Introduction
Development assistance holds promise for alleviating the death and suffering of impoverished children, women, and men from readily preventable and treatable conditions and to support global economic development, demographic sustainability, and political stability. Although the desirability of these goals is widely shared, there is little agreement on who should shoulder the financial responsibility or how best to use development assistance to achieve these goals.
How much financing should be provided and in what form, who is eligible, and what health areas and interventions should be prioritized? How should institutions balance the financing for current interventions and for future priorities? Should funding for research and development (R&D) be a health aid priority? And what exactly counts as health aid? Does a favorable loan to build a hospital in rural China count? How about in rural Mali? How much health aid flows through recognized channels, and how much falls outside well-documented channels? What criteria should be used to allocate scarce health aid resources? Which countries and populations have the strongest claims to assistance or favorable financing? This chapter provides frameworks for addressing these questions and understanding the crossroads for foreign aid to the health sector. This chapter does not provide a systematic review of current patterns of health aid allocation. The descriptive epidemiology of health aid—the patterns of sources, channels, flows, and targets of donor resources—is available from other sources, which we reference throughout this chapter. Instead, we address key questions that challenge our understanding of the present and planning for the future of international cooperation on health.
The first section addresses the measurement of health aid, including an overview of common definitions and measurements of how health aid flows, from whom, to whom, and to what intended ends. The section also summarizes recent efforts to reconsider the scope of health aid, including aid originating in non–Organisation for Economic Co-operation and Development (OECD) countries and support for R&D and other global public goods.
The second section addresses the normative landscape of health aid: What are the goals for the provision of health aid and the criteria guiding its allocation? We illustrate the role of the implicit and explicit goals of health aid, including the alleviation of death and suffering, human development, national relationships, global health equity, and international security. We also address how implicit and explicit goals guide the provision of health aid across regions and countries and across disease and intervention areas.
The third section provides two case studies that illustrate patterns of health aid sources and the breadth of health aid efforts. The fourth draws lessons learned from the experience with health aid and identifies guiding principles for organizing and implementing health aid resources. We end with a summary and recommendations for future health aid investments. Investing these resources wisely will play an important role in achieving a grand convergence in global health and a decent life for all (Jamison and others 2013).
TRACKING HEALTH AID
Health aid can be broadly defined as the transfer of resources from multilateral organizations, foundations, or governments to the health sector of a country or a population. Although much health aid is in the form of grants and in-kind gifts, some is in the form of concessionary agreements, loans, and preferable trade agreements. Beneath the broad definitions, however, lie several major challenges to the definitions and measurement of health aid.
Definitions
An important challenge to any discussion on prioritization of health aid is the lack of agreement on what exactly counts as health aid. This section describes two data sources that track health aid and highlights the differences between them.
The most detailed source of data on health aid comes from the OECD’s Development Assistance Committee (DAC). The DAC is charged with tracking and measuring all forms of donor financing, including official development assistance (ODA).1 ODA includes mostly grants and loans that are concessional in character and contain a grant element of at least 25 percent. The OECD maintains the Creditor Reporting System (CRS), a database of ODA coded into 36 broad sectors, including two sectors that are noted as “health” and “population and reproductive health.” The database contains information on grants and loans starting as early as 1973, but health aid data are sparse before 2000. Thirty donor nations (mostly members of the DAC); several multilateral organizations, such as the World Health Organization (WHO) and the African Development Bank; as well as the Bill & Melinda Gates Foundation provide specific information about the purpose, amount, and intended recipients of grants and loans qualifying as development assistance.
Because the CRS database has become an important public source of health aid data, its limitations deserve further mention. First, information about the purpose of an aid item may be too general for many health purposes. For example, the CRS lacks a code for reproductive, maternal, newborn, and child health. As a result, recent initiatives that aim to monitor the international flow of resources for such priorities use different measurements, producing different results (IHME 2016a; PMNCH 2014; Victora and others 2015). In addition, information on the purpose of a grant or loan in the CRS can be vague or short, often no longer than a few words, making it difficult to link the resources with their intended priorities.
The second data source contains development assistance for health, a term introduced by the Institute for Health Metrics and Evaluation (IHME) in 2009 that quickly became commonly used in the global health community. In contrast to ODA, development assistance for health includes financing from private sources and financial transfers that target the private sector such as advance market commitments. Unlike the CRS, which contains project-level information on more than 3 million projects, IHME’s data contain global health financing data aggregated by source, channel, recipient, and disease areas (IHME 2016a).
