillance Program include Clostridium difficile (C. difficile), vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), carbapenemase-producing Enterobacteriaceae (CPE) and carbapenemase-producing Acinetobacter (CPA).4 These microorganisms can survive for weeks on environmental surfaces or become airborne which serve as sources of transmission.5 Transmission of HAIs commonly occurs by direct contact with the contaminated environmental surfaces or through hospital staff or visitors who act as carriers.6 Thus, hand hygiene, proper cleaning of equipment and environments in the healthcare facilities, and monitoring infection are necessary to reduce HAIs, and to prevent the spread of pathogenic organisms.4
There are policies and best practice recommendations that describe the types of disinfectants and detailed protocols and procedures for routine and terminal cleaning and disinfection of the environments in healthcare settings.2 Given the increased awareness of the heterogeneity of the standard environmental cleaning and disinfection practices, whose outcomes are often suboptimal, several automated (non-manual) technologies including hydrogen peroxide (e.g., vapors or dry aerosols), and ultraviolet (UV) irradiation devices (e.g., continuous UV-C light, pulsed xenon UV light) have been developed for use in conjunction with the standard manual cleaning and disinfection.7 Two types of non-manual UV devices or units have been used for disinfection of air and surfaces in healthcare facilities.8,9 For air disinfection, the units can be either portable or housed atop a standard light fixture, and contain a fully shielded chamber with UV-C light bulb to prevent UV leakage, and fans, which draw air into the UV chamber through a filter and push air out into the occupied rooms.8 For surface disinfection, the devices are usually portable with UV-C light or pulsed xenon UV light, and can be placed in patient rooms after patient discharge and standard manual cleaning and disinfection.9 These new technologies have been demonstrated to be effective against pathogens (e.g., C. difficile, VRE, MRSA, and CPE) in healthcare facility environments.7,10,11 However, their clinical effectiveness in improving patient outcomes (e.g., reducing the rates of colonization and HAI) is less understood.
The aim of this report is to review the clinical effectiveness and evidence-based guidelines on the use of non-manual ultraviolet light disinfection for reducing rates of infection and colonization in healthcare facilities.
Research Questions
What is the clinical effectiveness of non-manual ultraviolet light disinfection for reducing rates of infection and colonisation in healthcare facilities?
What is the comparative clinical effectiveness of non-manual ultraviolet light disinfection methods versus accelerated hydrogen peroxide for reducing rates of infection and colonisation in healthcare facilities?
What are the evidence-based guidelines regarding ultraviolet light disinfection methods for reducing rates of infection and colonisation in healthcare facilities?
Key Findings
Low to very low quality evidence from inconsistent and mixed findings precludes a definitive conclusion regarding the clinical effectiveness of non-manual ultraviolet light disinfection in both air and surface for reducing rates of infection and colonization in healthcare facilities. Evidence regarding the comparative clinical effectiveness between non-manual ultraviolet light disinfection methods and accelerated hydrogen peroxide for reducing healthcare-acquired infections was not identified. The Health Quality Ontario guideline recommends against public funding of portable ultraviolet light surface-disinfecting devices for prevention of healthcare-acquired infections.
Methods
Literature Search Methods
A limited literature search was conducted on key resources including Medline, CINAHL, the Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. No filters were applied to limit the retrieval by study type. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2009 and March 8, 2019. Internet links were provided, where available.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Studies were excluded if they did not meet the selection criteria in and if they were published prior to 2009. Primary studies were excluded if they had been included in the identified SRs. Studies that did not report patient outcomes (e.g. HAIs) were excluded. Guidelines with unclear methodology or that were not clearly evidence-based were also excluded.
Critical Appraisal of Individual Studies
The AMSTAR-2 checklist was used to assess the quality of SRs.12 The critical appraisal checklists of Joanna Briggs Institute were used to assess the quality of the included RCTs and non-randomized studies.13 The quality of the evidence-based guidelines was assessed using AGREE II instrument.14 Summary scores were not calculated for the included studies; rather, a review of the strengths and limitations were described narratively.
Summary of Evidence
Quantity of Research Available
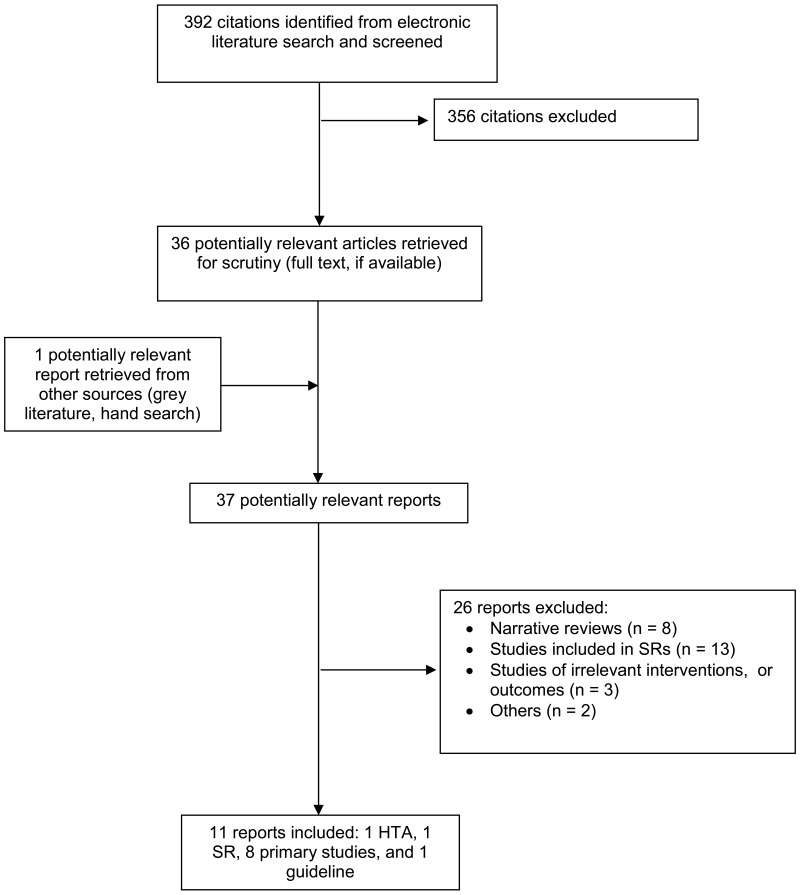
A total of 392 citations were identified in the literature search. Following screening of titles and abstracts, 356 citations were excluded and 36 potentially relevant reports from the electronic search were retrieved for full-text review. One potentially relevant publication was retrieved from the grey literature search. Of the 37 potentially relevant articles, 26 publications were excluded for various reasons, while 11 publications including one HTA, one SR, eight primary studies, and one guideline met the inclusion criteria and were included in this report. Appendix 1 presents the PRISMA flowchart of the study selection.
Summary of Study Characteristics
Systematic reviews and primary studies
The characteristics of the identified HTA15 and SR16 (), and primary studies17–24 () are presented in Appendix 2.
Study Design
The identified HTA15 and SR16 included RCTs and non-randomized studies. The literature search of major databases was from inception to January 23, 2017,15 or from inception to April 30, 2017.16
Eight additional primary studies were identified including one single-blinded, parallel RCT,22 two non-randomized studies with control,20,23 and five non-randomized studies of pre-post design.17–19,21,24
Country of Origin and Publication Year
The HTA15 was conducted by authors from Ontario, Canada. The SR16 was conducted by authors from USA. Both were published in 2018.
