Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016-2021.
Methods:
These bulletins are not systematic reviews and do not involve critical appraisal or include a detailed summary of study findings. Rather, they present an overview of the technology and available evidence. They are not intended to provide recommendations for or against a particular technology.
Literature Search:
A limited literature search was conducted using the following bibliographic databases: MEDLINE, Embase, PsychInfo, PubMed, and the Cochrane Library. Grey literature was identified by searching relevant sections of the Grey Matters checklist (https://www.cadth.ca/grey-matters). No methodological filters were applied. The search was limited to English-language documents but not limited by publication year. Regular alerts updated the search until project completion; only citations retrieved before March 13, 2019 were incorporated into the analysis.
Study Selection:
One author screened the literature search results and reviewed the full text of all potentially relevant studies. Studies were considered for inclusion if the intervention was esketamine (S-ketamine) and studied for treatment-resistant depression in a phase III trial. Conference abstracts and grey literature were included when they provided additional information to that available in the published studies.
Peer Review:
A draft version of this bulletin was reviewed by one clinical expert. The drug manufacturer also provided input on an earlier draft of this report.
Summary
- Major depressive disorder (MDD) is a common, debilitating, and recurrent mental health disorder. Approximately 10% to 30% of patients with MDD will not reach complete clinical remission despite multiple antidepressant pharmacologic approaches — this subpopulation is described as having treatment-resistant depression (TRD). TRD is a more challenging depressive disorder to treat and available therapies are often limited by significant psychological and physiological side effects.
- Esketamine is the S-enantiomer of racemic ketamine and is being developed as a nasal spray device for potential therapeutic use in patients with TRD. It was recently approved for marketing in the US and is under priority review at Health Canada.
- In one phase III trial, esketamine plus a newly initiated antidepressant demonstrated a statistically significant improvement in Montgomery-Åsberg Depression Rating Scale (MADRS) scores relative to placebo plus a newly initiated antidepressant. Esketamine plus a newly initiated oral antidepressant also statistically significantly reduced relapse events and delayed the occurrence of a relapse event.
- Similar to ketamine, dissociative effects, sedation, and elevations in blood pressure were reported. The majority of these side effects were mild or moderate in severity and resolved within a few hours of administration.
- Esketamine’s psychoactive properties give it the potential for addiction and abuse; policies to limit off-label use and guide esketamine distribution would be important to prevent diversion. Furthermore, there remains a lack of evidence comparing intranasal esketamine to current adjunctive strategies for TRD.
Background
Major depressive disorder (MDD) is a common, debilitating, and recurrent mental health disorder. MDD is characterized by symptoms of persistent low mood, changes in appetite and sleep, fatigue, loss of motivation, interest or pleasure, or feelings of worthlessness.1 It can also be associated with a substantial loss in productivity, quality of life, and increased mortality from suicide. MDD has a significant global burden, with an estimated 300 million people affected and reported as the leading cause of disability of all of the mental illnesses worldwide.2–4 Within Canada, approximately 11% (4.1 million) of the population have reported a major depressive episode in their lifetime.1,5 Even with available therapies, a significant proportion of patients will not reach complete clinical remission, with approximately 10% to 30% not responding to multiple antidepressants. 6,7
Treatment-resistant depression (TRD) is the clinical term used to define this subpopulation of MDD patients that have not achieved optimal response to conventional therapy. TRD presents an even greater burden, with an increased risk for subsequent relapses and suicide.8,9 Patients with TRD are also twice as likely to be hospitalized, incurring additional medical costs.8,10 Risk factors for TRD may include old age, high stress levels, concomitant psychiatric disorders, and a history of medication nonadherence.6
The most common definition of TRD is the inadequate response to two or more antidepressants.7,10,11 However, there is no clear consensus surrounding this definition, with only 17% of treatment studies closely matching this definition.10 Variations in this definition can be attributed to inconsistencies in the use of adjunctive therapies, variable dose and duration of an adequate treatment trial, and whether treatment failure occurred in the current depressive episode. As studies of emerging treatments utilize diverse definitions of TRD, it can be difficult to translate research findings into practical guidelines.
TRD is a more challenging depressive disorder to treat, with few strong recommendations in clinical practice guidelines. None of the available options have demonstrated superiority in improving clinical outcomes for patients with TRD.7 Available therapies are also often limited by significant side effects, such as weight gain and sedation. There remains an unmet need for a safe and effective treatment of TRD for which esketamine offers the potential to address.
The Technology
Janssen Research & Development is currently investigating esketamine for its potential therapeutic use in patients with MDD who are refractory to classical antidepressants.7 Esketamine is the S-enantiomer of racemic ketamine — a drug that has been used traditionally as a general anesthetic. In studies, sub-anesthetic intravenous doses of ketamine have demonstrated rapid antidepressant effects, as well as lessened fatigue and suicidality in patients with TRD and with minimal negative impacts on cognitive functioning.12,13 Due in part to these promising results and despite concerns around limited data on long-term safety and duration of effect, ketamine’s use for depression has expanded from research settings to specialized clinics in the US and Canada servicing this patient population.14,15
Esketamine is approximately three to four times more potent than R-ketamine, potentially translating into lower doses with fewer side effects compared with previously studied ketamine infusions.7,16,17 Esketamine’s antidepressant effects are not mediated by known mood-modulating pathways such as the monoamine, gamma-aminobutyric acid (GABA), or opioid axes. Although the specific mechanism of action has not been fully elucidated, it is postulated that esketamine acts primarily to block the N-methyl-D-aspartate (NMDA) receptor and activate the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor.