Largely lacking from both the CRS and IHME databases is information on investments in global public goods. Such goods include R&D for diseases that disproportionately affect people living in poor countries or for priorities with global benefits such as epidemic outbreak preparedness. The extent to which investments in global public goods should count as health aid is an area of active debate. The concept of health ODA plus attempts to provide a more complete picture of donor flows to global health by including flows to global functions (Schäferhoff and others 2015). Specifically, health ODA plus includes (1) health aid reported by donors to the OECD and (2) public funding for R&D for neglected diseases, including funding channeled by donors to organizations working on R&D without a specific focus on low- and middle-income countries (LMICs). For example, ODA plus includes financing by the Swedish government for research on antimicrobial resistance through the Karolinska Institute, as well as financing of research on a vaccine to prevent human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) through federal institutions such as the National Institutes of Health or private pharmaceutical and biotechnology companies. Because these resources do not flow to LMICs and support research priorities with global importance, this funding is not included in the CRS and IHME databases. The concept of health ODA plus posits that funding for health priorities that affect lower-income countries is a valuable component of health aid even if it does not flow directly to LMICs (and adds to the complexity of health aid measurements).
What other types of assistance are not measured or tracked reliably? Aid to priority areas such as neglected tropical diseases may be substantially underrepresented in the CRS and other data sets. Aid for noncommunicable diseases (NCDs) is not officially represented at all. Health aid from non-OECD countries such as Brazil, China, India, and South Africa is substantial, but these countries do not report to the DAC. Data on South-South cooperation are hard to track and may include items not considered aid by other definitions, even though it makes up an important supplement to more established forms of assistance (described in case study 2 in this chapter).
In addition, substantial amounts of aid, including health aid, flow between Arab nations and territories. Data on the magnitude or nature of this aid are very limited. Kuwait and the United Arab Emirates report to the CRS, and Qatar publishes aggregate information on aid provided mostly to other Arab nations (Kharas 2015). The United Arab Emirates has been increasing its ODA contributions since 2010, including a 608 percent increase in real terms in 2013 (mainly support to the Arab Republic of Egypt), of which a little less than 10 percent is designated for health. Support for health multilaterals is also growing. For example, Oman, Qatar, Saudi Arabia, and the United Arab Emirates all provide funding to Gavi, the Vaccine Alliance. Changing the CRS database to include reporting from non-DAC donor countries could add to our understanding of international cooperation and unmet needs. There is precedent for expanding the CRS database: several non-DAC donors already report ODA funding to the CRS, including Lithuania, Saudi Arabia, and Thailand. Expanding the global accounting of ODA—including health aid—would relieve areas of great uncertainty in deciding on resource allocations to countries and regions with limited information. For example, the resource flows to conflict regions such as Syria from non-OECD sources are largely unknown and likely substantial.
Sources and Flows of Health Aid
Growth in health aid slowed considerably between 2010 and 2015. During the “golden age of global health” (2000–10), health aid grew 11.4 percent a year on average. Since then, average growth has dropped to 1.2 percent. In 2015, health aid (as measured by the IHME) totaled US$36.4 billion, below the 2013 peak level of US$38.0 billion (see IHME 2016a). Much donor support for health originates in national budgets supported by taxes and other sources of national income in wealthier countries. Unlike domestic health spending that originates from governments that are, in many cases, accountable to the populations they serve, health aid is unstable. Because a donor government does not have the same fiduciary relationship to the population of another country as it does to its own population, health aid amounts and priorities may shift for reasons that have little to do with needs in the recipient country. The dependence of health aid on nonhealth priorities and dynamics that may be entirely exogenous to events in the recipient country makes health aid particularly vulnerable to swings. The impact of volatility may be particularly detrimental to funding streams that finance health services with few substitutes and long-term commitments, such as antiretroviral therapy.
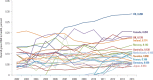
As shown in , substantial variation was experienced in the global increase in health aid. Between 2002 and 2014, some countries have given increasing amounts of health aid (for example, the United States), while others have given stable or declining amounts (Norway).
Health Aid as a Percentage of Total Aid for Major Country Donors, 2002–14.
Global political and economic cycles also shape donor priorities, with recessions leading governments to rearrange their spending priorities, often in ways that do not favor foreign aid. Following the 2007–08 recession, the 2010 health aid budget of OECD countries became more volatile and that of the United States stagnated. In 2005, several OECD countries committed to tethering foreign aid—including health aid—to a portion (0.7 percent) of their gross domestic product. If the recommendation to peg foreign aid to gross domestic product is followed, health aid will grow during economic booms and shrink during economic downturns, making future levels of commitment highly uncertain. Following the 2016 elections in the United States, the new administration expressed decreasing commitment to foreign aid programs, including explicit large reductions in health aid.
Private sources and foundations have been playing an increasingly important role in the health aid landscape. Overall, private sources made up more than 25 percent of health aid between 2010 and 2015, a relatively small component of direct contributions. The Bill & Melinda Gates Foundation was the third-largest overall contributor of health aid between 2010 and 2015, above most European countries (IHME 2016a). However, the influence of private aid transcends its direct financial contribution. Unencumbered by public interests, organizations such as the Bill & Melinda Gates Foundation and the Hewlett Foundation are at relative liberty to take strategic risks and set new agendas. The Bill & Melinda Gates Foundation, for example, provided critical early financing to Gavi, the Vaccine Alliance, and its emphasis on financing novel technological solutions for global health problems has generated funding for more than 1,000 exploratory high-risk, high-reward projects such as farming grasshoppers as a source of protein or developing odorants to block the ability of malaria-causing Anopheles mosquitoes to detect humans. The Hewlett Foundation has been a leader in advancing rigorous program evaluations to understand what works, such as through its support for the International Initiative for Impact Evaluation and leadership of the Effective Philanthropy Group.
