NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Minnesota Health Technology Advisory Committee. Minnesota Health Technology Assessments [Internet]. St. Paul (MN): Minnesota Department of Health; 1995-2001.
This publication is provided for historical reference only and the information may be out of date.
Executive Summary
The use of herbal products has increased rapidly over the last five years resulting in a record $9 billion in sales during 1999. This growth is being driven by a number of factors including aggressive marketing, low cost, easy access, minimal regulatory control, and a belief that because herbal products are "natural" they are safe. Herbal preparations are classified as dietary supplements and, therefore, are regulated by the Federal Drug Administration (FDA) under the Dietary Supplement Health and Education Act (DSHEA) of 1994. This act permits the sale of dietary supplements without the extensive premarket approval process required of new drugs. Concerns of inappropriate use, adverse reactions, questionable marketing, safety, and effectiveness have generated growing support to restore the FDA's original control of herbal products.
St. John's wort is one of many herbs that have been used for centuries. Currently, the most common use is in the treatment of mild depression. St. John's wort has been used effectively for the treatment of mild depression, but the exact mechanism of action is unknown. Some researchers believe that St. John's wort is a monoamine oxidase (MAO) inhibitor, while others suspect that high concentrations of flavonoids contribute to its antidepressant activity. Extracts from St. John's wort contain several active compounds, many of which have been shown to bind to the neuroreceptors in the brain.
To date, the majority of the studies are from Germany, consist of small sample sizes, and are short in duration. The classification of depressive disorders was inconsistent, and severity of depression did not always correlate with severity of symptoms according to scales. Different preparations for St. John's wort and the dose of the total extract varied considerably. However, the studies showed that there is evidence that St. John's wort is better than placebo and may work as well as other antidepressants in treating mild to moderate depressive disorders. One U.S. study found St. John's wort to be as least as effective as one selective serotonin reuptake inhibitor (SSRI) in the treatment of mild to moderate depression.
St. John's wort has not been systematically studied to document safety, side effects, and toxicity. Studies have shown St. John's wort to have fewer side effects than certain tricyclic antidepressants, however, additional scientific information on the interactions of St. John's wort with over-the-counter drugs, prescription drugs, other herbal products, and food needs to be studied.
Due to the limited amount of scientific information on St. John's wort and other herbal supplements, caution needs to be exercised in use of these products.
Conclusions
With the passage of DSHEA, the FDA has less stringent authority governing St. John's wort, and dietary supplements; therefore, clinical trials showing safety and effectiveness are not required. Variances exist in purity, strength, and potency.
Strong marketing and belief by consumers that herbal supplements are "natural," and therefore safe and effective, have resulted in increased use.
Herbal supplements are taken based on self-diagnosis, beliefs or customs, advice of friends, or advice of nontraditional providers. This has created the potential for severe problems by users.
Limited data exists demonstrating the effectiveness of St. John's wort with mild to moderate depression. Safety, interactions, and toxicity have not been fully studied. Clinically significant drug interactions have recently been reported with St. John's wort.
Recommendations
All persons using St. John's wort or herbal supplements should consult with their physician prior to using St. John's wort or other herbal supplements.
Additional data from well-designed, controlled studies is needed to determine the effectiveness and safety of St. John's wort and its potential interactions with other drugs.
Physicians should inquire if patients are taking any herbal supplements prior to prescribing any drug.
Pharmacists should inquire if patients are taking any herbal supplements prior to dispensing any drug.
Patients should be informed about possible complications, drug interactions, and food interactions that can occur with St. John's wort and other herbal supplements.
Patients who are currently taking indinavir, cyclosporine, or digoxin should not take St. John's wort. The use of St. John's wort has been shown to significantly decrease the concentration in the blood of indinavir (Crixivan®), cyclosporine, and digoxin.
Women should be informed that the effectiveness of oral contraceptives could be reduced by St. John's wort.
Due to a lack of clinical data, pregnant or nursing women should first contact their physician before taking St. John's wort or any herbal supplement.
Equal caution should be exercised when using herbal supplements and any drugs.
FDA rules should require herbal supplements to be systematically studied, (including interactions with other drugs) in order to protect the public from harm.
Introduction
Herbs have been in use worldwide for centuries. Today, the use of herbal products is common in approximately 80% of the world's population. 1 During the 1990s, the trend toward self-medication with herbal supplements, driven by strong marketing campaigns and consumerism, has increased in the United States. In 1996, sales of herbal supplements in the U.S. exceeded $1.2 billion and, in 1999, sales grew to an estimated $8.8 billion. 2 Herbal supplements are regulated in the U.S. by the Food and Drug Administration (FDA) as dietary supplements under the Dietary Supplement Health and Education Act (DSHEA). DSHEA has less stringent standards than those under which pharmaceutical drugs are regulated. Therefore, manufacturers of herbal supplements are not subject to the same approval process or quality control standards as pharmaceutical drug manufacturers.
It is estimated that more than 60 million people (23%) in the United States over the age of 18 use herbal supplements,3,4,5 or approximately 790,000 Minnesotans in 1998. The new attraction to herbals for Americans may stem from a person's desire to use something inexpensive, easily obtainable, and perceived as natural (not manufactured). Other attractions to herbal supplements may include a person's aversion to seeing a physician or it may be a part of his/her cultural background. Most herbal supplements are used after an individual has performed a self-diagnosis of his/her health needs. In a 1997 national phone survey, it was reported that 12 percent of 2055 respondents had used herbal supplements within the last year. Of these, 15 percent saw nontraditional providers and had a mean of 2.9 visits over that year. 5 In the U.S., an estimated 629 million visits to unconventional providers were made in 1997. 5 Physicians were not consulted in most of these instances, and fewer than three in ten people reported the use of any unconventional therapy to their physician.4,5
Due to the growing use of herbal supplements by the general public, an understanding of the benefits and risks of using herbal products should be part of a physician's base of knowledge. Lectures such as "Complementary and Alternative Pharmacotherapeutics" given at the University of Minnesota will help train physicians about herbs, but additional training is needed.6-7 Not only should there be an understanding of the properties of commonly used herbs, but physicians and pharmacists must be knowledgeable about the possible adverse results from mixing herbal products with each other or with prescription medications. Although there are few scientific studies on the toxicities of herbal products, there have been enough reported incidences of complications to establish that potential risks exist and should not be ignored2,7,9-15
FDA
Currently, herbal products (such as St. John's wort) are recognized by the FDA as dietary supplements and not as foods or drugs.1,3,16-18 A dietary supplement is any product taken by mouth that contains a so-called "dietary ingredient" and the label on the product clearly states that it is a dietary supplement. A "dietary ingredient" may include vitamins, minerals, herbs, and amino acids, as well as substances such as enzymes, organ tissues, metabolites, extracts, or concentrates. Dietary supplement ingredients may not be regulated as food additives.
From 1962 to 1994, the FDA regulated herbal supplements as pharmaceutical drugs.3,7 Before permitting the marketing of herbal products, the FDA required clinical trials to determine their effectiveness, safety, possible interactions with other substances, and appropriate dosage. In 1993, the FDA threatened to remove herbal supplements from the market altogether. 7
As a result of different and varying pressures, the U.S. Congress passed the Dietary Supplement Health and Education Act of 1994. This act permits the sale of dietary supplements without FDA approval provided that manufacturers do not claim to: restore normal function; correct abnormal function; treat, diagnose, cure, or prevent a specific disease or class of diseases; or mitigate conditions of health.17,18 Manufacturers may make claims associated with the maintenance of good health, structure, function, or mechanism, however, rules do not allow for implied disease claims. The promotion of unproven effects is allowed if there is an accompanying disclaimer stating that it has not been reviewed by the FDA for the purpose of medication. An FDA rule, effective February 7, 2000, changed the definition of disease so that it does not include "common conditions associated with natural states or processes that do not cause significant or permanent harm." This rule allows the manufacturers of dietary supplements to make claims for the treatment or mitigation of "natural states" such as hot flashes, mild memory problems, or hair loss associated with aging without proving that the products are safe or effective. However, as of March 23, 2000, the FDA will be requiring an information panel, entitled "supplement facts," to appear on all dietary supplements. This panel will be similar in format to the "nutrition facts" panel that now appears on most processed foods and will include serving size, nutrients, and other ingredients present in the product.
The Federal Trade Commission (FTC) primarily regulates the advertising and marketing of dietary supplements. In November 1998, the FTC issued the advertising guidelines for manufacturers and marketers of dietary supplements. The DSHEA does allow for the removal of herbal supplements from the market if health claims are made (as opposed to only structure or function claims) or if the FDA has proven the product unsafe.
Because herbal supplements are not regulated as drugs, manufacturers are able to sell herbal products without FDA approval and there is no requirement that manufacturers produce a product that meets set standards for uniformity and consistency. This raises concerns regarding variability of the same herbal product from one manufacturer to the next. Concerns include composition, quality, dosage, purity, and potency. Data are also lacking regarding safety, effectiveness, long-term toxicity, and adverse effects on specific populations (e.g., pregnant women, nursing mothers, and elderly patients with impaired liver function, cardiovascular problems, or other serious conditions).
With the increased use of herbal supplements, there is a growing concern that the FDA should reexamine the safety and efficacy of these products, as well as the distribution and marketing practices of their manufacturers. The original DSHEA law was not specific on a number of issues, so the FDA has developed rules and regulations to clarify the FDA's authority and the definitions of dietary supplements. Concern exists since the DSHEA does not permit the FDA the same regulatory powers for herbal supplements as for prescription drugs, and that herbal supplements are being promoted and used inappropriately.
On February 10, 2000, the FDA issued a warning letter to health professionals about St. John's wort. The letter stated: 'St. John's wort appears to be an inducer of an important metabolic pathway. Many prescription drugs used to prevent transplant rejection or pregnancy (oral contraceptives) are metabolized via this pathway; therefore, health care providers should alert patients about the potential drug interaction. Since herbal products are widely used in the United States and are available in various forms such as combination products and teas, it is important that health care professionals ask patients about concomitant use of products that contain John's wort (hypericum perforatum). This warning letter is based on an increasing volume of voluntary reports the FDA has been receiving over the last two years and upon a study completed by the National Institutes of Health (NIH).19,20,21
Background
St. John's wort (Hypericum perforatum), also known as Hypericum, Klamath weed and goat weed, is a perennial weed native to Europe and Asia that was brought to the United States by European colonists.16,22 The plant was named after John the Baptist in the first century and wort is an old English word meaning plant. St. John's wort has been used as an herbal medicine for centuries, both orally and topically, as an anti-inflammatory, sedative, analgesic, diuretic, antimalarial, and wound-healing agent.16,23,24 Greeks and Romans thought it could protect them from evil spirits and used it to "ward off" depressive illness.
While Germany and other European countries have long utilized St. John's wort as an antidepressant, 25 in the U.S. it reached a high level of interest 10 years ago after gaining some notoriety as an antiviral herb. 26 It is now being used in the U.S. for treatment of depression, anxiety, and insomnia. The use of St. John's wort for the treatment of depression has made it the number five selling herb in Europe and one of the top 10 herbs used in the U.S. and Minnesota.3,27 It is often referred to as "Nature's Prozac." 28 St. John's wort is still being used for wound healing, and a few studies have been undertaken to determine its effectiveness for this use.16 However, its most common use is for the treatment of mild depression and it is in this area that the vast majority of clinical research is being performed.22,28,29
In 1977, when the FDA regulated herbs as prescription drugs, it declared St. John's wort unsafe since cattle who ate large quantities of the plant developed a sensitivity to the sun accompanied by severe sunburn and blisters.10,16,27,30,31
Uses
St. John's wort has gained attention for treatment of depression, as a wound-healing agent and as an herb with potential antiviral benefits in the treatment of AIDS. 26 Its use in the treatment of mild depression is the most popular and will be the focus of this report.
