Abbreviations
- AAD
Antibiotic associated diarrhea
- CDI
Clostridium difficile infection
- C. difficile
Clostridium difficile
Context and Policy Issues
Antibiotics are frequently prescribed for a variety of conditions worldwide.1 Antibiotic use results in altering the gastrointestinal flora1,2 The changes in the gastrointestinal flora allows C. difficile to more easily colonize and infect patients.1 This gives rise to various symptoms, notably diarrhea which is referred to as antibiotic associated diarrhea (ADD). AAD occurs in 5% to 39% of patients treated with antibiotics.2 AAD may result in hospitalization and increased health care costs.3
Probiotics are live microorganisms which, when administered in sufficient quantities, counterbalance the changes in the gastrointestinal flora resulting from antibiotic use.3 This reduces the risk of colonization by other pathogenic bacteria and confers health benefits to the host.3 The most commonly tested probiotic species include the Lactobacillus genus, Bifidobacterium genus and Saccromyces genus.2 There is some debate surrounding the benefits of probiotics.
The purpose of this report is to review the evidence-based guidelines regarding the use of probiotics for the prevention, management, and treatment of AAD and C. difficile infection. A second CADTH report to be published subsequently will review the clinical effectiveness of probiotics
Research Question
What are the evidence-based guidelines regarding the use of probiotics for the prevention, management, and treatment of antibiotic-associated diarrhea and C. difficile infection?
Key Findings
One guideline recommended the use of probiotics for treatment of antibiotic associated diarrhea (varying strength of recommendation depending on the product).
Four guidelines did not recommend the use of probiotics for prevention of C. difficile infection; and two of these guidelines also did not recommend use of probiotics for treatment of C. difficile infection. One guideline mentioned that probiotics may be considered for prevention and treatment of C. difficle (weak recommendation)
Methods
Literature Search Methods
A limited literature search was conducted on key resources including PubMed, The Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases and a focused Internet search. Methodological filters were applied to limit retrieval to health technology assessments, systematic reviews, meta-analyses, randomized controlled trials and guidelines. The search was limited to English language documents published between January 1, 2013 and August 8, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2013. Guidelines that did not appear to be evidence based were excluded.
Critical Appraisal of Individual Studies
The included guidelines were assessed with the AGREE II instrument.4 Summary scores were not calculated for the included studies; rather, a review of the strengths and limitations of each included study were described narratively.
Summary of Evidence
Quantity of Research Available
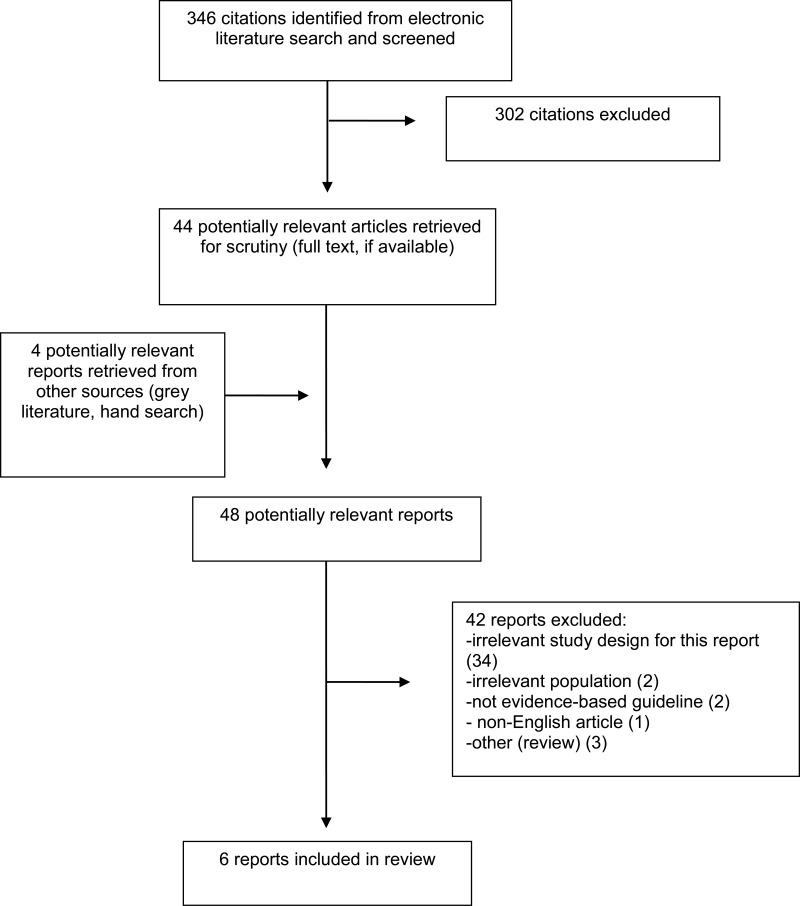
A total of 346 citations were identified in the literature search. Following screening of titles and abstracts, 302 citations were excluded and 44 potentially relevant reports from the electronic search were retrieved for full-text review. Four potentially relevant publications were retrieved from the grey literature search. Of these 48 potentially relevant articles, 42 publications were excluded for various reasons, while six publications met the inclusion criteria and were included in this report. These comprised six evidence-based guidelines.3,5–9
Appendix 1 presents the PRISMA flowchart of the study selection.
