Abbreviations
- ASA
Acetyl salicylic acid
- CADTH
Canadian Agency for Drugs and Technologies in Health
- hCG
Human chorionic gonadotrophin
- IV
Intravenous
- IVF
In-vitro fertilization
- IVIG
Intravenous immunoglobulin
- NICU
Neonatal intensive care unit
- NK
Natural killer cells
- NKT
Natural killer T-cells
- RCT
Randomized controlled trial
- RM
Recurrent miscarriage
- RPL
Recurrent pregnancy loss
- RRF
Recurrent reproductive failure
- RSA
Recurrent spontaneous abortion
- SCIG
Subcutaneous immunoglobulin
- TSA
Trial-sequential analyses
- URSA
Unexplained recurrent spontaneous abortion
Context and Policy Issues
Spontaneous abortion is common, with estimates of up to 30% of pregnancies lost before the sixth week of gestation, though most of these go both clinically and otherwise unrecognized.1 The incidence of clinically recognized spontaneous abortion is generally estimated to occur between one and two percent of known pregnancies.2 Spontaneous abortion that consecutively recurs three or more times for the same parents is referred to as recurrent spontaneous abortion(RSA).3 RSA has been associated with distress in those affected, causing grief and contributing to depression and anxiety.4,5
The cause of RSA is often difficult to identify, with half of all cases considered to be idiopathic.1,2 Known causes of RSA can include endocrine (including immunologic) and hematological disorders; anatomical defects, and; genetic and epigenetic factors.1,5 Individuals diagnosed with idiopathic RSA generally are known to generally have favourable prognoses and often do achieve successful pregnancy and live birth without treatment.6 Where the cause of RSA is known, treatment will vary according to the particular condition, and may include supportive care, low-dose aspirin and/or heparin, corticosteroids, metformin, in-vitro fertilization (IVF), surgical interventions, supplementation of progesterone or human chorionic gonadotrophin (hCG), suppression of luteinizing hormone, and intravenous immunoglobulin (IVIG).6
Immunoglobulin (also referred to as immune globulin or gamma globulin) is a purified blood product pooled from the plasma of healthy blood donors.7 Immunoglobulin may be administered as IVIG or as subcutaneous immunoglobulin (SCIG). In Canada, various preparations of immunoglobulin are approved specifically for use in patients with one or more of the following six conditions: primary immune deficiency, immune thrombocytopenic purpura, secondary immune deficiency states, chronic inflammatory demyelinating polyneuropathy, Guillain-Barré Syndrome, and multifocal motor neuropathy.8 The products approved for use are ANTHRASIL, Flebogamma, Octagam, Cutaquig (subcutaneous), and WinRho SDF.9,10 Others approved for marketing are Atgam, Cytogam, Gammagard, Gamunex, Hepagam B, Igivnex, Panzyga, Privigen, and Varizig.8,11
Between 1998 and 2006, Canada’s per capita use of IVIG grew 115%, which makes Canada one of the highest consumers of IVIG per capita worldwide.9,12,13 The belief is that much of this growth is attributable to an increase in off-label use of IVIG.10,12,13 A three month audit in 2007 conducted by the Ontario Regional Blood Coordinating Network found that: 50% of IVIG use was on-label; 40% was off-label but potentially clinically effective; and 10% was off-label and possibly not clinically effective.14 In Canada (except Quebec), Canadian Blood Services supplies IVIG to hospitals at no charge, however, there is no formal mechanism for oversight regarding IVIG use.9,10,14 Each dose of IVIG can cost between $550 and $2200 CAD per child and between $2000 and $8000 CAD per adult; this does not include other associated costs of treatment.13 From April 2005 to March 2006, IVIG use cost Canadian Blood Services $196.1 million CAD.9
IVIG has been identified as a potentially beneficial therapy for patients with RSA; thus, this report aims to synthesize available evidence on the clinical effectiveness of off-label use of IVIG for RSA. This report is complementary to a 2017 CADTH Rapid Response, Summary of Abstracts report: “Off-Label Use of Intravenous Immunoglobulin for Solid Organ Transplant Rejection, Paraneoplastic Disorders, or Recurrent Miscarriage: Clinical Effectiveness”.15
Research Questions
What is the clinical effectiveness of off-label use of intravenous or subcutaneous immunoglobulin for the treatment of recurrent spontaneous abortion?
Key Findings
Two systematic reviews, three randomized controlled trials, and four non-randomized, studies were identified describing the clinical effectiveness of intravenous immunoglobulin for the treatment of recurrent spontaneous abortion. One systematic review of good quality, one randomized controlled trial of good quality, two randomized controlled trials of moderate quality, and one non-randomized study of limited quality found no difference in live birth rates between patients with recurrent spontaneous abortion treated with intravenous immunoglobulin compared to placebo or other treatments. One systematic review of good quality, one non-randomized study of moderate quality and two nonrandomized studies of limited quality reported a significant improvement in rates of live birth for participants with recurrent spontaneous abortion treated with intravenous immunoglobulin compared to participants not receiving intravenous immunoglobulin. Of five included studies reporting on either adverse events or side effects, no serious adverse events were reported, with four studies reporting minor side effects in some patients treated with intravenous immunoglobulin compared to controls. Obstetric, perinatal, and neonatal outcomes were reported in four included studies, with no important clinical differences identified between treatment groups. The authors of most included studies suggested that additional evidence from larger, randomized studies remains necessary to reduce uncertainty concerning the clinical effectiveness of intravenous immunoglobulin to patients with recurrent spontaneous abortion.
Methods
Literature Search Methods
A limited literature search was conducted on key resources including PubMed, The Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. Methodological filters were applied to limit retrieval to health technology assessments, systematic reviews, meta-analyses, randomized controlled trials, and non-randomized studies. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2012 and October 26, 2017.
Selection Criteria and Methods
One reviewer screened all citations returned from the literature searches. In the first phase of screening, titles and abstracts were reviewed for relevance and those deemed to be potentially relevant were then retrieved15 and later assessed for eligibility by another reviewer using full-text.
The inclusion of sources at the full-text level of screening was based on the eligibility criteria outlined in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , did not use a comparative design, were duplicate publications, were systematic reviews whose included publications overlapped completely with other eligible systematic reviews, or were published prior to 2012.
Critical Appraisal of Individual Studies
Included systematic reviews were critically appraised by one reviewer using the AMSTAR 2 instrument.16 Both randomized and non-randomized studies were critically appraised using the Down’s and Black tool,17 No summary scores were calculated; rather, findings from critical appraisal were tabulated (Appendix 5) and a review of the strengths and limitations for each included study were described narratively.
Summary of Evidence
Quantity of Research Available
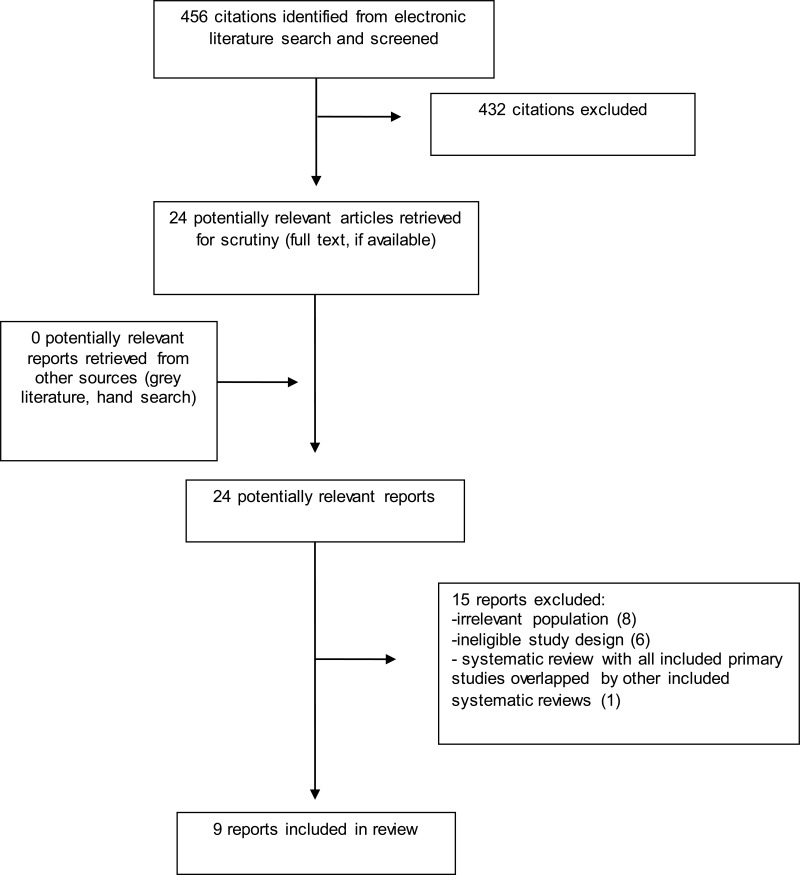
A total of 456 citations were identified in the literature search. Following screening of titles and abstracts, 432 citations were excluded and 24 potentially relevant reports from the electronic search were retrieved for full-text review. No potentially relevant publications were identified. Of the 24 potentially relevant articles, 15 publications were excluded for various reasons, and 9 publications were found to meet the review’s inclusion criteria, consequently being included within this report. These comprised two systematic reviews, three RCTs, and four non-randomized studies. Appendix 1 presents the PRISMA flowchart18 describing study selection. Additional references of potential interest are provided in Appendix 5.