Depression
St. John's wort, or an extract from the herb, is frequently being used for treatment of depression. Depression is a common disorder with an estimated lifetime prevalence of 17 percent in the United States.25,32,33 According to the National Mental Health Association the estimated number of people afflicted with clinical depression is over 19 million. 34 Depression is characterized by many subjective symptoms including despondency, loss of interest, irritability, eating disturbances, and disruption of sleeping patterns. Depression is generally considered to have three degrees of severity: mild, moderate, and severe. Mild depression, the most prevalent form of depression, does not interrupt daily activities whereas moderate depression occurs with more frequency and may sometimes interfere with daily activities. However, severe depression leads to extreme functional impairment and sometimes suicide.25,32,35
Medical researchers believe that depression is caused by a biochemical imbalance or by a combination of biochemical imbalances. One such imbalance is the deficiency of the neurotransmitters serotonin, dopamine, and norepinephrine. Neurotransmitters act as cellular messengers, enabling brain cells to communicate. They are secreted into the synapses, or spaces, between neurons and are "taken up" by receptors where they are subsequently stored or metabolized with the aid of an enzyme, monoamine oxidase. Compounds that interfere with or inhibit this process have been found to have a beneficial effect on depression. Another possible cause of depression is the over stimulation of the hypothalamic-pituitary-adrenal (HPA) axis by inflammatory cytokines, such as interleukins and tumor necrosis factors, resulting in the excessive production of cortisol. It is believed that cytokines are involved in cell-to-cell communication in the immune and central nervous system, and may play a role in depression as neuromodulators. Prolonged increases in cortisol levels are known to reduce serotonin production and sensitivity of serotonin receptors, as well as to inhibit the secretion of melatonin. Deficient levels of melatonin have been found in patients suffering from depression. 22
Several groups of antidepressant medications are used to treat depressive disorders: tricyclics, monoamine oxidase inhibitors (MAOI), and selective serotonin reuptake inhibitors (SSRI).25,36 Tricyclic antidepressants enhance the concentration of two neurotransmitter chemicals, norepinephrine and serotonin, and impede the reuptake process so that monoamines remain active longer. MAOIs regulate the metabolic degradation of norepinephrine, serotonin, and dopamine by inhibiting monoamine oxidase. SSRIs inhibit the uptake of serotonin. Bupropion, a newer antidepressant that is chemically unrelated to the other antidepressants, inhibits the uptake of norepinephrine and dopamine, more than serotonin, and does not inhibit MAO. 37
Wound Healing and Antiviral Activity
A burn ointment made from the extract taken from the flowers of St. John's wort has been
promoted as assisting in the healing of first-, second-, and third-degree burns. There are
references in literature which cite cases where first-degree burns healed in 48 hours, second-
and third-degree burns healed at least three times as rapidly as burns treated with
conventional methods and keloid formation was inhibited.
16
However, the most commonly cited reference is a single case report and not a scientific
study.
St. John's wort is also being used for cuts and abrasions and a few older
studies were found to support this use.26,38,39,40
Studies have shown two of hypericum's components, hypericin and pseudohypericin, inhibit a variety of encapsulated viruses.16,38,41 This antiviral activity is not completely understood. 40
Mechanisms of Action
The exact mechanism of action for St. John's wort, as an antidepressant, is unknown. There are several compounds found in extracts of the herb, including hypericin, hyperforin, pseudohypericin, xanthones, monoterpenes, β-sitosterol, flavonoids, bioflavonids, naphtodiantrons, melatonin, and catechin. Originally, hypericin was thought to be the primary active compound and is the substance from which extracts from the plant were and continue to be standardized. However, recent data from the NIH indicates that this may not be accurate and that some other compound, or combination of compounds, is responsible for its antidepressant effect.42,43 It has been shown that many of the active compounds in St. John's wort bind to the neuroreceptors in the brain. These neuroreceptors are thought to be involved in depression, and the binding inhibits uptake and causes destruction of neurotransmitters. In addition, one or more of its compounds seemingly suppress the stimulation of the HPA axis and the subsequent overproduction of cortisol, thus effectively increasing production and secretion of serotonin and melatonin.22,44
Early studies of the components of St. John's wort led researchers to be concerned that one of its mechanisms of action was as a monoamine oxidase inhibitor (MAOI). Subsequent study results have suggested that MAO inhibition can occur with very high concentrations of some components of St. John's wort, but these concentrations are not achieved with commercial preparations. In addition, the relative inhibitory potential of St. John's wort on the MAO system is well below that of the commonly used MAOIs.22,43,45 Some researchers suspected that high concentrations of flavonoids were a contributing reason for inhibition. Some studies report that St. John's wort extract inhibits serotonin expression.16,22
Findings
Depression
In a 1996 meta-analysis of 23 randomized clinical trials (Appendix I), Linde, et al. compared the effects of St. John's wort to placebo and tricyclic antidepressants. The trials involved more than 1757 patients with mild to moderate depressive disorders. Linde, et al. found that the St. John's wort extracts were superior to placebo and just as effective. With the tricyclic antidepressants, amitriptyline and imipramine, they found them to be slightly more effective, and had fewer side effects. Across all trials, patients improved 50 to 80 percent with St. John's wort, or at a rate similarly achieved with tricyclic antidepressants. In 13 of the 23 clinical trails that compared a single St. John's wort preparation with placebo, 55.1 percent of the subjects responded to St. John's wort, while 22.3 percent responded to placebo. The rate of side effects with St. John's wort was 4.1 percent and 4.8 percent with placebo.
Three clinical trials included in the meta-analysis compared St. John's wort (single preparation) with tricyclic antidepressants, resulting with 63.9 percent responding favorably to St. John's wort versus 58.5 percent for the tricyclic antidepressant. Of the 23 clinical trials, two compared a combination of St. John's wort and Valeriana with tricyclic antidepressants. Improvement was 67.7 percent for the combination and 50.0 percent for the antidepressants.
Several limitations existed in the clinical trails included in the 1996 meta-analysis comparing St. John's wort with tricyclic antidepressants. None of the studies were longer than 6 weeks in duration and all the antidepressants where given in daily amounts below or at the lower end of the usual dose range. 33 Linde, et al. concluded that additional long-term trials with standardized St. John's wort preparation methods and dose must be conducted. In addition, further studies are needed utilizing standard antidepressants in well-defined groups of patients, comparing different St. John's wort extracts and doses.30,36,46,47 It's argued that the length of the studies is no longer or shorter than the length of recommended treatment (5 to 6 weeks), whether for St. John's wort or other antidepressants. Prozac®, an SSRI, was approved based upon studies that had treatment times of 6 to 8 weeks. 48 Others argue that herbal supplements should not be held to a higher standard of evidence than prescribed medications. 49
A recent U.S. study (April 2000) of 30 patients found that the use of hypericum was at least as effective as sertraline, an SSRI, in the treatment of mild to moderate depression. 24 The study found that 47 percent of the patients receiving hypericum had significantly reduced Hamilton Rating Scale for Depression (HAM-D) scores as well as reduced Clinical Global Impression Scale scores (Appendix VI). Forty percent of those receiving sertraline also had significantly reduced scores.
Of the studies that have proven St. John's wort to be an effective antidepressant medication,24,25,33,36,47,50-52 the clinical trials have been of short duration, had few participants, and had no standardization of dosages. The classification of depressive disorders was inconsistent, and severity of depression did not always correlate with severity of symptoms according to scales. Different preparation methods for St. John's wort and the dose of the total extract varied considerably. 28 Due to the popularity of St. John's wort, the National Institute of Mental Health (NIMH) is presently recruiting patients for a clinical research study on the efficacy of St. John's wort for the treatment of depression. The three-year study, Protocol Number: 99-M-0151, will include 336 patients with major depression who will be randomly assigned to one of three treatments for an eight-week trail. One-third of the patients will receive St. John's wort, another third will be given a placebo, and the final third will take a SSRI. Patients responding positively will be followed for another 18 weeks to determine if those receiving St. John's wort have fewer relapses than patients given placebo. The results from this study will not be available until the year 2003. 53
Wound Healing
Wound healing studies have shown antimicrobial activity, minor antifungal properties, and effectiveness against gram-positive organisms. Studies have reported that hyperforin and adhyperforin have a greater effect than sulfonilamide against Staphyloccus aureus infection,16,27,50 and when used as an ointment, healing time and scarring from burns were reduced. 26 This healing property is attributed to the essential oil, phloroglucinols, and flavonoids. 16 Additional studies are needed to demonstrate St. John's wort as an antibiotic.
Antiviral Activity
In the late 1980s, excitement developed over the apparent activity of St. John's wort against the AIDS virus. Numerous studies were launched because of the herb's activity against a virus that was similar to the HIV virus. This excitement was enhanced since St. John's wort displayed few toxicity characteristics. Although earlier studies have been inconclusive,16,26 one clinical trial, published in March 1999, concluded that St. John's wort had no antiretroviral activity in the limited number of patients studied. The antiviral ability appears to involve a photoactivation process that forms singlet oxygen and inactivates viral fusion and syncytia formation. 16 Other theories and studies report hypericin as a potent inducer of glioma cell death due to inhibition of protein kinase C (PKC). 16 Further study is required in order to have a definitive understanding of the mechanisms involved and its effectiveness.
Dosage
There is no set dosage for St. John's wort when used as a treatment for depression. Studies have shown it to be effective in amounts of 200 to 900 mg with an optimum adult dosage at 900 mg (taken 300 mg three times daily and concentrations varying from 0.2 to 0.34 percent hypericum).16,46,51,54 Like other antidepressant medications, St. John's wort needs to be taken for 2 to 4 weeks to develop its mood-elevating effects. Daily doses are necessary to keep blood levels consistently above 6 ng/mL. For hypericin, peak plasma concentrations occur within 2.0 to 2.6 hours. After 14 days of repeated doses (3 × 300 mg/day), steady concentration levels occurred after four days. The peak concentration was 8.5 ng/ml, with trough concentrations 14 hours after the last dose being 5.3. 16
Safety
Due to differences in FDA regulations, St. John's wort has not been systematically studied to document safety and side effects. There is also a shortage of documented, scientific information on the interaction of St. John's wort with other over-the-counter drugs, prescription drugs, and other herbal products. For this reason, discussion between a patient and physician/pharmacist should occur prior to the use of St. John's wort.
The safety, effectiveness, long-term toxicity and adverse effects of St. John's wort for specific populations, such as pregnant women or nursing women, and elderly patients with impaired liver function, cardiovascular problems, or other serious conditions, has not been proven. Due to a lack of data, these persons should not use St. John's wort.
In an open trial of 3250 patients taking St. John's wort, only 2.4 percent reported side effects. 46 The most frequently reported side effects associated with St. John's wort are gastrointestinal symptoms, nausea, allergic reactions, fatigue, dizziness, and restlessness (Appendix II).
One component, hypericin, has been found to be photoactive and may cause photosensitivity, phototoxicity, or photoallergy in fair-skinned individuals. For this reason, persons consuming St. John's wort should use caution when exposed to the sun by wearing appropriate clothing and sunscreen.10,22,31,47
Prescription drugs are systematically studied for safety and efficacy in order to receive FDA approval. However, St. John's wort, which is an herbal supplement, does not need to be studied resulting in a limited number of documented adverse effects. Therefore, while prescription drugs may seem to have more adverse events associated them, this may only be a result of the closer scrutiny required of them by the FDA.
Side effects of tricyclic antidepressants and MAOIs including cardiac (i.e., tachycardia, postural hypotension, hypertension) and anticholinergic reactions are listed in Appendix II. St. John's wort has thus far proven to be free of any cardiac or anticholinergic side effects. 25 However, because of its possible MAOI properties, caution is warranted.46,55
Poison control centers, in a number of states, have been reporting adverse reactions to a variety of supplements. In New Mexico, during 1999, St. John's wort ranked first as the most reported herb for adverse reactions. The Pittsburgh Poison Control Center reports St. John's wort and ginseng as having the most reported herbal supplements incidences. 2
Interactions Drug
Serotonin syndrome, a potentially fatal side effect, can result when more than one antidepressant is taken at the same time. 20 Therefore, unless under physician care, it is advised not to use St. John's wort concurrently with other antidepressants.