Summary of Study Characteristics
Six relevant evidence-based guidelines.3,5–9 were identified. Study characteristics are summarized below and details are available in Appendix 2, and .
Study Design
In five guidelines3,5–7,9 a systematic literature search was conducted, and one guideline8 used a systematic review published by the Agency of Healthcare research and Quality. The method used for formulating the recommendations was by consensus in three guidelines,5,6,9 and unclear in three guidelines,3,7,8. The recommendations were graded in five guidelines.3,5–8 and not graded in one guideline.9
Country of Origin
Three guidelines were from the USA, one each published in 2018,6 2015,7 and 2013.8 Two guidelines were from the Netherlands, one each published in 2018,3 and 2013.5
Patient Population
The guidelines were relevant to patients who had CDI5–9 or AAD3 as a result of antibiotic use.
Interventions and Comparators
Five guidelines.5–9 mentioned probiotics in the recommendation but did not mention any specific types. One guideline3 recommended specific probiotic products (details presented in Appendix 4
).
Outcomes
Recommendations on use of probiotics for management of CDI.5–9 and AAD3 were presented.
Summary of Critical Appraisal
Critical appraisal of the studies is summarized below and details are available in, Appendix 3
.
In all the included guidelines.3,5–9 the scope, purpose, and the evidence associated with the recommendations were described. In four guidelines.5,7–9 the guideline development group comprised experts but details were not presented, in one guideline6 the guideline development group had multidisciplinary experts, and in one guideline3 the composition of the guideline development group was unclear. In five guidelines.3,5–7,9 a systematic literature search was conducted and in one guideline8 a literature search was not undertaken but rather a previously conducted systematic review was used. In all the guidelines it was unclear if patient input or resource implications had been considered.
Recommendations were graded in five guidelines,3,5–8 and not graded in one guideline.9 In three guidelines5,6,9 recommendations were formulated based on consensus, and in three guidelines3,7,8 the method for formulating guidelines was unclear. In one guideline5 it was mentioned that there were no conflicts of interest; in one guideline6 conflicts of interest were declared and a process was in place to address this; in three guidelines.3,8,9 conflicts of interest were declared and potential for bias was unclear; and in one guideline7 conflicts of interest were not presented. Although some elements of the quality assessment tool were not satisfied, considering that some key elements were satisfied, overall our confidence in the recommendations is not reduced.
Summary of Findings
A summary of the recommendations is presented in this section and details are available in Appendix 4, .
Guidelines
One guideline,3 recommends the use of Lactobacillus rhamnosus GG for the prevention of AAD (three star rating). Other probiotic products containing one or more of the following: Lactobacillus casei, Saccharomyces boulardii, Bifidobactirium bifidum, Bifidobacterium lactis, Enterococcus faecium, Lactobacillus acidophilus, Lactobactobacillus paracasei, Lactobacillus plantarum, Lactobacillus rhamnosus, Lactobacillus salivarous, Bifidobacterium longun, are recommended for treatment of ADD (one star rating)
Two guidelines6,8 do not recommend probiotic treatment for prevention of CDI, one guideline9 does not recommend probiotics for prevention or as an adjunct treatment for CDI, and one guideline5 does not recommend probiotics for the initial treatment of CDI. One guideline7 mentioned that probiotics may be useful in the prevention and treatment of C.difficle associated diarrhea and may be considered for recurrent and recalcitrant CDI (weak recommendation based on high quality evidence).