Summary of Study Characteristics
Study Design
Two systematic reviews(SR), published in 201519 and 201620 were identified. Both SRs sought randomized controlled trials (RCT) published until December, 2014,19 or until May, 2016.20 Each SR included 11 RCTs, and both used the Cochrane Risk of Bias tool to critically appraise included studies.19,20 There was overlap between nine of the included the studies in the two SRs, and this is detailed in Appendix 5.
Three randomized controlled trials21–23 (RCT) and four non-randomized, observational studies24–27 were also identified by this review. One RCT specified the duration of follow-up per outcome i.e., for women with live birth, women were followed throughout pregnancy and children were followed to two years; for patients with spontaneous abortion, follow-up was until loss of pregnancy and; for patients who did not conceive, follow-up ended at 12 months.21 The remaining studies included in this review either did not specify follow-up duration22,23,25–27 or did not report follow-up clearly.24 Of the non-randomized studies, two were prospective,24,26 and two were retrospective.25,27
Country of Origin
One SR was based in China,20 and the another SR was conducted by authors from Denmark.19 The RCTs were conducted in China,21 Denmark,22 and Iran.23 Two of the four non-randomized, observational studies were conducted in Spain,25,26 and the other two were conducted in Iran24 and Korea.27
Patient Population
One SR reported data from studies evaluating 582 pregnant patients with a history of ≥2 consecutive, recurrent, spontaneous abortions (RSA).20 The other SR reported on 531 people with a history of 3 or more consecutive, spontaneous abortions.
The three RCTs limited their recruitment to people with a history of 3 or more consecutive, spontaneous abortions. One RCT randomly allocated 192 participants to either the intervention or comparison arms of the study.21 Participants were recruited from a specialty miscarriage center in Korea and had a mean age of 31.2 years (SD ±3.7) in the intervention group and 31.6 (SD ±4.2) years in the comparison group (P = 0.81).21 Another RCT recruited and randomized 82 pregnant patients with secondary RSA from a recurrent miscarriage clinic in Denmark, with 42 patients allocated to the intervention arm (mean age 34.5 years, 95% CI 26.2-40.9) and 40 allocated to the comparison arm (mean age 34.2 years, 95% CI 26.1-41.0).22 The third RCT included in this review randomized 60 participants from a hospital-based Obstetrics and Gynecology department to either the intervention or comparison arms of the trial. The mean age of patients in the intervention arm of the study was 29.7 years (SD ±3.1), and in the comparison arm was 31.2 years (SD ±2.1).23
Both of the prospective, non-randomized studies limited recruitment to participants with a history of 3 or more consecutive, spontaneous abortions.24,26 One study was a controlled clinical trial of 94 participants recruited from a hospital-based infertility center in Iran with a mean age of 33.8 years (SD ±3.6) in the intervention arm and 34.1 years (±SD 3.4) in the comparison group. The other prospective, non-randomized study used a case-control design investigating 64 participants recruited from a hospital-based immunology clinic in Spain with either RSA (n=24) or recurrent implantation failure (n=40); the latter of which were not eligible for inclusion within the current review. Among the nested cohort of 24 eligible participants with RSA, those in the intervention group had a mean age of 35.7 years (SD ±4.4) and those in the comparison group had a mean age of 37 years (SD±5.1).26
Patients evaluated within the two retrospective, non-randomized studies had a history of either 2 or more 27 or 3 or more consecutive, RSA.25 The former reviewed medical records and stratified participants based on the findings of an etiological assessment of their history of RSA from a hospital-based Obstetrics and Gynecology department in Korea.27 The latter study examined 428 participants with recurrent reproductive failure – including 217 with RSA25 and a mean age of 36.48 (SD ±3.63, range 27 to 43) who were eligible for analysis in the current review.
Interventions and Comparators
The interventions of interest in one SR were IVIG before or during early pregnancy compared with placebo;20 and in the other SR were IVIG compared with placebo, no treatment, or standard care.19 Duration, dosing, and frequencies varied across included studies.19,20
The three RCTs all examined various regimens of IVIG compared with various active comparators.21–23 The Chinese trial administered pre-pregnancy treatment on a three-month alternating schedule (i.e., three months on treatment; three months off treatment) until either pregnancy was achieved or 24 months of alternating treatment was complete. Patients in the intervention arm received IVIG (25 grams (g)) on days eight, nine, or 10 of the menstrual cycle and every month until pregnancy was achieved, followed by once per week until 12 weeks gestation.21 The comparison group was treated with intravenous (IV) intralipid (20%; 250 millilitres (mL)) on day three of the patients’ menstrual cycles and every two weeks thereafter until pregnancy was achieved; at which time, the frequency of IV intralipid was increased to once per week until 12 weeks gestation.21 In the Danish trial, two different regimens of IVIG were administered across the almost-six years of the study, due to the drug brand becoming unavailable in 2011.22 From August 2008 to May, 2011, the intervention arm received eight infusions of IVIG (120 mg/ml) until between 14 and 15 weeks gestation with dose dependent on body weight 22 The comparison group received a placebo intervention comprised of eight infusions of albumin (5%) with dose dependent on body weight.22 As of May, 2011, patients in the intervention arm continued to receive eight infusions of IVIG (100 milligrams (mg)/mL) until between 14 and 15 weeks gestation, and the comparison group remained on a similar regimen of eight infusions to between 14 and 15 weeks gestation, with doses of both medications dependent on body weight22 (details provided in Appendix 2). The Iranian trial administered daily subcutaneous enoxaparin (40 mg) until 24 weeks gestation and daily aspirin (80mg) until 37 weeks gestation to both the intervention and comparison groups.23 In addition, the intervention group received IVIG (200 milligrams (mg)/kilogram (kg) body weight) each month until 24 weeks gestation.23
All of the non-randomized studies investigated IVIG (400 mg/kg body weight) using various regimens and comparators.24–27 The prospective, controlled clinical trial administered IVIG to patients in the intervention arm from confirmation of pregnancy to 32 weeks gestation, whereas patients in the comparison arm received standard care.24 Patients in the intervention arm of the prospective Spanish study received IVIG every three to four weeks until 13 weeks gestation, followed by 200 mg/kg monthly until 35 weeks gestation (IVF patients received an additional dose at both 24 hours and 15 days following confirmed pregnancy).26 There was no information reported concerning whether patients in the comparison group of this study received any treatment or not.26 In the Korean study, patients who had thrombophilia were administered daily, low-dose aspirin (100 mg) and subcutaneous, low-molecular-weight heparin (2,500 IU).27 Patients with cellular immune abnormality received IVIG once every three weeks from between four and six weeks to 30 weeks gestation. Patients with neither thrombophilia nor cellular immune abnormality received standard care.27 Finally, the retrospective Spanish study reported administration of IVIG once every three weeks in the first trimester of pregnancy, followed by 200 mg/kg every four weeks until between 35 and 36 weeks gestation, plus acetyl salicylic acid (100 mg; frequency and duration not reported), with additional doses (400 mg/kg) for IVF patients at 24 hours and 15 days following confirmed pregnancy.25 Patients in the comparison group received acetyl salicylic acid (100 mg) with or without an unspecified dose of low molecular weight heparin for patients with prothrombotic and/or cardiovascular risk factors.25
Outcomes
Live births and/or live birth rates were reported in all of the included studies.19–27 In one SR, live birth rate was defined as live births per pregnancies achieved and was analyzed using random effects models, and both cumulative meta-analyses and trial sequential analyses.20 Subgroup analyses included a comparison of patients with either primary (no history of live birth) or secondary (history of at least one live birth) RSA, and administration of IVIG prior to conception as opposed to following implantation of the embryo.20 Another SR stated its primary outcome of interest as no live birth, with secondary outcomes of interest including adverse events and quality of life analyzed using both meta-analyses and trial sequential analyses.19 Subgroup analyses in this SR focused on patients with primary versus secondary RSA and low- versus high-dose IVIG.19
The three RCTs all investigated live births, adverse events or side effects, and neonatal outcomes.21–23 Successful pregnancies were the primary outcome in one RCT and defined as those that reached 12 weeks gestation.21 This study also measured live births, side effects, and neonatal growth parameters (of which neither of the two latter outcomes/measures were pre-specified in detail), as well as cellular outcomes (i.e., nonclinical) that were not eligible for inclusion in this review 21 Another RCT calculated and compared live birth rate per treatment group as its primary outcome of interest, defined as the number of participants giving birth to a neonate surviving to at least 28 days of life over all those randomized.22 This study also recorded neonatal parameters, including birth weight, gestational length, Apgar scores of less than 10 and days in the NICU, as well as adverse events (not further detailed a priori).22 The remaining RCT included in this review investigated live birth (not further defined) as its primary outcome, as well as obstetric, perinatal, and neonatal outcomes, including preeclampsia, pre-term labour, gestational diabetes, low birth weight, congenital defects, and gestational age and side effects (including urticarial; bleeding; pain; irritation; hematoma at the injection site, and; ecchymosis).