Dextromethorphan, which is found in many cough suppressants, may result in serotonin syndrome when used with MAOIs such as phenelzine sulfate (Nardil®), tranylcypromine sulfate (Parnate®); or with SSRIs such as, paroxetine hydrochloride (Paxil®), fluoxetine hydrochloride (Prozac®), or sertraline hydrochloride (Zoloft®). Although there have been no confirmed reports, Dextromethorphan may also interact with St. John's wort due to pharmaceutical properties similar to antidepressants.20,56,57
St. John's wort should not be taken with amphetamines, meperidine, diet pills, asthma inhalants, nasal decongestants, or cold and hay fever medications.58
St. John's wort is an inducer of the cytochrome P450 3A4 enzyme system (CYP3A4).14,15,59 Drugs which are known substrates for CYP3A4 may also be subject to drug interactions with St. John's wort. Healthcare providers should be aware of the medications that are metabolized by CYP3A4.19,20,21
Oral contraceptives are metabolized via the CYP3A4 pathway and patients should be aware of this potential drug interaction. The Food and Drug Administration issued a public health advisory stating that oral contraceptives may potentially be less effective when taken with St. John's wort. 19
A 1999 single-blind, placebo-controlled parallel design study14 evaluated the effects of St. John's wort (92 mg of hypericin per 300 mg tablet of dried hypericum extract) on persons using digoxin (0.25 mg/d). Results showed a decrease in digoxin Cmax (peak plasma concentration), Ctrough (plasma concentration 24 after pervious dosing, and AUC(0-24) (area under the plasma concentration-time curve) values when compared to the group placebo. Comparison to the parallel placebo group after 10 days of co medication showed a reduction in Ctrough, Cmax, and AUC(0-24) of 33 percent (P=.0023), 26 percent (P=.0095), and 25 percent (P=.0035) respectively. After 10 days of co medication, Ctrough levels of digoxin were reduced by 37 percent (P=.0001) within the group using St. John's wort. Cmax levels and AUC(0-24) of digoxin were also reduced, 26 percent (P=.0013) and 28 percent (P=.0001) respectively. Use of St. John's wort did not affect the elimination half-life of digoxin. Data showed that a single dose of St. John's wort raised digoxin levels, suggesting that reduced digoxin levels were not due to impairment of absorption. It was hypothesized that the mechanism by which St. John's wort reduces serum digoxin levels may be induction of the transport protein, P-glycoprotein, which is important in the absorption of many drugs that are also metabolized by CYP3A4. There is also a threat of digoxin toxicity for patients who achieve therapeutic levels of digoxin while on St. John's wort once the St. John's wort is discontinued. 14
The concentrations of indinavir (Crixivan®), an HIV drug, and cyclosporine, used to suppress transplant rejection, are both lessened when patients are taking St. John's wort.14,19,21,59
St. John's wort increases the clearance of cyclosporine and indinavir. St. John's wort taken for 2 weeks with indinavir substantially decreased the AUC of indinavir plasma concentration by a mean of 57 percent and decreased the extrapolated minimum plasma concentration 8 hours after the dose by a mean of 81 percent. 20 St. John's wort has been associated with a drop in cyclosporine plasma concentrations, resulting in acute transplant rejection. In two cases, cyclosporine plasma levels had remained stable since the transplant was performed. After 3 weeks of using St. John's wort, the patients were admitted for acute heart rejection. No other abnormalities were found except a decrease in cyclosporine plasma levels. St. John's wort was stopped, and eventually plasma cyclosporine levels returned to baseline levels and remained within the therapeutic range.19,21,59
Foods
Questions remain about the interaction of St. John's wort and amino acids. While St. John's wort has not demonstrated significant MAOI activity, the potential may still exist. Therefore, it is generally recommended that St. John's wort should not be taken with food or drink containing the amino acids tyramine and tryptophan. The chemical tyramine appears naturally in a variety of foods, especially those that are aged or fermented. When combined with MAOIs, tyramine can cause a sudden, dangerous spike in blood pressure. Caution should be used with St. John's wort because of its possible MAOI properties.13,22,25,55 Tryptophan, which is found in high-protein foods, is the precursor of serotonin. The dietary sources for tryptophan include foods such as turkey, eggs, fish, dairy products, bananas, and walnuts. (See Appendix IV for a list of foods) Since there is some evidence that St. John's wort inhibits serotonin expression16,22 there should be an awareness of possible interaction.
Issues of Controversy
Herbal preparations because they are perceived as "natural," may give consumers a false sense that they are safer to use than synthetic prescription drugs. However, they may contain some of the same ingredients as prescription drugs and should be treated with the same caution. Although synthetic drugs are increasing, it is not widely known that plants were the basis for most prescription drugs. At one time, 86 of the 150 top-selling prescription drugs contained compounds derived from plants. Aspirin, digoxin, antibiotics, many anticholinergic agents, anticoagulants, antihypertensives, and some antineoplastic agents are all drugs that were once derived from plants. 3 Herbal supplements, as with manufactured drugs, can have the same potential benefits and risks associated with them. Therefore, all persons taking herbal supplements should consult their physician and exercise the same amount of caution given to prescription medications. 52
Patients and consumers should be strongly cautioned that herbal supplements might contain toxic materials. It has been found that up to 60 percent of Asian herbal products measured by weight, contain heavy metals such as lead, mercury, and arsenic, which were introduced to the product during the manufacturing process.2,29 Published reports concerning the mixing of herbal products, have caused serious problems, such as kidney failure.60
Voluntary reporting results in an underestimation of the number of incidences of adverse effects, limits the ability to identify all side effects, interactions, and toxicity of St. John's wort.
It is important to stay cognizant of new information as it becomes available. Appendix III is a listing of some web sites where information on St. John's wort and other herbal supplements can be found. Side effects, adverse reactions, FDA warnings, drug interactions, and uses for herbal supplements are some of the information that can be found.
Cost
No data currently exist evaluating the cost-effectiveness of St. John's wort for depression.
Comparing the cost of St. John's wort with other antidepressants is difficult due to varying dosages, strengths, and number of different medications available. The cost comparisons in this report are based upon antidepressant drug dosages and strengths from the 1999 Physicians' Desk Reference and St. John's wort dosages from the 1999 Physicians' Desk Reference for Nonprescription Drugs and Dietary Supplements.
Not all available prescription drugs are compared nor was a determination made whether the ones listed were more commonly used than others.
The price of St. John's wort and prescription drugs are reflected by the retail price obtained from local pharmacies, and may not reflect the cost to the patients. Third party payers do not pay for dietary supplements, and reimbursement for prescription drugs are dependent upon an individual's health plan benefits. Retail pricing varies from community to community and manufacturer to manufacturer. A cost comparison table can be found in Appendix V for the following:
St. John's wort
Hypericin - maintenance dose, one 300 mg (0.3% hypericin), three times a day. 46
Tricyclics
Imipramine Pamoate (Tofranil-PM®)- maintenance dose, 50 mg to 150 mg daily.
48
page 2092
Amitriptyline Hydrochloride (Elavil®)- maintenance dose, 50 mg to 100
mg daily.
48
page 3419
SSRIs
Fluoxetine Hydrochloride (Prozac°)- maintenance dose, 20 mg daily.
48
page 928
Paroxetine Hydrochloride (Paxil°)- maintenance dose, 30 mg daily.
48
page 3082
MAOIs
Phenelzine Sulfate (Nardil°)- maintenance dose, 45 mg daily. 48 page 2300
Conclusions
With the passage of DSHEA, the FDA has less stringent authority governing St. John's wort, and dietary supplements; therefore, clinical trials showing safety and effectiveness are not required. Variances exist in purity, strength, and potency.
Strong marketing and belief by consumers that herbal supplements are "natural," and therefore safe and effective, have resulted in increased use.
Herbal supplements are taken based on self-diagnosis, beliefs or customs, advice of friends, or advice of nontraditional providers. This has created the potential for severe problems by users.
Limited data exists demonstrating the effectiveness of St. John's wort with mild to moderate depression. Safety, interactions, and toxicity have not been fully studied. Clinically significant drug interactions have recently been reported with St. John's wort.
Recommendations
All persons using St. John's wort or herbal supplements should consult with their physician prior to using St. John's wort or other herbal supplements.
Additional data from well-designed, controlled studies is needed to determine the effectiveness and safety of St. John's wort and its potential interactions with other drugs.
Physicians should inquire if patients are taking any herbal supplements prior to prescribing any drug.
Pharmacists should inquire if patients are taking any herbal supplements prior to dispensing any drug.
Patients should be informed about possible complications, drug interactions, and food interactions that can occur with St. John's wort and other herbal supplements.
Patients who are currently taking indinavir, cyclosporine, or digoxin should not take St. John's wort. The use of St. John's wort has been shown to significantly decrease the concentration in the blood of indinavir (Crixivan®), cyclosporine, and digoxin.
Women should be informed that the effectiveness of oral contraceptives could be reduced by St. John's wort.
Due to a lack of clinical data, pregnant or nursing women should first contact their physician before taking St. John's wort or any herbal supplement.
Equal caution should be exercised when using herbal supplements and any drugs.
FDA rules should require herbal supplements to be systematically studied, (including interactions with other drugs) in order to protect the public from harm.
Appendix I: Summary of Studies
Appendix I: Summary of Studies
| Author Year | # in Study | # of weeks | Brand of St. John's wort parallel study | Daily dose | Response rate | Stat Sign | Side effects |
|---|---|---|---|---|---|---|---|
| Halama 1991* | 50 | 4 | Jarsin Placebo | 0.37 mg × 3 | 50% 0% | p<0.005 | 0% 0% |
| Harrer 1991* | 120 | 6 | Psychotonin M Placebo | 0.37 mg × 3 | 75% 25% | p<0.001 | 2% 0% |
| Schmidt 1989* | 40 | 4 | Psychotonin M Placebo | 0.37 mg × 3 | 62% 33% | p<0.01 | 0% 0% |
| Schlich 1987* | 49 | 4 | Psychotonin M Placebo | 0.25 mg × 3 | 68% 10% | p<0.05 | 0% 0% |
| Hoffman 1979* | 60 | 6 | Hyperforat Placebo | 0.3 mg × 3 | 61% 16% | na | 0% 0% |
| Ditzler 1994* | 60 | 8 | Nerapas** Placebo | 0.24 mg × 3 | 67% 33% | p<0.01 | No diff |
| Sommer 1994* | 105 | 4 | Jarsin 300 Placebo | 0.9 mg × 3 | 67% 28% | p<0.01 | No diff |
| Hubner 1994* | 40 | 4 | Jarsin 300 Placebo | 0.9 mg × 3 | 70% 47% | p<0.05 | No diff |
| Konig 1993* | 112 | 6 | extr. Z 90017 Placebo | 0.5-1.0 mg × 2 | 53% 53% | No diff | 22% 28% |
| Hansgen 1994* | 72 | 4 | Jarsin 300 Placebo | 0.9 mg × 3 | 81% 26% | p<.001 | 6% 3% |
| Lehrl 1993* | 50 | 4 | Jarsin Placebo | 0.37 mg × 3 | 42% 25% | No diff | NA |
| Schmidt 1993* | 65 | 6 | Jarsin 300 Placebo | 0.9 mg × 3 | 67% 27% | p<0.01 | 7% 10% |
| Quandt 1993* | 88 | 4 | Psychotonin M Placebo | 0.37 mg × 3 | 71% 7.7% | p<0.001 | 0% 0% |
| Osterheider 1992* | 47 | 8 | Psychotonin M Placebo | 0.25 mg × 3 | 0% 0% | No sign | na |
| Reh 1992* | 50 | 8 | Neuroplant Placebo | 0.5 mg × 2 | 80% 44% | p<0.02 | 0% 0% |
| Harrer 1994* | 102 | 4 | Jarsin 300 Maprotilin | 0.9 mg × 3 25.0 mg × 3 | 61% 67% | No sign | 25% 35% |
| Bergmann 1993* | 80 | 6 | Esbericum Amitryptilin | 0.25 mg × 3 10.0 mg × 3 | 84% 74% | No sign | 24% 58% |
| Vorbach 1994* | 135 | 6 | Jarsin 300 Imipramin | 0.9 mg × 3 25.0 mg × 3 | 82% 62% | No sign | 12% 16% |
| Kugler 1990* | 80 | 4 | Psychotonin Bromazepam | 0.37 mg × 3 | na | na | na |
| Werth 1989* | 30 | 2 | Psychotonin M Imipramin | 0.37 mg × 3 50.0 mg × 3 | 73% 60% | No sign | 7% 20% |
| Warnecke 1986* | 60 | 12 | Hyperforat Diazepam | 0.2 mg × 3 2.0 mg × 3 | 77.5% 50.0% | na | 0% 0% |
| Kniebel 1988* | 162 | 6 | Sedariston*** Amitryptilin | 0.1 mg × 2 25.0 mg × 3 | 88% 80% | p<0.05 | 10% 50% |
| Steger 1985* | 100 | 6 | Sedariston Amitryptilin | .01 mg × 2 100.0 mg × 1 | 70% 30% | P<0.0021 | 0% 0% |
| Brenner 2000 | 30 | 7 | Hypericum Sertraline | 1st wk 600 mg/d 6 wks 900 mg/d 1st wk 50 mg/d 6 wks 75 mg/d | 47% 40% | p<0.01 | 26% 47% |
| Witte 1995 | 97 | 6 | Psychotonin M Placebo | 0.5 mg × 2 | 79% 56% | p<0.02 | 0% 0% |
| Martinez 1993 | 20 | 4 | Jarsin 300 + light therapy | 300 Lux 3000 Lux | na | No sign | 0% 0% |
- 23
* Studies used in the 1996 Linde, et al meta-analysis.