Limitations
The AAD and CDI guidelines are based on limited available evidence on the use of probiotics for AAD or CDI. The probiotic products appear to have varied composition hence it is not clear which particular probiotic product is suitable for which category of patients or type of antibiotic used.
None of the identified guidelines were developed in Canada, hence it is possible that not all of the probiotic products mentioned in these guidelines are available in Canada.
Conclusions and Implications for Decision or Policy Making
Of the six relevant guidelines3,5–9 reviewed, one3 made recommendations regarding the management of AAD and five guidelines5–9 were for the management of CDI.
Overall, the majority of the recommendations do not support the use of probiotics for the prevention or treatment of CDI. One of the five guidelines pertaining to antibiotic-induced CDI recommends that probiotics be considered for prevention and management; the guideline development group considered this a weak recommendation.
One guideline,3 recommends the use of probiotics for treatment of AAD, with the strength of the recommendation varying depending on the specific probiotic product.
It is unclear whether all of the probiotic products recommended are available in Canada, thus it is unclear how generalizable the results are to the Canadian setting.
References
- 1.
Goldenberg
JZ, Yap
C, Lytvyn
L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children.
Cochrane Database Syst Rev. 2017;12:Cd006095. [
PMC free article: PMC6486212] [
PubMed: 29257353]
- 2.
Blaabjerg
S, Artzi
DM, Aabenhus
R. Probiotics for the prevention of antibiotic-associated diarrhea in outpatients-a systematic review and meta-analysis.
Antibiotics (Basel). 2017;6(4):21. [
PMC free article: PMC5745464] [
PubMed: 29023420]
- 3.
Agamennone
V, Krul
CAM, Rijkers
G, Kort
R. A practical guide for probiotics applied to the case of antibiotic-associated diarrhea in The Netherlands.
BMC Gastroenterol. 2018;18(1):103. [
PMC free article: PMC6091175] [
PubMed: 30078376]
- 4.
- 5.
Debast
SB, Bauer
MP, Kuijper
EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection.
Clin Microbiol Infect. 2014;20
Suppl 2:1–26. [
PubMed: 24118601]
- 6.
McDonald
LC, Gerding
DN, Johnson
S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA).
Clin Infect Dis. 2018;66(7):987–994. [
PubMed: 29562266]
- 7.
Steele
SR, McCormick
J, Melton
GB, et al. Practice parameters for the management of Clostridium difficile infection.
Dis Colon Rectum. 2015;58(1):10–24. [
PubMed: 25489690]
- 8.
Surawicz
CM, Brandt
LJ, Binion
DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections.
Am J Gastroenterol. 2013;108(4):478–498; quiz 499. [
PubMed: 23439232]
- 9.
Trubiano
JA, Cheng
AC, Korman
TM, et al. Australasian Society of Infectious Diseases updated guidelines for the management of Clostridium difficile infection in adults and children in Australia and New Zealand.
Intern Med J. 2016;46(4):479–493. [
PubMed: 27062204]
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Guidelines
View in own window
| Group and/or First Author, Year, Country | Objective | Guideline Development Group, Intended Users | Methodology |
|---|
| Agamenone,3 2018, The Netherlands | Aim: to provide a guide for the use of probiotics for the prevention of AAD | GDG was not specified.
Intended users: healthcare professionals and patients | Systematic literature search was conducted. Multiple databases were searched.
Articles were selected according to pre-defined inclusion and exclusion criteria. One person selected articles and extracted data.
Unclear how the recommendations were formulated.
Recommendations were graded. |
| ASCRS (Steele),7 2015, USA | Aim: to provide guidelines for the evaluation, management, and prevention of CDI | GDG comprised mainly of clinicians
Intended users: practitioners, health care workers, and patients | Systematic literature search was conducted. Multiple databases were searched. Other methodological details were not presented
The recommendations were formulated by the primary authors of the Guideline document and reviewed by the Clinical Practice Guidelines Committee, however the specifics of the method used was not described.
