NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Osborn D, Burton A, Walters K, et al. Primary care management of cardiovascular risk for people with severe mental illnesses: the Primrose research programme including cluster RCT. Southampton (UK): NIHR Journals Library; 2019 Apr. (Programme Grants for Applied Research, No. 7.2.)

Primary care management of cardiovascular risk for people with severe mental illnesses: the Primrose research programme including cluster RCT.
Show detailsThis section presents the full report of the methods and results of a systematic review that was conducted to inform the development of Primrose intervention and training programme.
Summary
Background
People with SMI are at increased risk of developing CVD and die at a younger age than the general population. This study evaluated and synthesised evidence from published systematic reviews and individual RCTs on the effectiveness of pharmacological and behavioural interventions for reducing modifiable CVD risk factors in people with SMI.
Methods
We searched The Cochrane Library for existing systematic reviews. We then searched the Cochrane Schizophrenia and Cochrane Depression, Anxiety and Neurosis Group Trial Registers between 1966 and 2014 for additional RCTs not included in the identified reviews. We searched for interventions to manage levels of cholesterol, diabetes mellitus, hypertension, weight, smoking and alcohol consumption.
Findings
Fifteen systematic reviews and 28 additional RCTs were included in the review, from 11,028 references. The synthesised data demonstrated good evidence of effective pharmacological and behavioural interventions for weight management and some evidence that combined pharmacological and behavioural interventions might be more effective than either alone. There was good evidence that pharmacological interventions were effective for smoking cessation, or reducing smoking, and some evidence for behavioural or combined interventions. Three studies reported effective interventions to reduce alcohol misuse. No studies were found on effective interventions targeting levels of cholesterol, diabetes mellitus or hypertension in SMI.
Conclusion
There is evidence that CVD risk attributable to weight and smoking can be managed effectively in SMI, using pharmacological and behavioural approaches. Future research should determine the effectiveness of interventions to manage cholesterol levels, diabetes mellitus, hypertension and alcohol misuse in this population.
Introduction
People with SMI die at a significantly younger age than the general population.124 The majority of these premature deaths (66%) are due to potentially preventable or treatable CVD.3,5
Increased mortality risk for CVD is seen in people with SMI compared with the general population. Those aged 50–75 years have a twofold increased risk of death from CVD, rising to threefold for those < 50 years old.3 There is evidence that CVD risk screening for people with SMI does not meet international or national guidelines.23,111
Increased risk for CVD is attributable to metabolic and anthropomorphic effects of antipsychotic medication as well as lifestyle factors including poor diet, low levels of physical activity, smoking and, in some cases, higher than recommended levels of alcohol consumption.
The challenge is to design and deliver effective interventions to prevent CVD in this group and this requires synthesis of evidence about modifying the different risk factors that contribute to CVD in SMI. Our overarching aim was to identify the best international evidence regarding effective CVD risk management for people with SMI to allow clinicians, policy-makers and researchers to meet the challenge of implementing and appraising CVD risk reduction strategies.
In primary care, multiple risk factors for CVD are often addressed in one consultation but existing reviews of pharmacological or behavioural interventions to reduce CVD risk in people with SMI tend to be limited to one or two contributing risk factors/behaviours (e.g. smoking52,95 diet and physical activity96–99 or alcohol use).100 Furthermore much existing research has largely been generated in secondary care rather than primary care where, in the UK, evidence-based guidelines suggest CVD risk in SMI should be managed.17 One review has synthesised evidence across CVD risk factors up to 2010.53 We aimed to update and improve on this review by including (1) more recently published studies, (2) existing systematic reviews and meta-analyses in addition to individual studies and (3) including only the highest-quality studies using a randomised experimental design. This review informed the development of a primary care-based intervention to reduce CVD risk.106
Methods
We searched for published systematic reviews and additional RCTs that were not included in existing systematic reviews.