Seven identified primary studies17–21,23,24 were conducted by authors from USA, and one study22 was from Spain. Seven studies17–23 were published in 2018, and one24 in 2011.
Study Setting, Target Rooms and Timing after Disinfection
The HTA15 and SR16 included studies assessing the intervention in the hospital setting. Various types of hospital were studied including community, academic, military, acute care and long-term care. The intervention sites were patient rooms, including bathrooms, and rooms in the intensive care units (ICUs) and non-ICUs. The year of intervention ranged from 2011 to 2014. The intervention was conducted after patients were discharged or transferred to other units.
The additional primary studies also assessed the intervention in various types of hospital setting, including tertiary-care, the Women and Children hospital, community, academic, and long-term acute care settings. The intervention sites were patient rooms, including bathrooms and common areas, in ICUs and non-ICUs. For surface disinfection,17–20 intervention was conducted after patients were discharged or transferred to other units. For air disinfection or purification,21–24 the intervention was applied while patients and staff were present.
Interventions and Comparators
Both the HTA15 and SR16 assessed the effectiveness of non-manual UV light surface-disinfecting devices for reducing HAIs. The UV devices were used in conjunction with standard hospital room cleaning and disinfection (i.e., manual cleaning), and were compared with manual cleaning done in the control groups or in the period before the intervention. Pulsed xenon UV devices and mercury bulb UV-C devices were included.
In the additional primary studies, four studies17–20 assessed non-manual UV devices (pulsed xenon or UV-C) for surface disinfection, and four studies21–24 assessed non-manual UV devices (UV-C) for air disinfection, in which the devices have a fully shielded UV-C bulb and fans that draw air in and out the irradiation chamber. All UV devices were used in conjunction with housekeeping protocols and standard manual cleaning established in the hospitals. Comparators were manual cleaning done in the control groups or in the period before the intervention. None of the studies reported detailed procedures of manual cleaning.
Outcomes
Both the HTA15 and SR16 evaluated HAIs as the outcome. Multidrug-resistant organisms included C. difficile, VRE, MRSA, and others.
The outcomes investigated in the additional primary studies included HAI or colonization, ventilator-associated pneumonia (VAP), catheter-associated urinary tract infection (CAUTI), central line-associated bloodstream infection (CLABSI), viral infection, length of hospital stay, and 30-day mortality. Common multidrug-resistant organisms investigated were C. difficile and VRE.
Treatment Duration
In the HTA,15 the length of application of the intervention was 7 months in the RCT, or ranged from 3 months to 27 months in the non-randomized studies. The periods before intervention in the non-randomized studies ranged from 3 months to 3 years. Length of application was not reported in the SR.16 For surface disinfection, the duration of UV treatment varied and was reported in two studies,17,18 but not in the others.19,20 For air disinfection, the UV unit was left running continuously in the occupied rooms.21–24
In the additional primary studies, the study periods in the controlled studies were 6 months20,23 and 5 years.22 For the non-randomized studies of pre-post design, the periods before intervention ranged from 6 months to 19 months, and the periods of intervention ranged from 6 months to 18 months.
Quality Appraisal Tools
The authors of the HTA15 assessed the quality of the included studies using the Cochrane Risk of Bias tool for RCTs, Effective Practice and Organization of Care (EPOC) tool for non-RCTs and for interrupted time-series studies, and The National Heart, Lung and Blood Institute quality assessment tool for before-after studies with no control groups. In the HTA, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework was used to evaluate the quality of the body of evidence for each outcome on the basis of the following considerations: risk of bias, inconsistency, indirectness, imprecision, publication bias, magnitude of effect, and dose-response gradient. The authors of the SR16 assessed the quality of the included studies using a published tool25,26 having items regarding sample representatives, bias and confounding, description of the intervention, outcomes and follow-up, and statistical analysis. Each item was scored 1 to 4, with 4 being highest quality.
Data Analysis and Synthesis
Giving the substantially clinical heterogeneity in study design and setting, interventions, comparators, and outcome measures, the authors of the HTA15 decided not to pool the data, but performed a qualitative synthesis of the included studies. The results were summarized and tabulated separately regarding the type of UV devices (i.e., pulsed xenon UV disinfecting devices and UV-C disinfecting devices) and outcome measures. The authors of the SR16 quantitatively synthesized data from included studies using meta-analysis approach, despite clinical heterogeneity. In the SR, subgroup analyses were performed based on baseline C. difficile infection rates, types of hospital, and studies reporting compliance monitoring process.
In the additional primary studies, appropriate statistical methods were used for comparisons of observations between intervention and comparator, or between pre- and post-intervention. The incidence of rate was calculated as number of new infections over the total number of patient days, usually expressed as number of cases per 1,000 patient days. Power analysis was not performed in all studies, except one.17
Funding
Both the HTA15 and SR16 received public funding for their work. Two identified primary studies17,22 were supported by public funding, while the rest of the studies18–21,23,24 did not report the source of funding or received the UV devices from the manufacturers.
Guidelines
The characteristics of the guideline27 are presented in in Appendix 2
Country of Origin
One evidence-based guideline from Health Quality Ontario, Canada27 was identified.
Objectives
The overall objective of the guideline27 was to provide recommendations related to the implementation of portable non-manual UV light surface-disinfecting devices for prevention of HAIs.
Target Users of the Guidelines
The guideline27 was targeted to healthcare professionals and funders, by providing objective advice for improving healthcare of Ontarians.
Methods Used to Formulate Recommendations
The Ontario Health Technology Advisory Committee reviewed the HTA15 conducted by Health Quality Ontario and made recommendations to the Minister of Health and Long-Term Care. The committee consisted of volunteer members across the province, including healthcare experts and patient perspective representatives.
Summary of Critical Appraisal
The quality assessment of the HTA15 and SR16 (), RCT22 (), non-randomized studies17–21,23,24 (), and guideline27 () are presented in Appendix 3.
Both the HTA15 and SR16 provided appropriate research questions, explanations for selection of the study designs for the inclusion, used comprehensive literature search strategies, described the included studies in adequate detail, and used satisfactory techniques for assessing the risk of bias in individual studies included in the review. It was unclear if the review authors of the HTA and SR performed study selection and data extraction in duplicate. Neither reported if the review methods had been established in a protocol prior to the conduct of the review, they did not provided a list of excluded studies, and did not report the source of funding for the included studies. While meta-analysis was performed in the SR,16 the authors of the HTA15 did not perform meta-analysis of the included studies, owing to substantial heterogeneity in study design and setting, interventions, comparators, and outcome measures. The authors of the HTA15 incorporated risk of bias and clinical heterogeneity in individual studies in the discussion and interpretation of the results, while the authors of the SR16 did not. The authors of the SR16 carried out an adequate investigation of publication bias. The HTA15 did not report potential sources of conflict of interest. Overall, the research methodology of the included HTA was more comprehensive and thorough than that of the SR, as it rated the evidence of each outcome using GRADE, and chose not to pool data from included studies due to clinical and methodological heterogeneity across studies.
The RCT22 was explicit in 11 of 13 items of the critical appraisal checklist covering adequate randomization, allocation concealment, similarity in baseline characteristics between groups, participant blinding, identical in treatment between groups other than the intervention of interest, no losses to follow-up, similar outcome measurement for treatment groups using reliable method and appropriate statistical analysis. It was unclear if blinding was applied to those delivering treatment and outcome assessors. The RCT had some risk in performance bias as because only patients were blinded.