Recommendations were graded using the GRADE system. |
| Australasian Society of Infectious Diseases (Trubiano)9 2016, Australia | Aim: to provided guidelines for the management of CDI in adults and children | GDG comprised experts in the area; details were not presented.
Intended users: Australasian clinicians | Systematic literature search was conducted using a single database (PubMed)
Recommendations were formulated based on consensus response achieved by discussion.
Recommendations were not graded |
| ESCMID (Debast)5, 2013, The Netherlands | Aim: to provide an overview of currently available treatment options for CDI, and to develop evidence-based update of treatment recommendations | GDG comprised experts in the area; details were not presented.
Intended users: those in clinical practice | Systematic literature search was conducted using PubMed and Google Scholar
Recommendations were formulated based on consensus using the method of Ullmann et al.
Recommendations were graded using GRADE system |
| IDSA & SHEA (McDonald)6 2018, USA | Aim: to update previous (2010) clinical practice guideline for CDI in terms of diagnosis, treatment, prevention of infection, and environmental management. | GDG comprised multidisciplinary experts (in areas of epidemiology, diagnosis, infection control, clinical management of patients
Intended users: those in clinical practice | Systematic literature search was conducted using multiple databases.
Recommendations were formulated based on consensus
Recommendations were graded using the GRADE system. |
| Surawicz,8 2013, USA | Aim: to provide guidelines for the diagnosis, treatment, and prevention of CDI | GDG comprised experts in the area; details were not presented.
Intended users: not specified | Method not described. It was mentioned however that it was based on a systematic review by AHRQ
The method for formulating the recommendations was not specified
Recommendations were graded using the GRADE system. |
AAD = antibiotic associated diarrhea; AHRQ = Agency for Healthcare Research and Quality; ASCRS = American Society of Colon and Rectal Surgeons; CDI = Clostridium difficile infection; ESCMID = European Society of Clinical Microbiology and Infectious Diseases; GDG = guideline development group, GRADE = Grades of Recommendation, Assessment, Development, and Evaluation; IDSA = Infectious Diseases Society of America; SHEA = Society for Healthcare Epidemiology of America
Table 3Grade of Recommendations and Level of Evidence for Guidelines
View in own window
| Grade of Recommendations | Strength of Evidence |
|---|
| Agamenone,3 2018, The Netherlands |
|---|
Three categories of recommendations.
Three star recommendation: significant effects for the reduction of AAD shown in at least three of the selected studies.
Two star recommendation: significant effects for the reduction of AAD shown in at least two of the selected studies.
One star recommendation: significant effects for the reduction of AAD shown in one study, a trend supported by two or more studies, or | NR |
| ASCRS (Steele),7 2015, USA |
|---|
GRADE system
1: Strong recommendation
2: weak recommendation | GRADE system
A: RCTs without important limitations or overwhelming evidence from observational studies
B: RCTs with important limitations (inconsistent results, methodological flaws, indirect or imprecise) or exceptionally strong evidence from observational studies
C: Observational studies or case series |
Implications of the recommendation grade assigned using GRADE:
1A: Strong recommendation, can apply to most patients in most circumstances without reservation
1B: Strong recommendation, can apply to most patients in most circumstances without reservation
1C: Strong recommendation but may change when higher quality evidence becomes available
2A: Weak recommendation, best action may differ depending on circumstances or patients’ or societal values
2B: Weak recommendation, best action may differ depending on circumstances or patients’ or societal values
2C: Very weak recommendations; other alternatives may be equally reasonable |
| Australasian Society of Infectious Diseases (Trubiano)9 2016, Australia |
|---|
| NR | NR |
| ESCMID (Debast)5, 2013, The Netherlands |
|---|
GRADE system
A = strongly supports recommendation of use
B = moderately supports recommendation of use
C = marginally supports recommendation of use
D = supports a recommendation against use | GRADE system
2a level
“I Evidence from at least one properly designed randomized, controlled trial.
II Evidence from at least one well-designed clinical trial, without randomization; from cohort or case–control analytic studies (preferably from more than one centre); from multiple time series; or from dramatic results of uncontrolled experiments.