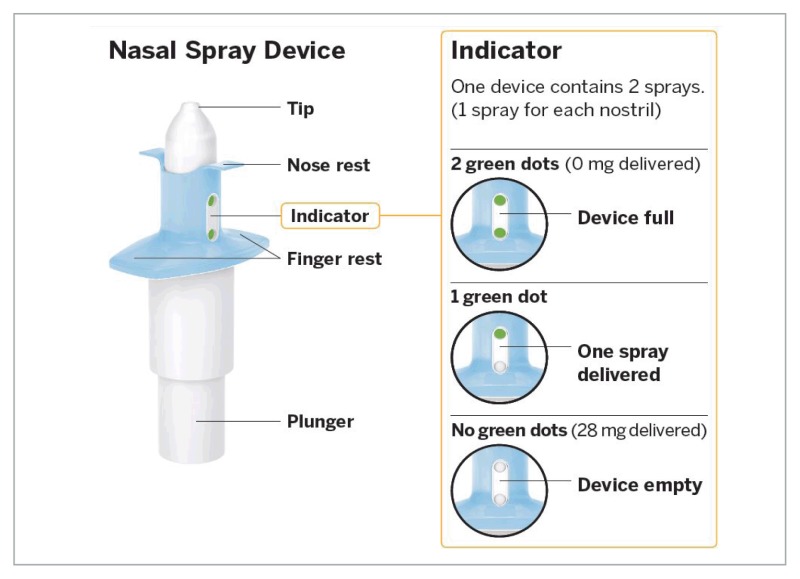
For TRD (defined by the manufacturer as MDD that has not responded adequately to at least two different antidepressants of adequate dose and duration in the current depressive episode), esketamine is formulated as a single-use nasal spray device.18 In contrast to intravenous ketamine, which is limited to outpatient clinics, intranasal administration is easier and could allow for increased access to therapy.19 Each device contains 28 mg of drug, delivered as one spray in each nostril.18 The maximum dose studied in clinical trials was 84 mg; therefore, multiple devices may be required. Priming of the device is not required but a five-minute rest period in-between each device is advised. For acute treatment, esketamine nasal spray requires administration twice weekly for four weeks during treatment induction, then weekly for another four weeks, followed by weekly or every other week during ongoing maintenance.18 According to the manufacturer, it is intended to be used under the direct supervision of a health care professional.
Regulatory Status
As of the publication of this report, esketamine is approved in the US for TRD; it is not approved for use in other countries or for any other indication. The manufacturer has filed for regulatory approval in Canada and the European Union for adults with TRD (defined as MDD patients who have not responded adequately to at least two different antidepressants of adequate dose and duration in the current depressive episode) based on five, phase III clinical trials (Janssen Inc., Toronto, ON: personal communication, 2019 Feb 1).20–25 Health Canada is currently reviewing esketamine as a priority submission.26
In the US, the FDA has recently approved the marketing of esketamine nasal spray, under the trade name Spravato, in conjunction with an oral antidepressant, “for the treatment of depression in adults who have tried other antidepressant medicines but have not benefited from them (treatment-resistant depression)”.27 It also received Breakthrough Therapy designation in 2016 from the FDA for MDD with imminent risk for suicide; this indication is not currently approved. Of note, the FDA grants a Breakthrough Therapy designation for drugs intended to treat a serious or life-threatening disease and where initial clinical data suggests that the drug may demonstrate substantial improvement over current therapies.28
Cost and Administration
The Canadian cost of esketamine is unavailable, as this product is not marketed in the country. For the US market, the manufacturer has announced that Spravato will be priced at $590 for a 56 mg dose and $885 for 84 mg.29
Esketamine is developed to be administered as a nasal spray. The dosage regimen used in the completed and ongoing phase III trials is esketamine 28 mg, 56 mg, or 84 mg intranasally twice weekly for four weeks, followed by once weekly for four weeks, then once weekly or once every other week for ongoing maintenance therapy.18,20–24,30,31 As a result, the cost of therapy will vary based on the dose used per session and the number of treatment sessions administered, both of which can differ from patient to patient.
The budget impact is unknown, as the Canadian acquisition cost is not yet available. The budget impact will largely depend on its sequential place in therapy within the context of other treatment options currently on the market for MDD.
Target Population
For the majority of patients with MDD, available medications, psychotherapy, and alternative therapies (e.g., electroconvulsive therapy, transmagnetic stimulation) are effective at relieving symptoms and achieving disease remission. However, MDD is a complex, heterogeneous disorder, and 10% to 30% of MDD patients do not respond to a sequence of multiple traditional antidepressant therapies.6,7
This subpopulation of patients with TRD could benefit from esketamine. However, the exact clinical need within this patient population is challenging to discern because of inconsistencies in the definitions of TRD, the variety of therapies currently available for TRD, the access to and reimbursement of non-pharmacological options, and the concurrent development of novel therapy options.
Current Practice
Because of limited evidence comparing available therapies, there is no standard treatment approach for TRD; therefore therapy should be individualized for each patient.6,7 The Canadian Network for Mood and Anxiety Treatments (CANMAT) guideline recommends making adjustments to therapy if there is no improvement within two to four weeks of initiating the antidepressant.11 The following options are recommended for managing an inadequate response:
- optimize the initial treatment
- switch the antidepressant class of drug
- add an adjunctive medication
- consider non-drug therapies.