Inclusion/exclusion criteria
Studies were included if they met the following criteria: (1) they used a randomised controlled design, (2) ≥ 50% of participants were adults with a diagnosis of schizophrenia, bipolar disorder or other non-organic psychotic disorders including schizoaffective disorder, (3) they evaluated interventions aimed at managing levels of cholesterol, diabetes mellitus, hypertension, weight, smoking and/or alcohol consumption, (4) title and abstract were written in English (papers were only excluded on the basis of being written in a non-English language where translation of the full text was not possible) and (5) were published between 1966 and 2014.
Search strategy
We conducted the search strategy in five stages in the following order: (1) we searched the Cochrane group publications lists and Cochrane Library database for systematic reviews, (2) an information specialist from the Cochrane Schizophrenia Group searched the Cochrane Schizophrenia Group Trials Register for RCTs with schizophrenia and psychosis populations, (3) we searched The Cochrane Library database for additional RCTs using an adapted search strategy to include populations with bipolar disorder provided by the Cochrane Depression, Anxiety and Neurosis Group, (4) an expert reference group searched for non-peer-reviewed literature, (5) experts in the field were asked to identify any relevant publications not captured by the electronic search strategies and (6) principal investigators identified on trials registers were contacted for relevant publications.
The following search terms were used:
Schizophrenia* OR severe mental illness* OR bipolar or mania* OR manic* OR hypomani* OR psychos* OR psychotic OR postpsychotic OR “post psychotic” OR “rapid cycling” OR schizoaffective OR bipolar OR mania* OR manic* OR hypomani*
AND
*physical* OR *cardio* OR *metabolic* OR *weight* OR *Tobacc* OR *Smok* OR *medical* OR *alcohol* OR *nutrition* OR *diet* OR *health* OR *diabete* OR *blood pressure* OR *hypertension* OR *cholesterol*OR *statin*
Screening
Titles and abstracts of reports of RCTs identified in the electronic searches were screened by four researchers (LA, AB, MH and VT; see Acknowledgements). References not meeting the inclusion criteria and duplicates (including individual RCTs in included systematic reviews) were removed. To assess reliability of screening, reviewers independently screened 10% of each other’s allotted references resulting in < 5% level of disagreement. Disagreements were resolved by discussion.
Full-text reports were obtained and screened by the same researchers using the criteria described above. Data were extracted from reports meeting the inclusion criteria.
Data extraction
Data within the RCTs were extracted using a modified template from Methods for the Development of NICE Public Health Guidance125 to record methodological (publication, design, etc.) and substantive characteristics (participant, setting, etc.) of included studies.
Quality assessment
The quality of evidence in included reports was assessed using The Cochrane Collaboration’s tool for assessing risk of bias.126
Data synthesis
Reference lists of published systematic reviews were cross-checked and reviews including the greatest number of relevant references were summarised. Papers from less comprehensive reviews were also included if they were not covered by the more comprehensive reviews. A meta-analysis of findings from individual RCTs was not possible owing to the heterogeneity of reporting, so a narrative synthesis of findings was created. Findings from RCTs with fewer than 10 participants, which may not be sufficiently powered to detect change, are summarised separately.
We performed a narrative synthesis of the evidence, first by selecting the most comprehensive systematic review and summarising this and all the additional trial results.
Findings
Fifteen systematic reviews and 28 additional individual RCTs of interventions met the inclusion criteria [Table 1 and Figure 2 for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of individual RCTs].
TABLE 1
Systematic reviews and RCTs identified by the search strategy
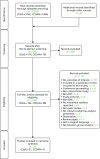
FIGURE 2
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of additional individual RCTs. CCDAN, Cochrane Depression, Anxiety and Neurosis Group; CSzG, Cochrane Schizophrenia Group.
No trials or reviews of interventions directly targeting levels of cholesterol, diabetes mellitus or hypertension in SMI were identified. Systematic reviews and individual RCTs meeting the inclusion criteria were therefore grouped into three categories of interventions: (1) weight, (2) smoking and (3) alcohol consumption.
In this section, we summarise the main findings of the systematic reviews, grouping the interventions according to their main target CVD risk factor and whether they were behavioural, pharmacological or both.