More than 300 foundations were registered with the U.S. Agency for International Development (USAID) in 2014, and most of them operate independently (USAID 2015). Their portfolios can be wide, including infectious diseases, reproductive health, and complementary areas such as education, health systems, and governance. One implication of the relatively small size of each foundation and their independent operation is that foundations commonly identify their own (often narrow) strategic focus rather than align their investments within a streamlined, global strategy.
Health financing is distributed unevenly across health areas. Since 2000, the launch year of the Millennium Development Goals (MDGs), the largest growth in health aid funding has been related to the control of infectious diseases, particularly HIV/AIDS and malaria. Child health and especially maternal and reproductive health have received more modest attention. (This trend has changed somewhat since 2010 following the launch of several global initiatives, such as the Group of Eight’s Muskoka Initiative on Maternal, Newborn and Child Health.) Health areas not targeted by the MDGs have received even less attention. These overlooked conditions include NCDs such as cardiovascular disease and cancers as well as neglected tropical diseases, even though the burden of these diseases is large in many aid-recipient countries.
Of the total amount of health aid in 2015, 30 percent and 28 percent were allocated to HIV/AIDS and to maternal, child, and newborn health, respectively, while 6 percent was targeted to malaria control, and only 1 percent to NCDs, even though NCDs are responsible for more deaths than any other major category in every region except Sub-Saharan Africa (Dieleman and others 2015). Box 16.1 discusses this issue in greater detail.

Funding for Noncommunicable Diseases.
Financing has shifted slightly with the launch of global initiatives focusing on child health, maternal health, and nutrition (Darmstadt and others 2014; Kirton, Kulik, and Bracht 2014). Mirroring these shifts, health aid for HIV/AIDS and tuberculosis has declined from peak levels in 2013 (IHME 2016a).
GOALS AND CRITERIA FOR ALLOCATING HEALTH AID
Donors’ and recipients’ normative views and goals inherently shape decisions about whether to provide aid, how much, in what form, to whom, toward what, and how (Centre on Global Health Security Working Group on Health Financing 2014). These views and goals underpin the variation in health aid across countries and partly explain, for example, why health aid per capita ranges from US$0.7 to US$32 in LMICs (IHME 2016a). This section examines stated and unstated goals underlying the allocation of health aid and discusses criteria for guiding the allocation of health aid resources across geographic and health areas.
Goals of Health Aid
Averting preventable deaths and suffering, especially in countries with limited domestic capacity to address health needs, is a shared goal of health aid providers and recipients. For example, the mission of the Global Fund is to invest the world’s money to defeat HIV/AIDS, tuberculosis, and malaria, and the global health mission of USAID is to support partner countries in preventing and managing major health challenges of poor, underserved, and vulnerable people (Global Fund 2016a; USAID 2012). Between 2000 and 2015, many donors also explicitly sought to help countries reach the MDGs on child mortality (MDG 4); maternal health (MDG 5); and HIV/AIDS, tuberculosis, and other major diseases (MDG 6) (Ravishankar and others 2009). These priorities and funding streams remained dominant even after the deadline for the MDG 2 at the end of 2015. At the same time, many donors cite broader goals for health aid, including goals related to poverty alleviation, economic growth, educational outcomes, and security. Starting in 2016 with the Sustainable Development Goals, health-related aims could be further integrated with broader development objectives.
Donors may also have goals that have less to do with recipient need and more to do with donor interests. These goals can occasionally be gleaned from revealed donor preferences without being made explicit. For example, some donors provide health aid to protect their own populations, such as targeting rapidly spreading infectious diseases, like Ebola virus disease; or to promote their political and economic interests (Berthélemy 2006; Hoeffler and Outram 2011). Irrespective of whether explicit or implicit goals are pursued, the Paris Declaration on Aid Effectiveness calls for donors to align their support, whenever possible, with recipient-country government priorities.
Criteria for Allocation across Geographic Areas
Guiding the allocation of health aid across countries or geographic areas is often of importance to donors. Recent and ongoing economic transitions, however, have made decisions about country allocation more difficult for donors seeking to direct health aid toward individuals or communities with large needs relative to their capacity (rather than to countries that may have large relatively well-off populations). Economic growth rates have been impressive in many countries, including many formerly low-income countries (LICs), over the past two decades, and many countries have moved from low-income to middle-income status, including populous countries such as China, India, and Nigeria. At the same time, many of these countries have pronounced inequalities in income and health. One consequence is that most of the world’s poor and the world’s disease burden are no longer located in LICs, but in middle-income countries (MICs) (IHME 2016b; Sumner 2012).