Optimizing treatment involves increasing the dose of the current antidepressant, as tolerated, and continuing treatment for an additional six to eight weeks.11 Switching to an antidepressant of a different class is an alternative for patients who had no response to therapy, or who were not tolerating the initial antidepressant. CANMAT advises this strategy may be best employed for patients in their first antidepressant trial and therefore may not be the optimal strategy for TRD (i.e., failed two or more antidepressants).
An adjunctive strategy involves adding a second medication to the initial antidepressant. This strategy includes both augmentation (i.e., adding a non-antidepressant medication) and combination (i.e., adding an antidepressant from a different class).11 There is low-quality evidence to suggest that adjunctive therapy is more effective than switching therapy in patients who had a partial response to the initial antidepressant and have residual symptoms.7,11,32 Adjunctive medications that are recommended to try first are the following atypical antipsychotic drugs: aripiprazole, quetiapine, and risperidone (off-label).11,33 If a patient fails to respond despite these first-line options, they can trial the following adjunctive treatments: bupropion, mirtazapine, brexpiprazole, olanzapine, lithium, and thyroid hormone.11 Lastly, non-drug approaches to be considered for patients with TRD include psychotherapy and neurostimulation (e.g., transcranial magnetic stimulation, electroconvulsive therapy).
Summary of Evidence
A total of five phase III trials evaluated the efficacy and safety of esketamine nasal spray in combination with a newly initiated oral antidepressant for patients with TRD; four were randomized, double-blind, active-controlled trials21–24 and one was an open-label safety study.20 There are an additional two phase III trials currently ongoing to further assess the efficacy and long-term safety of esketamine in this patient population.30,31 Details of these trials are summarized in Table 1.
Table 1
Summary of Esketamine Phase III Trials — Patient and Trial Characteristics
In the active-controlled studies, patients were randomized to receive either esketamine nasal spray plus a newly initiated oral antidepressant or placebo nasal spray plus a newly initiated oral antidepressant. All previous oral antidepressants to which patients did not respond were discontinued.
In all of the phase III trials, TRD was defined as nonresponse (i.e., 25% or less improvement) to two or more oral antidepressants in the current episode of depression, at a therapeutic dose for at least six weeks in duration. Across the trials, approximately 33% to 40% of randomized patients had previously failed four or more antidepressants.18 Patients from all phase III trials had severe depression (MADRS greater than 34) at baseline (Janssen Inc.: personal communication, 2019 Feb 1).18
All the completed phase III trials, with the exception of SUSTAIN 1, included a four-week screening period prior to randomization. This screening period allowed for the inclusion of patients who had previously failed one antidepressant to be randomized in the trial if they also demonstrated nonresponse to a second antidepressant treatment during the screening phase.
Patients were specifically excluded from any of the five studies if they had MDD with psychotic features, had previously demonstrated nonresponse to ketamine in the current depressive episode, had a history of suicidal ideations in the past six months, or had a history of a substance or alcohol use disorder. In trials with elderly patients (i.e., TRANSFORM-3, SUSTAIN 2), additional exclusion criteria included a Mini-Mental State Examination score of less than 25, neurodegenerative disorders, or clinically significant cardiovascular disorders.
Efficacy
The results of the phase III trials are summarized here and depicted in Tables 2 and 3. These results have not yet been fully published; the data were obtained from conference poster presentations and an FDA briefing report (Janssen Inc.: personal communication, 2019 Feb 1).18
Table 2
Summary of Key Efficacy Outcomes From Four of the Phase III Trials
Table 3
Efficacy Outcomes From the Maintenance Phase of SUSTAIN 1
MADRS
This metric describes depression severity. MADRS is a clinician-rated scale consisting of 10 domains (i.e., apparent sadness, reported sadness, inner tension, sleep, appetite, concentration, lassitude, interest level, pessimistic thoughts, and suicidal thoughts), for a total possible score of 60. Higher scores indicate more severe depression and thus a decrease in scores indicate improvement. In the TRANSFORM-2 study, esketamine plus a newly initiated oral antidepressant demonstrated a statistically significant superior change in the MADRS score at day 28 (mean difference −4.0, 95% CI: −7.31 to −0.64; P = 0.010) relative to placebo plus a newly initiated oral antidepressant. Furthermore, the mean difference between treatment groups was statistically significant for almost all the time points throughout the 28 days of treatment. In the TRANSFORM-1 and TRANSFORM-3 trials, there was no statistically significant difference between esketamine and placebo.
Response Rates
In the TRANSFORM-1 and TRANSFORM-2 trials with participants between 18 to 64 years of age, 53.1% to 69.3% of patients who received esketamine in combination with a new oral antidepressant were stable responders (i.e., defined as 50% or higher reductions in MADRS depression severity scores) by day 28. In comparison, 38.9% to 52% of patients who received placebo with a newly initiated antidepressant were stable responders. In the TRANSFORM-3 trial with elderly participants (65 years-old or more), the response rate was 27.0% in the esketamine plus antidepressant group compared with 13.3% in the group receiving placebo plus a new antidepressant.
Remission Rates
In the TRANSFORM-1 and TRANSFORM-2 trials, remission rates (i.e., defined as a MADRS score of 12 or less) ranged from 36.0% to 52.5% in the esketamine plus antidepressant group compared with 31% of patients in the placebo plus antidepressant group. As with the response rates, remission rates were lower in the elderly patients studied in the TRANSFORM-3 trial: 17.5% for the esketamine plus antidepressant group versus 6.7% for the placebo plus antidepressant group. No statistical tests were reported.