Risk of bias
Risk of bias was assessed in individual RCTs identified through the electronic searches (n = 28) and in an additional five RCTs that were taken from systematic reviews that were discarded from this review because the remaining studies had all been included in one of the 15 more comprehensive reviews, making a total of 33 assessments.
Three RCTs were rated as being at a high risk of bias. Of these, two were open-label studies of pharmacological interventions54,55 and one had high attrition, which differed across treatment groups.56
Seven RCTs were rated as being at a low risk of bias.57–63
The remaining 23 RCTs were rated as being at an unclear risk of bias. Although all studies reported using randomisation, 20 of the 23 RCTs did not adequately describe methods of allocation concealment and 17 out of 23 did not describe how participants were randomised.
Systematic reviews and randomised controlled trials targeting weight
Pharmacological interventions targeting weight
Systematic reviews of pharmacological interventions targeting weight
Four systematic reviews were identified which assessed pharmacological interventions to prevent antipsychotic-related weight gain in people with SMI.31,64,98,99 The most comprehensive review98 is summarised.
Maayan et al.98 included 32 studies (n = 1482 participants) of the effectiveness of 15 different medications on weight gain for people prescribed olanzapine, clozapine, risperidone, quetiapine or mixed first- or second-generation antipsychotics. Studies were conducted in the USA, Venezuela, Brazil, the UK, Finland, Turkey, Israel, Iran, China and South Korea. These studies explored a range of outcomes including weight, lipids and glucose.
Anthropometric outcomes
A pooled weight change of –1.99 kg (95% CI –2.77 to –1.20 kg) compared with placebo was reported after a mean of 13 weeks. Although modest and heterogeneous, greatest weight loss was reported for metformin (7 RCTs, n = 334, –2.94 kg, 95% CI –4.89 to –0.99 kg), followed by d-fenfluramine (1 RCT, n = 16, –2.60 kg, 95% CI –5.14 to –0.06 kg), sibutramine (2 RCTs, n = 55, –2.56 kg, 95% CI –3.91 to –1.22 kg) and topiramate (2 RCTs, n = 133, –2.52 kg, 95% CI –4.87 to –0.16 kg).
Metabolic outcomes
Compared with placebo, triglyceride levels decreased significantly more with metformin augmented with sibutramine (1 RCT, n = 28; weighted mean difference (WMD) –36.8 mg per 100 ml, 95% CI –63.94 to –9.66 mg per 100 ml; p = 0.008); metformin alone (2 RCTs, n = 109; WMD –28.07 mg per 100 ml, 95% CI –53.22 to –2.92 mg per 100 ml; p = 0.04) and fluvoxamine (1 RCT, n = 68, WMD –22.70 mg per 100 ml, 95% CI –44.59 to –0.81 mg per 100 ml; p = 0.04). Compared with placebo, LDL cholesterol end-point values were significantly lower only with sibutramine (1 RCT, n = 37, WMD –33.80 mg per 100 ml, 95% CI –60.41 to –7.19 ml; p = 0.01).
No significant effects on glucose levels were identified in placebo-controlled RCTs of metformin, sibutramine, metformin augmented with sibutramine, or rosiglitazone (Avandia®; GlaxoSmithKline plc, GSK House, Middlesex, UK).
Additional individual randomised controlled trials of pharmacological interventions targeting weight
A further 14 RCTs of pharmacological interventions to promote weight loss or prevent antipsychotic-related weight gain were identified by searching electronic databases.56–60,65–73 Five of these studies were trials of topiramate, two were of zonisamide and the others evaluated seven other pharmacological interventions. Findings from these studies, plus an additional study by Afshar et al.,61 included in a different systematic review64 are summarised below and in more detail (along with effect sizes where available) in Table 2.