** Combination product containing Hypericum, Valeria, Passiflorae, Coridalis cavae and Eschschdixiae Californiae.
*** Combination product containing Hypericum and Valeria.
na = not available.No sign = No statistical differences found.No diff = No differences found.
Appendix II: Side Effects, Adverse Effects, Precautions, and Warnings
Appendix II: Side Effects, Adverse Effects, Precautions, and Warnings
| Herb | Side and Adverse Effects | Precautions and Warnings |
|---|---|---|
| St. John's Wort* (Hypericum)
43
*While prescription drugs may seem to have more adverse events associated with them, this may only be a result of the closer scrutiny required of them by the FDA. | Stomach complaints, allergic reactions, fatigue, phototoxicity, photosensitivity, photoallergy, nausea, dizziness, tiredness, restlessness, headaches, vomiting, diarrhea, irritability, edema, erythema, rash, and rare cases of acute neuropathy. | Toxic effects have been reported in sheep and other grazing animals and were seen in the
form of facial edema and photosensitization. Photosensitization only seems to occur in
humans when the dosage taken is 30 to 50 times higher that recommended. Because of the
photosensitivity, persons of fair skin or who burn easily should use caution when exposed to
the sun. Interaction with other drugs can occur therefore taking other medications with hypericum is not recommended. The safety of using hypericum during pregnancy or lactation has not been proven so it should be avoided. The results are unclear about mixing alcohol and hypericum. Generally, consuming alcohol is not recommended while taking hypericum. FDA warnings indicate that St. John's wort is an inducer of the CYP 450 3A4 metabolic pathway. Many prescription drugs used to prevent conditions such as transplant rejection or pregnancy (oral contraceptives) are metabolized via this pathway, health care providers should alert patients about these potential drug interactions. |
| Antidepressant Tricyclics | ||
| Imipramine Pamoate (TOFRANIL-PM®) 37 | Cardiovascular: Orthostatic hypotension, hypertension tachycardia, palpitation,
myocardial infraction, arrhythmias, heart block, ECG changes, precipitation of congestive
heart failure, stroke. Psychiatric: Confusion (in elderly) with hallucinations, disorientation, delusions, anxiety, restlessness, agitation, insomnia and nightmares, hypomania, exacerbation of psychosis. Anticholinergic: Dry mouth and, rarely, associated sublingual adenitis, blurred vision, disturbances of accommodation, mydriasis, constipation, paralytic ileus, urinary retention, delayed micturition, dilation of the urinary tract. Neurological: Numbness, tingling, paresthesias of extremities, incoordination, ataxia, tremors, peripheral neuropathy, extrapyramidal symptoms, seizures, alterations in EEG patterns, tinnitus. Allergic: Skin rash, petechiae, urticaria, itching, photsensitization, edema (general or of face and tongue), drug fever, cross-sensitivity with desipramine. Hematologic: Bone marrow depression including agranulocytosis, eosinophilia, purpura, thrombocytopenia. Gastrointestinal: Nausea and vomiting, anorexia, epigastric distress, diarrhea, peculiar taste, stomatitis, abdominal cramps, black tongue. Endocrine: Gnecomastia in the male, breast enlargement and galactorrhea in the female, increased or decreased libido, impotence, testicular swelling, elevation or depression of blood sugar levels, inappropriate antidiuretic hormone (ADH) secretion syndrome. Other: Jaundice (simulating obstructive), altered liver function, weight gain or loss, perspiration, flushing, urinary frequency, drowsiness, dizziness, weakness and fatigue. | Though not indicative of addiction, abrupt cessation of treatment after prolonged therapy
may produce nausea, headache, and malaise. Other reported reactions include convulsions, emotional instability, syncope, and collapse. Extreme caution should be used when given to patients with cardiovascular disease due to the possibility of conduction defects, arrhythmias, congestive heart failure, myocardial infraction, strokes, and tachycardia. Patients with a history of seizure disorder should be monitored since this drug has been shown to lower the seizure threshold. Drug interactions with guanethidine clonidine, or similar agents; methylphenidate hydrochloride. May impair ability to operate automobiles or machinery. May enhance the CNS depressant effects of alcohol. Not to be used in children of any age. Possible benefits must be weighed against possible hazards to mother and child when used during pregnancy or lactation. May alter glycemic control of diabetic patients. Interaction with other drugs has been reported. |
| Amitriptyline Hydrochloride (ELAVIL®) 37 | Allergic: Skin rash, urticaria, photosensitization, edema of the face and
tongue. Anticholinergic: Dry mouth, blurred vision, disturbance of accommodation, constipation, paralytic ileus, urinary retention, dilation of urinary tract, increased ocular pressure, and mydriasis. Cardiovascular: Orthostatic hypotension, hypertension, tachycardia, palpitations, myocardial infraction, arrhythmias, heart block, stroke, syncope, and nonspecific ECG changes and changes in AV conduction. CNS and Neuromuscular: Confusional states, disturbed concentration, disorientation, delusions, hallucinations, excitement, anxiety, restlessness, insomnia, nightmares, numbness, tingling and paresthesias of the extremities, peripheral neuropathy, incoordination, ataxia, tremors, seizures, alteration in EEG patterns, extrapyramidal symptoms, including abnormal involuntary movements and tardive dyskinesia, dysarthria, nightmares, tinnitus, drowsiness, dizziness, weakness, fatigue, headache and syndrome of inappropriate ADH (antidiuretic hormone) secretion. Endocrine: Testicular swelling and gynecomastia in the male, breast enlargement and galactorrhea in the female, increased or decreased libido, lowering of blood sugar levels, and impotence. Gastrointestinal: Nausea, epigastric distress, vomiting, anorexia, stomatitis, peculiar taste, diarrhea, rarely hepatitis (including altered liver function and jaundice), parotid swelling, black tongue. Hematologic: Bone marrow depression, including agranulocytosis, leukopenia, eosinophilia, purpura, thrombocytopenia. Other: Weight gain or loss, increased perspiration, urinary frequency, and alopecia. Body as a Whole: Lupus-like syndrome (migratory arthritis positive ANA and rheumatoid factor). Digestive: Hepatic failure and ageusia. Withdrawal Symptoms: After prolonged administration, abrupt cessation of treatment may produce nausea, headache, and malaise. Gradual dosage reduction has been reported to produce, within two weeks, transient symptoms including irritability, restlessness, and dream and sleep disturbance. These symptoms are not indicative of addiction. Rare instances have been reported of mania or hypomania occurring within 2-7 days following cessation of chronic therapy with tricyclic antidepressants. | Abrupt cessation of treatment after prolonged administration may produce nausea,
headache, and malaise. These are not indicative of addiction. Should not be given concomitantly with MAOIs. May block the antihypertensive action of guanethidine or similarly acting compounds. Patients should have close supervision if using thyroid medication, alcohol, barbiturates and other CNS depressants, and disulfiram Patients with cardiovascular disorders should be watched closely. Close supervision is required when given to hyperthyroid patients or those receiving thyroid medication. Possible benefits must be weighed against possible hazards to mother and child when used during pregnancy or lactation. Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop. Not recommended for children under 12 years of age. Should be used with caution with patients with impaired liver function. Elevation and lowering of blood sugar levels have bee reported. Drug interaction can happen with drugs metabolized by P450 2D6. Clinically significant effects have been reported with tricyclic antidepressants when used concomitantly with cimetidine. Caution is advised if patients receive large doses of ethchlorvynol concurrently. May impair ability to operate automobiles or machinery. |
| Antidepressant SSRIs | ||
| Fluoxetine Hydrochloride (PROZAC®) 37 | Body as a Whole: Frequent -- chills. Infrequent -- chills and fever, face edema,
intentional overdose, malaise, pelvic pain, suicide attempt. Rare -- abdominal syndrome
acute, hypothermia, intentional injury, neuroleptic malignant syndrome, photosensitivity.
Cardiovascular System: Frequent -- hemorrhage, hypertension. Infrequent -- angina pectoris, arrhythmia, hemorrhage, congestive heart failure, hypotension, migraine, vascular headache. Rare -- atrial fibrillation, bradycardia, cerebral embolism, cerebral ischemia, cerebrovascular accident, extrasytoles, heart arrest, heart block, pallor, peripheral vascular disorder, phlebitis, shock, thrombophlebitis, thrombosis, vasospasm, ventricular arrhythima, ventricular extrasystoles, ventricular fibrillation. Digestive System: Frequent -- increased appetite. Infrequent -- aphthous stomatitis, dysphagia, cholelithiasis, colitis, eructation, esophagitis, gastritis, gastorenteritis, glossitis, gum hemorrhage, hyperchorhydria, increased salvation, liver function tests abnormal, melena, stomatitis, mouth ulceration, nausea/vomiting/diarrhea, stomach ulcer, thirst. Rare -- biliary pain, bloody diarrhea, cholecystitis, duodenal ulcer, enteritis, esophageal ulcer, fecal incontinence, gastrointestinal hemorrhage, hematemesis, hemorrhage of the colon, hepatitis, intestinal obstruction, liver fatty deposit, pancreatitis, peptic ulcer, rectal hemorrhage, salivary gland enlargement, stomach ulcer hemorrhage, tongue edema. Endocrine System: Infrequent -- hypothyroidism. Rare -- diabetic acidosis, diabetes mellitus. Hemic and Lymphatic System: Infrequent -- anemia, ecchymosis. Rare -- blood dyscrasia, hypochromic anemia, leukopenia, lymphocytosis, petechia, purpura, thrombocythemia, thrombocytopenia. Metabolic and Nutritional: Frequent -- weight gain. Infrequent -- dehydration, generalized edema, gout, hypercholesteremia, hyperlipemia, hypokalemia, peripheral edema. Rare -- alcohol intolerance, alkaline phosphatase increased, BUN increased, creatine phosphokinase increased, hyperkalemia, hyperuricemia, hypocalcemia, iron deficiency anemia, SGPT increased. Musculosketal System: Infrequent -- arthritis bone pain, bursitis, leg cramps, tenosynovitis. Rare -- arthrosis, chondrodystrophy, myasthenia, myopathy, myositis, osteomyelitis, osteoporosis, rheumatoid arthritis Nervous System: Frequent -- agitation, amnesia confusion, emotional lability, sleep disorder. Infrequent -- abnormal gait, acute brain syndrome, akathisia, apathy, ataxia, buccoglossal syndrome, CNS depression, CNS stimulation, depersonalization, euphoria, hallucinations, hostility, hyperkinesia, hypertonia, hypesthesia, incoordination, libido increased, myoclonus, nueralgia, neuropathy, neurosis, paranoid reaction, personality disorder, psychosis, vertigo. Rare -- abnormal electroencephalogram, antisocial reaction, circumoral paresthesia, coma, delusions, dysarthria, dystonia extrapyramidal syndrome, foot drop, hyperesthesia neuritis, paralysis, reflexes decreased, reflexes increased, stupor. Respiratory System: Infrequent -- asthma, epistaxis, hiccup, hyperventilation. Rare -- apnea, atelectasis, cough decreased, emphysema, hemoptysis, hypoventilation, hypoxia, larynx edema, lung edema, pneumothorax, stridor. Skin and Appendages: Infrequent -- acne, alopecia, contact dermatitis, eczema, maculopapular rash, skin discoloration, skin ulcer, vesiculobullous rash. Rare -- furunculosis, herpes zoster, hirsutism, petechial rash, psoriasis, purpuric rash, pustular rash, seborrhea. Special Senses: Frequent -- ear pain, taste perversion, tinnitus. Infrequent -- conjunctivitis, dry eyes, mydriasis, photophobia. Rare -- blepharitis, deafness, diplopia, exophthalmos, eye hemorrhage, glaucoma, hyperacusis, iritis, parosmia, scleritis, strabismus, taste loss, visual field defect. Urogenital System: Frequent -- urinary frequency. Infrequent -- abortion, albuminuria, amenorrhea, anorgasmia, breast enlargement, breast pain, cystitis, dysuria, female lactation, fibrocystic breast, hematuria, leukorrhea, menorrhagia, metrorrhagia, nocturia, polyuria, urinary incontinence, urinary retention, urinary urgency, vaginal hemorrhage. Rare -- breast enlargement, glycosuria, hypomenorrhea, kidney pain, oliguria, priapism, uterine hemorrhage, uterine fibroids enlarged. | Rash and possible allergic events, associated with fever, leukocytosis, arthralgias,