The direction of effect to indicate a clinical improvement was self-evident for the main outcomes of interest i.e., more live births and fewer adverse events/side effects were indicators of clinical benefit. The minimally important difference to detect a clinical effect was addressed by way of sample size calculations in three of the studies included in this review i.e., two RCTs22,23 and one non-randomized, observational study.24 One RCT specified that, to detect an assumed 70% versus 33% live birth rate in the intervention versus control groups, respectively, a required sample size of 41 patients per treatment group was needed.22 Another RCT reported that 30 patients would be needed per group to generate 80% power to detect a difference between groups.23 Finally, one nonrandomized, controlled clinical trial reported that, to identify an assumed difference of 75% versus 40% live birth rate between intervention and control groups, respectively, 44 study participants per group was necessary.24
Additional details regarding the characteristics of included publications are provided in Appendix 2.
Summary of Critical Appraisal
Systematic Reviews
Both systematic reviews included in this review19,20 demonstrated strengths and limitations. In terms of strengths, both SRs reported the conduct of study selection and data abstraction by two independent reviewers, and both described comprehensive literature searches with a detailed list of included studies. Both conducted critical appraisal of included studies and performed appropriate meta-analyses. However, whereas one SR made explicit mention of a protocol,19 the other did not.20 Protocols are an important feature of systematic reviews as they allow for the assessment of an a priori method and thus the extent to which risk of bias that may be present. Similarly, while one SR formally assessed publication bias,20 the other did not describe a formal assessment.19 Publication bias in SR is an important factor in understanding the extent to which findings from included, published studies may over represent a positive effect. Again, while one SR explicitly addressed publication status,19 the other did not,20 leaving uncertainty with regard to the latter SR as to whether its included studies are representative of the entire body of both published and unpublished evidence. Finally, while neither of the SRs reported sources of funding supporting the included studies, one reported the source of funding for the SR itself,19 whereas the other did not.20 Transparency with regard to funding support is a critical feature of SR, allowing the reader to assess the credibility and any potential conflict of interest for both the studies included in the SR, as well as the work completed on the SR itself.
Randomized Controlled Trials
Clarity of reporting is critical to a transparent assessment of the strengths and limitations of studies included in any review. Study reporting was clear for some criteria across the three RCTs included in this review, with main outcomes, patient characteristics, interventions, random variability and adverse events reported.21–23 Nonetheless, other criteria were not consistently reported across RCTs i.e., study aim and objectives were reported clearly in two21,22 but not in one of the RCTs;23 main findings and loss to follow-up were clearly reported in two 22,23 but not in one of the RCTs;21 probability values were clearly reported in one RCT,22 partially reported in another 21 and not clearly reported in the third RCT.23 Finally, as it concerned reporting, all three RCTs failed to provide an explicit list of principal confounders.21–23 Because some information was lacking from the reports of the studies included in this review, they could not be assessed in their entirety.
It was not possible to assess any of the items addressing external validity for the included RCTs, as details about the representativeness of subjects asked to participate; patients who consented to participate, and; the interventions administered, were either not reported or not reported in enough detail to assess.21–23 Because external validity could not be ascertained for the RCTs, it remains unclear whether their findings can appropriately be applied to other, similar patients.
An understanding of internal validity in general, and risk of bias in particular, is critical to informing the interpretation of a study. In this review, risk of bias was assessed as variable across the three RCTs i.e., while one of the trials demonstrated no evidence of a threat to internal validity by way of bias in its report of findings,22 both of the other RCTs were assessed as demonstrating some risk of bias.21,23 In terms of strengths, both RCTs demonstrated no evidence of unplanned analyses, used ostensibly appropriate statistical tests, and presented detailed descriptions of appropriate outcome measures.21,23 However, whereas one clearly reported consistent duration of patient follow-up23, the other did not.21 Further, both remaining RCTs did not describe any method to ensure patients or outcome assessors were blinded to the interventions and patient compliance with the interventions was not described.21,23 Thus, the effects reported in the two RCTs with a higher risk of bias should be interpreted with caution as this may have had an impact on the effects reported.
Risk of confounding is an important consideration in weighing whether the effect demonstrated in a study can be isolated to the intervention and comparator of interest as opposed to other, extraneous factors or characteristics. The risk of any confounding was assessed as being variably present across the RCTs included in this review.21–23 While all three trials allocated patients to treatment using randomization, 21–23 only one described randomization concealment.22 Likewise, while study subjects were apparently recruited from the same population over the same time period in all three studies, adjustment for known confounders was not explicitly addressed by any of the included RCTs.21–23 Lastly, loss to follow-up was clearly accounted for in two of the trials,22,23 while not clearly accounted for in one RCT.21 While all three of the trials were randomized, the limitations of each must be considered when weighing the internal validity of their findings.
Finally, power calculations are critical as part of considering adequacy of the sample size used in a study — which is a fundamental consideration in weighing the importance of its findings and conclusions, as it serves as an indicator of the probability of avoiding a Type II error (i.e., finding an apparent effect among the sampled patients in a study where no effect actually exists). While a power calculation was described in two of the RCTs,22,23 study power was not addressed in one of the trials,21 leaving the reader of the latter study less certain as to the potential for error in its reported findings.
Non-Randomized Studies
The study aims, objectives, and patient characteristics were clearly reported in all four nonrandomized studies, whereas a list of principal confounders and adverse events were not clearly reported in any of these four studies.24–27 Other reporting characteristics were reported variably with some of the four non-randomized studies clearly reporting main outcomes, interventions, main findings, random variability, loss to follow-up and probability values, and some either not reporting these criteria at all, or only partially.24–27 In particular, one study reported live birth in 121 of 217 enrolled patients with no clear explanation as to why this outcome was not reported in the full study population.25 Because at least some information was lacking from all of the reports of the non-randomized studies included in this review, the studies could not be assessed with regard to all critical appraisal criteria.
As with the RCTs in this review, all four non-randomized studies provided insufficient information as to the representativeness of their study participants,24–27 preventing an assessment of external validity. This is a particularly important limitation, as it leaves the reader unclear as to whether the reported findings can appropriately be applied to other, similar patients or not.
An assessment of bias in the four non-randomized studies indicated that all of the studies appeared to have implemented a consistent follow-up duration for study groups, ostensibly appropriate statistical tests, and outcome measures that were clearly described.24–27 Conversely, all four non-randomized studies reported no information regarding whether patients and outcome assessors were blind to the treatment allocation, as well as whether patients were compliant with the interventions to which they were allocated. Additional measures of internal validity specific to bias were reported variably across the four nonrandomized studies and are detailed in Appendix 3.
Internal validity was also considered in terms of confounding, with all four non-randomized studies included in this review demonstrating some limitations in their address of confounding.24–27 Perhaps most importantly, none of the non-randomized studies randomized patients to treatment, nor did they clearly report adjustment for potentially confounding variables,24–27 introducing a risk when considering whether the demonstrated effect can be attributed to the interventions of interest as opposed to other, extraneous factors or characteristics. Further, while two of the non-randomized studies in this review did report that patients from both study groups were recruited from the same centre within the same timeframe,24,27 two did not.25,26 Lastly, in three of the non-randomized studies, patients were explicitly allocated to treatment based on clinical or socioeconomic characteristics,25–27 and in the controlled clinical trial, patient allocation to the control group was described as voluntary.24 These methods for allocating study participants to treatment increase the risk of confounding and reduce certainty as to whether any effect demonstrated by the study was due to the interventions under study as opposed to extant differences between the patient groups being compared.