TABLE 2
Summary of findings from additional individual RCTs of pharmacological interventions targeting weight
Anthropometric outcomes in additional randomised controlled trials (two randomised controlled trials, n = 55, –2.56 kg, 95% CI –3.91 to –1.22 kg)
Three out of the five RCTs of topiramate reported a significant beneficial effect on BMI and/or weight loss.57,67,70 Two studies reported no significant effects of topiramate on weight loss or BMI,56,61 one of which was in comparison to sibutramine.56 Significant effects on BMI and/or weight and/or waist circumference were reported in two RCTs of zonisamide,58,59 one RCT of reboxetine plus betashistine72 and a RCT comparing metformin plus weekly diet and physical activity intervention with a weekly diet and physical activity intervention alone.71 No significant effects on BMI or weight were reported in RCTs of ramelteon,66 modafinil,69 atomoxetine,65 the supplement L-carnitine,68 ranitidine60 or pioglitazone.73
Metabolic outcomes
Only one of the five RCTs of topiramate examined the effect on lipids and reported a significant effect on preventing an increase in the level of total cholesterol compared with placebo.70 A single, small (n = 20) RCT of ramelteon reported a significant effect on reducing levels of cholesterol and reducing the cholesterol-to-HDL ratio.66 A RCT comparing metformin plus weekly diet and physical activity intervention with a weekly diet and physical activity intervention alone reported a significant reduction in the level of triglycerides in the metformin arm, but no effect on the levels of total, HDL, non-HDL and LDL cholesterol.71 A RCT comparing pioglitazone plus a manualised diet–exercise intervention with placebo plus a manualised diet–exercise intervention reported a significant effect on the levels of total and HDL cholesterol.73 Placebo-controlled RCTs of modafinil and atomoxetine reported no significant effect on lipids.65,69
Out of the eight RCTs examining the effect of pharmacological interventions on glucose58,61,65,66,69–71,73 one RCT of topiramate and one RCT of pioglitazone reported significant effects in reducing glucose.70,73
Out of the five RCTs examining the effect of pharmacological interventions on blood pressure,58,61,65,69,70 only one RCT (of topiramate) reported a significant reduction in systolic and diastolic blood pressure.70
Synthesising systematic review and individual randomised controlled trial findings
The most comprehensive systematic review98 reported the greatest effects for metformin on weight loss and blood lipids. Findings from more recently identified individual RCTs report varied effects for topiramate on weight management and limited evidence regarding topiramate and ramelteon on blood lipids.
Behavioural interventions targeting weight
Systematic reviews of behavioural interventions targeting weight management
We identified six systematic reviews of physical activity and/or diet interventions.75–78,96,97 The systematic review and meta-analysis by Bonfioli et al.96 provided the most comprehensive review of the literature and this is summarised here. Two RCTs identified in other reviews but not included in Bonfioli et al.96 are summarised together with additional individual trials identified by our search.
Bonfioli et al.96 reviewed 13 RCTs of behavioural interventions to prevent weight gain or promote weight loss. Studies were conducted in the USA (n = 4), Italy (n = 2), the UK (n = 1), Spain (n = 1), Sweden (n = 1), Switzerland (n = 1), Korea (n = 1), China (n = 1) and Australia (n = 1).
Interventions were delivered to both individuals (n = 4) and groups (n = 9) and included different combinations of dietary advice and diet programmes, physical activity, self-monitoring techniques (e.g. keeping diaries), goals and planning techniques (e.g. setting and reviewing goals) as well as general behavioural support approaches, such as motivational interviewing.
The meta-analysis reported an effect on BMI reduction favouring intervention groups compared with control (–0.98 kg/m2, 95% CI –1.31 to –0.65 kg/m2).
Subgroup analyses revealed that interventions delivered to individuals (–1.20 kg/m2, 95% CI –1.57 to –0.83 kg/m2), targeting weight gain prevention (–1.09 kg/m2, 95% CI –1.51 to –0.68 kg/m2) that include dietary (–1.31 kg/m2, 95% CI –1.78 to –0.83 kg/m2) or physical activity (–1.22 kg/m2, 95% CI –1.59 to –0.85 kg/m2) components had the greatest effect in reducing BMI.