edema, carpal tunnel syndrome, respiratory distress, lymphadenopathy, proteinuria and mild
transaminase elevation. In premarket trials, 2 patients developed a serious cutaneous systemic illness. Although rare, some patients have developed systemic events possibly related to vasculitis. Death has occurred in association with these systemic events. Anaphylactoid events, including bronchospasm, angioedema, and urticaria alone and in combination, have been reported. Pulmonary events, including inflammatory processes of varying histopathology and/or fibrosis, have been reported. These events have occurred with dyspnea as the only preceding symptom. Should be used with caution with patients who have seizures. Caution should be used when treating patients with concomitant systemic illnesses. May alter glycemic control of diabetic patients. Motor vehicle and machinery operation on may be affected. Patients who have become or are planning on becoming pregnant, or are nursing an infant should advise their physician. |
| Paroxetine Hydrochloride (PAXIL®) 37 | Body as a Whole: Frequent -- chills, malaise. Infrequent -- allergic reaction, carcinoma,
face edema, moniliasis, neck pain. Rare -- abscess, adrenergic syndrome, cellulitis, neck
rigidity, pelvic pain, peritonitis, ulcer. Cardiovascular System: Frequent -- hypertension, syncope, tachycardia. Infrequent -- bradycardia, condition abnormalities, electrocardiogram abnormal, hematoma, hypotension, migraine, peripheral vascular disorder. Rare -- angina pectoris, arrhythmia, atrial fibrillation, bundle branch block, cerebral ischemia, cerebrovascular accident, congestive heart failure, heart block, low cardiac output, myocardial infarct, myocardial ischemia, pallor, phlebitis, pulmonary embolus, supraventricular extrasystoles, throbophlebitis, thrombosis, varicose vein, vascular headache, ventricular extrasystoles. Digestive System: Infrequent -- bruxism, colitis, dysphagia, eructation, gastroenteritis, gingivitis, glossitis, increased salivation, liver function tests abnormal, mouth ulceration, rectal hemorrhage, ulcerative stomstitis. Rare -- aphthous stomatitis, bloody diarrhea, bulimia, cholelithiasis, duodenitis, enteritis, esophagitis, fecal impactions, fecal incontinence, gastritis, gum hemorrhage, hematemesis, hepatitis, ileus, intestinal obstruction, jaundice melena, peptic ulcer, salivary gland enlargement, stomach ulcer, stomatitis, tongue discoloration, tongue edema, tooth cavities, tooth malformation. Endocrine System: Rare -- diabetes mellitus, hyperthyroidism, hypothyroidism, and thyroiditis. Hemic and Lymphatic Systems: Infrequent -- anemia, leukopenia, lymphadenopathy, purpura. Rare -- abnormal erythrocytes, basophilia, eosinophilia, hypochromic anemia, iron deficiency anemia, leukocytosis, lymphedema, abnormal lymphocytes, lymphocytosis, microcytic anemia, monocytosis, normocytic anemia, thrombocythemia. Metabolic and Nutritional: Frequent -- edema, weight gain, weight loss. Infrequent -- hyperglycemia, peripheral edema, SGOT increased, SGPT increased, thirst. Rare -- alkaline phosphatase increased, bilirubinemia, BUN increased, creatinie phosphokinase increased, dehydration, gamma globulins increased, gout, hypercalcemia, hypercholesteremia, hyperkalemia, hyperphosphatemia, hypocalcemia, hypoglycemia, hypokalemia, hyponatremia, ketosis, lactic dehydrogenase increased. Musculoskeletal System: Frequent -- arthralgia. Infrequent -- arthritis. Rare -- arthrosis, bursitis, myositis, osteoporosis, generalized spasm, tenosynovitis, and tetany. Nervous System: Frequent -- amnesia, CNS stimulation, concentration impaired, depression, emotional lability, vertigo. Infrequent -- abnormal thinking, akinesia, alcohol abuse, ataxia, convulsion, depersonalization, dystonia, hallucination, hostility, hyperkinesia, hypertonia, hypesthesia, incoordination, lack of emotion, manic reaction, neurosis, paralysis, paranoid reaction. Rare -- abnormal electroencephalogram, abnormal gait, antisocial reaction, aphasia, choreoathetosis, circumoral paresthesias, delirium, delusions, diplopia, drug dependence, dysarthria, dyskinesia, euphoria, extrapyramidal syndrome, fasciculations, grand mal convulsion, hyperalgesia, hypokinesia, hysteria, libido increased, manic-depressive reaction, meningitis, myelitis, neuralgia, neuropathy, nystagmus, peripheral neuritis, psychosis, psychotic depression, reflexes decreased, reflexes increased, stupor, trismus, withdrawal syndrome. Respiratory System: Frequent -- cough increased, rhinitis. Infrequent -- asthma, bronchitis, dysponea, epistaxis, hyperventilation, pneumonia, respiratory flu, sinusitis, voice alteration. Rare -- emphysema, hemoptysis, hiccups, lung fibrosis, pulmonary edema, and sputum increased. Skin and Appendages: Frequent -- pruritus. Infrequent -- acne, alopecia, dry skin, ecchymosis, eczema, furunculosis, urticaria. Rare -- angioedema, contact dermatitis, erythema nodosum, erythema multiforme, fungal dermatitis, herpes simplex, herpes zoster, hirsutism, maculopapular rash, photosensitivity, seborrhea, skin discoloration, skin hypertrophy, skin ulcer, and vesiculobullous rash. Special Senses: Frequent -- tinnitus. Infrequent -- abnormality of accommodation, conjunctivitis, ear pain, eye pain, mydriasis, otitis media, taste loss, visual field defect. Rare -- amblyopia, anisocoria, blepharitis, cataract, conjunctival edema, corneal ulcer, deafness, exophthalmos, eye hemorrhage, glaucoma, hyperacusis, keratoconjunctivitis, night blindness, otitis externa, parosmia, photophobia, ptosis, and retinal hemorrhage. Urogenital System: Infrequent -- abortion, amenorrhea, breast pain, cystitis, dysmenorrhea, dysuria, hematuria, menorrhagia, nocturia, polyuria, urethritis, urinary incontinence, urinary retention, urinary urgency, vaginitis. Rare -- breast atrophy, breast carcinoma, breast enlargement, breast neoplasm, epididymitis, female lactation, fibrocystic breast, kidney calculus, kidney function, abnormal kidney pain, leukorrhea, mastitis, metrorrhagia, nephritis, oliguria, prostatic carcinoma, pyuria, urethritis, uterine spasm, urolith, vaginal moniliasis, vaginal hemorrhage. | Potential for interaction with MAOIs. Should not be taken in combination with a MAO, and
a 14-day waiting period is recommended between the discontinuation of one and the starting
of the other. There have been reports of serious, sometimes fatal reactions, including hypertherma, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include agitation progressing to delirium and coma. Should be used with caution on patients with history of mania and seizures. Older patients who are on diuretics or who are otherwise volume depleted should be watched for hyponatremia. Cases of abnormal bleeding have been reported (mostly ecchymosis and purpura). Caution is advisable when using with certain concomitant systemic illnesses. Patients should be cautioned about operating motor vehicle and machinery. Patients should avoid alcohol while taking the drug. Patients should advise their physician if they become or plan to become pregnant or if they are nursing an infant. |
| Antidepressant MAOI | ||
| Phenelzine Sulfate (NARDIL®) 37 | Common: Nervous System: Dizziness, headache, drowsiness, sleep disturbances (including insomnia and hypersomnia), fatigue, weakness, tremors, twitching, myoclonic movements, hyperreflexia. Gastrointestinal: Constipation, dry mouth, gastrointestinal disturbances, elevated serum transaminases (without accompanying signs and symptoms). Metabolic: Weight gain. Cardiovascular: Postural hypotension, edema. Genitourinary: Sexual disturbances, e.g., anorgasmia and ejaculatory disturbances. Less Common: Nervous System: Jitteriness, palilalia, euphoria, nystagmus, paresthesia. Gastrointestinal: Urinary retention. Metabolic: Hypernatremia. Dermatologic: Skin rash, sweating. Special Senses: Blurred vision, glaucoma. Less Frequent: Nervous System: Ataxia, shock-like coma, toxic delirium, manic reaction, convulsions, acute anxiety reaction, precipitation of schizophrenia, transient respiratory and cardiovascular depression followed by ECT. Gastrointestinal: Fatal progressive necrotizing hepatocellular damage in a few patients, reversible jaundice. Hematologic: Leukopenia. Immunologic: Lupus-like syndrome. Metabolic: Hypermetabolic syndrome (which may include but not limited to hyperpyrexia, tachycardia, muscular rigidity, elevated DK levels, metabolic acidosis, hypoxia, and coma, and may resemble an overdose). Respiratory: Edema of the glottis. General: Fever associated with increased muscle tone. Withdrawal may be associated with nausea, vomiting, and malaise. | The most serious changes involve changes in blood pressure. Occurrence of hypertensive crisis, which has sometimes been fatal. The following foods should be avoided; pickled herring, liver, dry sausage, broad bean pods, sauerkraut, cheese, beer and wines, alcohol-free and reduced-alcohol beer and wine products, yeast extract, meat extract, excessive amounts of chocolate and caffeine, and any spoiled or improperly refrigerated, handled, or stored protein-rich foods. Interaction with other medications. Not recommended for children under 16 years of age. Patients should advise their physician if they become or plan to become pregnant or if they are nursing an infant. Caution should be used when treating epileptic patients. Glucose metabolism may be affected, so caution is advised when treating diabetic patients. May cause excessive stimulation in schizophrenic patients; in manic-depressive states, may result in a swing from a depressive to a manic phase. Over stimulation may include increased anxiety, agitation, and manic symptoms. May cause restlessness or insomnia; some weakness, drowsiness, episodes of dizziness or dry mouth; nausea, diarrhea, abdominal pain or constipation. Tachycardia, significant anorexia, edema, palpitation, blurred vision, chills, and impotence have been reported. Headaches without blood pressure elevation have occurred. Rare instances of hepatitis and skin rash. Should be reserved for patients that do not respond to other antidepressants. Possible benefits must be weighed against possible hazards to mother and child when used during pregnancy or lactation. Patients should be warned about food that should not be eaten during use of the drug. Other drug and medication interactions have been reported. A hypertensive crisis is associated with the drug and has been fatal. Blood pressure should be followed closely. Glucose metabolism may be affected, so caution is advised when treating diabetic patients. Caution is advised with hyperthyroid patients. |
Appendix III: Selected Information Resources
Government Web sites
U.S. Food and Drug Administration http://www.fda.org
National Institutes of Health, Office of Alternative Medicine http://www.almed.od.nih.gov
U.S. Dept. Health and Human Services http://www.healthfinder.gov
U.S. Dept. of Agriculture National Library http://www.nal.usda.gov/fnic
Agency for Healthcare Research and Quality http://www.ahcpr.gov
FedWorld Information http://www.fedworld.gov
National Center for Complementary and Alternative Medicine http://www.nccam.nih.gov
Medical Information Web sites
Search Medical Libraries- http://directory.netscape.com/Top/Reference/Libraries/Medical_Libraries
Safemedication.com http://www.safemedication.com
Familydoctor.org http://www.medicinenet.com
Mayo Clinic http://www.mayohealth.com
Medscape http://www.medscape.com
Healtheon/WebMD http://www.webmd.com
AARP Pharmacy Service
RxList: The Internet Drug Index http://www.rxlist.com
Appendix IV: Foods Containing the AminoAcids Tryptophan and Tyrosine
Tyrosine
The following foods contain tyramine or bacteria with enzymes that can convert tyrosine to tyramine. These foods should be avoided by those displaying sensitivity to tyramine, or by those who are taking prescription monoamine oxidase (MAO) inhibitors. St. John's wort may contain a mechanism similar to a MAO inhibitor. Therefore, some doctors of natural medicine suggest that people using this herb avoid tyramine-containing foods.