Questions arise about the role of MICs with regard to health aid and, more generally, the central role currently given to mean national income, such as gross national income (GNI) per capita, in allocation decisions. Agreement is growing that GNI per capita is an inadequate basis for deciding which countries are eligible for health aid and how much each country should receive. Therefore, with respect to cross-country allocation of health aid, a central task for many donors in the coming years will be to consider resituating GNI per capita as one tool among several in the overall decision-making process.
The larger debate about how health aid can better target the communities and individuals in greatest need revolves around three broad approaches. One is determining whether GNI per capita thresholds should be used at all to determine eligibility for health aid. Many have called for donors to raise their thresholds, in effect reducing the role GNI per capita plays in determining eligibility. What other criteria should be used if GNI per capita does not provide an eligibility benchmark remains an open issue. Second, others argue for maintaining GNI per capita as a criterion, but supplementing it with criteria directly linked to health needs in the country. For example, the Global Fund hosted the Equitable Access Initiative in 2015, which concluded that countries’ health needs and fiscal capacity are important factors for donors to consider when allocating funds (Global Fund 2016b). Again, the specific metrics to use and how to integrate them remain open issues. Finally, some suggest that donors ought to go beyond countries and average measures such as GNI per capita and focus more on the subnational allocation of health aid. Options for linking eligibility and other allocation criteria directly to subnational units need more study.
Criteria for Allocation across Health Areas
Allocation across disease and health priorities requires additional consideration. Health aid resources cannot fully subsidize the health sector of even the poorest countries, and decisions for prioritizing disease areas and programs are unavoidable. In 2014, US$10.8 billion and US$10.1 billion were allocated to HIV/AIDS and maternal and child health, respectively, while US$2.4 billion was allocated to malaria and only US$475 million to NCDs (IHME 2016a). What accounts for such variation? What principles appear to guide—and ought to guide—the distribution of health aid?
Although some donors clearly state their general priorities, few provide the explicit criteria used to allocate health aid across disease areas. Perhaps the most straightforward way to prioritize financing decisions would be to allocate resources in proportion to the burden of disease such that if the death and disability from disease A is twice that from disease B, then twice the resources should go toward controlling disease A (Sridhar and Batniji 2008). While the equitability of this resource-allocation heuristic is appealing, its principal shortcoming is that, without considering the cost of reducing disease burden, allocation proportional to burden may not reduce as much disease burden as prioritizing diseases for which the most cost-effective interventions exist. Disease burden estimates can be useful for identifying the conditions causing the most mortality and morbidity, but they do not show where health aid resources could yield the greatest benefits (Bendavid and others 2015). For example, stroke is a leading cause of death and disability in China, but financing stroke treatment in China may yield relatively few benefits in comparison with treating and preventing tuberculosis (Coyle and others 2013; Prabhakaran, Ruff, and Bernstein 2015). To identify the investment priorities that provide the greatest benefits with the available health aid resources, information is needed on the cost-effectiveness of potential interventions. One of the principal objectives of the third edition of Disease Control Priorities is to provide this information.
A third proposed criterion for choosing disease priorities for health aid (in addition to disease burden and cost-effectiveness of interventions) would be to provide resources to the diseases the afflict the most ill, globally or nationally (Ottersen and others 2014). For example, priority could be assigned to interventions benefiting persons with lower healthy life expectancy. Although this criterion might yield different allocation guidance than a cost-effectiveness criterion, many interventions will score high on both—for example, cheap and highly effective interventions targeting potentially life-threatening conditions, such as diarrhea, malaria, and pneumonia, in children living in poverty.
Epidemiological and other transitions are creating new challenges for allocating health aid across disease areas. NCDs now account for almost 60 percent of the global burden of disease (Murray and others 2015), and 80 percent of NCD deaths occur in LMICs. Donors need to carefully balance their responses to NCDs with their responses to maternal, neonatal, and child health problems and with the unfinished agenda of infectious diseases. Weighing these choices may involve further inquiry into how criteria related to cost-effectiveness, disease burden, and the worse off can be specified and traded off. At the same time, transnational health threats, including pandemics and antimicrobial resistance, are increasingly being viewed as within the purview of health aid. Chapter 18 of Major Infectious Diseases (volume 6 of this series) on antimicrobial infections provides additional arguments supporting the role of health aid in curbing antimicrobial resistance (Miller-Petrie, Pant, and Laxminarayan 2017). What share of health aid should be allocated to these kinds of threats will be a key question. The interpretation and generation of cost-effectiveness estimates for interventions in these areas will also be important because such estimates are currently lacking or are highly uncertain.