Additional individual randomised controlled trials of behavioural interventions targeting weight
We identified a further nine RCTs of behavioural interventions to promote weight loss or prevent antipsychotic-related weight gain through searching electronic databases62,79–86 and an additional two RCTs in other reviews (Scocco et al.87 in Álvarez-Jiménez et al.78 and Jean-Baptiste et al.88 in Cabassa et al.75). Findings are summarised in more detail in Table 3, apart from four RCTs, which had < 10 participants in each trial arm and are summarised separately.78–80,88
TABLE 3
Summary of findings from additional individual RCTs of behavioural or combined pharmacological and behavioural interventions targeting weight
Anthropometric outcomes
One RCT of a diet and physical activity intervention with behavioural support that also encouraged patients to use a pedometer reported small but significant effects on reducing baseline measures in the intervention group of BMI (–0.32 kg/m2), weight (0.8 kg) and waist circumference (3.38 cm).83 A RCT of a diet, physical activity and behavioural support intervention also reported a significant reduction on waist circumference.79 One trial reporting significant effects on weight and BMI immediately after the intervention period reported no effect at 12 months.62
Three RCTs, one of a group diet and physical activity intervention, one providing free fruit and vegetables to participants for 6 months, and one of a nutrition, physical activity and contingency management intervention [two arms: (1) payment for weight loss and (2) payment for attendance] reported no effect on weight or BMI.82,84,86
Metabolic outcomes
Two RCTs, one of a nutrition, physical activity and behavioural support intervention, and one of nutrition, physical activity and contingency management, both reported no effect on the levels of triglycerides or total cholesterol.79,86
Three RCTs examined the effect of behavioural interventions on glucose: two reported no effect,82,86 and one reported a significant reduction in the intervention arm.62
One RCT examining the effects of interventions on blood pressure reported no effect.79
Randomised controlled trial with < 10 participants in trial arms
One RCT reported significant effects on weight. This involved an intensive, 16-week intervention of diet (including cooking demonstration, visits to supermarkets) physical activity (participants were given a pedometer) and behavioural support resulting in a –6.4 lb (–2.9 kg) weight change in the intervention group;88 however, only eight participants were included in the intervention arm.
Two RCTs reported no effect on BMI, weight or waist circumference80,81 and one of these RCTs did not have an effect on systolic blood pressure.81 One was an educational programme providing information and counselling on exercise and nutrition80 and the other was a 12-week exercise programme.81 A third RCT focusing on diet alone did not find an effect on weight gain.87
Combined pharmacological and behavioural interventions targeting weight
No systematic reviews of combined pharmacological and behavioural interventions targeting weight were identified.
Additional individual randomised controlled trials of combined pharmacological and behavioural interventions targeting weight
We did not identify any RCTs of combined pharmacological and behavioural interventions to promote weight loss or prevent antipsychotic-related weight gain through searching electronic databases, but we did identify one RCT,63 in a review focusing on behavioural interventions.77 This study compared metformin and a diet and physical activity intervention alone, in combination and with placebo and reported that diet and physical activity plus metformin were significantly superior to metformin or diet and physical activity alone in reducing fasting BMI, weight and fasting glucose.
Systematic reviews and randomised controlled trials targeting smoking
We identified three systematic reviews of pharmacological, behavioural or combined interventions to promote smoking cessation or reduction in smoking.52,89,95
Tsoi et al.95 was the most comprehensive review. There was one additional trial included in a different review which is described later alongside other additional trials we identified.91 Tsoi et al.95 reviewed 34 RCTs of pharmacological and/or behavioural interventions to promote smoking cessation, smoking reduction or relapse prevention (16 smoking cessation RCTs; nine smoking reduction RCTs; one relapse prevention RCT and eight RCTs reporting smoking outcomes in interventions aimed at other purposes). The majority of RCTs (28/34) were conducted in the USA.