All of the additional non-randomized studies with20,23 or without17–19,21,24 a control group provided appropriate research questions and objectives, measured the outcomes of participants in the same and reliable way, using appropriate statistical analysis. In all studies, it was unclear if participants between treatment groups were similar in characteristics, and received similar treatment and care other than the exposure or intervention of interest. It was also unclear if patients were lost to follow-up. Overall, these studies had high risk of bias in selection, performance, and detection.
The included guideline27 was explicit in terms of scope and purpose, stakeholder involvement (engaging healthcare experts and patient perspective representatives), clarity of presentation, and applicability. The guideline was also explicit in terms of rigour of development, except it was unclear if the guideline had been externally reviewed by experts prior to its publication, and whether there is a procedure for updating the guideline. It was also unclear about editorial independence of the guideline regarding potential influence of the funding body to the content of the guideline and competing interests of the guideline development group members.
Summary of Findings
The main findings and conclusions of the HTA15 and SR16 (), additional primary studies17–24 (), and guideline27 () are presented in Appendix 4.
Clinical Effectiveness
Non-manual UV light surface disinfection plus manual disinfection versus manual disinfection alone
Evidence regarding the clinical effectiveness of non-manual UV light surface disinfecting devices used in adjunct to standard hospital room cleaning disinfection (i.e., manual cleaning) compared to manual cleaning alone in reducing HAIs was derived from one HTA,15 one SR16 and four additional primary studies.17–20 The devices were operated after patients were discharged or transferred to other units.
C. difficile infection
The HTA15 included one RCT and two before-after studies for evaluating the use of mercury UV-C surface disinfecting devices on C. difficile infection rates. The RCT found that addition of UV-C room disinfection to standard manual cleaning did not show any reduction in hospital acquired C. difficile infection rates. The quality of this evidence was graded as low. Two pre-post studies reported that the use of UV-C devices in addition to manual cleaning was associated with a reduction in C. difficile infection rates in hospital. The quality of this evidence was graded as very low. The HTA also included six pre-post studies evaluating the use of pulsed xenon UV devices. All point estimates showed a reduction in hospital acquired C. difficile infection rates with the additional use of pulsed xenon UV disinfection, although statistically significant differences were not reached in two studies. The quality of this evidence was graded as very low.
The SR16 performed a meta-analysis of 11 studies, combining all study designs and types of UV devices. Results of the meta-analysis showed that using UV devices for surface disinfection after standard manual cleaning was associated with statistically significant reduction in C. difficile infection rates. In subgroup analyses, the statistically significant reduction in C. difficile infection rates was observed in studies having high baseline C. difficile infection rates (i.e., ≥ 1.5 / 1,000 patient days), but not in studies having low baseline C. difficile infection rates (<1.5 / 1,000 patient days), and in non-controlled studies, but not in controlled trials. Statistically significant reduction in C. difficile infection rates was observed regardless of whether or not studies reported compliance rates.
One pre-post study17 found no significant difference in hospital acquired C. difficile infection rates in a bone marrow transplant unit before and after implementation of standard manual cleaning with pulsed xenon UV surface disinfection. In contrast, another non-randomized study with a control group20 reported a significant reduction in C. difficile infection rates in hematology/bone marrow transplant and medical-surgery units having pulsed xenon UV surface disinfection compared to control units.
VRE infection
The HTA15 included one RCT and one before-after study evaluating the use of mercury UV-C surface disinfecting devices on VRE infection rates. Both studies showed a non-statistically significant reduction in hospital-acquired VRE infection rates with UV-C surface disinfection and standard manual cleaning compared to standard manual cleaning alone. The quality of this evidence was graded as low and very low. The HTA also included two pre-post studies evaluating the use of pulsed xenon UV devices that reported a significant reduction in hospital-acquired VRE infection rates after implementing UV surface disinfection in addition to standard manual cleaning compared to standard manual cleaning alone. The quality of this evidence was graded as very low.
The SR16 performed a meta-analysis on VRE infection rate using data from four studies, and found that the use of UV surface disinfecting devices after standard manual cleaning was associated with statistically significant reduction in hospital-acquired VRE infection rates.
In three additional primary studies, a significant reduction in VRE infection rates was observed in one study,20 but not in the other two,17,19 when evaluating the use of UV surface disinfecting devices (UV-C and pulsed xenon UV) in addition to standard manual cleaning.
MRSA infection
The HTA15 included one RCT and one before-after study evaluating the use of mercury UV-C surface disinfecting devices on MRSA infection rates. Both studies found no statistically significant difference in hospital-acquired MRSA infection rates with the use of UV-C surface disinfection and standard manual cleaning compared to standard manual cleaning alone. The quality of this evidence was graded as low and very low. The HTA also included three pre-post studies evaluating the use of pulsed xenon UV devices. These studies showed inconsistent results. One study reported a significant reduction in MRSA infection rates for pulsed xenon UV disinfection, while the point estimates of the other two studies favored standard manual cleaning. The quality of this evidence was graded as very low.
One additional pre-post study19 reported that the rate of MRSA infection was significantly reduced during UV-C disinfection intervention compared to pre-intervention.
Other HAIs
For mercury UV-C room disinfection, the HTA included one RCT and one pre-post study. The RCT found no cases of multidrug-resistant Acinetobacter infection or colonization after both treatment and control. The quality of this evidence was graded as low. The pre-post study found reduction in relative rates of infection with Acinetobacter baumannii or Klebsiella pneumonia after treated with UV-C disinfection, but the difference did not reach statistical significance. The quality of this evidence was graded as very low. For pulsed xenon UV room disinfection, the HTA included three pre-post studies. One pre-post study found that pulsed xenon UV disinfection significantly reduced Class I, but not Class II surgical site infection. The quality of this evidence was graded as very low. One pre-post study found no significant difference in any other HAI rates including VAP, CAUTI and CLABSI. The quality of this evidence was graded as very low. One pre-post study found that pulsed xenon UV disinfection significantly reduced the rate of multidrug-resistant gram-negative bacteria by 19%. The quality of this evidence was graded as very low.
One additional primary study19 found that UV-C disinfection was associated with significant reduction in relative rates of infection with Acinetobacter baumannii, but not with Klebsiella pneumonia or Pseudomonas aeruginosa. One pre-post study18 found that UV-C surface disinfection was associated with a 44% reduction in viral infection among pediatric patients in a pediatric long-term care facility.
Non-manual UV light air disinfection plus manual disinfection versus manual disinfection alone
The clinical effectiveness of non-manual UV light air disinfecting devices used in adjunct to standard hospital room cleaning disinfection (i.e., manual cleaning) compared to normal and manual cleaning alone in reducing HAIs was derived from four identified primary studies.21–24 The device is either portable or installed in the ceiling, and has a fully shielded chamber with a UV-C bulb and fans that produce continuous air flow in and out of the irradiation chamber. The devices were operated in the presence of patients and staff.
One pre-post study21 found a significant reduction in the overall HAI rates after installation of the UV-C air disinfecting devices in patient rooms of special care unit in a long-term acute care hospital. The overall HAI rate reduction was attributed mainly by the reduction of C. difficile infection and CAUTI, but not of MRSA, VRE or CLABSI.