III Evidence from opinions of respected authorities, based on clinical experience, descriptive case studies, or reports of expert committees.” Page 3 |
| IDSA & SHEA (McDonald)6 2018, USA |
|---|
GRADE system
Strong recommendation
Weak recommendation | GRADE system
Evidence level
Initial confidence in estimate of effect based on study type:
RCT – high confidence
Observational studies – low confidence.
Reasons for confidence level being lowered includes risk of bias, inconsistency, indirectness, imprecision, and publication bias.
Reasons for confidence level being increased includes large effect, dose response, and all plausible confounding and bias considered |
| Surawicz,8 2013, USA |
|---|
GRADE system
“The strength of a recommendation is graded as “strong”, when the evidence shows the benefit of the intervention or treatment clearly outweighs any risk, and as “conditional”, when uncertainty exists about the risk – benefit ratio.” Page 478 | GRADE system
“The quality of the evidence is graded as follows: “high”, if further research is unlikely to change our confidence in the estimate of the effect; “moderate”, if further research is likely to have an important impact and may change the estimate; and “low”, if further research is very likely to change the estimate”. Page 478 |
AAD = antibiotic associated diarrhea; ASCRS = American Society of Colon and Rectal Surgeons; ESCMID = European Society of Clinical Microbiology and Infectious Diseases; GRADE = Grades of Recommendation, Assessment, Development, and Evaluation; IDSA = Infectious Diseases Society of America; NR = not reported, RCT = randomized controlled trial; SHEA = Society for Healthcare Epidemiology of America;
Appendix 3. Critical Appraisal of Included Publications
Table 4Strengths and Limitations of Guidelines using AGREE II.4
View in own window
| Strengths | Limitations |
|---|
| Agamennone,3 2018, The Netherlands |
|---|
The scope and purpose were clearly stated. A systematic review was conducted using standard methodology Evidence was provided The document was likely externally reviewed as it was published in a journal Recommendations were not graded
|
Unclear if patient preferences were considered Unclear if resource implications were considered Unclear if a policy was in place for updating the guideline Conflicts of interests were declared; potential for bias seemed unlikely
|
| ASCRS (Steele),7 2015, USA |
|---|
The scope and purpose were clearly stated. GDG comprised mainly of clinicians A systematic review was conducted, however details of methodology were not presented Evidence was provided The document was likely externally reviewed as it was published in a journal Recommendations were graded using the GRADE system
|
Unclear if patient preferences were considered Unclear if resource implications were considered Unclear if a policy was in place for updating the guideline Conflicts of interest of the authors were not mentioned
|
| Australasian Society of Infectious Diseases (Trubiano)9 2016, Australia |
|---|
The scope and purpose were clearly stated. The guideline development group comprised experts in the area, details were not presented A systematic literature search was conducted using a single database (PubMed) Evidence was provided As this guideline is an update of a previous guideline, it is likely that a policy for updating is in place Conflicts of interest were declared. Some of the authors had association with industry. Potential for bias with respect to treatment with probiotics is unclear.
|
Unclear if the document was externally reviewed Unclear if patient preferences were considered Unclear if resource implications were considered Likely there was a policy for updating as the included guideline was an update Recommendations were not graded
|
| ESCMID (Debast)5, 2013, The Netherlands |
|---|
The scope and purpose were clearly stated. The guideline development group comprised experts in the area, details were not presented A systematic literature search was conducted using a single PubMed and Goggle Scholar. Method followed AGREE criteria Evidence was provided As this guideline is an update of a previous guideline, it is likely that a policy for updating is in place. Recommendations were graded using GRADE It was mentioned that the authors had no conflicts of interest.
|
Unclear if the document was externally reviewed Unclear if patient preferences were considered Unclear if resource implications were considered Likely there was a policy for updating as the included guideline was an update
|
| IDSA & SHEA (McDonald)6 2018, USA |
|---|
The scope and purpose were clearly stated. The guideline development group comprised multidisciplinary experts A systematic literature search was conducted between 2009 to 2016 using multiple databases Evidence was provided Recommendations were graded using GRADE. As this guideline is an update of a previous guideline, it is likely that a policy for updating is in place Conflicts of interest were declared. Some of the authors had association with industry. Potential for bias with respect to treatment with probiotics is unclear. However, there was a policy in place to handle conflicts of interest.
