NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009.
3.1. INTRODUCTION
The psychoactive effect of a drug usually refers to a chemical agent that exerts an action upon the central nervous system (CNS), alters brain function, and, consequently, produces a temporary change in an individual’s mood, feelings, perception, and/or behavior. Such agents may be prescribed as therapeutic medications or used (or abused) as recreational drugs. In each case, the subjective effects produced by such agents are generally not accessible to independent verification by an observer. However, methods were developed about 50 years ago whereby human subjects could self-rate their experiences on questionnaires after administration of a drug [1]. Generally, these self-inventories require subjects to provide information about themselves and are considered valuable because they venture “below the surface” to glean the effect of a drug on an individual. Also, they are convenient because they (usually) do not require the services of a group of raters or interviewers. Their chief disadvantage may be that individuals might not completely understand the effect of the drug or their drug “experience” and therefore might not always give an accurate report.
The drug discrimination (DD) paradigm is an assay of, and relates to, the subjective effect of drugs in nonhuman animals or humans. In a typical DD experiment, there are four basic components: (1) the subject, (2) the dose of drug that exerts an effect on the subject and precedes a response by the subject, (3) an appropriate (or correct) response, and (4) presentation of reinforcement.
SUBJECT → DOSE OF DRUG → RESPONSE → REINFORCEMENT
The drug effect that “leads to” a behavioral event (i.e., particular response) and signals that reinforcement is available is called the discriminative stimulus. A wide variety of psychoactive drugs can serve as discriminative stimuli (see below). In laboratory subjects, discriminative control by (usually) two treatments is established through the use of reinforcement (reward). When subjects receive a dose of a drug, it functions as a signal that prompts a correct behavioral response and results in the presentation of a reward. In other words, the effect of the drug is used as a “help” or “aid” to control appropriate behavioral responding by signaling that reinforcement is (or will be) available. Subjects are usually trained to distinguish administration of a particular dose of a particular drug (i.e., the training dose of a training drug) from administration of saline vehicle (i.e., usually a 0.9% sodium chloride solution that is often used as a solvent for many parenterally administered drugs). In a subject’s course of training sessions, the dose of drug is administered (i.e., drug sessions) and lever presses on the drug-designated lever (for that subject) produce reinforcement. In other training sessions, saline is administered (i.e., vehicle sessions) and responses on the (alternate) saline-designated lever produce reinforcement. The DD procedure can be characterized as a highly sensitive and very specific drug detection method that provides both quantitative and qualitative data on the effect of a training drug in relation to the effect of a test (i.e., challenge) agent. Historically, DD studies are linked by a common requirement that subjects must perform an appropriate (or correct) response that indicates a distinction was made between drug and nondrug conditions. As such, when employed with animals or humans, a subject’s response permits an experimenter to determine if a drug effect has been “perceived.” An excellent source of information on DD studies can be found at the Drug Discrimination Bibliography Web site (http://www.dd-database.org). The Web site, established and maintained by Drs. Ian P. Stolerman and Jonathan B. Kamien, is funded by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH) and contains close to 4000 DD references published between 1951 and the present. The citations include DD abstracts, journal articles, reviews, book chapters, and books. In addition, the Web site can be navigated to selectively retrieve references on particular training drugs, drug classes, test drugs, authors, and DD methodologies.
3.2. METHODS
3.2.1. Apparatus
Behavioral experiments with animals are often conducted in testing environments that eliminate or minimize the occurrence of extraneous events or conditions (e.g., loud sounds, lights, temperature changes, etc.). The experimental setting is also designed to make more likely the occurrence of a particular behavior. For example, placing a hungry rat in a small chamber in which a lever is the most prominent object increases the likelihood that the animal will press the lever, which will result in the delivery of a reward. Studies of DD are often conducted in standard two-lever operant chambers (Coulbourn Instruments, Model E10-10, Lehigh Valley, Pennsylvania, USA, or Med Associates, Model ENV-008, St. Albans, Vermont, USA) housed within light- and sound-attenuating outer chambers. Typically, one wall of each operant chamber is fitted with two levers and a device, centered equidistant between the levers, to deliver reinforcement. The reinforcement may be, for example, a 45-mg food pellet (e.g., Noyes Precision Pellets® PJAI-0045, Research Diets, Inc., New Brunswick, New Jersey, USA), sweetened condensed milk, or water (delivered in a 0.01 mL cup). An overhead 28-V house light illuminates each chamber. Solid-state and computer equipment are used to record lever presses, program the delivery of reinforcement, and record the number of reinforcements.