Pharmacological interventions targeting smoking
Systematic reviews of pharmacological interventions targeting smoking
Seventeen trials of pharmacological interventions were included in the Tsoi et al.95 review, a meta-analysis of seven placebo-controlled RCTs of bupropion showed that smoking cessation rates were significantly higher in treatment groups at the end of treatment [N = 7, n = 340; risk ratio (RR) 3.03, 95% CI 1.69 to 5.42] and a further meta-analysis of five RCTs confirmed this at 6 months (N = 5, n = 214, RR 2.78, 95% CI 1.02 to 7.58).
A meta-analysis of two trials comparing varenicline with placebo reported significantly higher smoking cessation rates in treatment groups at the end of treatment (N = 2, n = 137, RR 4.74, 95% CI 1.34 to 16.71).
Additional individual randomised controlled trials of pharmacological interventions targeting smoking
We identified one RCT in a systematic review of pharmacological and/or behavioural interventions.89 The RCT compared nicotine replacement therapy (NRT) with usual care and reported significant effects on point prevalence abstinence, carbon monoxide (CO) levels and cigarettes smoked per day.90 Findings are summarised in more detail in Table 4.
TABLE 4
Summary of findings from additional individual RCTs of pharmacological interventions targeting smoking
Behavioural interventions targeting smoking
Systematic reviews of behavioural interventions targeting smoking
Five trials of behavioural interventions were included in Tsoi et al.’s95 review. One trial compared a standard smoking cessation programme (American Lung Association programme) with a specialised group therapy intervention for people with schizophrenia and reported significantly higher abstinence rates 6 months post treatment in the standard programme (17.6% vs. 10.7%, p < 0.03). One trial compared a hospital staff-delivered group intervention with one lecture on the dangers of smoking and reported a significant reduction in cigarettes per day at 3 months [F(1,51) = 9.2; p < 0.05]. Other trials compared a single session of motivational interviewing vs. didactic psychoeducation vs. minimal intervention, active repetitive transcranial magnetic stimulation vs. sham repetitive transcranial magnetic stimulation, and high-intensity behaviour support plus NRT vs. low intensity monitoring and information plus NRT. All reported no significant increase in abstinence or reduction in cigarette use or expired CO levels.
Additional individual randomised controlled trials of behavioural interventions targeting smoking
No further RCTs were identified.
Combined pharmacological and behavioural interventions targeting smoking
Systematic reviews of combined pharmacological and behavioural interventions targeting smoking
Three trials of combined pharmacological and behavioural interventions were included in Tsoi et al.95 A contingent reinforcement intervention plus a nicotine patch was significantly better than both contingent reinforcement alone and minimal intervention in promoting smoking cessation at 9 months (50% vs. 28% vs. 10%).
Individual motivational interviewing plus cognitive-behavioural therapy (CBT) plus a nicotine patch was not effective in promoting smoking cessation compared with usual care but did have a significant effect on reducing cigarette consumption by 50% at 3 months (intervention vs. control, 42.5% vs. 15.7%; odds ratio 3.96, 99% CI 1.53 to 10.23; p < 0.001).
A four-arm RCT comparing financial incentives with no incentive and bupropion with placebo reported significantly reduced biomarkers for cigarette smoking [cotinine levels, F(3,144) = 6.40; p < 0.001)] and carbon monoxide levels [F(3,144) = 5.02; p < 0.01] in the financial incentive groups, but no added value of bupropion.
Additional individual randomised controlled trials of combined pharmacological and behavioural interventions targeting smoking
No further RCTs were identified.
Systematic reviews and randomised controlled trials targeting alcohol
Pharmacological interventions targeting alcohol
Systematic reviews of pharmacological interventions targeting alcohol
No systematic reviews of pharmacological interventions targeting alcohol were identified.
