Abbreviations
- MA
meta-analysis
- SIGN
Scottish Intercollegiate Guidelines Network
- SR
systematic review
Context and Policy Issues
Acute sore throat is a symptom that is often caused by an inflammation of the pharynx, tonsils or nasopharynx.1 In a Cochrane Systematic Reviews it was estimated that in patients who in the placebo group, 82% were symptom-free by one week (days six to eight).2 Most cases of acute sore throat are of viral origin and occur as a part of a common cold.1 On average adults would have two to four and children six to eight upper respiratory tract infections per year.1 In addition to viral pathogens, bacterial pathogens can also cause a pharyngeal infection, the most common causative pathogen being Streptococcus pyogenes. Groups C or G beta-hemolytic streptococci, Mycoplasma pneumoniae, and Chlamydia pneumoniae have also been suggested to be pathogens.1,3 A meta-analysis (MA) estimated that the prevalence of Streptococcus pyogenes in children during pharyngitis was approximately 20%.4 Most patients with acute sore throat present with symptoms which include pain on swallowing, headache and cough, and flu-like symptoms.3
In Australia, from April 2009 to March 2010, an inclusive throat complaint accounted for approximately 2% of reasons for an encounter with a general practitioner.5 Treatment options that have been used to relieve acute sore throat symptoms include non-pharmacological interventions (e.g. non-medicated lozenges, non-medicated mouthwashes), oral analgesia (e.g. aspirin, paracetamol, ibuprofen, and diclofenac potassium), medicated lozenges (e.g. lozenges containing benzocaine, hexylresorcinol or flurbiprofen), corticosteroids, antimicrobials, and throat sprays (e.g. chlorhexidine plus benzydamine throat spray).3 Benzydamine HCl 0.15% solution is an oral rinse that is currently indicated for the relief of pain in acute sore throat.6 There is some debate surrounding the benefits of benzydamine HCl 0.15% oral rinse for the relief of pain in acute sore throat.
The purpose of this report is to examine the clinical effectiveness, and evidence-based guidelines regarding the use of benzydamine oral rinse (0.15%) for pain relief in acute sore throat.
Research Questions
What is the clinical effectiveness of benzydamine oral rinse (0.15%) for pain relief in acute sore throat?
What are guidelines informing the use of benzydamine oral rinse (0.15%) for pain relief in acute sore throat?
Key Findings
No evidence was identified for the clinical effectiveness of benzydamine oral rinse (0.15%) for pain relief in acute sore throat. The Scottish Intercollegiate Guidelines Network published a guideline on the pain management for acute sore throat. However no recommendation was produced for the use of benzydamine due to the insufficiency of evidence to support a recommendation.
Methods
Literature Search Methods
A limited literature search was conducted on key resources including PubMed, the Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. No filters were applied to limit the retrieval by study type. The search was also limited to English language documents published between January 1, 2008 and August 28, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2013. Guidelines with unclear methodology were also excluded.
Critical Appraisal of Individual Studies
The included guideline was assessed with the AGREE II instrument.7 Summary scores were not calculated for the included guideline; rather, a review of the strengths and limitations of the included guideline was described narratively.
Summary of Evidence
Quantity of Research Available
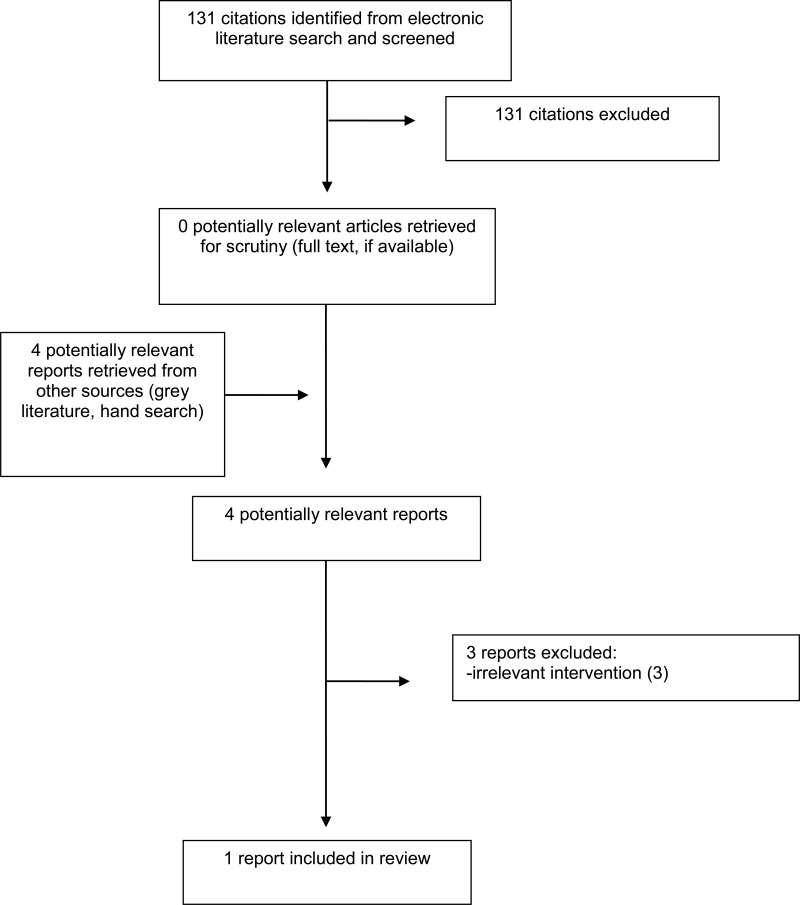
A total of 131 citations were identified in the literature search. Following screening of titles and abstracts, all citations were excluded and no relevant reports from the electronic search were retrieved for full-text review. Four potentially relevant publications were retrieved from the grey literature search for full text review. Of these potentially relevant articles, three publications were excluded for various reasons, and one publication (evidence-based guideline8) met the inclusion criteria and was included in this report. Appendix 1 presents the PRISMA9 flowchart of the study selection.