CASE STUDIES
This section presents two case studies illustrating the historical trajectory of health aid and the changing landscape of donor-recipient relationships. The first describes the role played by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and illustrates the tensions in setting priorities and strategies with ambiguous goals and motivations. While the PEPFAR case study delves into the challenges of archetypal health aid institutions, the second case study—describing China’s approach to development cooperation on health (South-South cooperation)—represents a complementary approach to health aid.
Case Study 1: PEPFAR
The spread of HIV/AIDS in Sub-Saharan Africa and the United States in the 1980s and 1990s preceded—and arguably caused—the expansion of health aid in the 1990s and 2000s. Health aid for HIV/AIDS increased from effectively zero in the mid-1990s to the largest single disease priority a decade later. The rapid global response was related to the spread of HIV/AIDS in Europe and the United States, where it became the leading cause of death among young men and created a groundswell of activism and growing recognition of the security and economic threats of infectious diseases in an increasingly globalized world. The United Nations General Assembly Declaration of Commitment on HIV/AIDS, endorsed in 2001, singled out HIV/AIDS as an exceptional priority.
That exceptionalism was backed by substantial increases in commitments and new disbursements toward global control of HIV/AIDS. The largest of those commitments, announced in early 2003, became PEPFAR. In this section, we draw on published materials and an interview with a former director of the Office of the U.S. Global AIDS Coordinator, the agency tasked with implementing PEPFAR, to understand historical and future trajectories of health aid and the challenges of identifying and standing by clear goals and criteria in aid allocation.
PEPFAR changed what was considered possible in health aid, directing billions of U.S. dollars annually toward a single issue in a small group of high-priority countries. The model adopted by PEPFAR involved rapid and concentrated deployment of resources as a response to a global public health emergency. The trade-offs of this approach included occasionally downplaying long-term considerations, such as international parity in resource allocation, that are more characteristic of multilateral organizations like the World Bank or United Nations agencies and that may lead to these thinly spread organizations’ relatively slow operations.
The program funded implementers with established track records, including multilateral U.S.-based organizations such as Columbia University, the Elizabeth Glaser Pediatric AIDS Foundation, the Harvard School of Public Health, and Catholic Relief Services. Driven by expediency, the first phase of implementation included capacity building and service provision that largely circumvented the public sector in partner countries and created a tension that is still evident today: success from PEPFAR’s perspective meant creating a parallel system of health care delivery. This allowed for short-term reduction in mortality, but created longer-term challenges. It took several years before PEPFAR prioritized capacity building in its partner countries and began moving U.S.-based partners to a technical assistance role. That model, in which in-country partners were supported to provide health services and the role of U.S.-based partners was more advisory, was viewed as more sustainable.
This tension between short-term goals and long-term vision is evident in many of PEPFAR’s decisions. As recently as 2016, efforts to shift contracts to in-country organizations were met with resistance from the original U.S.-based implementers. Shifting to in-country organizations was thought to enable further scale-up of services (by eliminating the payment of overhead to U.S.-based organizations) and to foster local capacity, sustainability, and competence (Vermund and others 2012). However, many of PEPFAR’s U.S.-based partners resisted the withdrawal of support, resulting in a gradual and (as of 2016) still-incomplete transition of implementation to local organizations.
Another example of an effort to bridge short-term and long-term goals is PEPFAR’s support for medical education in partner countries. Through a large grant program, PEPFAR supported the creation of a dozen medical training programs in Sub-Saharan African partner countries (Fogarty International Center 2015; Kim and Evans 2014). While this program reflects a commitment to creating long-term, in-country capacity, it also represents a rethinking of PEPFAR’s original priorities.
PEPFAR receives little credit for its attempts to balance short-term targets and long-range vision. These tensions were an integral part of PEPFAR’s implementation. In part because of the need for an epidemic control strategy that is responsive to a changing epidemic and in part because of changing leadership, PEPFAR has altered its strategy from responding to emergencies to increasing country ownership and integration, and, more recently, to achieving global public health goals that extend beyond HIV/AIDS control (Fauci and Folkers 2012).
The challenges facing PEPFAR’s strategic decisions possibly reflect its attempts to balance short-term and long-term strategic goals. For example, the U.S. Global AIDS Coordinator at the end of the George W. Bush administration was replaced swiftly after President Obama took office, and the future of PEPFAR was, for a while, highly uncertain (McNeil 2010). By 2017, PEPFAR had matured into an established health aid program with wide-ranging support and a broad mandate. From this position, it could adopt a long-term, stable set of guiding principles that could help relieve some of the pressures to shift strategies in response to leadership and funding changes.
Many see an opportunity for PEPFAR to leverage the infrastructure it created to focus on multiple diseases, including NCDs, and, in the process, to integrate with other health sectors (Fogarty International Center 2016). Although this may be an intuitive direction for improving the care of HIV/AIDS patients treated in PEPFAR-supported programs, it also signals a broadening of PEPFAR’s mandate at the same time that PEPFAR is poised to deepen its commitment to the highly ambitious goals of achieving both “90-90-90” (90 percent of persons with HIV/AIDS aware of their status, 90 percent in regular treatment, and 90 percent of those in treatment virally suppressed) and an “AIDS-free generation.” If “90-90-90” is achieved, 55 million individuals are estimated to need treatment by 2030, more than 3.5 times the number of people on treatment at the end of 2016 (Hoos, El-Sadr, and Dehne 2016). Successfully broadening and deepening its mandates, possibly with flat or declining resources, is likely to be among PEPFAR’s principal challenges.