Finally, sample size and study power were explicitly reported by one non-randomized study in this review,24 but not addressed in the other three.25–27
Additional details regarding the strengths and limitations of included studies are provided in Appendix 3.
Summary of Findings
Clinical Effectiveness – Benefit
Live Birth
All nine studies included in this review reported on live birth.19–27 Four of the included studies in this review reported that, overall, IVIG demonstrated a clinical benefit in terms of live birth,20,24–26 whereas five included studies found no significant effect of IVIG on live birth.19,21–23,27
Of the included SRs,19,20 nine of the primary studies were common to both (see Appendix 5). However, while one SR found a pooled effect of IVIG on live birth rates of borderline significance (risk ratio (RR) 1.25, 95% confidence interval (CI) 1.00 to1.56, P = 0.05),20 the other SR found that IVIG had no statistically significant effect (RR 0.92, 95% CI 0.75 to 1.12, P = 0.42).19 Subgroup analyses of primary and secondary RSA were conducted in both SRs, demonstrating that IVIG had no significant effect on live birth in patients with primary RSA.19,20 However, while subgroup analyses of patients with secondary RSA generated no significant finding in one SR,20 another SR demonstrated a trend toward benefit of IVIG on live birth in these patients (RR 0.77 95% CI 0.58 to1.02, P = 0.06).19 Other subgroup analyses included timing of IVIG administration (before conception or after implantation)20 and dose of IVIG (higher or lower than the median dose of 84 g administered across all of the included trials).19 Of patients receiving treatment prior to conception, a significant benefit of IVIG was demonstrated on live birth rates in one SR (RR 1.67, 95% CI 1.30 to 2.14, P < 0.0001) whereas no benefit of IVIG was evident in patients receiving IVIG after implantation.20 The subgroup analyses of IVIG by dose showed no statistically significant benefit with either higher or lower dose IVIG compared to patients not receiving IVIG.19 Authors of both SRs acknowledged that their findings were inconclusive and that more robust research is needed to elucidate any potential effect of IVIG on live birth for those with RSA.19,20
None of the three included RCTs identified a significant benefit of IVIG in terms of live birth, with both IVIG and non-IVIG treatment groups showing similar outcomes, and authors of all three trials concluding that IVIG was found to offer no benefit in comparison with placebo or other treatments.21–23 Similarly, one of the four included non-randomized studies identified no significant benefit of IVIG with regard to live birth,27 whereas three reported finding a significant effect.24–26 In the controlled clinical trial of 94 pregnant participants with RSA and cellular immune abnormality, authors reported live birth rates of 86% (38 of 44 patients) in the IVIG group versus 42% (21 of 50 patients) in the group receiving standard care (RR not reported (NR), P = 0.0006).24 Likewise, the non-randomized, prospective study of 24 patients with RSA found live birth rates of 95% (19 of 20 patients) in the IVIG-treated group compared with 50% (two of four patients) in the group not receiving IVIG (difference between groups not described statistically).26 Authors of all four non-randomized studies concluded by suggesting that there may be a benefit of IVIG for increasing live birth rates in women with RSA,24–27 with two of the studies emphasizing the importance of further research.24,27
Notably, of those four studies suggesting that IVIG offers benefit to participants with RSA in live births, three used non-randomized designs and were deemed during critical appraisal to have insufficiently addressed internal and external validity.24–26 Furthermore, the one SR that that reported a statistically significant benefit of IVIG may have been affected by one or more studies in particular, explicitly concluding that: “This significant observation after the inclusion of Lin et al. study is therefore not sufficiently convincing. Further trials are required to validate this observation.” (p. 723)20 Thus, while four of the nine studies in this review reported a benefit of IVIG in live birth for those with RSA, their findings should be interpreted with caution.
Clinical Effectiveness – Harm
Adverse events/Side effects
Five studies included in this review investigated either adverse events or side effects of IVIG treatment compared with placebo or other active treatment.19,21–24 In one SR, several meta-analyses comparing both serious adverse event rates in participants and their liveborn infants found no difference between groups treated with IVIG versus not treated with IVIG.19 While no adverse events in live-born neonates were reported in any of the studies examining this outcome in the SR, and a random-effects meta-analysis in participants treated with IVIG versus not treated with IVIG demonstrated no difference between groups, one fixed-effects meta-analysis indicated significantly more adverse events — including vaginal bleeding, rash, headache, fever, and itching — in participants treated with IVIG.19
Of the RCTs included in this review, all reported on either side effects or adverse events.21–23 One RCT found headache and mild fever in five and two patients, respectively, treated with IVIG; whereas no side effects were observed in patients treated with placebo.21 Another trial found statistically higher rates of skin rash with 40% IVIG patients versus 20% placebo patients affected, and higher rates of headache with 52% IVIG patients versus 30% placebo patients affected (RR NR, P = 0.04 for both adverse events).22 Yet another RCT observed fever and chills in one and palpitations in another of the study’s IVIG-treated patients, whereas the enoxaparin-treated patients were found to have ecchymosis (n=2) or induration at the site of injection (n=4) (difference between groups not described statistically).23
While one non-randomized, controlled clinical trial indicated that patients were monitored for any side effects, no report of side effects was included in the results.24
Obstetric and/or perinatal outcomes
Obstetric and perinatal harms were addressed by three studies included in this review.19,22,23 In one SR, some perinatal harms were reported as part of the study’s analysis of adverse events (i.e., no differences between groups in ‘serious adverse events’ including caesarean section and/or premature rupture of membranes).19 Two RCTs reported on rates of caesarean section, with both reporting no statistically significant difference between treatment groups.22,23 Finally, one of the RCTs included in this review also reported on intrauterine growth restriction, preeclampsia, and preterm delivery, and found no difference between groups based on their assigned treatment.23
Neonatal outcomes
Four studies included in this review evaluated outcomes in live-born neonates or infants.19,21–23 One SR reported these outcomes as part of their assessment of adverse events (as previously mentioned, no differences between groups in any ‘serious adverse events’ including admission to neonatal care unit, congenital malformations, mental retardation, and/or death).19 Of the three RCTs investigating neonatal harms, two reported no differences between groups based on treatment assignment.21,23 While another RCT similarly reported no differences between groups in most neonatal outcomes, a statistically significantly longer gestation was reported in the IVIG group i.e., 282 days in the IVIG group (95% CI 272 to 286) versus 272 days in the placebo group (95% CI 267 to 277), P = 0.02.22
Quality of Life
While one SR in this review sought data on quality of life in both pregnant participants and neonates/infants, none of the included studies in the SR addressed this outcome.19
Appendix 4 presents a tabulated summary of the main study findings and authors’ conclusions.
Limitations
There were some important limitations with the evidence identified in this review describing off-label IVIG for the treatment of RSA. The SRs included in this review generally used rigorous methods to meta-analyze many of the same studies; however, they produced discordant results concerning the benefit of IVIG. Nonetheless, the important limitations of one SR were acknowledged by its authors with regard to the observed effect being primarily driven by one or two studies, leading authors to conclude that their confidence in the findings was uncertain.20
Rigorous, randomized trial evidence was somewhat limited, such that three RCTs were found to be eligible. Additional evidence in this area is of limited methodological rigour, uses non-randomized designs, small sample sizes and/or does not employ the use of any comparison group against IVIG interventions. Notably, while all three RCTs included in this review found no comparative benefit of IVIG,21–23 three of four non-randomized studies conversely reported finding a benefit to patients with RSA.24–26 This may partly be a function of small sample sizes but may also betray risks to internal validity posed by the nonrandomized designs of the studies. While many of the primary studies in this review examined population groups with particular immunologic risk factors for RSA, additional research into the effect of IVIG in various risk strata (e.g., primary versus secondary RSA; various immunologic risk profiles) would likely reduce lingering uncertainty with regard to its benefit to some of these patient populations of particular interest.
Due to some discordance across studies in this review as to the findings generated and conclusions drawn by their authors, evidence addressing the use of off-label IVIG in patients with RSA appears underdeveloped, confirming that additional, rigorous research is needed to understand any potential effects. Further, as no evidence from North America was identified, and little from Western Europe, the generalizability of the findings reported in this review to the Canadian context may be limited. Consequently, the findings of this report should be interpreted with caution.
Conclusions and Implications for Decision or Policy Making
This review identified nine comparative studies evaluating the use of IVIG in patients with recurrent, spontaneous abortion (RSA). Two studies were systematic reviews (SR), each including 11 randomized controlled trials (RCT). Three studies were RCTs (not represented within the included studies of the two aforementioned SRs) that randomized patients to either intravenous immunoglobulin (IVIG) or non-IVIG therapy. Four studies used nonrandomized, observational designs investigating IVIG compared to treatment without IVIG in at least some patients who were eligible for this review. No evidence investigating the clinical effectiveness of SCIG in patients with RSA was identified.