Results From SUSTAIN 1 (ESKETINTRD3003):A Relapse Prevention Study
A relapse event was defined as at least two MADRS scores of 22 or greater within five to 15 days, or hospitalization for worsening depression, suicide attempt, suicide prevention, or completed suicide. According to the FDA, esketamine plus a newly initiated oral antidepressant statistically significantly delayed a relapse event, compared with placebo plus antidepressant, for both stable remitters (i.e., defined as 50% or higher reductions in MADRS score) and stable responders (i.e., defined as a MADRS score of 12 or less).18 The largest differences in relapse time between treatment groups occurred within the first two to four weeks. Esketamine also statistically significantly reduced the hazard for relapse for stable remitters (HR 0.49; 95% CI: 0.29 to 0.84, P = 0.003) and for stable responders (HR 0.30; 95% CI: 0.16 to 0.55, P < 0.001). Other efficacy outcomes data are summarized in Table 3.
Safety
Safety information for the completed phase III trials are reported here and in Table 2. As study results have not yet been fully published, the data were obtained from conference poster presentations and an FDA briefing report (Janssen Inc.: personal communication, 2019 Feb 1).18 An ongoing, open-label extension study is evaluating the safety of esketamine over five years following the completion of the earlier phase III efficacy trials; no results have been posted to date.30
Two (0.3%) deaths occurred in the optimization and maintenance phase of the SUSTAIN 2 trial — one due to acute respiratory and cardiac failure, and the second due to completed suicide (Janssen Inc.: personal communication, 2019 Feb 1). The FDA reports six deaths with esketamine during the development program; however, five of these deaths occurred after the drug was stopped.18 Three of these deaths were due to completed suicide, and three were due to one of following: a motor vehicle accident, sudden death, and a heart attack.
For patients in the esketamine treatment group, the frequency of serious adverse events ranged from 3.9% to 6.9%. Serious adverse events in the placebo group were only reported in TRANSFORM-3 (3.1%). These included:
- In TRANSFORM-3, three patients in the esketamine group reported anxiety, blood pressure increase, and hip fracture. Two patients in the placebo group reported feelings of despair and dizziness.
- In SUSTAIN 1, five patients reported one of the following adverse events: disorientation, hypothermia, lacunar stroke, sedation, and suicidal ideation; and one patient reported autonomic nervous system imbalance and simple partial seizure.
- In SUSTAIN 2 — a longer-term, open-label safety study that followed participants for up to one year — 55 patients reported 68 serious adverse events, with the most common events occurring in greater than two patients including depression, suicidal ideation, suicide attempt, nephritis, anxiety, and gastroenteritis. Of these serious adverse events, five were considered related to esketamine — anxiety, delusion, delirium, suicidal ideation, and suicidal attempt.
Across the phase III trials, a total of 71% to 90% of patients receiving esketamine experienced an adverse event. The frequency of adverse events in the comparator group was only reported in the TRANSFORM-3 trial as 60%. Adverse events most frequently reported included dizziness, nausea, dissociation, headache, vertigo, sedation, increased blood pressure, dysgeusia (altered sense of taste), hypoesthesia (reduced sense of touch), vomiting, and viral upper respiratory tract infection. Overall, these adverse events were less common in elderly patients than in adult patients. Blood pressure elevations, dissociative symptoms, and sedation occurred more often in the esketamine treatment group. These adverse events all occurred rapidly after receiving a dose of esketamine, peaked around 40 minutes, and resolved within four hours, 1.5 hours, and one hour, respectively. With subsequent esketamine dosing, the severity of dissociative symptoms appeared to lessen. No cases of psychosis, or ulcerative or interstitial cystitis, were reported.
The long-term safety study SUSTAIN 2 also reported moderate-to-severe nasal symptoms (e.g., taste disturbance, postnasal drip) in 11% of patients. In this study, 8.1% of patients experienced a urinary tract infection and 10.5% of all patients reported one the following urinary adverse events: dysuria (painful urination), pollakiuria (frequent daytime urination), micturition (frequent urination), urgency, and bladder pain. This safety study also reported that, regardless of age, cognitive functioning (e.g., memory, processing speed) remained stable throughout treatment.
In three of the phase III trials, discontinuation of esketamine due to adverse events ranged from 2.6% to 9.5% in the esketamine treatment group and 2.1% to 3.1% in the comparator group. The specific reasons for stopping treatment were not reported. Withdrawal due to adverse events was not reported for two of the studies.
Study Limitations
One major limitation of the evidence base presented in this bulletin is that all results were obtained from an FDA briefing report and unpublished conference posters that are not peer-reviewed and may include data gaps. Secondly, for most outcomes, the statistical significance was not calculated and it is unclear whether observed differences are clinically meaningful. Furthermore, the overall number of withdrawals in the long-term safety study was quite high, which could introduce bias to the results. In the induction phase, 198 (25.4%) patients withdrew, and of the 603 patients who entered the optimization and maintenance phase, 120 patients discontinued prior to study completion. Dissociation symptoms are a prominent side effect known to be associated with esketamine. These symptoms could have served as a cue to indicate to patients that they had received the drug, thus compromising the blinding of treatment allocation in the randomized trials.
Because of the trial design and choice of comparator, this evidence cannot be used to assess how esketamine compares with other options available to patients with TRD, such as adjunctive therapy. The generalizability of the study findings may also be limited because of the exclusion of patients with MDD with psychotic features.