Case Study 2: China’s Contributions to Global Health
Health aid is an integral part of China’s foreign aid, which it has been providing for more than 60 years, mostly as South-South partnerships (Zhou, Zhang, and Zhang 2015). Beginning in 1950 with aid to socialist neighboring countries and extending in the mid-1950s to LMICs in other regions, notably Africa, China has provided a large quantity of goods and materials in support of development projects.2 After the political and economic reform in 1978 and the subsequent rise in national income, China continued to expand the level of foreign aid and the diversity of aid forms. As of 2009, China’s total foreign aid equaled US$37.6 billion after increasing nearly 30 percent annually from 2003 to 2009 (China State Council 2011; Zhou, Zhang, and Zhang 2015). From 2010 to 2012, China contributed an additional US$14.4 billion in foreign aid (China State Council 2014). During this period, China focused more on LICs; basic infrastructure projects such as roads, ports, and water supply; social projects linked to personal welfare; and technical training (Zhou, Zhang, and Zhang 2015).
China’s health aid, although a small portion of overall Chinese foreign aid, increased over time, especially to Africa, with the launch of the Forum on China-Africa Cooperation. Unlike most OECD donors, China does not offer direct transfers to the health sector. It uses a project approach and provides health aid through grants. China’s in-kind health aid focuses more on specific aspects of the health system, such as the delivery of health care services; provision of essential medical products, procedures, and traditional Chinese medicine technologies; improvement of health infrastructure; development of a health workforce; and, more recently, malaria control and emergency response to the Ebola epidemic. The main focus is Africa, where almost 90 percent of the dispatched medical teams and 80 percent of donated health facilities—the dominant forms of China’s regular health aid—are targeted.
China’s variable aid components emerged gradually. In 1963, China first dispatched medical teams with donated drugs and medical equipment. Since 1970, China has constructed health facilities, and in 2000, it launched the Human Resources Development Fund for Africa. Since 2006, China has been involved in malaria control, and in 2014, it provided four rounds of emergency aid, totaling US$120 million, to control the Ebola outbreak in West Africa. Recently, to support the 2030 Agenda for Sustainable Development, a series of new initiatives has helped establish an African Union Center for Disease Control and regional medical research centers, assisted African countries to improve disease surveillance systems, and funded 100 maternal and child health projects for LMICs. China also contributes to the Global Fund; Gavi, the Vaccine Alliance; the WHO; the African Union; the World Food Programme; and the United Nations’ health programs. China’s normative approaches to health aid have also evolved, with more emphasis on mutually beneficial goals and shared development, while emphasizing noninterference in internal affairs and avoiding political conditions for aid.
Official data on the financial flows of China’s health aid are not available. According to Liu and others (2014), between 2007 and 2011, Chinese medical teams in Africa were equivalent to about US$60 million in aid annually, donated facilities were about the same, and total health aid to Africa averaged about US$150 million annually. However, these data include only central government health aid. They do not include basic salaries of medical team members, which are covered by their home hospitals; support provided by provincial governments to the medical teams they dispatch; scholarships for students from LMICs to study medicine in China, which are funded by the Ministry of Education; or R&D on neglected tropical diseases, which is funded by other sources.
China’s role in health and development is not limited to the direct provision of health aid through bilateral channels. Since the outbreak of severe acute respiratory syndrome, China has participated in global action on health security. China has also engaged in global health policy debates and worked with global health institutions. Although not counted as health aid by most historical yardsticks, these activities support shared global functions with benefits to LMICs.
GUIDING PRINCIPLES FOR THE NEXT DECADE
Health Aid Effectiveness
A growing body of evidence suggests that the surge in health aid, especially since 2000, has helped reduce the morbidity and mortality from many infectious diseases and the burden of child and maternal mortality in many LMICs, occasionally to levels approaching those in wealthier regions (Bendavid 2014b; Bendavid and Bhattacharya 2014). The declines in child mortality during the past 30 years coincided with the increase in health aid targeting the causes of child mortality such as vaccine-preventable illnesses. While this supports the role of health aid in the decline of child mortality, direct attribution is difficult because child mortality has declined for many reasons. The proliferation of effective organizations committed to expanding the provision of highly efficacious, low-cost child health goods, such as insecticide-treated bednets and vaccinations, suggests that health aid has played an important role, in addition to factors such as economic growth, improved education and nutrition, and the diffusion of knowledge such as the benefits of breastfeeding (Levine 2004). Health aid is associated with the convergence of mortality rates not only among different countries, but also within countries. The geographic and wealth distribution of child mortality has been narrowing within most aid-recipient countries, most precipitously after 2000, coinciding with the largest rise in health aid (Bendavid 2014a).
