Abbreviations
- ACR
American College of Radiology
- ASA
American Society of Anesthesiologists
- ASTM
American Society for Testing and Materials
- MRI
Magnetic resonance imaging
- NHMRC
National Health and Medical Research Council
- PICO
Patient, interventions, comparators, outcomes
- SMRI
Society for Magnetic Resonance Imaging
- RCT
randomized controlled trial
Context and Policy Issues
Magnetic resonance imaging (MRI) is a commonly used procedure in the diagnosis and evaluation of diseases. MRI uses a strong magnetic field and radio waves to produce a detailed cross-sectional 3-dimensional image of the patient’s internal organs and structures.1 While the relative safety and advantages of MRI over other imaging technologies resulted in its ever increasing use, there are a number of safety issues resulting from patient-related and equipment-related factors. Equipment with ferromagnetic property may pose a serious projectile hazard due to magnetic attraction, and should be permanently fixed or tethered to the floor or the wall.2 Patients may also suffer from thermal injury resulting from excessive heating of equipment during MRI, particularly with electrical conductive materials.2 Electromagnetic interference is another major concern that can cause device malfunction or lead to distortions or artefacts in the MRI image, therefore special protective cables or enclosures are used.2
MRI “safe” equipment is identified as having no ferromagnetic parts or radiofrequency interference.3 MRI “unsafe” equipment is comprised of ferromagnetic materials or those that cause radiofrequency interference.3 MRI “conditional” equipment may contain ferromagnetic parts but can operate properly at a safe distance from the MRI system.3 Equipment with MRI compatibility is necessary to operate in the MRI environment, which conventional accessories are not designed for.3 MRI scanners have magnets with different strengths, and compatibility should always be assessed from reliable sources. In addition, proper labeling of all equipment with specifications to operational conditions is mandatory.1
The American College of Radiology (ACR) published a guidance document on MR safety practices1 in which four zones of increasing magnetic sensitivity and barriers to access were described. Zone 1 and 2 constitute the freely accessible area outside the MRI environment and the interface separating the more restricted areas, respectively. Zone III is a restricted area within the MRI suite with strict control on ferromagnetic objects or equipment and should be under supervision of authorized personnel. Zone IV contains the MRI scanner; therefore the highest degree of restriction should be imposed to prevent any magnet-associated hazard.1
Monitoring of physiological parameters during an MRI examination is necessary for patients who are sedated or anesthetized, critically ill, or have underlying health issues.2 The Society for Magnetic Resonance Imaging (SMRI) recommends monitoring of patients at least visually or verbally, and using appropriate physiological monitors for high-risk patients or those unable to respond during MRI.5 In addition, some patients may require mechanical ventilation, which require the same safety considerations as monitoring.5 A number of guidelines are available to ensure the safety of patients and healthcare professionals working in an MRI unit.1,4,6–9 However, evidence for clinical effectiveness of equipment used in MRI settings is limited, particularly for ventilators and physiological monitors.
The objective of this report is to summarize the available evidence regarding the clinical effectiveness and evidence-based guidelines for MRI-compatible ventilators and physiological monitoring equipment for use during MRI in adult patients requiring ventilation.
Research Questions
What is the clinical effectiveness of magnetic resonance imaging (MRI)-compatible ventilators in adult patients requiring ventilation during an MRI exam?
What is the clinical effectiveness of physiological monitoring equipment used during a MRI exam in adult patients on a ventilator?
What are the evidence-based guidelines associated with the use of MRI-compatible ventilators for adult patients undergoing a MRI exam?
What are the evidence-based guidelines associated with the use of physiological monitoring equipment used for ventilated adult patients during an MRI exam?
Key Findings
No evidence regarding the clinical effectiveness of MRI-compatible ventilators and physiological monitors for ventilated patients during MRI was identified.
No evidence-based guidelines associated with the use of MRI-compatible ventilators and physiological monitoring for ventilated adult patients during MRI exams were found.
Methods
Literature Search Methods
A limited literature search was conducted on key resources PubMed, The Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. Methodological filters were applied to limit the retrieval to health technology assessments, systematic reviews, meta-analyses, randomized controlled trials, non-randomized studies, and guidelines. The search was limited to English language documents published between January 1, 2013 and May 23, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to 2013. Guidelines with no information on whether a systematic literature search was performed to collect evidence were also excluded.