One prospective, comparative RCT22 found no significant differences between UV-C technology between sterilizer rooms and control rooms in an ICU of cardiac surgery in patient colonization rates (any type of bacteria, gram-positive, gram-negative), HAI rates (total, VAP, urinary tract, catheter, blood, surgical site), ICU stay, total hospital stay and 30-day mortality rate.
One non-randomized study with a control group23 found that UV-C air disinfection in a wing of a long-term care ventilator unit significantly reduced the overall HAI rate (assessed based on antibiotic orders) compared to a control wing. However, no statistically significant differences between groups were observed for infection rates caused by multidrug-resistant organisms such as Acinetobacter, MRSA, VRE and C. difficile.
One pre-post study (one 6-month pre-period, and three consecutive 6-month post-periods), which evaluated the effect of UV-C air sterilizing device in the heating ventilation and air conditioning system on VAP in a neonatal ICU, found significant decrease in number of VAP cases and the number of antibiotics prescribed among high-risk neonatal patients (< 30 weeks gestation and ventilated for ≥ 14 days). However, these reductions were observed in the third 6-month period of the post-intervention, but not during the first and second 6-month periods. Similarly, the number of high-risk babies also dropped over time and significantly decreased in the third 6-month period of the post-intervention compared to pre-intervention. This situation could not rule out the possibility that VAP might have decreased over time because of reasons other than UV-C air sterilization.
Non-manual UV light disinfection versus accelerated hydrogen peroxide disinfection
No evidence could be identified for the comparative clinical effectiveness of non-manual UV light disinfection versus accelerated hydrogen peroxide disinfection for reducing rates of infection and colonization in healthcare facilities.
Guidelines
Based on the findings of its HTA,15 the Health Quality Ontario guideline27 recommends against public funding of portable of portable UV light surface-disinfecting devices for prevention of HAIs, as it was unclear if the technology is better than hospital standard cleaning and disinfection.
Limitations
The quality of evidence derived from primary studies included in the identified HTA and SR, as well as of those additionally identified studies in this report, were considered to be low to very low. Most studies were of pre-post design, of which confounding variables such as patient characteristics, infection control practices, and methods of delivery of care between before- and after-intervention periods were not identified and controlled. As the study investigators and outcomes assessors were not blinded and the hospital manual cleaning protocols were not often described, it was unclear if the reduction of HAIs reported in some studies was actually associated with implementation of non-manual UV devices. Given substantial clinical and methodological heterogeneity among studies, regarding patient characteristics, settings, hospital types and units, target rooms, types of UV devices, and manufacturer’s disinfecting protocols, the authors of the HTA decided not to combine data from included studies, while meta-analysis was conducted by the authors of the SR. Despite the difference in data analysis, both HTA and SR found that the statistically significant reduction in C. difficile infection rates associated with UV surface disinfection was observed in non-controlled studies (i.e., pre-post studies), but not in controlled trials. Mixed findings were also observed among additional identified studies regarding the use of non-manual UV devices for surface or air disinfection. The conclusions of studies18–21,23,24 having some connection with the manufacturers were in favor of the UV devices, while those17,22 receiving public funding did not find any additional benefit in reducing HAIs compared to standard manual cleaning. As most studies, including those of the HTA and SR, were conducted in the US and no studies were from Canada, the findings could not be generalizable to the Canadian context, as it is difficult to know if the treatment practices and manual cleaning and disinfection protocols are similar among hospitals in US and Canada. This review could not identify any studies comparing non-manual disinfecting methods with accelerated hydrogen peroxide systems for reducing HAIs.
Conclusions and Implications for Decision or Policy Making
Given the study limitations, the clinical effectiveness of non-manual UV light disinfection for reducing HAIs remains inconclusive. In addition, the Health Quality Ontario guideline does not recommend public funding of portable UV light surface-disinfecting devices for prevention of HAIs, as it was uncertain if UV technology used in conjunction with standard manual cleaning and disinfection is better than standard manual cleaning and disinfection alone for preventing HAIs. Future controlled trials with high degree of internal validity and power analysis would reduce the uncertainty regarding the effectiveness of this technology.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
Dancer
SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination.
Clin Microbiol Rev. 2014;27(4):665–690. [
PMC free article: PMC4187643] [
PubMed: 25278571]
- 8.
Memarzadeh
F, Olmsted
RN, Bartley
JM. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: effective adjunct, but not stand-alone technology.
Am J Infect Control. 2010;38(5 Suppl 1):S13–24. [
PMC free article: PMC7115255] [
PubMed: 20569852]
- 9.
- 10.
Doll
M, Morgan
DJ, Anderson
D, Bearman
G. Touchless Technologies for Decontamination in the Hospital: a Review of Hydrogen Peroxide and UV Devices.
Curr Infect Dis Rep. 2015;17(9):498. [
PubMed: 26252970]
- 11.
Weber
DJ, Rutala
WA, Anderson
DJ, Chen
LF, Sickbert-Bennett
EE, Boyce
JM. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials.
Am J Infect Control. 2016;44:(5 Suppl):e77–84. [
PMC free article: PMC7132689] [
PubMed: 27131140]
- 12.
- 13.
- 14.
- 15.
Health Quality O. Portable Ultraviolet Light Surface-Disinfecting Devices for Prevention of Hospital-Acquired Infections: A Health Technology Assessment.
Ont Health Technol Assess Ser. 2018;18(1):1–73. [
PMC free article: PMC5824029] [
PubMed: 29487629]
- 16.
Marra
AR, Schweizer
ML, Edmond
MB. No-Touch Disinfection Methods to Decrease Multidrug-Resistant Organism Infections: A Systematic Review and Meta-analysis.
Infect Control Hosp Epidemiol. 2018;39(1):20–31. [
PubMed: 29144223]
- 17.
Brite
J, McMillen
T, Robilotti
E, et al. Effectiveness of ultraviolet disinfection in reducing hospital-acquired Clostridium difficile and vancomycin-resistant Enterococcus on a bone marrow transplant unit.
Infect Control Hosp Epidemiol. 2018;39(11):1301–1306. [
PMC free article: PMC8524758] [
PubMed: 30226124]
- 18.
Pavia
M, Simpser
E, Becker
M, Mainquist
WK, Velez
KA. The effect of ultraviolet-C technology on viral infection incidence in a pediatric long-term care facility.
Am J Infect Control. 2018;46(6):720–722. [
PubMed: 29550083]
- 19.
Raggi
R, Archulet
K, Haag
CW, Tang
W. Clinical, operational, and financial impact of an ultraviolet-C terminal disinfection intervention at a community hospital.
Am J Infect Control. 2018;46(11):1224–1229. [
PubMed: 29934205]
- 20.
Sampathkumar
P, Folkert
C, Barth
JE, et al. A trial of pulsed xenon ultraviolet disinfection to reduce Clostridioides difficile infection.
Am J Infect Control. 2018;27:27. [
PubMed: 30502111]
- 21.
Ethington
T, Newsome
S, Waugh
J, Lee
LD. Cleaning the air with ultraviolet germicidal irradiation lessened contact infections in a long-term acute care hospital.
Am J Infect Control. 2018;46(5):482–486. [
PubMed: 29290480]
- 22.
Heredia-Rodriguez
M, Alvarez-Fuente
E, Bustamante-Munguira
J, et al. Impact of an ultraviolet air sterilizer on cardiac surgery patients, a randomized clinical trial.
Med Clin (Barc). 2018;151(8):299–307. [
PubMed: 29807859]
- 23.