In this review, the authors of four included studies found that IVIG may offer a beneficial clinical effect to patients with RSA in terms of live birth;20,24–26 whereas five studies found no clinical benefit of IVIG compared with placebo or other treatments.19,21–23,27 Of the four studies reporting a beneficial effect of IVIG, three were non-randomized studies with important limitations — particularly as it concerns the risk of confounding and potential threats to external validity.24–26 One SR reported finding a clinical benefit of marginal statistical significance, but authors advised caution in the interpretation of their results based on the impact of one or more included studies in the meta-analyses.20 Of relevance to this, one SR that was excluded from this review due to complete overlap in its primary studies with the two included SRs concluded that there was no beneficial effect of IVIG on live birth.28 Other outcomes investigated were adverse events, including obstetric, perinatal and neonatal outcomes; though, no serious side effects or other adverse events were observed.19,21–24
Notably, the literature in this area does highlight the potential benefit of IVIG in particular subgroups of patients with RSA e.g., those with RSA known to be of immunologic etiology2,29,30 and those with secondary RSA.31,32 In this review, three non-randomized studies of limited quality addressed the effect of IVIG in the context of RSA with immunologic abnormalities;24,25,27 whereas two reported a significant benefit of IVIG on live birth,24,25 one reported no benefit.27 Nonetheless, given the important methodological limitations of these studies, their results should be interpreted with caution. As it concerns secondary RSA, two SRs of good quality reported subgroup analyses of live birth showing no benefit of IVIG to secondary RSA patients.19,20 In addition, one RCT of good quality in this review limited its recruitment to secondary RSA patients, finding no effect of IVIG on live birth.22
In conclusion, evidence informing the use of IVIG for patients with RSA is inconsistent with regard to its effect on live birth. While four studies in this review suggested a benefit of IVIG for patients with RSA, five studies of higher quality found no effect. All of the included studies demonstrated some risk of bias due to uncertain external validity (representativeness) and some studies demonstrated a risk of confounding, in particular. One SR reported a beneficial effect of IVIG with marginal statistical significance, but cautioned the interpretation of their findings due to the impact of one or more included primary studies. Consequently, the findings summarized within this review should be interpreted with caution, and the clinical effectiveness of IVIG for RSA remains unclear. Further evidence from larger, more robust studies — particularly those that focus on particular subgroups of RSA patients — remains necessary to reduce uncertainty.
References
- 1.
- 2.
Yamada
H, Deguchi
M, Maesawa
Y, Nakajima
Y, Nishino
Y, Tanimura
K, et al. Mediumdose intravenous immunoglobulin therapy for women with six or more recurrent miscarriages. J Reprod Immunol. 2015
Jun;109:48–51. [
PubMed: 25747500]
- 3.
- 4.
Griebel
CP, Halvorsen
J, Golemon
TB, Day
AA. Management of spontaneous abortion. Am Fam Physician. 2005
Oct
1;72(7):1243–50. [
PubMed: 16225027]
- 5.
Arias-Sosa
LA, Acosta
ID, Lucena-Quevedo
E, Moreno-Ortiz
H, Esteban-Perez
C, ForeroCastro
M. Genetic and epigenetic variations associated with idiopathic recurrent pregnancy loss. J Assist Reprod Genet [Internet]. 2018
Mar [cited 2018 May 3];35(3):35566. Available from:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5904072- 6.
- 7.
- 8.
- 9.
Hume
HA, Anderson
DR. Guidelines for the use of intravenous immune globulin for hematologic and neurologic conditions. Transfus Med Rev. 2007
Apr;21(2 Suppl 1):S1–S2. [
PubMed: 17397766]
- 10.
Constantine
MM, Thomas
W, Whitman
L, Kahwash
E, Dolan
S, Smith
S, et al. Intravenous immunoglobulin utilization in the Canadian Atlantic provinces: a report of the Atlantic Collaborative Intravenous Immune Globulin Utilization Working Group. Transfusion. 2007
Nov;47(11):2072–80. [
PubMed: 17958537]
- 11.
- 12.
Robinson
P, Anderson
D, Brouwers
M, Feasby
TE, Hume
H, IVIG Hematology and Neurology Expert Panels. Evidence-based guidelines on the use of intravenous immune globulin for hematologic and neurologic conditions. Transfus Med Rev. 2007
Apr;21(2 Suppl 1):S3–S8. [
PubMed: 17397767]
- 13.
Feasby
T, Banwell
B, Benstead
T, Bril
V, Brouwers
M, Freedman
M, et al. Guidelines on the use of intravenous immune globulin for neurologic conditions. Transfus Med Rev. 2007
Apr;21(2 Suppl 1):S57–107. [
PubMed: 17397768]
- 14.
- 15.
- 16.
- 17.
- 18.
Liberati
A, Altman
DG, Tetzlaff
J, Mulrow
C, Gotzsche
PC, Ioannidis
JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
- 19.
- 20.
Wang
SW, Zhong
SY, Lou
LJ, Hu
ZF, Sun
HY, Zhu
HY. The effect of intravenous immunoglobulin passive immunotherapy on unexplained recurrent spontaneous abortion: a meta-analysis. Reprod Biomed Online. 2016
Dec;33(6):720–36. [
PubMed: 27720163]
- 21.
Meng
L, Lin
J, Chen
L, Wang
Z, Liu
M, Liu
Y, et al. Effectiveness and potential mechanisms of intralipid in treating unexplained recurrent spontaneous abortion. Arch Gynecol Obstet. 2016
Jul;294(1):29–39. [
PubMed: 26671484]
- 22.
Christiansen
OB, Larsen
EC, Egerup
P, Lunoee
L, Egestad
L, Nielsen
HS. Intravenous immunoglobulin treatment for secondary recurrent miscarriage: a randomised, doubleblind, placebo-controlled trial. BJOG [Internet]. 2015
Mar [cited 2018 Feb 1];122(4):500–8. Available from: 10.1111/1471-0528.13192/epdf [
PubMed: 25412569] [
CrossRef]
- 23.
- 24.
Ahmadi
M, Aghdam
SA, Nouri
M, Babaloo
Z, Farzadi
L, Ghasemzadeh
A, et al. Intravenous immunoglobulin (IVIG) treatment modulates peripheral blood Th17 and regulatory T cells in recurrent miscarriage patients: Non randomized, open-label clinical trial. Immunol Lett. 2017
Dec;192:12–9. [
PubMed: 29030251]
- 25.
Ramos-Medina
R, Garcia-Segovia
A, Gil
J, Carbone
J, guaron de la
CA, Seyfferth
A, et al. Experience in IVIg therapy for selected women with recurrent reproductive failure and NK cell expansion. Am J Reprod Immunol. 2014
May;71(5):458–66. [
PubMed: 24612159]
- 26.
Moraru
M, Carbone
J, Alecsandru
D, Castillo-Rama
M, Garcia-Segovia
A, Gil
J, et al. Intravenous immunoglobulin treatment increased live birth rate in a Spanish cohort of women with recurrent reproductive failure and expanded CD56(+) cells. Am J Reprod Immunol. 2012
Jul;68(1):75–84. [
PubMed: 22509929]
- 27.
Lee
SK, Kim
JY, Han
AR, Hur
SE, Kim
CJ, Kim
TH, et al. Intravenous immunoglobulin G improves pregnancy outcome in women with recurrent pregnancy losses with cellular immune abnormalities. Am J Reprod Immunol. 2016
Jan;75(1):59–68. [
PubMed: 26510488]
- 28.
- 29.
Stricker
RB, Winger
EE. Update on treatment of immunologic abortion with low-dose intravenous immunoglobulin. Am J Reprod Immunol. 2005
Dec;54(6):390–6. [
PubMed: 16305665]
- 30.
Kiprov
DD, Nachtigall
RD, Weaver
RC, Jacobson
A, Main
EK, Garovoy
MR. The use of intravenous immunoglobulin in recurrent pregnancy loss associated with combined alloimmune and autoimmune abnormalities. Am J Reprod Immunol. 1996
Oct;36(4):22834. [
PubMed: 8911631]
- 31.
Sapir
T, Carp
H, Shoenfeld
Y. [Intravenous immunoglobulin (IVIG) as treatment for recurrent pregnancy loss (RPL)]. Harefuah. 2005
Jun;144(6):415–20, 454, 453. Hebrew. [
PubMed: 15999561]
- 32.