Additional references of potential interest are provided in Appendix 5.
Summary of Study Characteristics
Additional details regarding the characteristics of included publications are provided in Appendix 2.
Study Design
No relevant studies that examined the clinical effectiveness of benzydamine oral rinse (0.15%) for pain relief in acute sore throat were identified.
One evidence-based guideline was identified regarding the use of benzydamine for pain relief in acute sore throat.8 The guideline was developed by the Scottish Intercollegiate Guidelines Network (SIGN), and published in 2010. This guideline was an update of one previous guideline on the management of sore throat and indications for tonsillectomy which was published in 1999 (SIGN 34: Management of sore throat and indications for tonsillectomy). The guideline working group consisted of independent experts from a broad range of medical expertise (e.g., otolaryngologists, general practitioners, pediatricians, anesthetists, surgeons, nurses) and, overall, the guidelines broadly covered management of sore throat and indications for tonsillectomy.8
Guideline recommendations were based on an evidence review. The evidence was graded on a 4-point scale in which 1++ corresponded to high quality MA, systematic review (SR) of randomized controlled trials of randomized controlled trials with a very low risk of bias. Class 4 corresponded to evidence of the lowest quality and highest risk of bias, expert opinion. The strength of the recommendations, classified as Level A evidence (based on the highest quality evidence) to Level D (based on lowest quality evidence), were based on the level of evidence available. Good practice points corresponded to clinical experience from the guideline development group.8
Country of Origin
The evidence-based guideline was developed by SIGN in Scotland.8
Patient Population
The guideline applies to children and adults with acute and recurrent sore throat, and it covers diagnosis, pain management, antibiotic use, indications for surgical management and postoperative care.8 The guideline indicated that there is no accepted definition of ‘childhood’ exists in Scots law or NHS Scotland. However, the upper cut-off ages used in studies of children included in the guideline varied from 12 to 16. Also, it was indicated that recommendations concerning tonsillectomy in childhood apply to ages 4 to 16.8 Only recommendations related to pain management are relevant to the current report. The aim of the guideline is to suggest an approach to the management of acute sore throat in general practice and to provide criteria for referral for tonsillectomy in recurrent tonsillitis. The intended users are general practitioners, nurses, pediatricians, pharmacists, otolaryngologists, anesthetists, public health specialists, and patients with recurrent sore throat and their careers.8
Interventions and Comparators
The guideline considered aspects of the diagnosis, and pain management for acute and recurrent sore throat including several pharmacological treatments (e.g., paracetamol, ibuprofen, adjuvant compounds to painkillers, topical sprays, Chinese medicines, and adjunctive therapies [benzydamine [topical agents], sprays, lozenges, gargles, and steroids]).8
Outcomes
The outcomes of interest in the guideline were clinical effectiveness and safety (efficacy included reducing fever, headache, achiness, and throat pain; and safety included gastrointestinal adverse effects, nausea, and diarrhea).
Summary of Critical Appraisal
Additional details regarding the strengths and limitations of included guideline are provided in Appendix 3.