Kane
DW, Finley
C, Brown
D. UV-C light and infection rate in a long term care ventilator unit. Can J Infect Control. 2018;33(1):44–48.
- 24.
Ryan
RM, Wilding
GE, Wynn
RJ, Welliver
RC, Holm
BA, Leach
CL. Effect of enhanced ultraviolet germicidal irradiation in the heating ventilation and air conditioning system on ventilator-associated pneumonia in a neonatal intensive care unit.
J Perinatol. 2011;31(9):607–614. [
PubMed: 21436785]
- 25.
Aboelela
SW, Saiman
L, Stone
P, Lowy
FD, Quiros
D, Larson
E. Effectiveness of barrier precautions and surveillance cultures to control transmission of multidrug-resistant organisms: a systematic review of the literature.
Am J Infect Control. 2006;34(8):484–494. [
PubMed: 17015153]
- 26.
Cohen
CC, Cohen
B, Shang
J. Effectiveness of contact precautions against multidrug-resistant organism transmission in acute care: a systematic review of the literature.
J Hosp Infect. 2015;90(4):275–284. [
PMC free article: PMC4486607] [
PubMed: 26051927]
- 27.
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Studies
Table 2Characteristics of Included Systematic Reviews
View in own window
| First Author, Publication Year, Country, Funding | Objectives, Types and Numbers of Primary Studies Included, Quality Assessment Tool, Databases and Search Date | Characteristics | Interventions; Length of Application | Outcomes |
|---|
Health Quality Ontario15 Canada Funding: Public | Objectives: To evaluate the effectiveness and budget impact of portable UV light surface-disinfecting devices for reducing hospital-acquired infections. 10 studies included (1 RCT, 1 interrupted time series, 8 before-after) Study quality was assessed using Risk of Bias tool for RCTs, Effective Practice and Organisation of Care (EPOC) tool for non-RCTs and for interrupted time-series studies, and The National Heart, Lung and Blood Institute quality assessment tool for before-after studies with no control groups. The GRADE framework was used to evaluate the quality of the body of evidence for each outcome on the basis of the following considerations: risk of bias, inconsistency, indirectness, imprecision, publication bias, magnitude of effect, and dose-response gradient. MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, National Health Service Economic Evaluation Database (NHSEED), Database of Abstracts of Reviews of Effects (DARE), and Cumulative Index to Nursing & Allied Health Literature (CINAHL) Search date: Inception to January 23, 2017 | Hospital type: Community, academic, military, acute care, long-term care Intervention site: Patient rooms in the ICUs and non-ICUs Year of intervention: 2011 to 2014 | UV devices: pulsed Xenon UV light, UV-C radiation (mercury bulb) UV devices were used as adjunct to standard hospital room cleaning and disinfection (i.e., manual cleaning) and compared with manual cleaning done in the control groups or in the period before the interventions Length of application:
- –
RCT: 7 months for each strategy - –
Non-randomized studies:
| Healthcare-acquired infections:
- –
Clostridium difficile - –
Vancomycin-resistant Enterococcus (VRE) - –
Methicillin-resistant Staphylococcus aureus (MRSA) - –
Other multidrug-resistant organisms
|
Marra et al., 201816 USA Funding: VA Health Services Research and Development award | Objectives: To determine the impact of no-touch disinfection methods to decrease health-care associated infections. 20 studies included
- –
13 studies on UV light (1 CT, 1 RCT, 11 before-after) published from 2013 to 2017 - –
7 studies on HP vapor (1 prospective cohort, 6 before-after) published from 2008 to 2016
Study quality was assessed using a published tool25,26 having items regarding sample representatives, bias and confounding, description of the intervention, outcomes and follow-up, and statistical analysis. Each item was scored 1 to 4, with 4 being highest quality. Reviewers assessed the scores and provided an overall statement such as “completely adequate”, “partially adequate”, “inadequate, not stated or impossible to tell” or “not applicable”.PubMed, CINAHL, CDSR, DARE and EMBASE Search date: Inception to April 2017 | Hospital type: Community, academic, military, acute care, long-term care Intervention site: Patient rooms in the ICUs and non-ICUs Year of intervention:
- –
UV light: 2011 to 2014 - –
HP vapor: 2005 to 2012
| Interventions:
- –
Type of UV light: pulsed Xenon UV light, UV-C radiation (mercury bulb) - –
HP vapor disinfection system
The interventions were used as adjunct to standard hospital room cleaning and disinfection (i.e., manual cleaning) and compared with manual cleaning done in the control groups or in the period before the interventionsLength of application: NR | Healthcare-acquired infections:
- –
Clostridium difficile - –
Vancomycin-resistant Enterococcus (VRE)
|
CT = clinical trial; GRADE = Grading of Recommendations Assessment, Development, and Evaluation; HP = hydrogen peroxide; ICUs = intensive care units; NR = not reported; RCT = randomized controlled trial; UV = ultraviolet
Table 3Characteristics of Included Primary Studies
View in own window
| First Author, Publication Year, Country, Funding | Study Setting and Design | Target rooms and Timing after disinfection | Non-Manual UV Device | Comparators | Clinical Outcomes |
|---|
| Surface disinfection |
|---|
Brite et al., 201817 USA Funding: New York State Department of Health, Healthcare-associated infection Prevention Project and the MSKCC Core Cancer Center | Bone marrow transplant unit (25 beds, single-patient rooms) of a 474-bed tertiary-care cancer center Interrupted time series Before: 19 months Washout: 1 month After: 12 months | Rooms of patients diagnosed with CDI and other hospital rooms After discharge or transfer | PX-UV device (Xenex) UV disinfection was used after standard terminal (manual) cleaning according to manufacturer’s recommendations Length of cycle: 5 minutes Duration: minimum, 5 minutes per position | Standard terminal (manual) cleaning:
- –
C. difficile: hypochlorite solution (bleach) - –
Other rooms: quaternary ammonium
| Hospital-acquired colonization or infection with VRE and C. difficile |
Pavia et al., 201818 USA Funding: NR; UV-C device was provided by Clorox Healthcare | Toddler unit of 97-bed children hospital Pre-post Before: 12 months After: 12 months | Five of 12 toddler unit rooms, bathrooms and common areas (2 or 3 treatments per week) Patients were removed from areas prior to UV-C treatment | UV-C device UV disinfection was used after standard (manual) cleaning according to manufacturer’s recommendations | Standard terminal (manual) cleaning: quaternary ammonium | Hospital-acquired viral infection rates |
Raggi et al., 201819 USA Funding: Clean Sweep Group, Inc. | Community hospital (337 beds) Pre-post Before: 12 months After: 12 months | All patient rooms After discharge or transfer | UV-C device (Skytron) UV disinfection was used after standard terminal (manual) cleaning according to manufacturer’s recommendations | Standard terminal (manual) cleaning: NR on type of disinfectants | Hospital-acquired infection rates of 5 multidrug resistant bacteria (Acinetobacter baumannii, Klebciella pneumonia, MRSA, VRE, and Pseudomonas aeruginosa) |
Sampathkumar et al., 201820 USA Funding: NR; PX-UD devices were provided by Xenex | Tertiary care hospital (Mayo Clinic, 2059 beds) Non-randomized design with control Study period: 6 months | UV disinfection: 3 units (2 hematology bone marrow transplant units and a medical surgical unit) Control: 3 similar units After discharge or transfer | PX-UV device (Xenex) UV disinfection was used after standard terminal (manual) cleaning according to manufacturer’s recommendations | Standard terminal (manual) cleaning:
- –
All patient rooms in the hematology and bone marrow transplant units were cleaned with hypochlorite solution (bleach) daily. - –
Only rooms of patients with known C. difficile infection were cleaned with bleach
| C. difficile infection |
| Air disinfection |
|---|
Ethington et al, 201821 USA Funding: NR, UV-C device was provided by American Green Technology | Long-term acute care hospital (123 beds) Pre-post Before: 12 months After: 12 months | Special care unit (16 rooms). All rooms are negative pressure with single beds | UV-C device (Vidashield) used in conjunction with established housekeeping protocols The devices were installed in the ceiling of the occupied patient rooms, hallway and biohazard room. Each device has a fully shielded chamber with a UV-C bulb, and fans that pull air to the irradiation chamber and push the air back to the room. | Normal light and established housekeeping protocols for occupied patient rooms and standard terminal (manual) cleaning at patient discharge | Hospital-acquired infection rates with C. difficile, catheter-associated urinary tract infection, central line-associated bloodstream infection, MRSA, VRE |
Heredia-Rodriguez et al., 201822 Spain Funding: Healthcare Research fund at Instituto de Salud Carlos III, and the Health Management at the Healthcare Regional Ministry of Junta de Castilla y Leon | University hospital RCT Study period: 5 years (January 2011 to January 2016) | Intensive care unit of cardiac surgery (10 single rooms) | Portable UV-C air sterilizer (Medixair) (5 rooms; 522 patients) Each device produces continuous airflow and has a four 25 watts UV low pressure fluorescent lamps that are completely shielded. | Without the device (5 rooms; 575 patients) | - –
Hospital-acquired infection rates after cardiac surgery - –
Length hospital stay - –
30-day mortality rate
|
Kane et al., 201823 USA Funding: NR | Long-term care ventilator unit (full-time mechanical ventilation patients aged > 18 years) Non-randomized design with control Study period: 6 months | One wing (40 patients): all rooms had UV-C units Control wing (46 patients): no UV-C units | UV-C device (VidaShield) with fully shielded UV-C bulb has fans that continuously draw air in and out the irradiation chamber. UV disinfection was used in adjunct with standard terminal (manual) cleaning according to manufacturer’s recommendations. | Standard terminal (manual) cleaning: NR on type of disinfectants After discharge or transfer | Hospital-acquired infection rates (measured based on antibiotic orders) |
Ryan et al., 201124 USA Funding: NYSTAR Center for Advanced Technology in Biomedical and Bioengineering, Department of Pediatrics, SUNY at Buffalo, and eUVGI technology and installation and environmental sample collection from Vigilair Systems | Women and Children’s Hospital of Buffalo Pre-post Before: 6 months After: Three consecutive 6 months | Neonatal intensive care unit | UV-C air sterilizer (Enhanced UV germicidal irradiation; Sterile-Aire) installed in the heating ventilation air conditioning system | Before UV-C air sterilizer device installation | - –
Tracheal microbial load (colonization) - –
Ventilator-associated pneumonia
|
CDI = Clostridium difficile infection; NR = not reported; PX-UV = pulsed-xenon ultraviolet radiation; RCT = randomized controlled trial; UV = ultraviolet; UV-C = continuous UV radiation; VRE = vancomycin-resistant enterococcus
Table 4Characteristics of Included Guidelines
View in own window
| First Author, Society/Group Name, Publication Year, Country, Funding | Intended Users/Target Population | Intervention and Practice Considered | Major Outcomes Considered | Evidence Collection, Selection and Synthesis | Recommendations Development and Evaluation | Guideline Validation |
|---|
| Health Quality Ontario27, 2018 | Intended users: Healthcare professionals and funders Target population: All patients admitted in hospitals | Portable UV light surface-disinfecting devices | Hospital-acquired infections | Systematic methods used to search for evidence were reported The level of evidence and grade of recommendations were assessed using GRADE | The Ontario Health Technology Advisory Committee reviewed the HTAs conducted by Health Quality Ontario and made recommendations to the Minister of Health and Long-Term Care. The committee consisted of volunteer members across the province, including healthcare experts and patient perspective representatives. | No guideline validation was reported |
GRADE = Grading of Recommendations Assessment, Development, and Evaluation; HTA = health technology assessment; UV = ultraviolet
Appendix 3. Quality Assessment of Included Studies
Table 5Quality Assessment of Systematic Reviews
View in own window
| AMSTAR 2 Checklist12 | Health Quality Ontario15 | Marra16 et al., 2018 |
|---|
| 1. Did the research questions and inclusion criteria for the review include the components of PICO? | Yes | Yes |
| 2. Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? | No | No |
| 3. Did the review authors explain their selection of the study designs for inclusion in the review? | Yes | Yes |
| 4. Did the review authors use a comprehensive literature search strategy? | Yes | Yes |
| 5. Did the review authors perform study selection in duplicate? | Unclear | Unclear |
| 6. Did the review authors perform data extraction in duplicate? | Unclear | Unclear |
| 7. Did the review authors provide a list of excluded studies and justify the exclusions? | No | No |
| 8. Did the review authors describe the included studies in adequate detail? | Yes | Yes |
| 9. Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review? | Yes | Yes |
| 10. Did the review authors report on the sources of funding for the studies included in the review? | No | No |
| 11. If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results? | NA | Yes |
| 12. If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? | NA | No |
| 13. Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? | Yes | No |
| 14. Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? | Yes | No |
| 15. If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? | NA | Yes |
| 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? | No | Yes |
AMSTAR = Assessing the Methodological Quality of Systematic Reviews; NA = not applicable
Table 6Quality Assessment of Randomized Controlled Trials
View in own window
| JBI Critical Appraisal Checklist for RCT13 | Heredia-Rodriguez22 et al., 2018 |
|---|
| 1. Was true randomization used for assignment of participants to treatment groups? | Yes |
| 2. Was allocation to treatment groups concealed? | Yes |
| 3. Were treatment groups similar at the baseline? | Yes |
| 4. Were participants blind to treatment assignment? | Yes |
| 5. Were those delivering treatment blind to treatment assignment? | Unclear |
| 6. Were outcomes assessors blind to treatment assignment? | Unclear |
| 7. Were treatment groups treated identically other than the intervention of interest? | Yes |
| 8. Was follow up complete and if not, were differences between groups in terms of their follow up adequately described and analyzed? | Yes |
| 9. Were participants analyzed in the groups to which they were randomized? | Yes |
| 10. Were outcomes measured in the same way for treatment groups? | Yes |
| 11. Were outcomes measured in a reliable way? | Yes |
| 12. Was appropriate statistical analysis used? | Yes |
13. Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial? | Yes |
JBI = Joanna Briggs Institute; RCT = randomized controlled trial
Table 7Quality Assessment of Non-Randomized Studies
View in own window
| JBI Critical Appraisal Checklist for Non-Randomized Studies13 | Brite17 et al., 2018 | Ethington 21 et al., 2018 | Kane23 et al., 2018 | Pavia18 et al., 2018 | Raggi19 et al., 2018 | Sampathkumar et al., 201820 | Ryan24 et al., 2011 |
|---|
| 1. Is it clear in the study what is the ‘cause’ and what is the ‘effect’ (i.e. there is no confusion about which variable comes first)? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Were the participants included in any comparisons similar? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 3. Were the participants included in any comparisons receiving similar treatment/care, other than the exposure or intervention of interest? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 4. Was there a control group? | No | No | Yes | No | No | Yes | No |
| 5. Were there multiple measurements of the outcome both pre and post the intervention/exposure? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Was follow up complete and if not, were differences between groups in terms of their follow up adequately described and analyzed? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| 7. Were the outcomes of participants included in any comparisons measured in the same way? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Were outcomes measured in a reliable way? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Was appropriate statistical analysis used? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
JBI = Joanna Briggs Institute
Table 8Quality Assessment of Guidelines
View in own window
| AGREE II checklist14 | Health Quality Ontario27 |
|---|
| Scope and purpose | |
| 1. Objectives and target patients population were explicit | Yes |
| 2. The health question covered by the guidelines is specifically described | Yes |
| 3. The population to whom the guidelines is meant to apply is specifically described | Yes |
| Stakeholder involvement | |
| 4. The guideline development group includes individuals from all relevant professional groups | Yes |
| 5. The views and preferences of the target population have been sought | Yes |
| 6. The target users of the guideline are clearly defined | Yes |
| Rigour of development | |
| 7. Systematic methods were used to search for evidence | Yes |
| 8. The criteria for selecting the evidence are clearly described | Yes |
| 9. The strengths and limitations of the body of evidence are clearly described | Yes |
| 10. The methods of formulating the recommendations are clearly described | Yes |
| 11. The health benefits, side effects, and risks have been considered in formulating the recommendations | Yes |
| 12. There is an explicit link between the recommendations and the supporting evidence | Yes |
| 13. The guideline has been externally reviewed by experts prior to its publication | Unclear |
| 14. A procedure for updating the guideline is provided | Unclear |
| Clarity of presentation | |
| 15. The recommendations are specific and unambiguous | Yes |
| 16. The different options for management of the condition or health issue are clearly presented | Yes |
| 17. Key recommendations are easily identified | Yes |
| Applicability | |
| 18. The guideline describes facilitators and barriers to its application | Not appplicabble |
| 19. The guidelines provides advice and/or tools on how the recommendations can be put into practice | Yes |
| 20. The potential resource (cost) implications of applying the recommendations have been considered | Yes |
| 21. The guideline presents monitoring and/or auditing criteria | Not applicable |
| Editorial independence | |
| 22. The views of the funding body have not influenced the content of the guideline | Unclear |
| 23. Competing interests of guideline development group members have been recorded and addressed | Unclear |
Appendix 4. Main Study Findings and Author’s Conclusions
Table 9Summary of Findings of Systematic Reviews
View in own window
| Main Study Findings | Author’s Conclusions |
|---|
| Health Quality Ontario 201815 |
|---|
Mercury UV-C room disinfection and standard terminal (manual) cleaning versus standard terminal (manual) cleaning alone
C. difficile infection rate:
- –
One cluster RCT (low quality evidence)
- –
Two pre-post studies (very low quality evidence)
Combined HAI and colonization relative rate:
- –
One cluster RCT (low quality evidence)
- –
One pre-post study (very low quality evidence)
MRSA infection rate:
- –
One cluster RCT (low quality evidence)
- –
One pre-post study (very low quality evidence)
VRE infection rate:
- –
One cluster RCT (low quality evidence)
- –
One pre-post study (very low quality evidence)
Other HAI rates:
- –
One RCT found no cases of multidrug-resistant Acinetobacter infection or colonization after both treatment and control (low quality evidence) - –
One pre-post study found reductions in relative rates of infection with Acinetobacter baumanni or Klebsiella pneumonia after treated with UV-C disinfection, but the difference did not reach statistical significance (very low quality evidence)
Pulsed xenon UV room disinfection and standard terminal (manual) cleaning versus standard terminal (manual) cleaning alone
C. difficile infection rate:
- –
Six pre-post studies (very low quality evidence)
RR (95% CI) = 0.37 (0.02 to 6.89); P = 0.51 RR (95% CI) = 0.59 (0.41 to 0.86); P = 0.005 RR (95% CI) = 0.43 (0.24 to 0.77); P = 0.005 RR (95% CI) = 0.78 (0.61 to 1.01); P = 0.06 RR (95% CI) = 0.83 (0.7 to 0.97); P = 0.02 RR (95% CI) = 0.47 (0.26 to 0.86); P = 0.015 versus 1 year prior
Combined HAI and colonization relative rate:
- –
Three pre-post studies (very low quality evidence)
RR (95% CI) = 1.17 (0.50 to 2.76); P = 0.72 RR (95% CI) = 0.71 (0.55 to 0.91); P = 0.01 RR (95% CI) = 0.80 (0.73 to 0.88); P < 0.001
MRSA infection rate:
- –
Three pre-post studies (very low quality evidence)
RR (95% CI) = 1.26 (0.34 to 4.75); P = 0.75 RR (95% CI) = 1.20 (0.75 to 1.91); P = 0.45 RR (95% CI) = 0.73 (0.58 to 0.92); P = 0.007
VRE infection rate:
- –
Two pre-post studies (very low quality evidence)
Other HAI rates:
- –
One pre-post study found that pulsed xenon disinfection significantly reduced Class I surgical site infection, but not Class II surgical site infection (very low quality evidence) - –
One pre-post study found no significant difference in any other HAI rates including VAP, CAUTI, CLABSI (very low quality evidence) - –
One pre-post study found that pulsed xenon disinfection significantly reduced the rate of multidrug-resistant gram-negative bacteria by 19% (P = 0.04) (very low quality evidence)
| “We are unable to make a firm conclusion about the effectiveness of this technology on HAIs given the very low quality of evidence.”15 p.3 |
| Marra et al., 201816 |
|---|
UV light no-touch technology (UV-C and PX-UV) and standard terminal (manual) cleaning versus standard terminal (manual) cleaning alone
C. difficile infection rate:
- –
Overall (11 studies): RR (95% CI) = 0.64 (0.49 to 0.84); I2 = 0%; P = 0.0010 - –
Subgroups based on baseline C. difficile infection rates:
- –
Subgroups based on study design:
- –
Subgroups based on types of hospital:
- –
Subgroups based on studies reporting compliance rates:
VRE infection rate:
- –
Overall (4 studies): RR (95% CI) = 0.42 (0.28 to 0.65); I2 = 0%; P < 0.0001
| “Ultraviolet light no-touch disinfection technology may be effective in preventing C. difficile infection and VRE infection.”16 p.20 |