Appendix 1. PRISMA Diagram18 Describing Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Characteristics of Included Systematic Reviews and Meta-Analyses
View in own window
| First Author, Publication Year, Country | Study Designs and Numbers of Primary Studies Included | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes |
|---|
Wang.201620
China | 11 RCTs | Unreported number of female participants with ≥2 consecutive, spontaneous abortions (582 of whom achieved pregnancy) | IVIG before or during early pregnancy
vs.
Placebo |
Live birth rate
Random effects model Cumulative meta-analyses
Subgroup analyses
|
Egerup, 201519
Denmark | 11 RCTs | 531 female participants with ≥3 consecutive, spontaneous abortions | IVIG
vs.
Placebo, no intervention, or treatment as usual |
No live birth Adverse events
Obstetric: (serious) caesarean sections, premature rupture of membranes prior to gestational week 28, hospitalization due to infusionrelated symptoms; (non-serious) vaginal bleeding, rash, headache, fever, and itching In neonates: (serious) admission to neonatal care unit, congenital malformations, mental retardation, death (non-serious) none found
Quality of life
Subgroup analyses
|
IVIG = intravenous immunoglobulin; RCT = Randomized controlled trial; RSA = Recurrent spontaneous abortion, vs. = versus
Table 3Characteristics of Included Primary Clinical Studies
View in own window
| First Author, Publication Year, Country | Study Design | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes, Length of Follow-Up |
|---|
| Randomized Controlled Trials |
|---|
Meng, 201621
China | Single-centre, parallel-group, RCT stratified by prior number of miscarriages | Patients
192 non-pregnant patients with ≥3 consecutive, spontaneous abortions randomized
Mean age, years (±SD)
Intervention = 31.2 years (3.7)
Comparison = 31.6 (4.2)
Setting
Miscarriage center | IVIG (25 g) day 8, 9, or 10 of menstrual cycle and every month until pregnancy achieved, followed by once per week to 12wks gestation
vs.
IV intralipid (20%; 250 mL) on day 3 of menstrual cycle and every 2wks until pregnancy achieved, followed by IV intralipid (20%; 250mL) once per week to 12wks gestation
Pre-pregnancy treatments were administered monthly for 3 mos, then discontinued for 3 mos, and alternated every 3 mos following until pregnancy was achieved or until 24 mos |
Live birth rate Side effects Neonatal growth
Height, weight, head and chest circumference at 6, 12, 18 and 24 mos
Follow-up = For pts with live birth, infants followed for 2yrs; for pts with pregnancy loss, until loss of pregnancy; for pts who did not conceive, within 12mos |
Christiansen, 201522
Denmark | Single-centre, parallel-group, double-blind, placebo-controlled RCT stratified by prior number of miscarriages | Patients
82 female participants with ≥3 consecutive, spontaneous abortions secondary to at least 1 birth (>28wks gestation) randomized upon confirmation of pregnancy
Mean age, years (95% CI)
Intervention = 34.(26.2-40.9)
Comparison = 34.2 (26.1-41.0)
Setting
Recurrent miscarriage clinic | From August 2008 to May, 2011: 8 infusions of IVIG (120 mg/mL) (i) for pts <75kg, 24 g (200 mL), and (ii) for pts ≥75 kg, 36 g (300 mL) until 14 to 15 wks gestation
vs.
Placebo i.e., 8 infusions of albumin (5%) dependent on weight (200mL or 300mL) until 14-15wks gestation
From May 2011 to April 2014:
8 infusions of IVIG (100 mg/ml) (i) for pts <75kg, 25 g (250 ml), and (ii) for pts ≥75 kg, 35 g (350 ml) until 14 to15 wks gestation
vs.
8 infusions of placebo i.e., albumin (5%) dependent on weight (250mL or 350mL) until 14 to 15wks gestation |
Live births Adverse events
Follow-up = NR |
Nazari, 201523
Iran | Single-centre, parallel-group RCT | Patients
60 pregnant participants with ≥3 consecutive, spontaneous abortions randomized
Mean age, years (±SD)
Intervention = 29.7 (3.1)
Comparison = 31.2 (2.1)
Setting
Hospital-based
Obstetrics and
Gynecology department | IVIG (200 mg/kg body weight) every month to 24wks gestation, plus subcutaneous enoxaparin (40 mg) every day to 24 wks gestation, plus aspirin (80mg) every day to 37 wks gestation
vs.
Subcutaneous enoxaparin (40 mg) every day to 24wks gestation, plus aspirin (80mg) every day to 37wks gestation |
Live births Obstetric, perinatal and neonatal outcomes
Side effects
Urticaria, bleeding, pain, irritation, hematoma at injection site, ecchymosis
Follow-up = NR |
| Prospective Non-Randomized Studies |
|---|
Ahmadi, 201724
Iran | Controlled clinical trial | Patients
94 pregnant participants with ≥3 consecutive, spontaneous abortions and cellular immune abnormality
Mean age, years (±SD)
Intervention = 33.8 (3.6)
Comparison = 34.1 (3.4)
Setting
Hospital-based infertility center | IVIG (400 mg/kg body weight) every 4wks from confirmation of pregnancy to 32 wks gestation
vs.
Standard care |
Follow up = NR |
Moraru, 201226
Spain | Prospective cohort (nested within casecontrol) | Patients
24 pregnant participants with ≥3 consecutive, spontaneous abortions and NK or NKT-like cells
Mean age, years (±SD)
Intervention = 35.7 (4.4)
Comparison = 37 (5.1)
Setting
Hospital-based immunology clinic | IVIG (400 mg/kg body weight) every 3 to 4 wks to 13 wks gestation, followed by 200 mg/kg every month to 35wks gestation, with or without an additional dose (400 mg/kg) 24hrs and 15 days following confirmed pregnancy (IVF patients only)
vs.
Unspecified treatment |
Follow-up = NR |
| Retrospective Non-Randomized Studies |
|---|
Lee, 201627
Korea | Retrospective cohort | Patients
189 female participants with ≥2 consecutive, spontaneous abortions, with or without cellular immune abnormality
Mean age
NR by intervention and comparison groups
Setting
Hospital-based Obstetrics and Gynecology department | IVIG (400 mg/kg body weight) every 3wks from 4 to 6 wks to 30 wks gestation (for pts with cellular immune abnormality), with or without low-dose aspirin and low-molecular-weight heparin (for pts at risk of thrombophilia)
vs.
Standard care (for pts without cellular immune abnormality), with or without low-dose aspirin and low-molecularweight heparin (for pts at risk of thrombophilia) |
Follow-up = NR |
Ramos-Medina, 201425
Spain | Retrospective cohort (nested within casecontrol) | Patients
217 female participants (with live birth results reported for 121 only) with ≥3 consecutive, spontaneous abortions and NK or NKT-like cells
Mean age, years (±SD, range)
Total cohort = 36.48 (3.63, 27 to43)
Setting
Multiple participating (N=NR) centers of unspecified type | IVIG (400 mg/kg body weight) every 3 wks (first trimester only); 200 mg/kg every 4 weeks (to 35 to36wks gestation) plus acetyl salicylic acid (100 mg; scheduled NR), with or without an additional dose (400 mg/kg) 24hrs and 15 days following confirmed pregnancy (IVF patients only)
vs.