The SIGN guideline8 was based on a systematic literature review of reports published from 2000 to 2008 to update previous guideline. Patient-relevant issues were included in the search criteria and, as part of the supporting material, a detailed search strategy was provided for each study design type (i.e., SR, randomized controlled trials, and observational studies). The specific selection criteria and how evidence was synthesized were not provided. The guideline development involved disease experts who declared their potential conflict of interests (unpublished) and patient representatives who provided their views and preferences. The draft guideline underwent public consultation and external specialist review. The guideline developers noted that it would be considered for renewal in three years although no specific process was provided for updating these guidelines. No details on whether a new set of updates are being planned or in progress were available. However, the SIGN website does acknowledge that some of the recommendations in this guideline may be out of date.
One of the main limitations of this guideline was the lack of detail provided on how the systematic literature review was conducted. For instance, the number of articles identified from the literature review and the inclusion/exclusion criteria to screen articles were not provided. This lack of clarity surrounding how the literature was searched makes it difficult to assess in terms of the quality of the systematic review that informed the subsequent development of the guideline recommendations. For instance, the potential for publication bias in the systematic review is uncertain.
Summary of Findings
Clinical effectiveness of benzydamine oral rinse (0.15%) for pain relief in acute sore throat
No relevant evidence regarding the clinical effectiveness of benzydamine oral rinse (0.15%) for pain relief in acute sore throat was identified; therefore, no summary can be provided.
Guidelines for informing the use of benzydamine oral rinse (0.15%) for pain relief in acute sore throat
A summary of the recommendations is presented in this section and details are available in Appendix 4.
One evidence-based guideline, produced by SIGN in Scotland was identified that provides recommendations for the pain management for acute sore throat, including benzydamine.8 With respect to adjunctive therapy including benzydamine topical agents, the guideline did not provide a recommendation due to the insufficiency of evidence to support a recommendation.
Limitations
The main limitation of this review is the paucity of available evidence. No evidence was identified regarding the clinical effectiveness of benzydamine oral rinse (0.15%) for pain relief in acute sore throat. The single evidence-based guideline identified was developed with the Scottish health care system in mind, these results may not be generalizable to the Canadian setting. Although rigorous methodology was generally followed to provide recommendations based on the best available evidence, given the lack of literature identified from the systematic review on the use of benzydamine for pain relief in acute sore throat, no recommendations were provided. The guideline considered benzydamine (topical agents) as adjunctive therapy and no details were provided as for what is the strength or format of the benzydamine treatment was considered in the guideline, hence it cannot be ascertained exactly what the product was, and whether benzydamine oral rinse (0.15%) was considered as an adjunctive therapy in this guideline. Lastly, the guideline did not provide recommendation on the use of benzydamine for the pain management for acute sore throat due to lack due to the insufficiency of evidence to support a recommendation.
Conclusions and Implications for Decision or Policy Making
No evidence was identified for the clinical effectiveness of benzydamine oral rinse (0.15%) for pain relief in acute sore throat. A single evidence-based guideline was identified that considered providing recommendations regarding the use of benzydamine for pain relief in acute sore throat.8 However no recommendation was produced for the use of benzydamine due to the insufficiency of evidence to support a recommendation.8 Therefore, well-designed studies are needed to determine the clinical effectiveness regarding the use of benzydamine oral rinse (0.15%) for pain relief in acute sore throat, and the evidence-based guidelines need to be developed for the Canadian setting.
References
- 1.
Pelucchi
C, Grigoryan
L, Galeone
C, Esposito
S, Huovinen
P, Little
P, et al. Guideline for the management of acute sore throat.
Clin Microbiol Infect. 2012;18 Suppl 1:1–28. [
PubMed: 22432746]
- 2.
- 3.
- 4.
Kronman
MP, Zhou
C, Mangione-Smith
R. Bacterial prevalence and antimicrobial prescribing trends for acute respiratory tract infections.
Pediatrics. 2014;134(4):e956–965. [
PubMed: 25225144]
- 5.
- 6.
- 7.
- 8.
- 9.
Liberati
A, Altman
DG, Tetzlaff
J, Mulrow
C, Gotzsche
PC, Ioannidis
JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publication
Table 2Characteristics of Included Guideline
View in own window
| Intended Users, Target Population | Intervention and Practice Considered | Major Outcomes Considered | Evidence Collection, Selection, and Synthesis | Evidence Quality Assessment | Recommendations Development and Evaluation | Guideline Validation |
|---|
| Scottish Intercollegiate Guidelines Network8 |
|---|
Target population: Acute and recurrent sore throat in children and adults
Intended Users: General practitioners, nurses, pediatricians, pharmacists, otolaryngologists, anesthetists, public health specialists, patients with recurrent sore throat and their carers. | For the management of acute sore throat: Analgesic (paracetamol, ibuprofen, adjuvant compounds to painkillers [e.g. caffeine], topical sprays, Chinese medicines); adjunctive therapies (benzydamine (topical agents), sprays, lozenges (e.g. Fisherman’s Friends), gargles, steroids) Antibiotics (Penicillin V, macrolides, cefalexin, amoxicillin, coamoxiclav) | Clinical effectiveness and safety (including reducing fever, headache, achiness, and throat pain; gastrointestinal adverse effects, nausea, diarrhea) | A systematic review of the literature was carried. Electronic database searches, the date range covered was January 2000-December 2008. Internet searches were carried out on various websites. The main searches were supplemented by material identified by individual members of the guideline development group.