Concurrent Developments
Other Drugs for Treatment-Resistant Depression
Orally administered ketamine has recently been investigated for TRD in a phase I proof-of-concept study.34 In this study, oral ketamine decreased depressive symptoms and was well-tolerated.
In addition to ketamine and esketamine, there are several other compounds in development for TRD:
- Tulrampator (CX-1632, RespireRx) is an orally administered drug that acts on the AMPA receptor. It is being investigated in a phase II trial for TRD.51
Other Esketamine Indications
In addition to TRD, intranasal esketamine is being investigated in phase III trials for patients with MDD who are at imminent risk for suicide.52–54 Suicide remains a significant global burden, accounting for 1.5% of worldwide mortality.55 This indication received a breakthrough designation from the FDA in 2016. Results of a double-blind, randomized phase II trial comparing esketamine 84 mg with placebo twice weekly demonstrated statistically significant improvements in suicidal thoughts within four hours; however, these effects were not sustained at 24 hrs.54 Similarly, esketamine statistically significantly improved depressive symptoms at four hours and approximately 24 hours; however, these effects were not sustained at day 25.
Inhaled esketamine is also being investigated in a phase II study for treatment-resistant bipolar depression.56
Implementation Issues
Cited concerns surrounding intravenous ketamine for depression may inform potential uptake issues for esketamine. One key concern with ketamine is its safety profile, specifically dissociative and cognitive side effects, sedation, elevations in blood pressure, and toxicity to the urinary system.57 With the exception of cognitive dysfunction, these side effects were also reported with intranasal esketamine use (Janssen Inc.: personal communication, 2019 Feb 1). However, the majority of the urinary adverse events were mild and resolved without intervention. Dissociative symptoms, blood pressure elevations, and sedation were reported to resolve within a few hours but may require patient monitoring shortly after drug administration. Ketamine’s potential for addiction and diversion was another important concern.57 However, post-marketing data suggests that ketamine abuse is in fact relatively uncommon.18 Much like ketamine, esketamine has psychotogenic properties that also make it susceptible to abuse; risk management could still be an important consideration.7,16,58
Cautions currently exist surrounding driving after receiving ketamine, given the potential for dissociative and sedative side effects.18,57 A small, double-blind, randomized phase I study assessed esketamine’s impact on driving performance.59 Healthy patients received single doses of esketamine 84 mg intranasally, oral placebo, and mirtazapine 30 mg orally in a crossover design. There were no statistically significant differences between esketamine and placebo groups in their driving performance (i.e., measured by the weaving of a car) eight hours after drug administration, whereas mirtazapine had a significant detrimental impact on driving relative to placebo. However, the generalizability of these results to chronic esketamine use is limited. Given the prevalence of dissociation and sedation in the larger phase III trials, the risk for potential accidents and the impact on driving may still be of concern.
Although esketamine is formulated in a more convenient nasal spray device, it must be administered under direct medical supervision. This could present a challenge for patients, as it would require repeat visits to the physician’s office on a bi-weekly basis. Because of the potential driving impairment shortly after esketamine administration, travelling to the physician’s clinic and back may be problematic for patients, especially in areas with limited public transportation. The need for medical supervision also poses the question of organizing the distribution of this drug in Canada: if patients are required to obtain the medication at their retail pharmacy, policies may be required to ensure that the patient does not self-administer the medication.
There is also the potential for off-label use, as esketamine is being investigated for not only unipolar TRD but also for depression with imminent risk of suicide and treatment-resistant bipolar depression. There is also the risk of off-label use for patients with non–treatment-resistant MDD.
While there are ongoing long-term safety studies, further research is required to inform the appropriate duration of esketamine treatment and whether it can eventually be tapered or discontinued. Furthermore, there is a lack of evidence comparing intranasal esketamine to current adjunctive strategies for TRD.
Footnotes
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein do not necessarily reflect the views of Health Canada, Canada’s provincial or territorial governments, other CADTH funders, or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Contact ac.htdac@stseuqer with inquiries about this notice or legal matters relating to CADTH services.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
References
- 1.
- Lam RW, McIntosh D, Wang J, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 1. disease burden and principles of care. Can J Psychiatry. 2016;61(9):510–523. [PMC free article: PMC4994789] [PubMed: 27486151]
- 2.
- Depression and other common mental disorders: global health estimates. Geneva (CH): World Health Organization; 2017. [Accessed 2019 Apr 1]. https://apps
.who.int /iris/bitstream/handle /10665/254610/WHO-MSD-MER-2017 .2-eng.pdf;jsessionid =073A899990718FDDE11F70671E1D49D1?sequence=1 . - 3.
- Collins PY, Patel V, Joestl SS, et al. Grand challenges in global mental health. Nature. 2011;475(7354):27–30. [PMC free article: PMC3173804] [PubMed: 21734685]
- 4.
- Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. [PubMed: 23993280]
- 5.
- Patten SB, Lee RC. Epidemiological theory, decision theory and mental health services research. Soc Psychiatry Psychiatr Epidemiol. 2004;39(11):893–898. [PubMed: 15549241]
- 6.
- Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–388. [PMC free article: PMC3363299] [PubMed: 22654508]
- 7.
- Garay RP, Zarate CA Jr, Charpeaud T, et al. Investigational drugs in recent clinical trials for treatment-resistant depression. Expert Rev Neurother. 2017;17(6):593–609. [PMC free article: PMC5418088] [PubMed: 28092469]
- 8.
- NIHR Innovation Observatory. Esketamine for treatment-resistant depression. 2017. [Accessed 2019 Jan 29]. http://www
.io.nihr.ac .uk/report/esketamine-for-treatment-resistant-depression/ - 9.