Acetyl salicylic acid (100 mg) with or without low molecular weight heparin (dose unspecified; for pts with prothrombotic and/or cardiovascular risk factors) |
Follow-up = NR |
CI = Confidence interval; hrs = hours; IV = Intravenous; IVF = In-vitro fertilization; IVIG = Intravenous immunoglobulin; g = grams; kg = kilograms; mg = milligrams; mL = milliletres; mos = months; n/N = Number; NICU = Neonatal intensive care unit; NK = Natural killer cells; NKT = Natural killer T-cells; NR = Not reported; pts = patients; RCT = randomized controlled trial; SD = Standard deviation; vs. = versus; wks = weeks
Appendix 3. Critical Appraisal of Included Publications
Table 4Strengths and Limitations of Systematic Reviews and Meta-Analyses using AMSTAR 216
View in own window
| Strengths | Limitations |
|---|
| Wang, 201620 |
|---|
Study selection and data abstraction performed in duplicate Comprehensive literature search Included studies list provided Study characteristics detailed Critical appraisal of included studies completed and considered in the interpretation of findings Meta-analyses conducted appropriately Risk of publication bias formally assessed
| No mention of an a priori design or protocol Consideration of publication status not reported Excluded studies list (with reasons) not included Sources of funding/support neither reported for the SR nor for its included studies
|
| Egerup, 201519 |
|---|
Explicit mention of a review protocol Study selection and data abstraction performed in duplicate Comprehensive literature search Publication status explicitly considered Included studies list provided Study characteristics detailed Critical appraisal of included studies completed and considered in the interpretation of findings Meta-analyses conducted appropriately Source of funding/support for the SR reported
| Excluded studies list (with reasons) not included No description of publication bias assessment (i.e., cursory mention in discussion only) Sources of funding/support for included studies not reported
|
Table 5Strengths and Limitations of Clinical Studies using Down’s & Black Checklist17
View in own window
| Strengths | Limitations |
|---|
| Randomized Controlled Trials (RCTs) |
|---|
| Meng, 201621 |
|---|
Reporting
Aim and objectives, main outcomes, patient characteristics, interventions, random variability and adverse events clearly reported
Internal validity – bias
No evidence of unplanned analyses Statistical tests appear appropriate Outcome measures clearly reported
Internal validity – confounding
| Reporting
External validity
Internal validity – bias
Variability in follow-up duration Blinding of study patients and outcome assessors not reported Intervention compliance not reported
Internal validity – confounding
Concealment of randomization not reported Intention-to-treat analyses not reported Loss to follow-up inadequately accounted for
Power
|
| Christiansen, 201522 |
|---|
Reporting
Aim and objectives, main outcomes, patient characteristics, interventions, main findings, random variability, adverse events, loss to follow-up and probability values clearly reported
Internal validity – bias
Study subjects and outcome assessors blinded to intervention allocation No evidence of unplanned analyses Consistent follow-up for both study groups Statistical tests appear appropriate No evidence of a lack of compliance with the interventions Outcome measures clearly reported
Internal validity – confounding
Study subjects recruited from the same centre over the same period of time Study subjects were randomized to treatment Randomization was concealed Loss to follow-up accounted for
Power
| Reporting
External validity
Internal validity – confounding
|
| Nazari, 201523 |
|---|
Reporting
Main outcomes, patient characteristics, interventions, main findings, random variability, adverse events and loss to follow-up reported
Internal validity – bias
No evidence of unplanned analyses Consistent follow-up for both study groups Statistical tests appear appropriate Outcome measures clearly reported
Internal validity – confounding
Study subjects recruited from the same centre over the same period of time Study subjects were randomized to treatment Loss to follow-up accounted for
Power
| Reporting
Study aim, objectives and probability values not clearly described List of principal confounders not clearly reported
External validity
Internal validity – bias
Internal validity – confounding
|
| Non-Randomized Studies |
|---|
| Ahmadi, 201724 |
|---|
Reporting
Study aim/objective, main outcomes, patient characteristics, interventions, main findings, random variability, loss to follow-up and probability values reported
Internal validity – bias
No evidence of unplanned analyses Consistent follow-up for both study groups Statistical tests appear appropriate Outcome measures clearly reported
Internal validity – confounding
Power
| Reporting
External validity
Internal validity – bias
Internal validity – confounding
|
| Lee, 201627 |
|---|
Reporting
Internal validity – bias
Consistent follow-up for both study groups Statistical tests appear appropriate Outcome measures clearly reported
Internal validity – confounding
| Reporting
Main outcomes partially reported List of principal confounders, random variability, adverse events, loss to follow-up and probability values not clearly reported
External validity
Internal validity – bias
Study subjects and outcome assessors not blinded Unclear whether unplanned analyses were undertaken Intervention compliance not reported
Internal validity – confounding
Study subjects not randomized to treatment Adjustment for confounding not reported Losses to follow up excluded from the analysis
Power
|
| Ramos-Medina, 201425 |
|---|
Reporting
Aim and objectives, main outcomes, patient characteristics, interventions, and probability values reported
Internal validity – bias
No evidence of unplanned analyses Consistent follow-up for both study groups Statistical tests appear appropriate Outcome measures clearly reported
| Reporting
Random variability reported inconsistently List of principal confounders, main findings, adverse events, and loss to follow-up not clearly reported
External validity
Internal validity – bias
Internal validity – confounding
Unclear whether subjects from both study groups were recruited from the same centre Study subjects recruited at various points in time Study subjects not randomized to treatment Adjustment for confounding not reported Loss to follow-up not reported
Power
|
| Moraru, 201226 |
|---|
Reporting
Internal validity – bias
Consistent follow-up for both study groups Statistical tests appear appropriate Outcome measures clearly reported
| Reporting
Main outcomes, interventions, list of principal confounders, main findings, random variability, adverse events, loss to follow-up, and probability values not clearly reported
External validity
Internal validity – bias
Study subjects and outcome assessors not blinded Intervention compliance not reported Unclear whether unplanned analyses were undertaken
Internal validity – confounding
Unclear whether subjects from both study groups were recruited from the same centre Study subjects recruited at various points in time Study subjects not randomized to treatment Adjustment for confounding not reported Loss to follow-up not reported
Power
|
RCT = Randomized controlled trial
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 6Summary of Findings for Included Systematic Reviews and Meta-Analyses
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Wang, 201620 |
|---|
Primary analyses
Live birth rate, all patients
Live births/pregnancies achieved (%)
Random effects comparison of IVIG vs. placebo, RR (95% CI)
1.25 (1.00-1.56) Difference between groups
Cumulative meta-analyses, RR (95% CI)
1.25 (1.00-1.56) Difference between groups
Subgroup analyses
Live birth rate, primary vs. secondary RSA
Primary RSA (5 studies, 183 patients)
Secondary RSA (6 studies, 220 patients)
Live births/pregnancies achieved (%)
Fixed effects comparison of IVIG vs. placebo, RR (95% CI)
1.26 (0.99-1.61) Difference between groups
Cumulative meta-analyses, RR (95% CI)
NR (NR) Difference between groups
Live birth rate, IVIG before conception vs. following implantation
Before conception (4 studies, 213 patients)
Live births/pregnancies achieved (%)
Meta-analysis, RR (95% CI)
1.67 (1.30-2.14) Difference between groups
Following implantation (7 studies, 369 patients)
Live births/pregnancies achieved (%)
Meta-analysis, RR (95% CI)
1.10 (0.93-1.29) Difference between groups
| “The 11 included studies were highquality trials with low risk of biases. Of the 582 women who achieved pregnancy, 353 had live births. Our analysis showed that IVIG might be of beneficial value to treat unexplained RSA. Cumulative and TSA indicated the need for more clinical trials to validate the effectiveness of IVIG as its therapeutic value is still inconclusive.” (p. 732) |
| Egerup, 201519 |
|---|
Primary analyses
No live birth (11 studies, 531 patients)
IVIG, N/patients evaluated (%)
No IVIG, N/patients evaluated (%)
Meta-analysis, RR (95% CI)
0.92 (0.75-1.12) Difference between groups
Adverse events (AEs)
Serious AEs in participants with RSA (8 studies, 381 participants)
IVIG, N/ participants evaluated (%)
No IVIG, N/ participants evaluated (%)
Meta-analysis, RR (95% CI)
1.06 (0.67-1.67) Difference between groups
Serious AEs in live-born infants (9 studies, 286 infants)
IVIG, N/ infants evaluated (%)
No IVIG, N/ infants evaluated (%)
Meta-analysis, RR (95% CI)
1.18 (0.58-2.42) Difference between groups
AEs in participants with RSA (9 studies, 451 participants)
IVIG, N/ participants evaluated (%)