Summary of Evidence
Quantity of Research Available
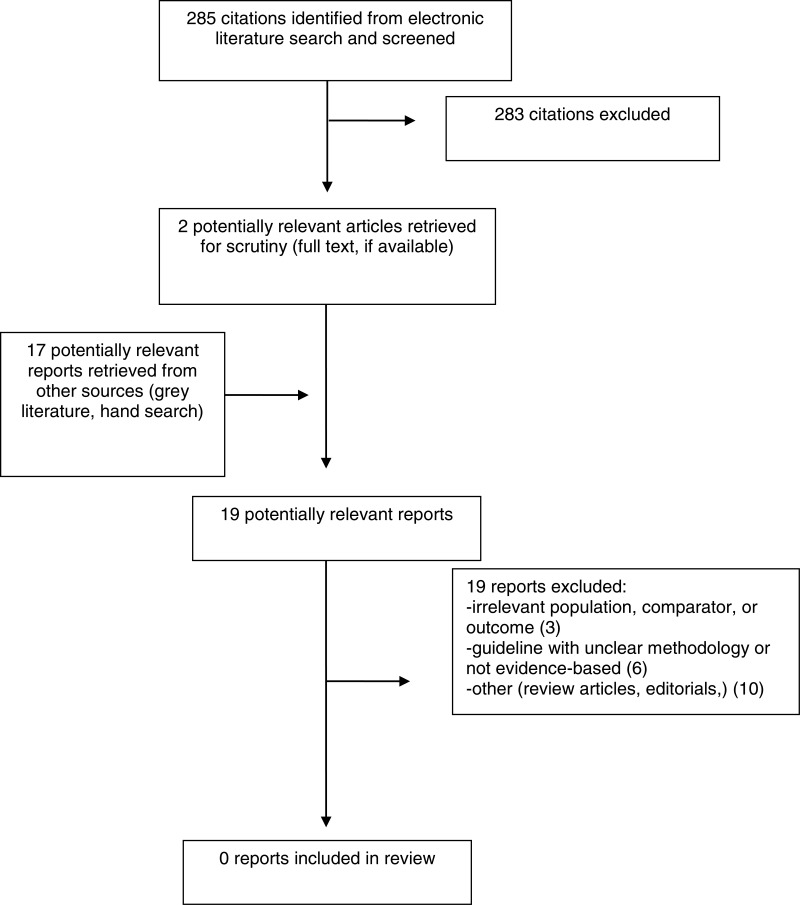
A total of 285 citations were identified in the literature search. Following screening of titles and abstracts, 283 citations were excluded and two potentially relevant reports from the electronic search were retrieved for full-text review. In addition, 17 potentially relevant publications were retrieved from the grey literature search for full text review. None of these potentially relevant articles met the inclusion criteria described in . Appendix 1 presents the PRISMA flowchart of the study selection. Additional references of potential interest are provided in Appendix 5.
Summary of Findings
Question 1. What is the clinical effectiveness of MRI-compatible ventilators in adult patients requiring ventilation during an MRI exam?
No relevant evidence regarding the clinical effectiveness of MRI-compatible ventilators was identified; therefore, no summary can be provided.
Question 2. What is the clinical effectiveness of physiological monitoring equipment used during a MRI exam in adult patients on a ventilator?
No relevant evidence regarding the clinical effectiveness of physiological monitoring equipment for ventilated patients during MRI was identified; therefore, no summary can be provided.
Question 3. What are the evidence-based guidelines associated with the use of MRI-compatible ventilators for adult patients undergoing a MRI exam?
No relevant evidence-based guidelines associated with the use of MRI-compatible ventilators were identified; therefore, no summary can be provided.
Question 4. What are the evidence-based guidelines associated with the use of physiological monitoring equipment used for ventilated adult patients during an MRI exam?
No relevant evidence-based guidelines associated with the use of physiological monitors for ventilated patients during MRI were identified; therefore, no summary can be provided.
Conclusions and Implications for Decision or Policy Making
No relevant clinical studies or guidelines were identified from a five-year review of the literature that provided information on the clinical effectiveness, safety and practice guidelines for MRI-compatible ventilators and physiological monitoring among adult patients requiring ventilation during MRI. The paucity in evidence warrants further research in this area. One practice advisory, which did not meet in the inclusion criteria of this review, was primarily targeted towards anesthesiologists to ensure safe practices within an MRI environment and provided information on the safety of physiological monitors.10 The authors of the advisory indicated that the practice advisory report was not intended as standards or guidelines; instead a set of systematically developed recommendations that may be adopted, modified, or rejected according to the clinical needs and constraints of the local institution. Evidence from several non-comparative observational studies and case reports included in the advisory suggest that monitoring equipment are not associated with serious harms involving the MRI scanner or the individual, other than occasionally evoking a burning sensation or interference with the MRI scan. However, the evidence is not specific to patients on ventilation, and there is significant uncertainty in the quality of the included studies due to a lack of study quality assessment and adequate details. The authors recommended that MRI-compatible ventilators should be available in the unit; however, this appears to be applicable to any equipment used during MRI without any supporting evidence for clinical benefits. The advisory also recommends that anesthesiologists should ensure safety for all personnel within the MRI location, and responsible for inspecting and labeling all equipment for MRI compatibility, communicating with other healthcare professionals in case patients require ventilation, monitoring or other steps, providing anesthetic care, and management of any emergencies. With no recently published comparative data or guidelines on the safety and clinical effectiveness of MRI-compatible ventilators and physiological monitors for ventilated patients, there is a significant dearth in the evidence to inform policy decisions.
References
- 1.
Expert Panel on MR Safety, Kanal
E, Barkovich
J, Bell
C, Borgstede
JP, Bradley
WG
Jr., et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530. 10.1002/jmri.24011 Accessed 2018 Jun 19. [
PubMed: 23345200] [
CrossRef]
- 2.
Shellock
FG. Monitoring patients in the MR environment.
MRISafety.com. Shellock R and D Services; 2018.
http://www.mrisafety.com Accessed 2018 Jun 19.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
Practice advisory on anesthetic care for magnetic resonance imaging: an updated report by the American Society of Anesthesiologists Task Force on Anesthetic Care for Magnetic Resonance Imaging. Anesthesiol. 2015;122(3):495–520. [
PubMed: 25383571]
Appendix 1. Selection of Included Studies
Appendix 1. Additional References of Potential Interest
Guideline without the population of interest
Guidelines with unclear methodology, not evidence-based, without information specific to the research questions, or published before 2013
Expert Panel on MR Safety, Kanal
E, Barkovich
J, Bell
C, Borgstede
JP, Bradley
WG
Jr., et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37:501–530. 10.1002/jmri.24011 Accessed 2018 Jun 19. [
PubMed: 23345200] [
CrossRef]
Canadian Guidelines/HTA reports
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
MRI-compatible ventilators and physiological equipment for patients undergoing MRI exams: a review of clinical effectiveness and guidelines. Ottawa: CADTH; 2018 Jun. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.