3.2.2. Subjects
Table 3.1 shows that different species have been used as subjects in DD studies. To date, the rat has been used most often as the experimental subject in DD citations. Also of interest is the number of studies that cited humans as the experimental subjects. It is noted that DD procedures for humans are similar to those used for laboratory animals, but are adjusted to the uniqueness of humans. For example, drugs are usually administered under double-blind conditions and money typically serves as reinforcement for correct responses. In addition, many human DD studies include questionnaire data on subjective effects of the administered agent(s) [12,13]. In a DD study with animals or humans as subjects, however, the learning of a DD involves appropriate responses for the presentation of reinforcement under the pharmacological effect of different treatments.
TABLE 3.1
Species Used as Subjects in Drug Discrimination Experiments
3.2.3. Operant Training
The discriminative stimulus effect of a drug is most frequently established via operant conditioning, a learning paradigm in which a subject emits a response that is followed closely by reinforcement. In general, operant behavior is “controlled” by its consequences. For example, a hungry rat may “act on” or “operate on” its environment and press a lever, which closes a switch and activates a food dispenser or liquid dipper to produce a pellet in a tray or liquid (e.g., sweetened milk) in a small cup. The operant is the behavior just prior to the reinforcement. In such a situation, the rat’s press of the lever, which is followed by reinforcement, may result in an increased probability of the animal pressing the lever in the future. In practice, however, operant conditioning is the study of behavior maintained by schedules of reinforcement, which are defined as the delivery of reinforcement to a subject according to some well-defined rule. In DD applications, the animals’ opportunity to press a lever under a schedule of reinforcement gives them, in effect, “communication” to the investigator of “how a drug affects their CNS.” It is also noted that schedules of reinforcement are used with humans and the pattern of responding is generally similar to those obtained with nonhuman animals.
An animal’s initial training in a DD two-lever operant task begins with “magazine training,” which involves training the subject to eat from a food tray or drink from a dipper cup and, consequently, for it to learn that the noise made by the activation of the (mechanical) delivery device indicates the imminent presentation of “compensation.” At the beginning of the study, the experimenter teaches the rats to press a lever for reinforcement with the technique of successive approximation or “shaping.” The latter procedure involves the reinforcement of initial behavior that may only be vaguely similar to the final desired response (i.e., lever pressing); reinforcement continues for variations in behavior that come closer to pressing the lever. For example, an experimenter may begin with the presentation of reward (e.g., via a hand switch that bypasses a schedule of reinforcement) to a rat every time it turns toward a lever. Later, only movements that bring it closer to a lever are reinforced. After the rat has learned to approach a lever, it is not reinforced until it touches the lever. Eventually, the rat’s behavior will have been so shaped that it will readily press a lever when put in the chamber. (It is noted, however, that when a relatively large number of animals are used in an experiment, shaping can be a very time-consuming procedure that requires much patience on the part of the experimenter.) When every press of the lever is followed by reinforcement, the organism is said to be on a continuous reinforcement schedule. As a consequence of such a schedule, the number of reinforcements could become quite high and might lead to decreased responding over time because of satiation. However, it is not necessary to reinforce every response in order to maintain responding. That is, an animal can be reinforced intermittently (i.e., part of the time). The intermittent schedule can be based on a portion of responses or on a time interval. The two most common schedules of reinforcement are ratio and interval schedules, each of which can be fixed (unvarying) or variable (random). In DD studies, the fixed ratio (FR) and variable (random) interval (VI or RI) schedules of reinforcement are used extensively and are discussed here. In an FR schedule the subject must complete a fixed number of responses in order to obtain reinforcement. In an FR 10 schedule, for example, every 10th response is reinforced. In VI schedules, the length of time elapsing before reinforcement is delivered varies around the mean value specified by the schedule. On a VI 15 sec schedule, reinforcement is available, on average, after 15 sec have elapsed since the last reinforcement but may be available, for example, as shortly as 2 sec later, or not until 60 sec have elapsed. The first response after a time interval has elapsed produces reinforcement for the organism. Once the rat learns to press on the right-side lever and the left-side lever under the schedule of reinforcement (e.g., FR or VI), DD training begins.
3.3. DRUGS AS STIMULI
Table 3.2 lists some of the drugs, from several different pharmacological and chemical classes, that have been shown to serve as stimuli. In most of these studies, either the FR or VI schedule of reinforcement was used to establish the discrimination. Although DD techniques have varied, it is typical that an organism is taught to respond on one lever (e.g., right-side lever) when a dose of training drug is administered before a training session and on another lever (e.g., left-side lever) when vehicle (e.g., saline) is given. Correct responses are intermittently reinforced by delivery of food (e.g., pellet, sweetened milk). The organism’s eventual learning of the correct response in a typical two-lever choice task involves its determination that the effect produced by the administration of the dose of the training drug on certain days is distinct from that produced by the injection of vehicle on other days.