- Thase ME. Treatment-resistant depression: prevalence, risk factors, and treatment strategies. J Clin Psychiatry. 2011;72(5):e18. [PubMed: 21658343]
- 10.
- Gaynes BN, Asher G, Gartlehner G, et al. Technology assessment program project ID. PSYT0816. Rockville (MD): Agency for Healthcare Research and Quality; 2018. [Accessed 2019 Jan 28]. Definition of treatment-resistant depression in the medicare population. https://www
.cms.gov/Medicare /Coverage/DeterminationProcess /downloads/id105TA.pdf . [PubMed: 30260611] - 11.
- Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. Can J Psychiatry. 2016;61(9):540–560. [PMC free article: PMC4994790] [PubMed: 27486148]
- 12.
- CADTH Rapid response report: summary with critical appraisal. Ottawa (ON): CADTH; 2017. [Accessed 2019 Jan 28]. Ketamine for treatment-resistant depression or post-traumatic stress disorder in various settings: a review of clinical effectiveness, safety, and guidelines. https://www
.cadth.ca /sites/default/files /pdf/htis/2017/RC0855 %20Ketamine%20for%20Resistant %20Depression%20Final.pdf . [PubMed: 29533569] - 13.
- Serafini G, Howland RH, Rovedi F, Girardi P, Amore M. The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol. 2014;12(5):444–461. [PMC free article: PMC4243034] [PubMed: 25426012]
- 14.
- Ketamine Clinics of Los Angeles. Ketamine clinics of Los Angeles. 2019. [Accessed 2019 Apr 1]. https://www
.ketamineclinics.com/ - 15.
- Canadian Rapid Treatment Center of Excellence. Understanding intravenous ketamine infusion therapy. 2019. [Accessed 2019 Apr 1]. https://www
.crtce.com/ - 16.
- Gould TD, Zarate CA Jr, Thompson SM. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu Rev Pharmacol Toxicol. 2019;59:213–236. [PMC free article: PMC6364552] [PubMed: 30296896]
- 17.
- Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37(7):865–872. [PMC free article: PMC4933765] [PubMed: 27018176]
- 18.
- Center for Drug Evalulation and Research. FDA briefing document: Psychopharmacologic Drugs Advisory Committee (PDAC) and Drug Safety and Risk Management (DSaRM) Advisory Committee Meeting. Silver Spring (MD): U.S. Food and Drug Administration (FDA); Feb 12, 2019. [Accessed 2019 Feb 13]. https://www
.fda.gov/downloads /AdvisoryCommittees /CommitteesMeetingMaterials /Drugs /PsychopharmacologicDrugsAdvisoryCommittee/UCM630970.pdf . - 19.
- Andreassen OA. Nasal administration of esketamine-a promising new antidepressant [abstract]. Acta Neuropsychiatr; Presented at 59th Annual Meeting of Scandinavian College of Neuropsychopharmacology; 2018 Apr 11–13; Aarhus, Denmark. 2018. [Accessed 2019 Apr 1]. pp. 1–23. https://www
.cambridge .org/core/services/aop-cambridge-core /content /view/B1568F88C93EBF4E12820A1BB1755A65 /S0924270818000121a .pdf/abstracts_scandinavian _college_of _neuropsychopharmacology _scnp_59th_annual _meeting_11_13_april _2018_aarhus_denmark.pdf . - 20.
- Janssen Research & Development, LLC. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018. [Accessed 2019 Jan 25]. NCT02497287: A long-term, safety and efficacy study of intranasal esketamine in treatment-resistant depression (SUSTAIN-2). https:
//clinicaltrials .gov/ct2/show/NCT02497287 . - 21.
- Janssen Research & Development, LLC. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Jan 25]. NCT02417064: A study to evaluate the efficacy, safety, and tolerability of fixed doses of intranasal esketamine plus an oral antidepressant in adult participants with treatment-resistant depression (TRANSFORM-1). https:
//clinicaltrials .gov/ct2/show/NCT02417064 . - 22.
- Janssen Research & Development, LLC. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018. [Accessed 2019 Jan 25]. NCT02418585: A study to evaluate the efficacy, safety, and tolerability of flexible doses of intranasal esketamine plus an oral antidepressant in adult participants with treatment-resistant depression (TRANSFORM-2). https:
//clinicaltrials .gov/ct2/show/NCT02418585 . - 23.
- Janssen Research & Development, LLC. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018. [Accessed 2019 Jan 25]. NCT02422186: A study to evaluate the efficacy, safety, and tolerability of intranasal esketamine plus an oral antidepressant in elderly participants with treatment-resistant depression (TRANSFORM-3). https:
//clinicaltrials .gov/ct2/show/NCT02422186 . - 24.
- Janssen Research & Development, LLC. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Jan 25]. NCT02493868: A study of intranasal esketamine plus an oral antidepressant for relapse prevention in adult participants with treatment-resistant depression (SUSTAIN-1). https:
//clinicaltrials .gov/ct2/show/NCT02493868 . - 25.
- Johnson & Johnson. Janssen submits European Marketing Authorisation Application for esketamine nasal spray for treatment-resistant depression. Oct 10, 2018. [Accessed 2019 Feb 19]. https://www
.jnj.com/janssen-submits-european-marketing-authorisation-application-for-esketamine-nasal-spray-for-treatment-resistant-depression . - 26.