Additional individual randomised controlled trials of pharmacological interventions targeting alcohol
We identified five RCTs of pharmacological interventions to reduce alcohol misuse in people with SMI54,55,81,92,93 (Table 5). Two compared naltrexone plus behavioural support with behavioural support alone. They reported contrasting findings: one reported a significant effect on drinking days (number of days on which participants drank) and number of drinks,91 and one reported no effect on these outcomes.92 A placebo-controlled RCT of naltrexone plus disulfiram alone or in combination reported a significant increase on days abstinent and reduction in heavy drinking days (days on which five or more drinks consumed) when compared with no drug.55 Two RCTs of acamprosate54,93 reported no effect on frequency of alcohol, and one reported a significant reduction on number of drinks per week54 but included only nine participants.
TABLE 5
Summary of findings from additional individual RCTs of pharmacological and behavioural interventions targeting alcohol
Behavioural interventions targeting alcohol
Systematic reviews of behavioural interventions targeting alcohol
We identified one systematic review of RCTs of 32 behavioural interventions to reduce alcohol or other substance misuse (n = 3165).94 Interventions included motivational interviewing and CBT alone and in combination, integrated and non-integrated models of care and skills training. Studies were conducted mostly in the USA (n = 19), six in Australia, three in the UK and one each from Denmark, Germany, Ireland and Switzerland. There were no significant effects of any intervention on outcome measures of alcohol or substance use apart from one motivational interviewing intervention on alcohol abstinence at 6 months (n = 28, RR 0.36, 95% CI 0.2 to 0.8; number needed to treat = 2, 95% CI 2 to 5) though this study was rated as ‘very low quality of evidence’.
Additional individual randomised controlled trials of behavioural interventions targeting alcohol
No further RCTs were identified.
Combined pharmacological and behavioural interventions targeting alcohol
No systematic reviews or RCTs were identified.
Interpretation
Summary of main findings
We performed a comprehensive review of international evidence regarding interventions to reduce cardiovascular risk in people with SMI. We included existing systematic reviews as well as more recent RCTs. We found evidence for effective pharmacological and behavioural interventions to decrease weight and smoking, but limited evidence on effective interventions to manage alcohol misuse. We did not find RCT evidence regarding interventions directly targeting cholesterol levels, hypertension, diabetes mellitus or CVD multiple risk factors.
Regarding pharmacological interventions for managing weight, metformin appeared to be most effective over an average 13-week period, with a modest weight loss of nearly 3 kg on average.98 Evidence from the 10 more recent individual trials focused mainly on topiramate, which was also shown to be similarly effective in managing weight,57,67,70 although adverse effects may limit its tolerability.17 Incidentally, it also prevented an increase in the level of cholesterol.70
For behavioural interventions for managing weight, those aimed at individuals (rather than groups), with both dietary and physical activity components were most effective in reducing BMI.96 No trials were identified that assessed the effectiveness of physical activity alone. The most effective interventions to reduce BMI were those combining dietary, physical activity and behavioural support.63,83,85 although adverse effects may limit its tolerability.17 There was some evidence of effectiveness of behavioural interventions on metabolic outcomes with one of three RCTs reporting a significant effect on plasma glucose levels.62
The one trial of a combined pharmacological and behavioural (diet and physical activity) intervention was significantly more effective in reducing BMI, weight and fasting glucose than the pharmacological or behavioural components of the intervention alone.63
Smoking
A meta-analysis demonstrated significant effects of bupropion on smoking abstinence and reduction.95 One trial of NRT reported significant effects on abstinence, number of cigarettes smoked and CO levels.90
One trial of a standardised smoking cessation programme included in a published review was significantly more effective than a SMI-tailored intervention in promoting abstinence.95 Limited conclusions can be drawn from the two RCTs of behavioural interventions identified in this review as their findings conflict: one reported significant effects on reducing smoking; the other reported no significant effect on abstinence.95
One RCT comparing contingency management and bupropion alone or in combination reported a significant effect of contingency management on CO levels but no additional effects of bupropion.95
Most of the trials of smoking cessation or reduction interventions included in the review and RCTs identified by this review were conducted in secondary care. It may be that delivering these interventions to patients in primary care who are likely to be more stable may result in a better response.