Dairy Products—American, processed, blue*, boursault*, brick (natural), brie, camembert*, cheddar*, Emmenthaler*, Gruyere, Mozzarella, parmesan, Romano, sour cream, Roquefort*, Stilton*, Swiss.
Alcoholic Beverages -- Ale, beer (including some non-alcoholic beers), red wine (especially Chianti)*, port*, reisling*, Sauternes*, Sherry*, Vermouth*, distilled spirits.
Meat/Fish -- Beef or Chicken Liver*, other meats, fish (un-refrigerated, fermented), fermented sausages (bologna, pepperoni, salami, summer sausage)*, game meat*, meat tenderizer, meat extracts, caviar, salted herring and other dried fish, pickled herring (spoiled)*, shrimp paste.
Fruit/Vegetables -- Avocados (especially over-ripe), bananas, bean curd, canned figs (overripe), miso soup, red plums, raisins, sauerkraut*, soy sauce, spinach, teriyaki, tomatoes, yeast extracts* (Marmite, etc.).
Foods Containing Other Vasopressors -- Fava beans (over-ripe); dopa*; Coffee, tea, colas: caffeine; Chocolate: phenyl ethylamine.
* contain high to very high amounts of tyramine
Tryptophan
Tryptophan appears in diary foods, nuts, and fowl. Listed below
is the tryptophan content per 1 gram of the high protein food.
Tyrosine
| FOODS FROM PLANTS | |
| Almonds | 9.4 mg |
| Garbanzo, dry-raw | 8.0 mg |
| Beans, soy, cooked | 15.0 mg |
| Carrots, cooked | 7.8 mg |
| Oatmeal, cooked | 16.0 mg |
| Potato, baked | 10.0 mg |
| Rice, brown, cooked | 10.8 mg |
| FOODS FROM ANIMALS Chicken, breast 12.0 mg Cow, beef roast 10.7 mg Milk, skim 13.9 mg High-tryptophan protein foods include plain yogurt, skinless chicken and turkey |
Almonds, cashews, peanuts, and sunflower seeds are high-tryptophan vegetable proteins.
Appendix V: Cost Comparison
Table 7 Appendix V: Cost Comparison
| Herb/Antidepressant | Quantity Daily Dose | Pharm A | Pharm B | Grocery Store C | Cost per Tablet | Cost per 30 Day Month |
|---|---|---|---|---|---|---|
| St. John's Wort (0.3% Hypericin) | ||||||
| Manufacture A | Qty 36 300 mg × 3 | $6.99 | $0.19 | $17.10 | ||
| Manufacture B | Qty 36 300 mg 3 | $11.57 | $0.32 | $28.10 | ||
| Qty 60 300 mg × 3 | $5.97 | $0.09 | $8.10 | |||
| Qty 120 300 mg × 3 | $8.74 | $0.07 | $6.30 | |||
| Manufacture C | Qty 100 300 mg × 3 | $12.81 | $0.13 | $11.70 | ||
| Manufacture D | Qty 50 300 mg × 3 | $6.99 | $0.14 | $12.60 | ||
| Manufacture E | Qty 72 300 mg × 3 | $6.49 | $0.09 | $8.10 | ||
| Tricyclics | ||||||
| Imipramine Pamoate (TOFRANIL-PM®) | Qty 100 50 mg 1 | $30.69 | $29.71 | $0.31 $0.30 | $9.31 $9.00 | |
| Amitriptyline Hydrochloride (ELAVIL®) | Qty 100 25 mg × 2 | $50.99 | $52.62 | $0.50 $0.53 | $30.00 $31.80 | |
| SSRIs | ||||||
| Fluoxetine Hydrochloride (Prozac®) | Qty 100 20 mg × 1 | $237.69 | $261.54 | $2.38 $2.62 | $71.40 $78.60 | |
| Paroxetine Hydrochloride (PaxilJ) | Qty 100 20 mg × 1 | $223.69 | $233.97 | $2.24 $2.34 | $67.20 $70.20 | |
| MAOI | ||||||
| Phenelzine Sulfate (Nardil®) | Qty 100 45 mg × 1 | $55.39 | $56.54 | $0.55 $0.57 | $16.50 $17.10 | |
Appendix VI: Description of Hamilton Rating Scale and Clinical Global Impressions Scale
The Hamilton Rating Scale for Depression (HAM-D) 55
The HAM-D is designed to measure the severity of illness in patients already diagnosed as having depression. The HAM-D offers high validity and reliability in measuring response to treatment.
HAM-D scale contains items that assess somatic symptoms, insomnia, working capacity and interest, mood, guilt, psychomotor retardation, agitation, anxiety, and insight. The 31-item HAM-D includes items used to rate the reversed vegetative symptoms of depression, such as oversleeping, overeating, and weight gain, which are particularly common in younger people.
There is an initial interview conducted. Patients are given time to answer questions, speak of problems, and ask questions. Subsequent assessments can be briefer and more focused. Some symptoms are difficult to elicit enough information from the patient to permit full quantification. If a symptom is present, score 2; if absent, score 0; and if doubtful or trivial, score 1. For those symptoms where more detailed information can be obtained, the score is expanded: 2 indicate mild symptoms, 3 moderate symptoms, and 4 severe symptoms. The higher the score the more severe the depression is considered to be.
Successful therapy should result in a lower score in subsequent testing. Experts agree that a reduction in the total score of at least 50% is necessary to consider a treatment effective.
The Clinical Global Impressions Scale (CGI or CGIS) 56
The CGI consists of three global scales (items) that have been designed to measure the severity, global improvement, and efficacy of treatment of patients who already have been diagnosed as having depression. It was developed during some collaborative schizophrenic studies and is used with the General Scoring Sheet.
Severity of illness is the first scale in the CGI. A rating is filled in by the investigator at the start of treatment based on a 0-7 point weighted scale. It goes from not assessed (0), to among the most extremely ill patients (7). During the initial assessment, the other two scales of the CGI may be omitted by marking them with 0 (not assessed).
Global Improvement is the 2nd scale in the CGI. Total overall improvement is judged by whether or not, in the judgment of the assessor, the improvement is entirely due to the drug treatment. It is also a 0-7 point weighted scale, going from not assessed (0) to very much worse (7) respectively.
The last scale is the Efficacy Index. This scale has the assessor select from a matrix of four terms for Side Effects (Y axis) and four terms for Therapeutic Effects (X axis). The Efficacy Index (EI) is then determined by dividing the therapeutic score (TS) by the side effect (SE) score.
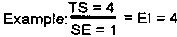
Successful therapy should result in a lower overall score in subsequent testing. Experts agree that a reduction in the total score of at least 50% is necessary to consider a treatment effective.
Appendix VII: Public Comments
The following statements were submitted to HTAC during the public comment period on this assessment. The workgroup and full Committee reviewed each statement and incorporated them into the report as the Committee deemed appropriate.
| Date sent: | Wed, 11 Oct 2000 10:23:39-0500 |
| To: | richard.carlson@health.state.mn.us |
| From: | Gregory Plotnikoff (plotn002@tc.umn.edu) |
| Subject: | SJW |
Richard Carlson
Project Team Leader
Health Technology Advisory
Committee
121 E. 7th Place, Suite 400
St. Paul, MN 55164-0975
Dear Richard-
Congratulations to you and your team on producing such a fine and substantive report! Thank you for sending me a copy and inviting me to comment on this important report.
In regards to constructive feedback, I have a few thoughts. One is minor and two are neither major nor minor but are only submitted for completeness. One concern I have is in the major category because you haven been so thorough, so committed to public safety and have demonstrated a great deal of hard work in this report.
The minor issue is the misspelling of my name in footnote 2.
For your consideration are 1) articles of related interest to side effects, and, 2) articles/letters of related interest to the subject of standardization.(I recognize that you may already have these but I only had up to footnote 27 in my packet).
Regarding drug interactions:
Induces photosensitivity
Photochem Photobiol Rev
1980;5:229-35.
J Alt Comp Ther 1999;5:397-98.
Pharmacopsychiatry
1997;30(suppl.2):S94-S101.
Induces cytochrome P450 (CYP3A4)
Lancet 1999;354:2014
Clin Pharmacol Ther
2000;67:451-57.
Induces P-glycoprotein
Clin Pharmacol Ther 1999;66:338-45.
Lowers serum levels of Idinavir
Lancet 2000;355:547-8.
Lowers serum levels of
digoxin
Clin Pharmacol Ther 1999;66:338-45.
Lowers serum levels of
theophylline
Ann Pharmacother 1999;33:502.
Reduces anticoagulant effect of
warfarin
Lancet 2000;355:576-77.
May precipitate serotonin syndrome with
SSRI's
J Geriatr Psychiatry Neurol 1999;12:7
(Because of the P450 effect, drugs which are known substrates for this isozyme include some nonsedating antihistamines, oral contraceptives, anti-retrovirals, antiepileptics, calcium channel blockers, cyclosporine, chemotherapeutic agents, macrolide antibiotics and antifungals)
I believe that standardization is an important issue that deserves to be addressed. Standardized to an ingredient with some activity (e.g. hypericin) may not capture the most important ingredient(s) such as hyperforin. (citations: Planta Med 1998; 64: 291-4, Life Sci 1998;63: 499-510, Pharmacopsychiatry 1997; 2:108-12, Arzneimittelforschung 2000;50:415-9) This confounds clinical studies, their meta-analyses and our understanding of risk/benefit ratios.
To understand physician concerns on standardization, see the discussion in both Gorski's and Menke's letters to the editor of the BMJ in 320 (7242): 1141a regarding Phillip M, Kohnen R, Hiller K-O "Hypericum extract versus imipramine or placebo..." (BMJ 1999;319: 1534-1539) as well as the authors' response. (all three are available at: http://www.bmj.com/cgi/content/full/320/7242/1141/a)
The not-so-minor concern that I have is the warning about the use of tyramine containing foods. Your information is quite thorough and accurate but rests upon the assumption that SJW has achievable MAO inhibitor properties.
This is a significant assumption in your report and must be scrutinized using the scientific literature. To date, to the best of my knowledge, there has been no demonstration of achievable in vitro MAO inhibition or in vivo MAO inhibition despite widespread/global use of the SJW herb. The ingredients believed to be most active, hypericin and hyperforin, have significantly greater affects on other neurotransmitter-related systems and at achievable serum levels. Hence the previous MAO inhibition concerns are considered excessive and unsupportable by numerous scientific authorities including the American Botanical Council/Herb Research Foundation, FDA, etc.
Your conclusions and recommendations on page 12-13 are right on target. As you have stated so well, St. John's Wort should not be taken with any prescription medications. Pregnancy and SJW use has not been addressed and may be important to some readers.
Again, congratulations! Please let me know if I can support your work in any way.
Sincerely,
Greg Plotnikoff
Gregory A. Plotnikoff, MD, MTS
Medical Director,
Center for Spirituality and
Healing
Assistant Professor,
Clinical Medicine and Pediatrics
University
of Minnesota Academic Health Center
FUMC Box 505
420 Delaware Street
SE
Minneapolis, MN 55455
VM: 612-626-3817
Fax:612-626-5280
| From: | "Ila Harris" <IHARRIS@FAMPRAC.UMN.EDU> |
| To: | richard.carlson@health.state.mn.us |
| Subject: | St. John's wort monograph |
Richard,
Thank you for your invitation to review the St. John's wort monograph. I have
reviewed it and have several comments. They are attached in a Word document. Please let me know
if you have any questions or comments. I will be out of town until Mon, Nov 27. Please send me a
quick email to let me know you received this email and was able to open the document OK.
Thanks, Ila Harris
Ila Mehra Harris, Pharm.D., BCPS
University of Minnesota
Bethesda
Clinic
580 Rice St.
St. Paul, MN 55103
P (651) 227-6551
F (651)
227-1804
General Comments
Overall, it was a good and complete monograph. However, I do
have many comments. My reference list ended at #42. I often found myself going back to the
references to check a citation and found that I didn't have the citation because it was after
#42. Therefore, it was impossible to double check those statements.
Also, check www.consumerlabs.com to see if they have evaluated SJW (I cannot do this right now b/c my internet is down) and include it in the monograph. This will be helpful to know which SJW products passed their testing.
The comments listed are categorized by subheading.
Executive Summary
Paragraph 1, line 5:
Delete "Therefore, are
regulated by the FDA under the DSHEA of 1994." This is misleading. The FDA is only able to step
in and take action to recommend a dietary supplement to be removed from the market if it is a
significant and imminent hazard to consumers.