|
Unclear if patient preferences were considered Unclear if resource implications were considered Unclear if the document was externally reviewed. It was reviewed by appropriate committees and Boards of IDSA and SHEA.
|
| Surawicz,8 2013, USA |
|---|
The scope and purpose were clearly stated. The guideline development group comprised experts in the area but details were not provided A systematic review does not appear to have been conducted however the evidence from a systematic review by AHRQ was used. Evidence was provided As this was published in a journal it is likely that it was externally reviewed Conflicts of interest were declared. Some of the authors had association with industry. Potential for bias with respect to treatment with probiotics is unclear.
|
Unclear if patient preferences were considered Unclear if resource implications were considered Unclear if there was a policy in place for updating
|
ASCRS = American Society of Colon and Rectal Surgeons; CDI = Clostridium difficile infection; ESCMID = European Society of Clinical Microbiology and Infectious Diseases; GRADE = Grades of Recommendation, Assessment, Development, and Evaluation; IDSA = Infectious Diseases Society of America; SHEA = Society for Healthcare Epidemiology of America;
Appendix 4. Recommendations
Table 5Recommendations and Associated Evidence
View in own window
| Evidence | Recommendations |
|---|
| Agamennone,3 2018, The Netherlands |
|---|
| Based on systematic review and meta-analysis of the evidence identified, there was a suggestion of reduction in the incidence of AAD. | List of recommended probiotics (brand name: probiotic strains and CFU per dose) for AAD
Three star recommendation:
Microbial Platinum:
Lactobacillus rhamnosus GG, 3.3 × 1010 CFU per dose
Culturelle:
Lactobacillus rhamnosus GG, 1.0 × 1010 CFU per dose
One star recommendations:
Actimel (dairy product):
Lactobacillus casei DN-114001, 1.0 × 1010 CFU per dose
Probioticum:
Saccharomyces boulardii, 2.5 × 109 CFU per dose
Winbiotic Pro-AD:
Bifidobacterium bifidum W23, 1.1 × 109 CFU per dose
Bifidobacterium lactis W51, 1.1 × 109 CFU per dose
Enterococcus faecium W54, 1.1 × 109 CFU per dose
Lactobacillus acidophilus W37, 1.1 × 109 CFU per dose
Lactobacillus acidophilus W55, 1.1 × 109 CFU per dose
Lactobacillus paracasei W20, 1.1 × 109 CFU per dose
Lactobacillus plantarum W62, 1.1 × 109 CFU per dose
Lactobacillus rhamnosus W71, 1.1 × 109 CFU per dose
Lactobacillus salivarius W24, 1.1 × 109 CFU per dose
Probactial Duo:
Saccharomyces boulardii, 6.0 × 109 CFU per dose
Lactobacillus acidophilus NCFM, 2.1 × 109 CFU per dose
Lactobacillus paracasei Lpc-37, 2.1 × 109 CFU per dose
Bifidobacterium lactis Bi-04, 2.1 × 109 CFU per dose
Bifidobacterium lactis Bi-07, 2.1 × 109 CFU per dose
Imutis:
Saccharomyces boulardii 6.0 × 109 CFU per dose
Lactobacillus acidophilus 2.0 × 109 CFU per dose
Lactobacillus rhamnosus 3.0 × 109 CFU per dose
Bifidobacterium longum, 2.0 × 109 CFU per dose
Advanced Multi-Billion Dophilus:
Lactobacillus acidophilu, LA-5, 1.3 × 109 CFU per dose
Lactobacillus paracasei L CASEI 431, 1.3 × 109 CFU per dose
Lactobacillus rhamnosus GG, 1.3 × 109 CFU per dose
Bifidobacterium lactis BB-12, 1.3 × 109 CFU per dose |
| ASCRS (Steele),7 2015, USA |
|---|
| Two systematic reviews suggested reduced incidence of C. difficile associated diarrhea with the use of probiotics. Two systematic reviews suggested that only specific probiotic strains such as Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Saccharomyces boulardii are effective in the prevention of C. difficile infection. The authors further mentioned that that there was still uncertainty regarding the optimal agent, the length of therapy, and the dose. | Recommendation:
“Probiotics may be useful in the prevention and treatment of C difficile-associated diarrhea.” Page 15
Recommendation grade: 2A (weak recommendation based on high quality evidence) |
| Australasian Society of Infectious Diseases (Trubiano)9 2016, Australia |
|---|
