5.2. In adults (including older persons) and adolescents with bone metastases, what is the evidence for the use of bisphosphonates or monoclonals compared with each other or no treatment or other bisphosphonates in order to prevent and treat pain
The systematic review team have divided Key Question 5.2 into five sections: bisphosphonates versus placebo, comparisons of bisphosphonates, monoclonal antibodies (hereafter monoclonals) versus placebo, comparisons of monoclonals, and bisphosphonates versus monoclonals.
5.2.1. Bisphosphonates vs. Placebo
Forty eligible studies compared bisphosphonates to placebo (see Evidence Profile 5.2.1).81–120 Most study participants had either breast or prostate cancer. Fifteen of the studies were restricted to people (women or men) with breast cancer (or included mostly people with breast cancer). Ten studies were restricted to men with prostate cancer. Two additional studies included mostly people with breast or prostate cancer. The third most common cancer across studies was lung cancer. Thirteen studies evaluated clodronate, nine zoledronate, five each ibandronate and pamidronate, and one each etidronate and risendronate.
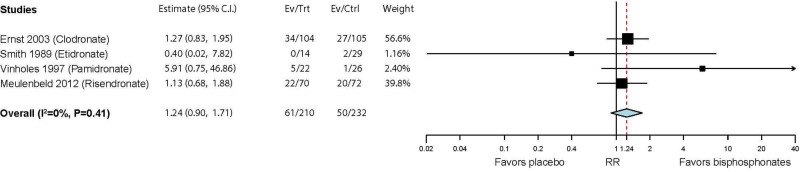
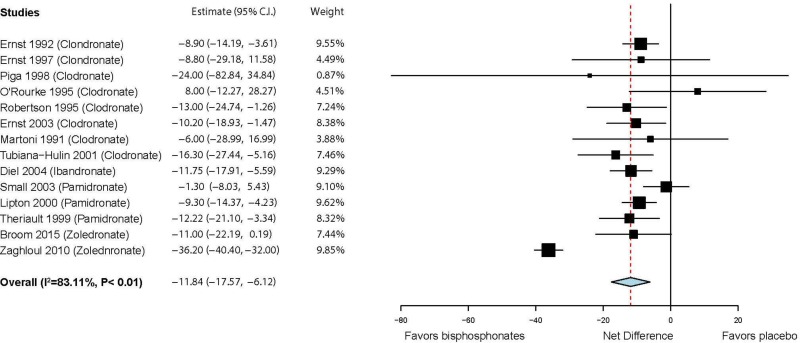
There is moderate strength of evidence of greater pain relief with use of bisphosphonates compared with placebo among patients with painful bone metastases. Seven trials evaluated categorical pain relief; however, four evaluated improvements in pain (e.g., reductions of at least 2 points on a 5 point pain scale)89,99,109,117 and three evaluated complete pain relief.86,96,107 The studies were mostly vague about whether they were assessing overall cancer pain or metastatic bone pain. Four studies evaluated clodronate and one each etidronate, pamidronate, and risedronate. Although favoring use of bisphosphonates, no statistically significant difference in complete relief of pain (RR 1.61; 95% CI 0.89, 2.93) or pain improvement (RR 1.24; 95% CI 0.90, 1.71) were found (see and below). Fourteen trials evaluated pain on continuous scales (which were each converted to a 100 point scale, with 100 = worst pain).83,85,87–89,97,98,101,104,105,108,111,113,119 Six studies evaluated clodronate, three pamidronate, and one each ibandronate and zoledronate. The studies, overall, indicated statistically significant improvement in pain, with an overall net difference of −11.8 (95% CI −17.6, −6.1) (See below).
No study evaluated speed of pain relief. A single study provided low strength of evidence suggesting no significant difference in duration of pain relief between risendronate and placebo in people with prostate cancer. The study reported HR = 1.27 (95% CI 0.84, 1.92), favoring placebo (3.4 month median duration with risendronate, 5.5 months with placebo).