The specific selection criteria and how evidence was synthesized were not provided. | Guideline recommendations were based on an evidence review. The evidence was graded on a 4-point scale in which 1++ corresponded to high quality MA, SR of randomized controlled trials of randomized controlled trials with a very low risk of bias. Class 4 corresponded to evidence of the lowest quality and highest risk of bias, expert opinion. | The guideline development involved disease experts who declared their potential conflict of interests (unpublished) and patient representatives who provided their views and preferences. | The draft guideline underwent public consultation and external specialist review. The guideline developers noted that it would be considered for renewal in three years although no specific process was provided for updating these guidelines. |
MA = meta-analyses; SR = systematic reviews
Appendix 3. Critical Appraisal of Included Publication
Table 3Strengths and Limitations of Guideline using AGREE II7
View in own window
| Item | Guideline |
|---|
| SIGN8 |
|---|
| Domain 1: Scope and Purpose |
|---|
| 1. The overall objective(s) of the guideline is (are) specifically described. | ✓ |
| 2. The health question(s) covered by the guideline is (are) specifically described. | ✓ |
| 3. The population (patients, public, etc.) to whom the guideline is meant to apply is specifically described. | ✓ |
| Domain 2: Stakeholder Involvement |
|---|
| 4. The guideline development group includes individuals from all relevant professional groups. | ✓ |
| 5. The views and preferences of the target population (patients, public, etc.) have been sought. | ✓ |
| 6. The target users of the guideline are clearly defined. | ✓ |
| Domain 3: Rigour of Development |
|---|
| 7. Systematic methods were used to search for evidence. | ✓ |
| 8. The criteria for selecting the evidence are clearly described. | x |
| 9. The strengths and limitations of the body of evidence are clearly described. | x |
| 10. The methods for formulating the recommendations are clearly described. | ✓ |
| 11. The health benefits, side effects, and risks have been considered in formulating the recommendations. | x |
| 12. There is an explicit link between the recommendations and the supporting evidence. | ✓ |
| 13. The guideline has been externally reviewed by experts prior to its publication. | ✓ |
| 14. A procedure for updating the guideline is provided. | ✓ |
| Domain 4: Clarity of Presentation |
|---|
| 15. The recommendations are specific and unambiguous. | ✓ |
| 16. The different options for management of the condition or health issue are clearly presented. | ✓ |
| 17. Key recommendations are easily identifiable. | ✓ |
| Domain 5: Applicability |
|---|
| 18. The guideline describes facilitators and barriers to its application. | ✓ |
| 19. The guideline provides advice and/or tools on how the recommendations can be put into practice. | ✓ |
| 20. The potential resource implications of applying the recommendations have been considered. | ✓ |
| 21. The guideline presents monitoring and/or auditing criteria. | ✓ |
| Domain 6: Editorial Independence |
|---|
| 22. The views of the funding body have not influenced the content of the guideline. | ✓ |
| 23. Competing interests of guideline development group members have been recorded and addressed. | ✓ |
AGREE II = Appraisal of Guidelines for Research and Evaluation – II; SIGN = Scottish Intercollegiate Guidelines Network; ✓ = yes; X = no or unclear.
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 4Summary of Recommendations in Included Guideline
View in own window
| Evidence | Recommendations |
|---|
| Scottish Intercollegiate Guidelines Network8 |
|---|
Evidence for adjunctive therapy (benzydamine [topical agents], sprays, lozenges [e.g. Fisherman’s Friends], gargles, steroids):
“No good quality evidence on the effectiveness of nonprescription throat sprays, lozenges and gargles was identified. No studies provided evidence of lasting benefit. No trials compared these products with conventional analgesics.” (P. 9) | Recommendation:
“There is insufficient evidence to support a recommendation.” (p. 9) |
Appendix 5. Additional References of Potential Interest
Clinical Practice Guidelines – irrelevant intervention
Pelucchi
C, Grigoryan
L, Galeone
C, et al. Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012;18 Suppl 1:1–28. [
PubMed: 22432746]
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Benzydamine for acute sore throat: A review of clinical effectiveness and guidelines. Ottawa: CADTH; 2018 Sep. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.