Changing Aid Commitments
Economic development of aid recipients, changing distribution of disease burden, and growing recognition of the importance of global functions are creating new conditions and new opportunities that would intuitively lead to shifts in the allocation and emphasis of health aid. As countries are increasingly able to finance the delivery of health goods, and mortality from causes financed by health aid continue to decline (for example, vaccine-preventable illnesses or malaria), the allocation of health aid resources may be better used to address new priorities.
Outside of a spike in funding earmarked for Ebola response, health aid funding remained largely flat between 2010 and 2016. Unless new resources become available, any increases in financing of some priorities will require trade-offs and the deprioritization of existing high priorities. This is a challenging endeavor for some streams of health aid, where resources are tied up in long-term commitments. A striking example of this limited flexibility is the financing of antiretroviral therapy (ART) for millions of persons living with HIV/AIDS. ART is costly, life saving, and lifelong, and efforts to move ART programs from donor to domestic funding have been met with vociferous resistance (McNeil 2010).
Liberating aid committed from long-term programs would bring flexibility in responding to new challenges and opportunities, but the transition will be gradual and may not be feasible in the near term. In the meantime, resources could be diverted from low-value priorities lacking long-term commitments with relatively low opportunity cost. It could be expedient to start examining such priorities before tackling entitlements and long-term commitments.
Increasing the domestic ownership of health investments is one way to shift the allocation of health aid commitments. National governments in aid-recipient countries can finance some if not most health care delivery for their own populations. In the past 20 years, health aid grew, in part, because many countries did not adequately finance priorities that donors perceived as urgent (for example, HIV/AIDS) or exceptionally high value (for example, vaccinations). However, as countries continue to develop economically, including many in Latin America, South and South-East Asia, and Sub-Saharan Africa, the domestic resources dedicated to supporting health care could grow with, or even faster than, general economic growth (Moon and Omole 2013; Resch, Ryckman, and Hecht 2015). Additional domestic resources could finance goods and services, including child health, maternal health, reproductive health, and the prevention and treatment of some infectious diseases such as soil-transmitted helminths and malaria.
Which Health Aid Investments Work?
Health aid would have more impact if resources were guided by evidence of effectiveness and cost-effectiveness. A proliferation of randomized field trials during the past two decades has added a new layer of specificity to the evidence on what works for health improvements in LMICs. However, similar to the role of randomized clinical trials in clinical medicine, the interventions examined in each trial are specific, and the study populations may not be broadly representative. This limited generalizability notwithstanding, the widespread popularity of randomized controlled trials could point to other ways in which evidence could improve health aid.
Randomized evaluations could be incorporated into the design of major programs. Currently, most randomized evaluations are organized by academic institutions and result in attempts to infer generalizable insights about the process of successful development from high-quality evidence in specific instances. Despite the proliferation of randomized evaluations, however, concerns about generalizability of trial insights have only increased over time (Deaton 2009; Pritchett 2004). A shift in focus would greatly improve their utility: randomized evaluations could replace traditional monitoring and evaluation. Trials provide credible estimates of the effectiveness of specific interventions and the mechanisms of action. They are less biased than traditional monitoring and evaluation and could be streamlined so that routine field evaluations could be carried out. Using rigorous evidence to guide the allocation of health aid would lend credibility, improve resource allocation, and ultimately improve health.
Randomized trials are not the only approach to discovering “what works.” They are part of a broader context of scientific understanding and discovery. For many issues in global health, randomized trials may not be feasible for practical or ethical reasons. For example, studying the effect of good governance on health is not readily amenable to randomized assignment (Kudamatsu 2012). For such questions, observational analyses are the only way to discover meaningful insights. The accumulation of evidence is a gradual process, but lessons learned through cumulating evidence have been important in guiding interventions that save many lives (Glassman and Levine 2016).
Identifying Investment Opportunities
The burden (or projected burden) of disease is a predominant consideration in choosing new health aid investments, with high-burden conditions arguably deserving more attention than low-burden conditions. However, efficient distribution of resources is also needed. To allocate resources efficiently, the cost-effectiveness of available interventions must be taken into account. For example, coronary bypass surgery may be an efficacious option for a high-burden condition, but it is not cost-effective relative to preventing coronary artery disease (Basu, Bendavid, and Sood 2015).
Interventions that are similarly cost-effective may have different effectiveness (and different costs). Decision makers may have to choose among options that provide greater benefits to fewer people and similarly cost-effective options that provide fewer benefits to more people. A stylized example is a trade-off between two interventions with similar cost-effectiveness. Intervention A averts 1.0 disability-adjusted life year per person, while intervention B averts only 0.1 disability-adjusted life year per person; intervention A also costs 10 times more than intervention B to treat one person. With a fixed budget, choosing intervention A yields the same population-level benefits at the same cost as intervention B, and while only one-tenth of the people can be treated, people successfully treated with intervention A will realize greater gains (on average) than those treated with intervention B (Rose 2001). An efficiency (cost-effectiveness) framework cannot distinguish between the two interventions. The greater number of beneficiaries could advantage intervention B under an equity framework, but the greater effectiveness of intervention A may reduce the uncertainty about impact, which may be an important consideration in some circumstances.