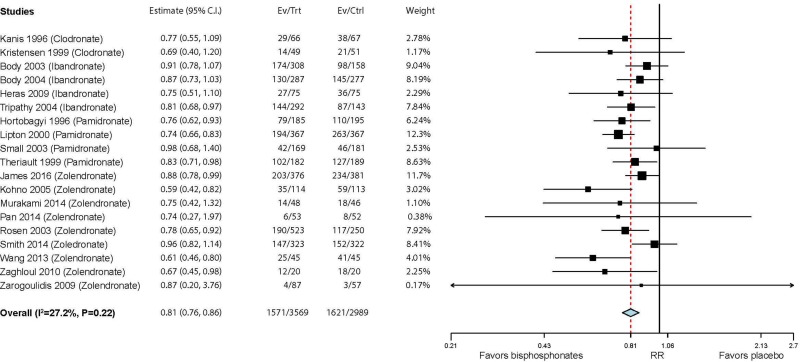
Twenty-five studies evaluated the various skeletal-related events.85,90,92–95,97,100,102,103,105,108,110,114,118–128 Fourteen of the studies included people with breast cancer (or mostly breast cancer), four prostate cancer, three lung cancer (or mostly lung cancer), and one bladder cancer. Nine of the studies evaluated zoledronate, five ibandronate, and four each clodronate and pamidronate. Overall, the studies provided moderate strength of evidence that bisphosphonates reduce the risk of skeletal-related events. The six studies that reported hazard ratios for time to first skeletal-related event (any) in comparisons of zoledronate (4 studies) or ibandronate (2 studies) found a statistically significant benefit of bisphosphonates over placebo (HR = 0.71; 95% CI 0.61, 0.84).82,90,92,106,110,119 Eighteen studies found a reduction in risk of any skeletal-related event yielding a summary RR of 0.81 (95% CI 0.76, 0.86) (see below).81,82,90–95,97,100,106,108,110–112,118–120
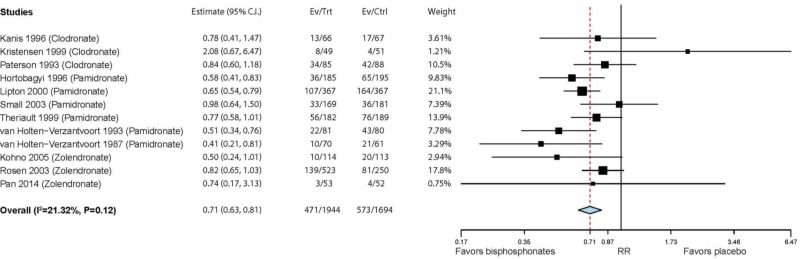
Twelve trials also found a reduction in risk of fracture with bisphosphonates (RR = 0.75; 95% CI 0.67, 0.84) (see below). Eight trials nominally favored bisphosphonates to reduce the risk of spinal cord compressions (RR = 0.74; 95% CI 0.49, 1.12) (see below). The three zoledronate studies together found a statistically significant reduction in risk of spinal cord compression (RR = 0.52; 95% CI 0.27, 0.99), but this result was not significantly different than the nonsignificant summary of the pamidronate studies (RR = 1.07; 95% CI 0.60, 1.90; P=0.72 between studies of different medications).
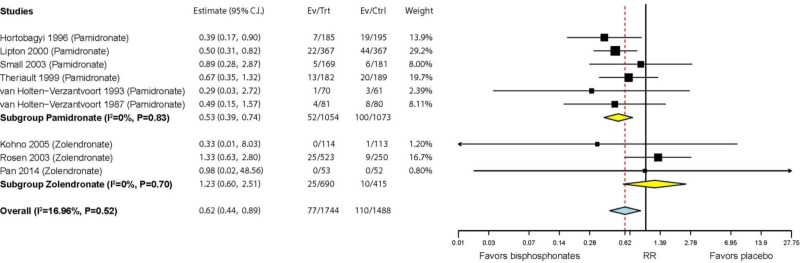
The 12 studies that reported on bone radiotherapy found a significantly reduced risk with bisphosphonates (RR = 0.71; 95% CI 0.63, 0.81) (see below). Nine studies also found a significantly reduced risk of bone surgeries with bisphosphonates (RR = 0.62; 95% CI 0.44, 0.89) (see below). A significantly greater risk reduction was found in the four studies of pamidronate (RR = 0.53; 95% CI 0.39, 0.74) than the two studies of zoledronate (RR = 1.23; 95% CI 0.60, 2.51; P=0.042 between studies of different medications).