Paragraph 1, line 7:
DESHA should be changed to DSHEA.
Change wording.
"...permits the sale of dietary supplements by the FDA..." is incorrect. The FDA does not sell
dietary supplements.
Paragraph 4, line 3:
Incorrect. There is now more information regarding drug
interactions with St John's wort.
Executive Summary; Recommendations
Statement 6: Add Oral Contraceptives to
the list of what not to take with St. John's Wort. (or if pts take St. John's Wort when they are
taking OCs, they should use backup contraception).
Introduction
Paragraph 1, line 6:
Change wording. Misleading that
FDA regulates dietary supplements. FDA and DSHEA are separate. Could change it to: " Herbal
supplements are classified as dietary supplements under the DSHEA".
FDA
Paragraph 1, line 2:
Add "s" to food and drug to make them
plural.
Paragraph 2, line 1:
Take out "a" and add an "s" to drug to make drugs.
Paragraph 3, line 6:
After sentence ending in "mechanism", add this sentence: "New
rules do not allow for implied disease claims."
Paragraph 6, line 4:
Delete "market products" and change to "make claims for treating
or mitigating." Manufacturers were always allowed to market these products, but the difference
now is that actual claims can be made for these conditions because they are now not considered
diseases. Previously, only structure and function claims could be made.
Paragraph 6, line 4:
Incorrect. The FDA soon after removed morning sickness from the
list because they realized this would promote use of supplements in pregnancy that the effects
on the fetus were unknown.
Paragraph 6, lines 1-8:
Move these 2 sentences to paragraph 3, after the sentence
ending in "medication."
Paragraph 7, line 1:
Change "doctors" to "health professionals."
Wound Healing and Antiviral Activity
Is this topical or oral use? Please
specify.
Mechanisms of Action
Paragraph 1, line 1:
Add hyperforin to the
list of active ingredients.
Depression
Paragraph 1, line 4:
Change placebos to placebo.
Paragraph 1, line 5:
Remove hyphen in between side and effects.
Remove comma
after amitriptyline.
Paragraph 1, lines 9 and 10:
Change placebos to placebo.
Wound Healing
Topical or oral? Please specify.
Antiviral Activity
Line 5:
Should the references just be 18 and 21?
Dosage
Lines 7-8:
Please provide more information/clarify. First,
where is the reference for this statement? (very important to have). Secondly, what is the peak
concentration seen with St. John's Wort? Thirdly, change the wording because it is confusing;
"daily doses" keep the levels > 6 ng/ml: this could be taken as once daily doses. Why
then is BID necessary? Please clarify.
Drug Interactions
Paragraph 2, line2:
Change pheneizine to
phenelzine (spelling error).
Paragraph 2, line 3:
Venlafaxine is not actually classified as an SSRI.
Paragraph 3:
Clarify what diet pills (now there are things such as Xenical), clarify
asthma inhalants (many are available), clarify cold and hay fever medications (many different
are available, including nonsedating antihistamines such as Claritin). Also, are the
interactions listed significant? I didn't have reference list past #42 so I couldn't look at the
citations. I don't think some of these are clinically important.
Paragraph 4, line 7:
Add comma after HIV drug.
Paragraph 4, line 9:
Specify that SJW induces CYP 450 3A4
Paragraph 4, lines 11-12:
Provide more information about these interactions, such as
SJW decreases efficacy of lovastatin and warfarin. "Cancer medications" is too vague. Does SJW
really interact with all cancer treatments?
Foods
The information under here is not true and not the consensus. St.
John's wort only has minor MAOI activity. Food interactions are actually not a concern with any
scientific validity. The "Natural Medicines Comprehensive Database" states that "Theoretically,
concomitant use of large amounts of SJW and tyramine-containing foods might cause a hypertensive
crisis. However, very large amounts of SJW are likely to be required to cause this interaction."
The text "Herbal Medicinals: A Clinician's Guide (Miller LG, Murray WJ, ed) states that "No
drug-food interactions have been documented despite purported MAOI activity." And "Due to SJW's
low degree of MAO inhibitory activity, the use of a tyramine-free diet is not currently
advocated. But the use of excessive amounts of any food or medication on a tyramine-free diet is
not recommended." This needs to be changed.
Issues of Controversy
Paragraph 1, line 7:
Change consult their
"physician" to consult a "health care professional". Also, add a comment that consumers should
be aware that not all physicians, pharmacists, and other health professionals are knowledgeable
in this area.
Paragraph 3, line 1:
Change "mortality and morbidity" to "adverse effects."
Paragraph 4:
Add that info on other herbal meds can be found at these web
sites.
Cost
Under MAOIs, correct spelling: phenelzine.
Conclusions
Add that depression can be a serious condition and people
should not treat it on their own. Could also be a symptom of another disease (e.g.,
hypothyroidism).
Recommendations
6th statement: add oral contraceptives to the list of drugs
that they should avoid SJW if they are taking. OR, add statement that they should use backup
contraception while they take SJW.
Appendix I
Add reference # to the table.
Vorbach 1993 #2: Response rate column is blank, but yet p < 0.05. Please fill in response rate column.
Kriebel 1988: is this correct, that 88% vs 80% was p < 0.05 (sedariston was significantly better than amitriptyline??? The %s look pretty close.
Brenner 2000: is this correct, that hypericum was significantly more effective than sertraline (47% vs. 40%, p < 0.01)?
Appendix II
Unfair. Prescription drugs have much
longer adverse effect lists because of the FDA approval process and b/c they are tested for side
and adverse effects. St. John's Wort has a much shorter list because of this, not because it
definitely has fewer side effects. This must be discussed somewhere. Under SJW, add that it is a
CYP 450 3A4 inducer. Why is Etrafon included in this table? The perphenazine in it is an
antipsychotic drug and clouds the side effect profiles because it has many additional side
effects than the TCA. Delete it. If you want to include another agent, include a secondary amine
TCA such as desipramine or nortriptyline, which have less severe side effects than the tertiary
amine imipramine. Phenelzine sulfate (Nardil) is under the same column as SSRIs, under
paroxetine. Move it so it is under MAOIs.
Consumers Healthcare Products Association®
October 31, 2000
Ms. Nancy Cusick
Health Technology Advisory Committee
121 East 7th Place,
Suite 400
St. Paul, Minnesota 55101
Re: HTAC Preliminary Report on St. John's Wort
Dear Ms. Cusick:
These comments are provided in response to the announcement
of the Minnesota Health Technology Advisory Committee (HTAC) in the October 2, 2000 State
Register regarding its preliminary report on St. John's Wort. The Consumer Healthcare Products
Association (CHPA) is pleased to have this opportunity to offer its comments with regard to the
recommendations of that report.
In many respects the CHPA and its members are in agreement with the preliminary findings of the HTAC. A number of CHPA members manufacture or distribute dietary supplement products that contain St. John's Wort and they share the HTAC's concerns that some dietary supplements may interact with prescription medicines. According, CHPA agrees that:
- Persons should discuss their use of dietary supplements with a healthcare professional, especially if they are currently taking any prescription or nonprescription medicines.
- Physicians should inquire whether their patients are taking any dietary supplements before prescribing prescription drugs.
- Patients (and their doctors) should be informed about possible complications, side effects or interactions that may occur between prescription drugs and nonprescription drugs, dietary supplements and even foods.
- Based on the current evidence, patients who are currently taking indinavir or cyclosporin should avoid taking St. John's Wort because of apparent interactions between these prescription drugs and St. John's Wort that may affect their metabolism by the body.
In fact, the CHPA has already developed a voluntary program among its members that calls for the following statement on the labeling of any dietary supplement that contains St. John's Wort:
If you are taking a prescription drug, ask a health professional.
Since the initiation of this voluntary program by the CHPA on April 2, 2000, CHPA has achieved 100% compliance among its members who manufacture or distribute these products. In June, CHPA petitioned the federal Food & Drug Administration (FDA) to establish a regulation that would require this labeling statement on all dietary supplements containing this ingredient. The agency has yet to respond to this petition, but CHPA urges the Minnesota HTAC to likewise contact FDA and extend its support for this petition. (A copy of CHPA's voluntary program, a press release and our petition to the FDA are enclosed.)
Similarly, CHPA's concerns about a lack of clinical data regarding possible effects of certain dietary supplements on a developing fetus or newborn led the association to initiate another voluntary label warning earlier this year. In February, CHPA adopted a voluntary program to label dietary supplements with the following precaution: "If you are pregnant or nursing a baby, ask a health professional." Like the St. John's Wort warning, we have achieved 100 percent compliance among our members with this label statement.
Dietary Supplements Are Not Prescription Drugs And Should Not Be Regulated The Same Way.
However, CHPA is concerned about the recommendation that was proposed by the HTAC. The State Register indicates that the HTAC recommends, " The same FDA rules governing prescription drugs should also be required for herbal supplements." It is not apparent from the Register announcement exactly what rules governing prescription drugs the HTAC is referring to. (There are regulations for advertising, labeling, off-label usage, Prescription-only dispensing, manufacturing practices, security & storage, etc.) Surely, the HTAC is not suggesting that St. John's Wort should be considered a prescription drug in all respects, but that is not clear. There are a host of differences between prescription medicines, and dietary supplements, that strongly counsel against identical regulatory treatment for the two categories of products. For instance:
- Today's consumers are taking greater control of their health care. Dietary supplements, along with traditional nonprescription, over-the-counter medicines, provide consumers with valuable self-care options that are safe, accessible, convenient, and cost-effective. The importance of access to self-healthcare opportunities that save money and empower consumers should not be underestimated.
- Dietary supplements are considered a subset of foods under the federal Dietary Supplement Health & Education Act (DSHEA) and are regulated by the Food and Drug Administration (FDA) pursuant to the U.S. Food, Drug, and Cosmetic Act. Under federal law, the following items are considered dietary supplements:
- vitamin and mineral products;
- herbals or other botanicals;
- amino acids; and
- other substances used to supplement the diet.
It is estimated that approximately 70 percent of Americans are currently using vitamin and mineral supplements and 25 percent are using herbal products. - FDA has authority to take action against any dietary supplement product that is found to be unsafe or that makes unsubstantiated claims or unapproved drug claims. In addition, the Agency has legal authority to move against any dietary supplement that presents a significant or unreasonable risk of injury or illness. While the standards for prescription drugs and supplements are different, there is no lack of enforcement authority for supplements, and FDA commissioner Jane Henney has told Congress she had sufficient statutory authority to regulate supplements.
- Unlike prescription drugs, and advertising and marketing of dietary supplements is primarily regulated by the Federal Trade Commission (FTC). In November 1998, FTC issued advertising guidelines for manufacturers and marketers of dietary supplements. The guidance document reaffirms longstanding policy and practices that advertising for all products -- including dietary supplements -- must be truthful, not misleading, and substantial. The FTC has taken numerous enforcement actions against mis-marketed dietary supplements in the past year, with the gratitude and encouragement of reputable industry members.
- FDA has extensive authority over dietary supplements (albeit, different authority than that granted to it for prescription drugs). Specifically, the FDA has the power to:
- Stop any company from selling a dietary supplement that is toxic or unsanitary [see Food Drug and Cosmetic (FDC) Act, Section 402(a)];
- Stop the sale of a dietary supplement that has false or unsubstantiated claims [see FDC Act, Section 403(r)(6)];
- Take action against dietary supplements that pose "a significant unreasonable risk of illness or injury: [see FDC Act, Section 402(f)];
- Stop any company making a claim that a product cures or treats a disease [see FDC Act, Section 201(g)];>
- Stop a new dietary ingredient from being marketed if FDA does not receive enough safety information in advance [see FDC Act, Section 413];
- Require dietary supplements to meet strict manufacturing requirements (Good Manufacturing Practices), including potency, cleanliness, and stability [see FDC Act, Section 402(g)].
- FDA is expected to publish by year's end, proposed regulations to establish Good Manufacturing Practices (GMPs) specifically geared to dietary supplements to assure consistent product quality. The dietary supplement industry, including CPHA, has submitted comments to FDA supporting good manufacturing standards for these products. Prescription drug manufacturers already have GMP regulations in place, but they are hardly appropriate to the supplement industry whose raw materials are often naturally occurring botanicals, herbals, and mineral supplements as opposed to carefully synthesized chemicals in a lab.