| The authors mentioned that a previous Australian guideline did not recommend probiotic therapy for prevention or adjunctive treatment option for CDI. A Cochrane review and some reports did not support probiotic therapy. Further a revised Cochrane systematic review and two recent RCTs were reviewed. One large RCT did not show any benefit in preventing CDI with a probiotic mixture of lactobacilli and bifidobacteria. | Recommendation:
“While probiotics may be of some value in limited studies, we do not recommend probiotic therapy use in prevention or adjunctive treatment in CDI.” Page 483 |
| ESCMID (Debast)5, 2013, The Netherlands |
|---|
Findings for probiotic treatment for CDI appear to be conflicting. One meta-analysis concluded that moderate quality evidence suggested benefit with probiotic prophylaxis for CDI. Whereas a Cochrane systematic review concluded that evidence was insufficient to recommend use of probiotics, in general, as an adjunct to antibiotic treatment for CD diarrhea
One RCT and one evidence-based review based on subgroup analysis showed efficacy with adjunct probiotic for recurrent CDI but not for initial CDI, on comparing relapse rates. | Recommendation:
‘There is insufficient evidence to support administration of probiotics, toxin-binding resins and polymers, or monoclonal antibodies.” Page 10
For the recommendation on the adjunct therapy for initial CDI, the quality of evidence is level 1, and the strength of probiotic recommendation is level D, Page 14, 18 |
| IDSA & SHEA (McDonald)6 2018, USA |
|---|
| Several meta-analyses suggested that probiotics may prevent CDI when given to patients on antibiotics, who do not have a history of CDI. However, in the studies with the greatest influence on the meta-analyses results, the placebo arm had CDI rates much higher than that would be expected for the patient population studied, potentially biasing the results towards benefit with probiotics. Furthermore, the organisms present in the probiotic formulations have the potential of causing infections in hospitalized patients (from three publications). | Recommendation:
“There are insufficient data at this time to recommend administration of probiotics for primary prevention of CDI outside of clinical trials (no recommendation).” Page e30 |
| Surawicz,8 2013, USA |
|---|
One meta-analysis concluded that S. boulardii was effective for C. difficile disease, however a Cochrane systematic review concluded that there was insufficient evidence to recommend probiotics, in general as an adjunct to antibiotics in the treatment of CDI.
There is no strong evidence to support use of probiotics for the treatment of RCDI, and only weak evidence regarding the efficacy of S. bourlardi.
Evidence on decrease in CDI with probiotics is limited One RCT showed that a probiotic yoghurt drink (containing Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophiles) decreased the risk of CDI, however this RCT had with a small number of patients and also the placebo arm patients experienced greater rate of CDI than normally expected, potentially the results towards benefit with probiotics. Another study found capsules containing Lactobacillus acidiphilus and Lactobacillus casei were effective in preventing both AAD and CDI in hospitalized patients. | Recommendation:
“There is limited evidence for the use of adjunct probiotics to decrease recurrences in patients with RCDI. (Moderate recommendation, moderat-equality evidence)” Page 487
“Although there is moderate evidence that two probiotics (L. rhamnosus GG and S. boulardii) decrease the incidence of antibiotic associated diarrhea, there is insufficient evidence that probiotics prevent CDI. (Strong recommendation, low-quality evidence)” Page 491 |
AAD = antibiotic associated diarrhea, ASCRS = American Society of Colon and Rectal Surgeons; CDI = Clostridium difficile infection; ESCMID = European Society of Clinical Microbiology and Infectious Diseases; IDSA = Infectious Diseases Society of America; RCDI = recurrent CDI; RCT = randomized controlled trial; SHEA = Society for Healthcare Epidemiology of America;
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Probiotics for antibiotic-associated diarrhea and Clostridium difficile Infection: a review of guidelines. Ottawa: CADTH; 2018 Sep. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.