- Government of Canada. Drug and health product submissions under review (SUR): esketamine hydrochloride. 2019. [Accessed 2019 Mar 4]. https://www
.canada.ca /en/health-canada/services /drug-health-product-review-approval /submissions-under-review.html . - 27.
- U.S. Food and Drug Administration. News release: FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic. Mar 5, 2019. https://www
.fda.gov/NewsEvents /Newsroom/PressAnnouncements /ucm632761.htm . - 28.
- Center for Drug Evalulation and Research. Expedited programs for serious conditions – drugs and biologics. Silver Spring (MD): U.S. Food and Drug Administration; 2014. [Accessed 2019 Jan 28]. Guidance for industry. https://www
.fda.gov/downloads /Drugs/GuidanceComplianceRegulatoryInformation /Guidances/UCM358301.pdf . - 29.
- Reuters. J&J prices ketamine-like depression treatment at $590–$885 for two doses. Mar 6, 2019. [Accessed 2019 Apr1]. https://www
.reuters.com /article/us-johnson-johnson-fda-pricing /jj-prices-ketamine-like-depression-treatment-at-590-885-for-two-doses-idUSKCN1QN2AX . - 30.
- Janssen Research & Development, LLC. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Jan 25]. NCT02782104: A long-term safety study of intranasal esketamine in treatment-resistant depression (SUSTAIN-3). https:
//clinicaltrials .gov/ct2/show/NCT02782104 . - 31.
- Janssen Research & Development, LLC. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Jan 25]. NCT03434041: A study to evaluate the efficacy, pharmacokinetics, safety and tolerability of flexible doses of intranasal esketamine plus an oral antidepressant in adult participants with treatment-resistant depression. https:
//clinicaltrials .gov/ct2/show/NCT03434041 . - 32.
- Gaynes BN, Dusetzina SB, Ellis AR, et al. Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32(1):114–119. [PubMed: 22198447]
- 33.
- AG-risperidone: 0.25mg, 0.5mg, 1mg, 2mg, 3mg and 4mg tablets [product monograph]. Boucherville (QC): Angita Pharma Inc.; Jan 11, 2019. [Accessed 2019 Jan 31]. https://pdf
.hres.ca/dpd_pm/00049156.PDF . - 34.
- Domany Y, Bleich-Cohen M, Tarrasch R, et al. Repeated oral ketamine for out-patient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. Br J Psychiatry. 2019;214(1):20–26. [PubMed: 30246667]
- 35.
- Naurex, Inc, an affiliate of Allergan plc. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Feb 1]. NCT03560518: Study of rapastinel as monotherapy in patients with MDD. https:
//clinicaltrials .gov/ct2/show/NCT03560518 . - 36.
- Naurex, Inc, an affiliate of Allergan plc. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Feb 1]. NCT03675776: Study of rapastinel as monotherapy in patients with major depressive disorder (MDD). https:
//clinicaltrials .gov/ct2/show/NCT03675776?term =rapastinel&recrs =a&phase =2&rank=3 . - 37.
- Wilkinson ST, Sanacora G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug discovery today. 2018;14:14. [PMC free article: PMC6397075] [PubMed: 30447328]
- 38.
- Axsome Therapeutics, Inc. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018. [Accessed 2019 Feb 1]. NCT02741791: A study to assess the efficacy and safety of AXS-05 in subjects with treatment resistant major depressive disorder (STRIDE-1). https:
//clinicaltrials .gov/ct2/show/NCT02741791?term =axsome&rank=2 . - 39.
- Allergan. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Feb 1]. NCT03738215: Efficacy, safety and tolerability of cariprazine as an adjunctive treatment to antidepressant therapy (ADT) in patients with major depressive disorder (MDD) who have had an inadequate response to antidepressants alone. https:
//clinicaltrials .gov/ct2/show/NCT03738215?term =Cariprazine&cond= %22Depression %22&phase =2&rank=4 . - 40.
- Allergan. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Feb 1]. NCT03739203: The object of this study is to evaluate the efficacy, safety and tolerability of cariprazine as an adjunctive treatment to antidepressant therapy (ADT) in patients with major depressive disorder (MDD) who have had an inadequate response to antidepressants alone. https:
//clinicaltrials .gov/ct2/show/NCT03739203?term =Cariprazine&cond= %22Depression %22&phase =2&rank=5 . - 41.
- Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399–408. [PMC free article: PMC5813086] [PubMed: 29119217]
- 42.
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016;3(7):619–627. [PubMed: 27210031]
- 43.
- Murrough James. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018. [Accessed 2019 Feb 1]. NCT01882829: Nuedexta in treatment-resistant major depression. https:
//clinicaltrials .gov/ct2/show/NCT01882829 . - 44.
- Avanir Pharmaceuticals. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2017. [Accessed 2019 Feb 1]. NCT02153502: Efficacy, safety, and tolerability study of AVP-786 as an adjunctive therapy in patients with major depressive disorder with an inadequate response to antidepressant treatment. https:
//clinicaltrials .gov/ct2/show/NCT02153502 . - 45.
- National Institute of Mental Health (NIMH). ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Feb 1]. NCT02484456: Antidepressant effects of the glycine receptor antagonist AV-101 (4-chlorokynurenine) in major depressive disorder. https:
//clinicaltrials .gov/ct2/show/NCT02484456 . - 46.
- VistaGen Therapeutics, Inc. ClinicalTrials.gov. Bethesda (MD): U.S. National Library Medicine; 2019. [Accessed 2019 Feb 1]. NCT03078322: AV-101 as adjunct antidepressant therapy in patients with major depression (ELEVATE). https:
//clinicaltrials .gov/ct2/show/NCT03078322?term =AV-101&phase =12&rank=1 . - 47.