No IVIG, N/ participants evaluated (%)
Random effects meta-analysis, RR (95% CI)
1.55 (0.89-2.69) Difference between groups
Fixed effects meta-analysis, RR (95% CI)
1.54 (1.13-2.11) Difference between groups
AEs in infants (4 studies, N infants = NR)
Premature birth, <37wks (10 studies, 284 participants with RSA)
IVIG, N/participants evaluated (%)
No IVIG, N/participants evaluated (%)
Difference between groups, RR (95% CI)
Low birth weight (7 studies, 238 infants)
IVIG, N/infants evaluated (%)
No IVIG, N/infants evaluated (%)
Meta-analysis, RR (95% CI)
1.02 (0.43-2.45) Difference between groups
Quality of life (QoL)
Participants with RSA (0 trials, 0 patients) Infants (0 trials, 0 patients)
Subgroup analyses
No live births, primary vs. secondary RSA
Primary RSA (6 studies, 181 patients)
No live birth/patients evaluated (%)
Meta-analysis, RR (95% CI)
1.32 (0.88-1.98) Difference between groups
Secondary RSA (6 studies, 220 patients)
No live birth/patients evaluated (%)
Meta-analysis, RR (95% CI)
0.77 (0.58-1.02) Difference between groups
No live births, higher vs. lower dose IVIG (< vs. ≥ median dose of all trials i.e., 84g)
Higher dose IVIG vs. no IVIG
No live birth, RR (95% CI)
0.85 (0.64–1.12) Difference between groups
Adverse events, RR (95% CI)
2.33 (1.00–5.46) Difference between groups
Lower dose IVIG vs. no IVIG
No live birth, RR (95% CI)
1.19 (0.81-1.75) Difference between groups
Adverse events, RR (95% CI)
0.85 (0.46-1.58) Difference between groups
| “Based on our results, we have insufficient evidence to recommend or refute IVIg for women with RM. Treatment with IVIg compared with placebo seems to increase the risk of adverse events. Subgroup analysis suggests that women with secondary RM seemed most likely to obtain a potential beneficial effect of IVIg, however, trial sequential analysis showed that insufficient information is presently accrued. IVIg should therefore only be administered in randomised clinical trials, clearly explaining the potential risks to participants. According to our subgroup analyses, women with secondary RM are the group, which most likely should be included in such trials.” (p. 17/21) |
CI = Confidence interval; IVIG = intravenous immunoglobulin; NR = Not reported; RCT = Randomized controlled trial; RM = Recurrent miscarriage; RR = Relative risk; RSA = Recurrent spontaneous abortion; TSA = Trial sequential analysis
Table 7Summary of Findings for Included Primary Clinical Studies
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Randomized Controlled Trials (RCTs) |
|---|
| Meng, 201621 |
|---|
Successful pregnancy, n/pts evaluated (%) *
Difference between groups
Live births, n/pts evaluated (%)
Difference between groups
| “There were no statistically significant differences in successful pregnancy rates between the two groups… Intralipid can be used as an alternative treatment to IVIG for URSA, and its potential mechanism of action may occur by regulating NK cell function and promoting trophoblast invasion.” (p. 29) |
| Christiansen, 201522 |
|---|
Live births, intention-to-treat analyses, n/pts evaluated (%)
Difference between groups
Live births, per-protocol analyses, n/pts evaluated (%)
Difference between groups
Adverse events, n/pts evaluated (%)
Skin rash
Difference between groups
Headache
Difference between groups
Perinatal and neonatal outcomes
Emergency cesarean section, n/pts evaluated (%)
Difference between groups
Elective cesarean section
Difference between groups
All neonatal measures (with the exception of gestational length at delivery), difference between groups
Gestational length at delivery in singletons, days (95% CI)
Difference between groups
| “In this trial, which is the largest so far, IVIg did not increase the live birth rate in patients with secondary recurrent miscarriage and the treatment cannot be recommended in clinical practice.” (p. 500) |
| Nazari, 201523 |
|---|
Live births, n/patients evaluated (%)
Difference between groups
Adverse events
Difference between groups
All perinatal and neonatal outcomes
Difference between groups
| “As >87% of live births occurred in both of our study groups, we demonstrated the high effectiveness of these two therapeutic regimens in patients with recurrent abortion. The incidence of abortion in the group receiving aspirin and enoxaparin alone versus the group receiving aspirin and enoxaparin and IVIG was not statistically significant … The combination regimen of heparin and aspirin could be considered as a standard treatment protocol in idiopathic recurrent abortion. Due to the high cost of IVIG and its complications in patients taking this drug, its use should be discontinued. Furthermore, further studies with a greater sample size are recommended before and after pregnancy.” (pp. S19, S20) |
| Non-Randomized Studies |
|---|
| Ahmadi, 201724 |
|---|
Live births, n/patients evaluated (%)
Difference between groups
| “In the current study, it was found that the administration of IVIG in RM women with cellular immune cells abnormalities during pregnancy… may be associated with successful pregnancy outcome. However further studies are needed to further clarify and elucidate this important issue.” (p. 18) |
| Lee, 201627 |
|---|
Live births, n/patients evaluated (%)
Difference between groups
| “In conclusion, IVIG treatment can improve the pregnancy outcome especially in women with RPL and cellular immune abnormalities. Treatment based on thorough evaluation of the underlying etiology including cellular immunity and thrombophilia may lead to a significantly improved live birth rate in women with RPL. Further studies are warranted to elucidate unknown mechanisms in reproductive failure.” (p. 66) |
| Ramos-Medina, 201425 |
|---|
Live births, n/patients evaluated (%) *
ASA (with or without low-molecular weight heparin)
Difference between groups
| “Immunomodulation with IVIg in our selected group of RRF patients with immunologic alterations enhanced clinical pregnancy and live birth rates. Our results may facilitate the design of future clinical trials of IVIg in this pathology.” (p. 458) |
| Moraru, 201226 |
|---|
Live births, n/patients evaluated (%)
Difference between groups
| “Intravenous immunoglobulin therapy for women with RRF and NK or NKT-like cell expansion was a safe and beneficial therapeutic strategy that associated with high clinical pregnancy and live birth rates.” (p. 75) |
- *
As reported in Figure 1 (p. 33), which does not correspond with the data as reported in the narrative (p. 33)
- *
Of 217 patient enrolled, data on live birth are reported for 121 only; no rationale for this is clearly reported
ASA = acetyl salicylic acid; CI = Confidence interval; hrs = hours; IV = Intravenous; IVF = In-vitro fertilization; IVIG = Intravenous immunoglobulin; g = grams; kg = kilograms; mg = milligrams; mL = millilitres; mos = months; n/N = Number; NICU = Neonatal intensive care unit; NK = Natural killer cells; NKT = Natural killer T-cells; NR = Not reported; NS = Not significant; RCT = randomized controlled trial; RM = Recurrent miscarriage; RPL = Recurrent pregnancy loss; RR = Relative risk; RRF = Recurrent reproductive failure; SD = Standard deviation; TSA = Trial-sequential analyses; URSA = Unexplained recurrent spontaneous abortion vs. = versus; wks = weeks
Appendix 5. Overlap between Included Systematic Reviews
Table 8Primary Study Overlap between Included Systematic Reviews
View in own window
| Primary Study Citation | Systematic Review Citation |
|---|
| Egerup, 201519 | Wang, 201620 |
|---|
| Christiansen, 1995 | X | X |
| Christiansen, 2002 | X | X |
| Christiansen, 2014 | X | X |
| Coulam, 1995 | X | X |
| German RSA/IVIG 1994 | X | X |
| Jablonowska, 1999 | X | X |
| Lin & Li, 2015 | | X |
| Liu & Chen, 2010 | | X |
| Mahmoud, 2002 | X | |
| Perino, 1997 | X | X |
| Stephenson, 1998 | X | X |
| Stephenson, 2010 | X | X |
| Triolo, 2003 | X | |
Appendix 6. Additional References of Potential Interest
Non-comparative Studies
Cohen
BM, Machupalli
S. Use of gammaglobulin to lower elevated natural killer cells in patients with recurrent miscarriage. J Reprod Med. 2015
Jul;60(7–8):294–300. [
PubMed: 26380487]
Manfredi
G, Dell’Aera
L, Liguori
R. Overcoming recurrent spontaneous abortions in women suffering from IgG subclass deficiency: high efficiency of low dose intravenous immunoglobulins treatment. Eur Ann Allergy Clin Immunol. 2015
May;47(3):91–4. [
PubMed: 25951147]
Nyborg
KM, Kolte
AM, Larsen
EC, Christiansen
OB. Immunomodulatory treatment with intravenous immunoglobulin and prednisone in patients with recurrent miscarriage and implantation failure after in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2014
Dec;102(6):1650–5. [
PubMed: 25256927]
Yamada
H, Deguchi
M, Maesawa
Y, Nakajima
Y, Nishino
Y, Tanimura
K, et al. Mediumdose intravenous immunoglobulin therapy for women with six or more recurrent miscarriages. J Reprod Immunol. 2015
Jun;109:48–51. [
PubMed: 25747500]
Non-Systematic Literature Review
Coulam
CB, Acacio
B. Does immunotherapy for treatment of reproductive failure enhance live births?
Am J Reprod Immunol. 2012
Apr;67(4):296–304. [
PubMed: 22340745]
Mekinian
A, Cohen
J, ijotas-Reig
J, Carbillon
L, Nicaise-Roland
P, Kayem
G, et al. Unexplained recurrent miscarriage and recurrent implantation failure: is there a place for immunomodulation?
Am J Reprod Immunol. 2016
Jul;76(1):8–28. [
PubMed: 26847715]
About the Series
CADTH Rapid Response Report: Summary with Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Off-label use of intravenous immunoglobulin for recurrent spontaneous abortion: a review of clinical effectiveness. Ottawa: CADTH; 2018 May. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.