Alcohol
There was less evidence regarding alcohol in SMI. Two trials of acamprosate reported no significant effects on frequency of alcohol use.54,93 Two RCTs compared naltrexone plus behavioural support with behavioural support alone; one reported a significant effect in reducing quantity and frequency of use,91 while the other reported no effect. One RCT reported no differentiation in the effectiveness of naltrexone over disulfiram.55
A published review of 32 interventions reported that only one intervention based on motivational interviewing was effective in promoting abstinence but was rated as very low quality of evidence.94
Clinical implications
CVD risk could be reduced in people with SMI by delivering combined pharmacological and behavioural interventions for both smoking and weight reduction. The exact content of interventions could be tailored to the preference of the individual as a variety of elements have been shown to be effective, mostly for individuals but also in groups.
There is little specific evidence regarding the effectiveness of interventions to reduce other CVD risk factors commonly found in SMI, including dyslipidaemia, diabetes mellitus and hypertension; however, there is evidence from the general population on what interventions might work to reduce and manage CVD risk. Algorithms are also available to guide treatment recommendations for reducing CVD risk in the general population in both Australia and the UK.38
Our research findings are in line with recent international guidelines in both England17 and Scotland127 regarding the management of physical health in people with psychosis or bipolar disorder. The lack of evidence regarding managing lipids, diabetes mellitus and hypertension in SMI requires attention. We need to know whether this disadvantaged group of people are accessing and receiving the best interventions to prevent CVD, and then whether interventions, such as statins and antihypertensive drugs, are decreasing excessive rates of CVD. This will require international research to determine whether or not these effective interventions are actually being offered and accepted by people with SMI and ultimately to assess whether or not the stubborn mortality gaps for people with SMI can be diminished in years to come.
Review limitations
It is not straightforward to synthesise all the evidence regarding CVD risk management in SMI. The individual studies frequently have their own design problems and limitations, and evidence is extremely heterogeneous in terms of interventions, settings, target groups and outcome measures. Factors limiting interpretation of findings included small sample sizes, varying or undefined inclusion criteria, setting, short length of follow-up and completeness of intervention description.
Nine of the 23 RCTs not included in the systematic reviews of pharmacological or behavioural interventions to manage weight were tested on SMI patients with a minimum BMI or minimum weight gain since commencing antipsychotic medication (e.g. BMI of > 25 kg/m2 was one of the inclusion criteria).56–58,65–68,79,83 This limits the generalisability of findings to all SMI patients and highlights a reactive rather than proactive approach to weight management in SMI.
Most of the trials identified by this review were conducted in secondary care limiting their generalisability to other settings, such as primary care.
A further limitation is the length of time between intervention end and collection of follow-up data. As is the nature of pharmacological interventions, follow-up data were collected at the end of the intervention which was, on average, 13 weeks after baseline assessment (aside from one that collected data 4 weeks after intervention end). Of the behavioural and combined pharmacological and behavioural interventions the average length of time between intervention end and collection of follow-up data was 19 weeks. This is a relatively short amount of time in the lifespan of people with SMI, so conclusions regarding longer term effectiveness cannot be made.
There was a lack of detail used to describe behavioural interventions. This problem has been acknowledged in the wider literature on interventions to change behaviour.128 Although guidance exists to promote detailed reporting to allow replication;129 for some interventions, replication would not be possible based on the descriptions in published reports we have reviewed.
Conclusion
Although we can be optimistic about the efficacy of interventions to address smoking and weight gain in SMI, we also reveal a lack of evidence for managing a range of CVD risk factors including lipids, diabetes mellitus and hypertension as well as a lack of evidence on managing multiple CVD risk factors. These are the challenges for clinical researchers and service planners alike.
- Systematic review of pharmacological and behavioural interventions for reducing ...Systematic review of pharmacological and behavioural interventions for reducing cardiovascular disease risk in people with severe mental illnesses - Primary care management of cardiovascular risk for people with severe mental illnesses: the Primrose research programme including cluster RCT
- Profile neighbors for GEO Profiles (Select 957169) (199)GEO Profiles
Your browsing activity is empty.
Activity recording is turned off.
See more...