Effectiveness and cost-efficiency are important criteria for health aid (Denny and Emanuel 2008), but aid displacement is also a consideration. Health aid flowing to disease areas from which domestic resources could easily be diverted is likely to lead to displacement, possibly outside the health sector. This is especially true if the aid recipient believes that the sum total of health aid and domestic resources flowing to the same area exceeds the social optimum. The evidence for health aid displacement is consistent with this process (Lu and others 2010). To prevent or reduce the likelihood of displacement, donors might fund interventions for diseases that are relatively underfunded.
Using health aid to fund cost-effective interventions for underfunded high-burden diseases could yield high returns. Local context will determine the appeal of a particular intervention, given that the burden, cost (cost-effectiveness), domestic prioritization, and effectiveness of an intervention are locally determined. Future work comparing the appeal of interventions based on local conditions could have important implications for health aid decisions.
Investments in Global Functions
The Lancet Commission on Investing in Health made the case that, as LMICs undergo economic growth, the value of health aid investments in “global functions”—that is, the provision of global public goods and protection against global cross-border health threats (Jamison and others 2013)—might become more appealing in comparison with country-specific investments. This concept has been echoed in several high-impact policy analyses (Blanchet and others 2014; Centre on Global Health Security Working Group on Health Financing 2014; Frenk and Moon 2013; Ottersen and others 2014).
Based on work by the Lancet Commission on Investing in Health, one study estimated how much donors spend on global functions versus how much they spend on country-specific support (Schäferhoff and others 2015). Global functions were characterized by their ability to address transnational issues and were divided into those providing global public goods (conducting R&D of new health tools, generating and sharing knowledge), those managing cross-border externalities (preparing for outbreaks, tackling antimicrobial resistance), and those fostering leadership and stewardship (convening leaders to build consensus). Country-specific support, in contrast, tackles current health priorities that justify international collective action. The study found that about one-fifth of health ODA plus was for key global health functions, with the rest channeled to country-specific support. Strengthening donor support for global functions could have several benefits that are not immediately obvious.
First, every country benefits from investments in global health, and the costs of inaction are potentially very high—for example, a severe influenza pandemic could result in as much as US$3 trillion in global losses (Gostin and Friedman 2015). The returns on investing in R&D are potentially among the largest of all investments in global health, but actual investments in R&D for neglected and poverty-related diseases are limited. For example, a 70 percent efficacious vaccine would reduce new HIV/AIDS infections by 44 percent (Harmon and others 2016), leading to large reductions in incidence and potential epidemic control. The WHO has therefore called for a doubling of current R&D expenditures for poverty-related and neglected diseases—from US$3 billion to US$6 billion a year, approximately 3 percent of total health R&D (Consultative Expert Working Group on Research and Development: Financing and Coordination 2012). Market-shaping activities such as advanced market commitments also have led to important gains, especially in the fields of immunization and diagnostics. However, only a small fraction of current health aid has market-shaping effects.
Second, enhanced capacity for global disease surveillance and detection and improved international coordination are important for responding to emerging health threats, such as the Ebola outbreak in West Africa. Donors invested less than US$1 billion in 2013 for management of cross-border externalities (including outbreak preparedness but also environmental challenges and other global threats). In the years leading up to the Ebola outbreak, the WHO’s budget for outbreak and crisis response was cut from US$469 million in 2012–13 to US$241 million in 2014–15. A pandemic of larger proportions could be extraordinarily costly, estimated at about US$500 billion per year in losses (Fan, Jamison, and Summers 2016). On the other hand, implementing a framework to improve preparedness for such an event is estimated to cost about US$4.5 billion a year and could lead to large savings (Sands, Mundaca-Shah, and Dzau 2016).
Third, investments in global functions would help address the “middle-income country dilemma”: although most of the poor now live in pockets of poverty in MICs and face high mortality rates, these countries are considered to be sufficiently wealthy to finance health care for their entire populations and are therefore commonly not eligible for health aid. Poor individuals in MICs would benefit from donor support for global functions, such as R&D, knowledge sharing, market shaping, and better systems for controlling and managing outbreaks. China and India, for example, would substantially benefit from collective purchasing of commodities, market shaping to reduce drug prices, and international efforts to control multidrug-resistant tuberculosis. These countries would also benefit from greater global leadership and dialogue on topics such as how to fight the double burden of infectious and noncommunicable diseases, how to design and implement taxation polices to increase domestic financing, and how to engage in cross-sectoral work, including human rights and education.