These are just a few of the reasons that identical regulatory treatment of prescription drugs and dietary supplements are ill advised. There are many regulatory proposals on specific issues that would improve manufacturing quality, provide wider margins of safety, and strengthen consumer confidence in the dietary supplement marketplace -- initiatives that supported by the industry. However, a blanket statement that these over-the-counter products should be regulated identically with prescription medicines does not serve consumers or the industry. Therefore, CHPA opposes this recommendation and requests that it is stricken from the HTAC's final report.
The CHPA would be pleased to provide further information to the HTAC on any of the matters raised in these comments. If I can be of further assistance in this matter, please do not hesitate to contact me.
Sincerely yours,
Steven M. Mister
Vice President & Associate General
Counsel
Enclosures
CHPA/smm/s
References
- 1.
- Cott JM Natural Product Formulations Available in Europe for Psychotropic Indications Psychopharmacology Bulletin 1995314:745–750. [PubMed: 8851648]
- 2.
- Gugliotta G Health Concerns Grow Over Herbals as Industry Booms Analysis Suggests Rising Toll in Illness and Death. Washington Post. 03-19-2000;A01
- 3.
- Plotnikoff G, George J Herbalism in Minnesota: What Should Physicians Know? Minnesota Medicine 05-199982May:1–29. [PubMed: 10337136]
- 4.
- Eisenberg DM, Davis RB, Ettner SL, et al Trends in Alternative Medicine Use in the United States, 1990-1997 Journal of the American Medical Association 11-11-199828018:1569–1575. [PubMed: 9820257]
- 5.
- Eisenberg DM, Kessler RC, Foster C, et al Unconventional Medicine in the United States The New England Journal of Medicine 01-28-19933284:246–252. [PubMed: 8418405]
- 6.
- George BA, Axtel S Herbs in Medical School Education Minnesota Medicine 05-1999825:20–21.
- 7.
- Wetzel MS, Eisenberg DM, Kaptchuk TJ Courses Involving Complementary and Alternative Medicine at US Medical Schools JAMA 09-02-19982809:784–787. [PubMed: 9729989]
- 8.
- Tyler VE What Pharmacists Should Know About Herbal Remedies Journal of the American Pharmaceutical Association 1999NS361:29–37. [PubMed: 8835439]
- 9.
- Upton R St. John's Wort: Hypericum perforatum. American Herbal Pharmacopoeia and Therapeutic Compendium. 07-1997;1:3–32.
- 10.
- The Dietary Supplement Health and Education Act of 1994. Available at: http://www
.crnusa.org/dshea.htm Accessed on: 10-26-1999. Summary and Analysis. - 11.
- Federal Register 21 CFR Part 101. 04-29-1998;RIN 0910-AA59:Regulation on Statements Made for Dietary Supplements Concerning the Effect of the Product on the Structure or Function of the Body.
- 12.
- FDA Public Health Advisory: Risk of Drug interactions with St John's Wort a Dietary Supplement Indinavir And Other Drugs. Available at: http://www/fda
.gov/cder /drug/advisory/stjwort.htm Accessed on: 12-07-2000. - 13.
- Henney J Risk of Drug Interactions With St. John's Wort Journal of the American Medical Association 04-05-200028313:
- 14.
- Miller ND St. John's Wort (Hypericum perforatum): Clinical Effects on Depression and Other Conditions Alternative Medicine Review 199831:18–26. [PubMed: 9600023]
- 15.
- Fong H Botanicals In Health and Disease. Quality, Research and Regulatory Aspects in the United States. 1999;April 15, 1999 presentation at the 28th Annual Current Issues in Nutrition Conference. Herbal Remedies—The Promise, The Concern, The Science.
- 16.
- Brener R, Azbel V, Madhusoodanan S, et al Comparison of an Extract of Hypericum (LI 160) and Sertraline in the Treatment of Depression: A Double-Blinded, Randomized Pilot Study Clinical Therapeutics 03-10-200022(4):411–419. [PubMed: 10823363]
- 17.
- Josey ES, Tackett RL St. John's Wort: a new alternative for depression? International Journal of Clinical Pharmacology and Therapeutics 1999373:111–119. [PubMed: 10190758]
- 18.
- Locher CP, Burch MT, Mower HF, et al Anti-microbial activity and anti-complement activity of extracts obtained from selected Hawaiian medicinal plants. Journal of Ethopharmacology. 1999;49:23–32. [PubMed: 8786654]
- 19.
- Foster S 101 Medicinal Herbs: An Illustrated Guide. Interweave Press. 11-1998:192–193.
- 20.
- Nash JM Nature's Prozac? Time 09-199715012:80–81.
- 21.
- de Smet PA, Nolen WA St. John's wort as an antidepressant. British Medical Journal. 08-03-1996;313:241–242. [PMC free article: PMC2351659] [PubMed: 8704522]
- 22.
- Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). Fourth Edition Washington DC, American Psychiatric Association. 02-1998. pp. 1–11.
- 23.
- Linde K, Ramirez G, Mulrow C, et al St. John's wort for depression-an overview and meta-analysis of randomized clinical trials. British Medical Journal. 07-03-1996;313:253–258. [PMC free article: PMC2351679] [PubMed: 8704532]
- 24.
- Depression: What You Need to Know.-Fact Sheet. Available at: http://www
.nmha.org/infoctr /factsheets/21.cfm Accessed on: 10-30-2000. - 25.
- Pear R Mental Disorders Common, U.S, Says; Many Not Treated. Available at: http://www
.nytimes.com/ .121399mental-health.html Accessed on: 01-13-1999. - 26.
- Deltito J, Beyer D The scientific, quasi-scientific and popular literature on the use of St. John's Wort in the treatment of depression. Journal of Affective Disorders. 1998;51:345–351. [PubMed: 10333988]
- 27.
- Plain Talk about Depression. Available at: http://www
.pharminfo .com/disease/mental/dep_info.html Accessed on: 10-26-1999. - 28.
- Schempp CM, Pelz K, Wittmer A, et al Antibacterial activity of hyperforin from St. John's wort, against multiresistant Staphylococcus aureus and gram-positive bacteria Lancet 06-19-19993539203:–. [PubMed: 10382704]
- 29.
- Schempp CM, Winghofer B, Ludtke R, et al Topical application of St. John's wort (Hypericum perforatum L.) and of its metabolite hyperforin inhibits the allostimulatory capacity of epidermal cells. British Journal of Dermatology. 2000;142:979–984. [PubMed: 10809859]
- 30.
- Meruelo D, Lavie G, Lavie D Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin. Proc.Natl.Acad.Sci. 07-1988;85:5230–5234. [PMC free article: PMC281723] [PubMed: 2839837]
- 31.
- Suzuki O, Katsumata Y, Oya M, et al Inhibition of Monoamine Oxidase by Hypericin. Planta Medica. 1984:272–274. [PubMed: 6484033]
- 32.
- Cott JM In Vitro Receptor Binding and Enzyme Inhibition by Hypericum perforatum Extract Pharmacopsychiat 199930supplement:108–112. [PubMed: 9342770]
- 33.
- Denke A, Schempp H, Weiser D, et al Biochemical Activities of Extracts from Hypericum perforatum L Arzneimittel-Forschung 2000505:415–419. [PubMed: 10858868]
- 34.
- Thiede HM, Walper A Inhibition of MAO and Comt by Hypericum Extracts and Hypericin Journal of Geriatric Psychiatry and Neurology 10-199471:S54–S56. [PubMed: 7857510]
- 35.
- The Medical Letter St. John's Wort The Medical Letter on Drugs and Therapeutics 11-21-1997391014:107–108. [PubMed: 9398822]
- 36.
- Rey J, Walter G Hypericum perforatum (St. John's Wort) in depression: pest or blessing? Alternative Medicine. 12-1998;169:583–586. [PubMed: 9887899]
- 37.
- 1999. Physicians' Desk Reference (PDR) 53rd ed. Montvale, NJ, Medical Economics Company; pp. 924–928, 2091-2093, 2299-2301, 3078-3083, 3418-3420.
- 38.
- St. John's Wort Hypericum perforatum. Available at: http://tnp
.com/substance.asp?ID=89 Accessed on: 06-19-2000. - 39.
- Castleman M 1991. The Healing Herbs: The Ultimate Guide to Curative Power of Nature's Medicines; Emmaus, PA, Rodale Press; pp. 321–325.
- 40.
- Gaster B, Holroyd J St John's Wort for Depression-A Systematic Review Archives Internal Medicine 01-24-20001602:152–156. [PubMed: 10647752]
- 41.
- NIH Serotonin Transporter Availability and Mood State in Normal Volunteers Taking Hypericum Perforatum (St. John's wort). Available at: http:
//clinicalstudies .info.nih.gov/detail/A_1999-M-0151 .html Accessed on: 08-09-2000. - 42.
- Kerb R, Brockmoller J, Staffeldt B, et al Single-Dose and Steady-State Pharmacokinetics of Hypericin and Pseudohypericin Antimicrobial Agents and Chemotherapy 09-1996409:2087–2093. [PMC free article: PMC163478] [PubMed: 8878586]
- 43.
- PDR for Nonprescription drugs and dietary supplements. 20th ed. Montvale, NJ, Medical Economics Company. 1999.
- 44.
- Ernst E, Rand JI, Barnes J, et al Adverse effects profile of the herbal antidepressant St. John's wort (Hypericum perforatum L.). Eur J Clin Pharmacol. 1998;54:589–594. [PubMed: 9860144]
- 45.
- Arya OS, Ford JH An investigation of the type of photosensitization caused by the ingestion of St. John's wort (Hypericum Perforatum) by calves Journal of Comparative Pathology 01-1981911:135–141. [PubMed: 7343570]
- 46.
- Kroll D St. John's Wort: An Example of the Problems with Herbal Medicine Regulation in the United States. Available at: http://www
.pharminfo .com/disease/mental/dep_info.html Accessed on: 10-26-1999. - 47.
- Herbal remedy for eczema linked to kidney failure. Minneapolis Star & Tribune. 08-18-1999;A1
- 48.
- Piscitelli S, Burstein A, Chaitt D, et al Indinavir concentrations and St. John's Wort Lancet 02-12-20003559203:547–550. [PubMed: 10683007]
- 49.
- Graedon J, Graedon T Herb Library-St. John's Wort Hypericum Perforatum. Available at: http://www
.healthcentral .com/peoplepharmacy /pp_herblibrary/herblibrary.cfm Accessed on: 06-19-2000. - 50.
- Zal HM Five Herbs for Depression Consumer Reports 12-19993912:3343–3349.
- 51.
- Johne A, Brockmoller J, Bauer S, et al Pharmacokinetic interaction of digoxin with an herbal extract from St. John's wort (hypericum perforatum). Clinical Pharmacology & Therapeutics. 1999;66:338–345. [PubMed: 10546917]
- 52.
- Roby CA, Anderson GD, Kantor E, et al St John's Wort: Effect on CYP3A4 activity Clinical Pharmacology & Therapeutics 05-2000675:451–457. [PubMed: 10824623]
- 53.
- Ruschitzka F, Meier P, Turina M, et al Acute heart transplant rejection due to Saint John's Wort Lancet 02-12-20003559203:548–549. [PubMed: 10683008]
- 54.
- Hirshon Plant Poisoning, Herbs. Available at: http://www
.emedicine .com/emerg/topic449.htm Accessed on: 05-22-2000. - 55.
- The Hamilton Rating Scale for Depression (HAM-D). Available at: http://www
.wellbutrin-sr .com/hcp/eval/eval.htm Accessed on: 12-01-2000. - 56.
- Guy W Clinical Global Impressions. New Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology. 1976:218–222.
- Executive Summary
- Introduction
- FDA
- Background
- Uses
- Findings
- Dosage
- Safety
- Issues of Controversy
- Cost
- Conclusions
- Recommendations
- Appendix I: Summary of Studies
- Appendix II: Side Effects, Adverse Effects, Precautions, and Warnings
- Appendix III: Selected Information Resources
- Appendix IV: Foods Containing the AminoAcids Tryptophan and Tyrosine
- Appendix V: Cost Comparison
- Appendix VI: Description of Hamilton Rating Scale and Clinical Global Impressions Scale
- Appendix VII: Public Comments
- References
- St. John's Wort - Minnesota Health Technology AssessmentsSt. John's Wort - Minnesota Health Technology Assessments
Your browsing activity is empty.
Activity recording is turned off.
See more...