CAUTI = Catheter-associated urinary tract infection; CI = confidence interval; C. difficile = Clostridium difficile; CLABSI = Central line-associated bloodstream infection; HAI = hospital-acquired infection; IRR = incidence rate ratio; MDRO = multidrug-resistant organisms; MRSA = methicillin resistant Staphylococcus Aureus; OR = odds ratio; PX-UV = pulsed xenon ultraviolet radiation; RR = relative risk; SD = standard deviation; UV = ultraviolet; UV-C = continuous ultraviolet radiation; VAP = ventilator-associated pneumonia; VRE = vancomycin-resistant enterococcus
Table 10Summary of Findings of Included Primary Studies
View in own window
| Main Study Findings | Author’s Conclusions |
|---|
| Brite et al., 201817 |
|---|
Post-intervention (PX-UV) versus pre-intervention in a bone marrow transplant unit
Length of hospital stay (median, SD): 29.48 (16.41) versus 24.33 (12.59) days Monthly incidence rate of infection
- –
C. difficile: 9.3 per 1,000 patient days versus 7.1 per 1,000 patient days; P = 0.503 - –
VRE: 12.2 per 1,000 patient days versus 9.7 per 1,000 patient days; P = 0.4389
Interrupted time series analysis Level change after UV cleaning
- –
C. difficile: IRR (95% CI) = 0.51 (0.13 to 2.11); P = 0.356 - –
VRE: IRR (95% CI) = 1.34 (0.37 to 4.80); P = 0.652
Trend change after UV cleaning
- –
C. difficile: IRR (95% CI) = 1.08 (0.89 to 1.31); P = 0.413 - –
VRE: IRR (95% CI) = 0.96 (0.81 to 1.14); P = 0.625
Hospital-acquired incidence rate of infectiony
- –
C. difficile: 1.411 per 1,000 days versus 1.114 per 1,000 days; P = 0.70 - –
VRE: 3.0236 per 1,000 days versus 3.6588 per 1,000 days; P = 0.60
Manual cleaning, hand hygiene compliance, antibiotic utilization: no difference between two periods
| “Utilization of UV disinfection to supplement routine terminal cleaning of rooms was not effective in reducing hospital acquired VRE and C. difficile among stem cell transplant recipients”17 p.1301 |
| Pavia et al., 201818 |
|---|
Post-intervention (UV-C) versus pre-intervention in a pediatric long-term care facility
Hospital acquired viral infections
| “The results suggest that UV-C technology is a potentially important component of eliminating the environment as a source of viral infections”18 p.720 |
| Raggi et al., 201819 |
|---|
Post-intervention (UV-C) versus pre-intervention at a community hospital
Overall HAI incidence rates (per 1,000 patient days): 3.94 versus 4.87; P = 0.006 HAI incidence rates with MDRO (per 1,000 patient days)
- –
Acinetobacter baumannii: 0.16 versus 0.34; P = 0.03 - –
Klebsiella pneumonia: 1.22 versus 1.16; P = 0.36 - –
MRSA: 0.98 versus 1.42; P = 0.02 - –
Pseudomonas aeruginosa: 1.16 versus 1.29; P = 0.22 - –
VRE: 0.45 versus 0.68; P = 0.05
Emergency department admissions: 297.9 minutes versus 296.2 minutes; P = 0.18 Direct cost saving: $1,219,878 over a 12-month period calculated from the reduction of hospital length of stay
| “The UV-C disinfection was associated with a statistically significant facility-wide reduction of multidrug-resistant HAIs and generated substantial direct cost savings without adversely impacting hospital operations”19 p.1224 |
| Sampathkumar et al., 201820 |
|---|
PX-UV disinfection in 3 units (2 hematology and bone marrow transplant units and a medical-surgery unit) versus 3 control units (same type of patients)
C. difficile infection rates (per 10,000 patient days):
- –
Before intervention (21 months): 21.3 versus 26.1; P = 0.17 - –
Intervention (6 months): 11.2 versus 28.7; P = 0.03
VRE infection rates in hematology and bone marrow transplant units only (per 10,000 patient days):
- –
Before intervention (21 months): 25.6 versus 46.0; P = 0.002 - –
Intervention (6 months): 12.3 versus 32.5; P = 0.02
| “The addition of UV disinfection to terminal cleaning has resulted in a reduction in C. difficile infection in our hospital that has sustained over several months. During the pilot phase on units with a VRE surveillance program, we also saw a reduction in VRE acquisition.”20 p.3 |
| Ethington et al., 201821 |
|---|
| Post-intervention (UV-C air sterilizer) versus pre-intervention in ICU
| “Continuous shielded UV-C reduced airborne bacteria and may lower the number of HAI, including those caused by contact pathogens.”21 p.482 |
| Heredia-Rodriguez et al., 201822 |
|---|
UV-C air sterilizer rooms versus control rooms in ICU of cardiac surgery
Patient colonization rates (%)
- –
Any type of bacteria: 40.4 versus 43.1 - –
Gram-positive: 21.6 versus 24.3 - –
Gram-negative: 18.8 in both groups
HAI rates (%)
- –
Total: 14.0 versus 15.5; P = 0.45 VAP: 4.6 versus 5.0; P = 0.77 - –
Urinary tract: 2.9 versus 2.6; P = 0.78 - –
Catheter: 1.4 versus 1.6; P = 0.71 - –
Blood: 2.4 versus 2.8; P = 0.78 - –
Surgical site: 2.8 versus 3.5; P = 0.56
Stay in the ICU (mean days, SD): 4.6 ± 8.2 versus 4.6 ± 7.3; P = 0.98 Total hospital stay (mean days, SD): 18.3 ± 5.5 versus 19.2 ± 18.6; P = 0.38 30-day mortality rate (%): 3.83 versus 6.4; P = 0.053
| “Novel UV-C technology had not been shown to significantly reduce nosocomial infections or mortality rates in cardiac surgery patients”22 p.299 |
| Kane et al., 201823 |
|---|
UV disinfecting (UV-C air sterilizer) wing versus control wing in long-term care ventilator unit
Overall infection rate based on antibiotic ordered: 12.5 ± 2.12 per 1,000 patient days versus 17.5 ± 2.81 per 1,000 patient days; P = 0.022 Types of infection-causing organisms: Acinetobacter, MRSA, VRE and C. difficile Infection rates caused by those organisms: No statistically significant difference between groups (P > 0.05). The authors suggested that the non-significant difference was due to relatively small sample size (n = 81), which is underpowered.
| “Findings suggest that continuous exposure to UV-C treated air reduces HAI. Shielded UV-C units in patient rooms may be an effective non-staff intervention dependent method for reducing HAI.”23 p.44 |
| Ryan et al., 201124 |
|---|
| Post-intervention (UV-C air sterilizer) versus pre-intervention in neonatal ICU
*P < 0.011 compared to pre-intervention | “Enhanced ultraviolet germicidal irradiation (eUVGI) decreased heating ventilation and air conditioning system microbial colonization and was associated with reduced newborn intensive care unit environment and tracheal microbial colonization. Significant reduction in VAP and antibiotic use were also associated with eUVGI in this single-center study.”24 p.607 |
CAUTI = Catheter-associated urinary tract infection; CI = confidence interval; C. difficile = Clostridium difficile; CLABSI = Central line-associated bloodstream infection; HAI = hospital-acquired infection; IRR = incidence rate ratio; MDRO = multidrug-resistant organisms; MRSA = methicillin resistant Staphylococcus Aureus; OR = odds ratio; PX-UV = pulsed xenon ultraviolet radiation; SD = standard deviation; UV = ultraviolet; UV-C = continuous ultraviolet radiation; VAP = ventilator-associated pneumonia; VRE = vancomycin-resistant enterococcus
Table 11Summary of Findings of Included Guidelines
View in own window
| Recommendations |
|---|
| Health Quality Ontario 201827 |
|---|
| “Health Quality Ontario, under the guidance of the Ontario Health Technology Advisory Committee, recommends against publicly funding portable ultraviolet light surface-disinfection devices for prevention of hospital-acquired infections”27 p.1 |
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Non-Manual Ultraviolet Light Disinfection for Hospital Acquired Infections: A Review of Clinical Effectiveness and Guidelines. Ottawa: CADTH; 2019 Apr. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.