Context and Policy Issues
Otitis media is diagnosed by visualizing an infected middle ear with inflammation.1 Patients usually present with mild to severe otalgia and can have other symptoms including fever. Among children, otitis media is among the most common causes for physician visits in developed countries; approximately 80% will have at least one episode by the time they are aged three.2 Though rarer, the incidence of otitis media among adults is still estimated at 1%.3 Most infections are bacterial with Streptococcus pneumoniae and Haemophilus influenza being the most common perpetrators. Antibiotics are commonly prescribed.4
The choice of antibiotic is based on knowledge of the most common organisms responsible for ear infections. It should thus be active against, at least, Streptococcus pneumoniae and Haemophilus influenza. Amoxicillin or amoxicillin-clavulanate has been recommended as the first line treatment.1,3 Amoxicillin, like penicillin, belongs to a class of drugs called beta-lactams. This class includes a broad range of antibiotics that operate by inactivating enzymes in bacterial cell membranes.5 While popular, the most commonly reported antimicrobial allergies are to beta-lactams.6 Alternatives among patients with allergies are thus necessary.
Another class of antibiotics, fluoroquinolones, may provide a suitable alternative to beta-lactams. Fluoroquinolones were initially ineffective against Streptococcus pneumoniae and thus not recommended. In the late 1990s, newer, more effective fluoroquinolones emerged and prescribing increased alongside.7 However, toxicities with newer agents have been widely reported and the risk associated with fluoroquinolones, such as joint toxicity in children, may outweigh the benefits.8 A recent review on acute otitis media in adults recommended two fluoroquinolones only, levofloxacin or moxifloxacin, as they are active against respiratory pathogens. However due to their boxed warning of potentially serious side effects, they were suggested as a last resort.1
The extent to which fluoroquinolones are clinically effective or cost-effective for patients with otitis media unable to take beta-lactams remains unclear. This review aims to synthesize evidence for the clinical effectiveness and cost-effectiveness of fluoroquinolones for the treatment of otitis media in patients unable to take beta-lactams antibiotics, and to examine evidence-based guidelines for this population.
Research Questions
What is the clinical effectiveness of fluoroquinolones for the treatment of otitis media in patients unable to take beta-lactams antibiotics?
What is the cost-effectiveness of fluoroquinolones for the treatment of otitis media in patients unable to take beta-lactams antibiotics?
What are the evidence-based guidelines regarding the use of fluoroquinolones for the treatment of otitis media in patients unable to take beta-lactams antibiotics?
Key Findings
No evidence regarding the clinical effectiveness or cost-effectiveness of fluoroquinolones to treat otitis media in patients unable to take beta-lactam antibiotics was identified. Further, no guidelines regarding the use of fluoroquinolones for the treatment of otitis media in patients unable to take beta-lactams antibiotics were found.
Methods
Literature Search Methods
A limited literature search was conducted on key resources including Medline via OVID, the Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. No filters were applied to limit the retrieval by study type. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2009 and March 29, 2019.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2009. Guidelines with unclear methodology were also excluded.
Critical Appraisal of Individual Studies
No relevant studies or guidelines were identified.
Summary of Evidence
Quantity of Research Available
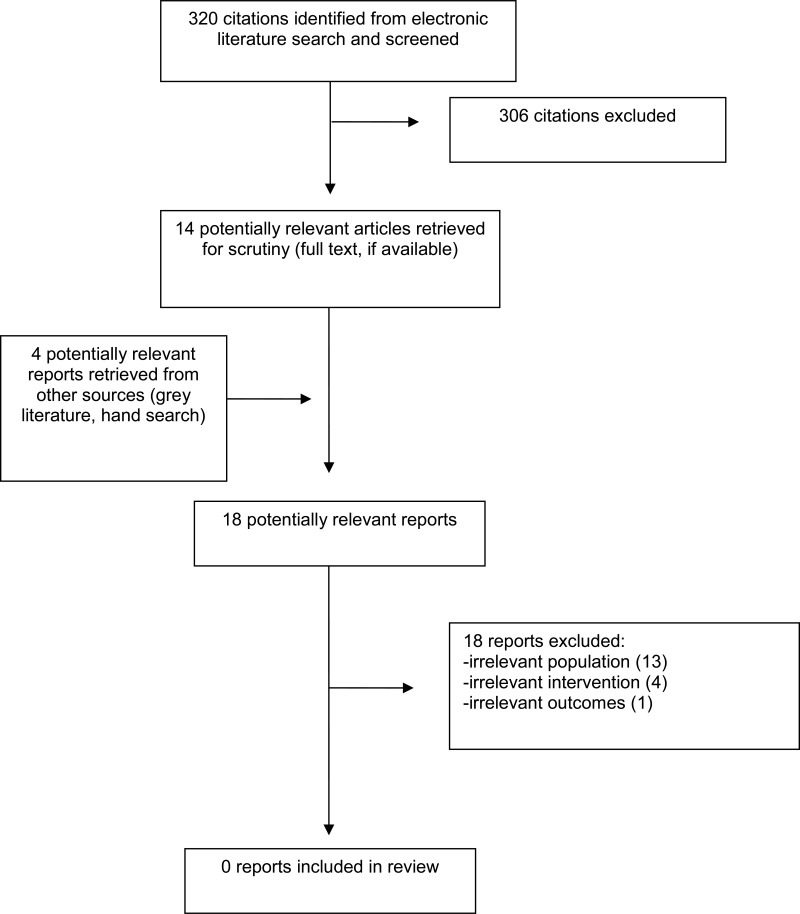
A total of 320 citations were identified in the literature search. Following screening of titles and abstracts, 306 citations were excluded and 14 potentially relevant reports from the electronic search were retrieved for full-text review. Four potentially relevant publications were retrieved from the grey literature search for full text review. Of these potentially relevant articles, all publications were excluded for various reasons. Appendix 1 presents the PRISMA9 flowchart of the study selection. Additional references of potential interest are provided in Appendix 2.