- Charite University. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Feb 1]. NCT02456948: Adjunct minocyline in treatment-resistant depression (Mino-TRD). https:
//clinicaltrials .gov/ct2/show/NCT02456948 . - 48.
- Pakistan Institute of Living and Learning. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2016. [Accessed 2019 Feb 1]. NCT02263872: Minocycline as an adjunct for the treatment of depressive symptoms: pilot randomized controlled trial. https:
//clinicaltrials .gov/ct2/show/NCT02263872 . - 49.
- Brigham and Women’s Hospital. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018. [Accessed 2019 Feb 1]. NCT02660528: Tocilizumab augmentation in treatment-refractory major depressive disorder. https:
//clinicaltrials .gov/ct2/show/NCT02660528 . - 50.
- Janssen-Cilag International N.V. European Union Clinical Trials Register. Amsterdam (NL): European Medicines Agency; 2015. [Accessed 2019 Feb 1]. 2014-005206-37: A double-blind, placebo-controlled, multicenter study of sirukumab as adjunctive treatment to a monoaminergic antidepressant in adults with major depressive disorder. https://www
.clinicaltrialsregister .eu/ctr-search /search?query=2014-005206-37 . - 51.
- Servier. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2018. [Accessed 2019 Feb 1]. NCT02805439: Efficacy and safety of S 47445 versus placebo as adjunctive treatment in depressed patients not fully recovered from depressive symptoms with a current antidepressant treatment. https:
//clinicaltrials .gov/ct2/show/NCT02805439?term =NCT02805439&rank=1 . - 52.
- Janssen Research & Development, LLC. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Feb 1]. NCT03039192: A study of the efficacy and safety of intranasal esketamine in the rapid reduction of symptoms of major depressive disorder, in adult at imminent risk for suicide (Aspire I). https:
//clinicaltrials .gov/ct2/show/NCT03039192 . - 53.
- Janssen Research & Development, LLC. ClinicalTrials.gov. Bethesda (MD): U.S. National Library of Medicine; 2019. [Accessed 2019 Feb 1]. NCT03097133: A study to evaluate the efficacy and safety of intranasal esketamine in addition to comprehensive standard of care for the rapid reduction of the symptoms of major depressive disorder, including suicidal ideation, in adult participants assessed to be at imminent risk for suicide (Aspire II). https:
//clinicaltrials .gov/ct2/show/NCT03097133 . - 54.
- Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620–630. [PubMed: 29656663]
- 55.
- O’Connor RC, Nock MK. The psychology of suicidal behaviour. Lancet Psychiatry. 2014;1(1):73–85. [PubMed: 26360404]
- 56.
- Celon Pharma SA. EU Clinical Trials Register. Amsterdam (NL): European Medicines Agency; 2018. [Accessed 2019 Feb 10]. 2018-002669-20: A multicentre, double-blind, randomised, placebo - controlled phase II study to assess efficacy, safety and pharmacokinetics of inhaled Esketamine in subjects with treatment-resistant bipolar depression. https://www
.clinicaltrialsregister .eu/ctr-search /search?query =eudract_number:2018-002669-20 . - 57.
- Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M. A review of ketamine abuse and diversion. Depress Anxiety. 2016;33(8):718–727. [PubMed: 27328618]
- 58.
- Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sanchez E, Gutierrez-Rojas L, Meana JJ. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS drugs. 2018;32(5):411–420. [PubMed: 29736744]
- 59.
- van de Loo AJ, Bervoets AC, Mooren L, et al. The effects of intranasal esketamine (84 mg) and oral mirtazapine (30 mg) on on-road driving performance: a double-blind, placebo-controlled study. Psychopharmacology (Berl). 2017;234(21):3175–3183. [PMC free article: PMC5660834] [PubMed: 28755104]
Table 4
Number of Patients With Adverse Events in the Phase III Trials
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review Inclisiran: A Small Interfering RNA Molecule for Treating Hypercholesterolemia.[CADTH Issues in Emerging Healt...]Review Inclisiran: A Small Interfering RNA Molecule for Treating Hypercholesterolemia.Klinovski M, Boucher M, Perras C, Grobelna A. CADTH Issues in Emerging Health Technologies. 2016
- Review An Overview of Liquid Biopsy for Screening and Early Detection of Cancer.[CADTH Issues in Emerging Healt...]Review An Overview of Liquid Biopsy for Screening and Early Detection of Cancer.Cowling T, Loshak H. CADTH Issues in Emerging Health Technologies. 2016
- Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.[Med J Aust. 2020]Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Osborne SR, Alston LV, Bolton KA, Whelan J, Reeve E, Wong Shee A, Browne J, Walker T, Versace VL, Allender S, et al. Med J Aust. 2020 Dec; 213 Suppl 11:S3-S32.e1.
- Gene expression profiling for guiding adjuvant chemotherapy decisions in women with early breast cancer: an evidence-based and economic analysis.[Ont Health Technol Assess Ser....]Gene expression profiling for guiding adjuvant chemotherapy decisions in women with early breast cancer: an evidence-based and economic analysis.Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2010; 10(23):1-57. Epub 2010 Dec 1.
- Esketamine for Treatment-Resistant Depression - CADTH Issues in Emerging Health ...Esketamine for Treatment-Resistant Depression - CADTH Issues in Emerging Health Technologies
Your browsing activity is empty.
Activity recording is turned off.
See more...