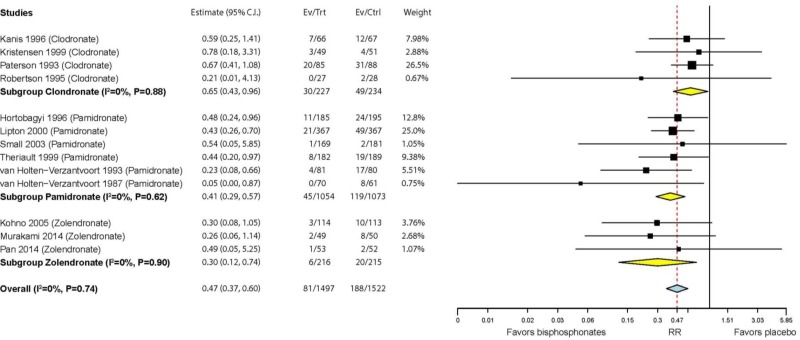
Thirteen studies reported on risk of hypercalcemia with bisphosphonates (see below). Overall, bisphosphonates lowered the risk of hypercalcemia compared with placebo (RR = 0.47; 95% CI 0.37, 0.60). The studies of zoledronate (RR = 0.30; 95% CI 0.12, 0.74) and pamidronate (RR = 0.41; 95% CI 0.29, 0.57) showed a nominally stronger effect on hypercalcemia than studies of clodronate (RR = 0.65; 95% CI 0.43, 0.96), but the differences among studies of different medications were not statistically significant (P=0.072).
Five studies provide varying strength of evidence that bisphosphonates do not affect quality of life compared with placebo.84,85,89,92,105 The studies evaluated clodronate (3 studies), ibandronate (1 study), and zoledronate (1 study). The five studies provided very low strength of evidence of no significant difference in changes in quality of life scores measured on a variety of scales (summary net difference on a 0 to 100 [best] scale = 8; 95% CI −6, 22), but one study provided moderate strength of evidence of reduced and delayed deterioration in quality of life with clodronate (RR = 0.81; 95% CI 0.67, 0.99 and HR = 0.71; 95% CI 0.56, 0.92).84
Two studies provided very low to low strength of evidence of small improvements in functional outcomes with bisphosphonates compared with placebo.92,97 One study each found net differences (all transformed to 100 point scale where 100 = best score) in ECOG performance status of −7.7 (95% CI −17.0, 1.7), in FACT-P physical well-being of 1.4 (95% CI 0.5, 3.3), in FACT-P social well-being of 1.8 (95% CI 1.0, 2.6), and in FACT-P functional well-being of 1.8 (95% CI 0.6, 2.9). However, it should be noted that these confidence intervals are estimated from reported data and for the FACT-P scores, the study implied they found no significant differences between zoledronate and placebo.
Four studies explicitly reported on the risk of osteonecrosis of the jaw.83,99,106,116 Across the studies, there were no occurrences of this adverse event with either bisphosphonates (N=460) or placebo (N=450).
Evidence Profile 5.2.1. Bisphosphonates vs. Placebo (PDF, 406K)
Scores from individual studies have been transformed to a uniform 0–100 scale (100 = worst).