Summary of Findings
Clinical Effectiveness of Fluoroquinolones
No relevant evidence regarding the clinical effectiveness of fluoroquinolones for the treatment of otitis media in patients unable to take beta-lactams antibiotics was found; therefore, no summary can be provided.
Cost-Effectiveness of Fluoroquinolones
No relevant evidence regarding the cost-effectiveness of fluoroquinolones for the treatment of otitis media in patients unable to take beta-lactams antibiotics was found; therefore, no summary can be provided.
Guidelines Regarding Fluoroquinolones
No relevant guidelines for the treatment of otitis media in patients unable to take beta-lactams antibiotics were found; therefore, no summary can be provided.
Conclusions and Implications for Decision or Policy Making
It was not possible to determine the clinical effectiveness or cost-effectiveness of fluoroquinolones for treatment of otitis media in patients unable to take beta-lactam antibiotics. No relevant evidence-based guidelines were identified. Future studies are needed to determine whether fluoroquinolones are a suitable alternative in patients who are unable to take beta-lactam antibiotics in order to guide decision-making.
References
- 1.
Limb
CL, Lustig
LR, Durand
ML. Acute otitis media in adults. In: Post
TW, ed.
UptoDate. Waltham (MA): UptoDate; 2019:
www.uptodate.com. Accessed 2019 Apr 23.
- 2.
Teele
DW, Klein
JO, Rosner
B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study.
J Infect Dis. 1989;160(1):83–94. [
PubMed: 2732519]
- 3.
Pelton
S. Acute otitis media in children: treatment. In: Post
TW, ed.
UptoDate. Waltham (MA): UptoDate; 2019:
www.uptodate.com. Accessed 2019 Apr 23.
- 4.
Gonzales
R, Malone
DC, Maselli
JH, Sande
MA. Excessive antibiotic use for acute respiratory infections in the United States.
Clin Infect Dis. 2001;33(6):757–762. [
PubMed: 11512079]
- 5.
Letourneau
AR. Beta-lactam antibiotics: mechanisms of action and resistance and adverse effects. In: Post
TW, ed.
UptoDate. Waltham (MA): UptoDate; 2019:
www.uptodate.com. Accessed 2019 Apr 23.
- 6.
Lee
CE, Zembower
TR, Fotis
MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance.
Arch Intern Med. 2000;160(18):2819–2822. [
PubMed: 11025792]
- 7.
Chen
DK, McGeer
A, de Azavedo
JC, Low
DE. Decreased susceptibility of streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network.
N Engl J Med. 1999;341(4):233–239. [
PubMed: 10413735]
- 8.
Hooper
DC. Fluoroquinolones. In: Post
TW, ed.
UptoDate. Waltham (MA): UptoDate; 2019:
www.uptodate.com. Accessed 2019 Apr 23.
- 9.
Liberati
A, Altman
DG, Tetzlaff
J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
Appendix 1. Selection of Included Studies
Appendix 2. Additional References of Potential Interest
Guidelines excluded due to unclear methodology (though they have some mention of using fluoroquinolones among patients unable to take beta lactams)
Limb
CL, Lustig
LR, Durand
ML. Acute otitis media in adults. In: Post
TW, ed. UptoDate. Waltham (MA): UptoDate; 2019:
www.uptodate.com. Accessed 2019 Apr 23.
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Fluoroquinolones for the treatment of otitis media: a review of clinical effectiveness, cost-effectiveness, and guidelines. Ottawa: CADTH; 2019 Apr. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.