Evidence-to-Decision table 5.2.1. In adults (including older persons) and adolescents with bone metastases, what is the evidence for the use of bisphosphonates compared no treatment in order to prevent and treat pain? (PDF, 488K)
5.2.2. Comparisons of Bisphosphonates
Seven eligible studies compared different bisphosphonates (see Evidence Profile 5.2.2) in patients with various cancers with bone metastases–mostly breast, prostate, and non-small cell lung cancer127,136; Francini, 2011 #235;Choudhury, 2011 #236;Wang, 2013 #237;Barrett-Lee, 2014 #238;von Au, 2016 #239}. The studies evaluated clodronate, ibandronate, pamidronate, and zoledronate. Study participants were generally older, with study mean ages ranging from 53 to 73 years old. As will be shown, the evidence is relatively sparse, with only seven studies evaluating four bisphosphonates. There are six possible pairwise comparisons (e.g., clodronate vs. ibandronate, clodronate vs. pamidronate, …). With more studies reporting on the same outcomes, network meta-analysis may be feasible in the future. Given these limitations, the evidence is of low or very low strength, as will be elaborated. For these reasons, there are not six separate evidence profiles (for each pairwise comparison) and no relative effects (e.g., RR) for these pairwise comparisons. Instead, absolute event rates (or within-arm changes) are provided for each of the four medications.
With only two or three studies evaluating pain control, there is low strength of evidence of no differences in relief of pain or mean changes in pain scores across the different bisphosphonates. From one study, pain relief on ibandronate (6%) was less common than on other bisphosphonates (15–26% in one or two studies for each medication). Changes in pain (as a continuous measure from 0 to 100 [worst]) were similar for each of the four bisphosphonates (−3.3 to −5.0). The studies did not report on speed of pain relief. Two studies provided very low strength of evidence regarding duration of pain relief. One study found no difference in average duration of pain relief in patients with a variety of cancers (about half with lung cancer) between ibandronate (5.5 months) and pamidronate (5.2 months).137 One study reported that in patients with prostate cancer those taking clodronate had longer duration of pain relief (13 months) than those taking zoledronate (9 months, P=0.03).138
Six studies reported on skeletal-related events. However, the studies had serious methodological limitations, sparsely reported on any give comparison across the four bisphosphonates, and were generally small resulting in imprecision. Thus, there is very low strength of evidence overall regarding skeletal-related events. Broadly similar percentages of people had any skeletal-related event across bisphosphonates (18–26%, no data on pamidronate). Within studies, fracture rates were mostly similar between bisphosphonates, except in one study of people with breast cancer in which 16% of those taking clodronate had fractures compared with 7% taking pamidronate (P=0.03). Three studies found no significant differences in rates of spinal cord compression across bisphosphonates. Two studies no significant differences in rates of bone radiotherapy across bisphosphonates. Three studies found no significant differences in rates of bone surgery across bisphosphonates.
Three studies reported on rates of hypercalcemia across bisphosphonates. Two of these found no differences in risk of hypercalcemia between ibandronate (10.7%) and zoledronate (9.3%) in one study, and between clodronate (2.9%) and zoledronate (1.4%) in the other. The third study, however, reported the hypercalcemia rate in the zoledronate group (28%%) was lower than with ibandronate (45%; RR = 0.64; 95% CI 0.39, 1.03) or with pamidronate (50%; RR = 0.57; 95% CI 0.35, 0.91). Three studies reported rare rates of osteonecrosis of the jaw for clodronate (1.5%), ibandronate (0.7%), and zoledronate (1.2), providing low strength of evidence.
Evidence Profile 5.2.2. Comparison of Bisphosphonates (PDF, 624K)
Evidence-to-Decision table 5.2.2. In adults (including older persons) and adolescents with bone metastases, what is the evidence for the use of bisphosphonates compared to other bisphosphonates in order to prevent and treat pain? (PDF, 485K)
5.2.3. Monoclonals vs. Placebo
A single eligible study compared monoclonals to placebo (Evidence Profile 5.2.3). The study evaluated tanezumab in adults with prostate cancer, breast cancer, renal cell carcinoma, or multiple myeloma with painful bone metastases (mean age 56 years, range 32 to 77).141
The study provided very low strength of evidence of no difference in average or worst pain between groups (between group differences −2.6 [95% CI −11.8, 6.6] and −0.1 [95% CI −9.3, 9.1], respectively), and in percentage of people who achieve pain relief (by at least 50%) (RR = 1.38 [95% CI 0.55, 3.49]).
The study did not report on speed of pain relief, duration of pain relief maintenance, quality of life, or functional outcomes.
The study provided very low strength of evidence regarding skeletal-related events, reporting only that 1 of 29 (3.4%) patients in the tanezumab arm had a femur fracture but, implicitly, none of the 30 people on placebo had a fracture (although one had undefined metastatic disease progression).
No study reported on osteonecrosis of the jaw.
Evidence Profile 5.2.3. Monoclonals vs. Placebo (PDF, 517K)
Evidence-to-Decision table 5.2.3. In adults (including older persons) and adolescents with bone metastases, what is the evidence for the use of monoclonal antibodies (monoclonals) compared to no treatment in order to prevent and treat pain? (PDF, 507K)
5.2.5. Monoclonals vs. Bisphosphonates
Nine eligible trials compared monoclonal antibodies and bisphosphonates (Evidence Profile 5.2.5).142–150 All evaluated the monoclonal denosumab; most evaluated zoledronate. but also pamidronate, or a variety of bisphosphonates (based on local practice). Studies included patients with metastatic bone lesions, mostly from breast or prostate cancer, but also non-small cell lung cancer, multiple myeloma, and other cancers. Three trials with identical protocols,146–148 except for which cancers were eligible, were separately conducted and reported, but also combined and reported in a summary article.142 Patient ages varied widely across studies.
One study provided low strength of evidence for pain relief and time until pain relief (speed) and very low strength of evidence for quality of life.150 The study included people with either breast cancer or multiple myeloma and compared denosumab and zoledronate. The study found no difference in the percentage of people who had decreases in their pain scores of at least 2 (of 10) points (RR = 0.89; 95% CI 0.67, 1.10); they did not evaluate complete pain relief. The study also found no difference in average time until this pain outcome was reached (2.7 vs. 2.6 months). The study also found no significant difference in quality of life, as assessed by an improvement of at least 5 (of 108) points in FACT-G (Functional Assessment of Cancer Therapy-General; RR = 1.08; 95% CI 0.95, 1.23). No study evaluated pain reduction maintenance.
The studies provide (mostly) high strength of evidence favoring denosumab over bisphosphonates to prevent skeletal-related events. Across six studies, rates of any skeletal-related event (summary RR = 0.86; 95% CI 0.81, 0.91), fracture (summary RR = 0.88; 95% CI 0.78, 0.96), bone radiation therapy (summary RR = 0.80; 95% CI 0.73, 0.88), and hypercalcemia (summary RR = 0.58; 95% CI 0.34, 0.81) were statistically significantly more common among those treated with bisphosphonates. Spinal cord compression and bone surgery were rarer events, but also occurred less frequently among patients taking denosumab, although the differences were nonsignificant in a single study reporting spinal cord compression (RR = 0.88; 95% CI 0.65, 1.20) and bone surgery (RR = 0.87; 95% CI 0.62 to 1.23). Because only a single study reported these outcomes, they were deemed to have moderate strength of evidence.
Two studies provided low strength of evidence for functional outcomes. The studies both reported that people taking denosumab had better functional outcomes than those on zoledronate, although in both studies the differences were not statistically significant. The studies evaluated time to increase (worsening) in interference due to pain (16 vs 14.9 months) and ECOG performance status (RR = 1.07 [95% CI 0.99, 1.16]). Three studies provide high strength of evidence regarding the risk of osteonecrosis of the jaw. The adverse event was more common with denosumab than bisphosphonates, with a summary RR = 1.40 (95% CI 0.92, 2.13).
Evidence Profile 5.2.5. Monoclonals vs. Bisphosphonates (PDF, 544K)
Evidence-to-Decision table 5.2.5. In adults (including older persons) and adolescents with bone metastases, what is the evidence for the use of monoclonal antibodies (monoclonals) compared to bisphosphonates to prevent and treat pain? (PDF, 525K)