TABLE 3.2
A Partial List of Drugs that Have Been Used as the Discriminative Stimulus in Drug Discrimination Experiments
In general, there has been good agreement between species on (1) whether or not a particular drug can function as a discriminative stimulus, and (2) results obtained with test (or challenge) agents (see stimulus generalization tests below). The present survey describes some training and test results from rats trained to discriminate diazepam (3.0 mg/kg) from vehicle (one drop of Tween 80 per 10 mL distilled water) under an FR 10 schedule of reinforcement. For these studies, 12 experimentally naïve, male albino Sprague Dawley rats (Charles River Labs, Wilmington, Massachusetts, USA) weighing 325–350 g at the beginning of the experiment were used. Rats were housed individually and had free access to water, but were gradually food restricted to approximately 80% of their free-feeding weights before training began. The colony room was kept at a constant temperature (approximately 21–23°C) and humidity (~ 50%); lights were turned on from 0600 to 1800 hr.
3.3.1. Discrimination Training Procedure
The dose-of-a-drug versus saline treatment paradigm constitutes the single most widely used DD procedure and its properties have been documented best. In the present example, the rats’ training sessions are preceded by an intraperitoneal (IP) injection of either 3 mg/kg of diazepam (dose is based on weight of base) or vehicle (one drop of Tween 80 per 10 mL of distilled water) with only the stimulus-appropriate lever present (i.e., left- or right-side lever). A pre-session injection interval (PSII) of 15 min is used; during this interval the animals are in their home cages. The route of administration and the PSII for the drug and its vehicle are typically chosen on the basis of the known pharmacokinetic properties and/or behavioral effect(s) of the drug. Training sessions are of 10 min duration, 5–7 days per week. For a particular session, just one of the two levers (i.e., the treatment-appropriate lever) is programmed to deliver reinforcement; presses on the incorrect lever have no programmed consequence. For six of the rats, responses on the right-side lever are reinforced after drug administration, while responses on the left-side lever are reinforced after vehicle administration; lever response conditions are reversed for the remaining six rats. In addition, lever assignments for a particular operant chamber are alternated (e.g., first animal in chamber 1 is assigned left-side lever as drug lever and right-side lever as saline lever; second animal in chamber 1 is assigned right-side lever as drug lever and left-side lever as saline lever, etc). The latter tactic is important because it has been observed that rodents may learn to use olfactory hints (or cues) that remain in the operant chamber from preceding animals [46]. In addition, diazepam or vehicle is administered on a random schedule with the constraint that no more than two consecutive sessions with the drug or vehicle can occur; an equal number of drug and vehicle sessions occur. The experimenter will note that initial injections of the dose of training drug might hinder or disrupt the animals’ pressing of the drug-designated lever. Animals should develop behavioral tolerance to the disruptive effects of the drug (i.e., diazepam) and will, over time, perform the task. Animals do not, however, develop tolerance to the stimulus effect of the training dose of the training drug. If tolerance did develop, then the dose of the drug would not continue to serve as a discriminative stimulus and the animals’ performance would significantly decline.
3.3.2. Discrimination Data
3.3.2.1. Percent Drug Lever Responding
An animal’s degree of progress in learning the DD is determined by an evaluation of its distribution of presses on the two levers. In particular, an animal’s learning of the discrimination can be evaluated either prior to, or up to, the delivery of the first reinforcement. Thus, when an FR 10 schedule of reinforcement is used, DD learning can be assessed for each subject by dividing the number of responses that occurred on the drug-designated lever by the total number of responses that occurred on both levers up to the delivery of the first reinforcement; percent of responses on the drug-appropriate lever is then obtained by multiplying the value by 100. For instance, assume that a rat has the right-side lever designated as the diazepam-appropriate lever. On a Monday, the animal is injected with 3 mg/kg of diazepam, placed in its assigned operant chamber, and proceeds to press the left-side lever 9 times and the right-side lever 10 times; food reward (in this example) could be presented after the 10th right-side lever press. For this day, discriminative control would be assessed at 53% diazepam-appropriate responding (i.e., × 100). On Tuesday, this same rat is injected with vehicle, placed into its designated chamber, and presses the right-side lever 4 times and the left-side lever 10 times; food is presented after the 10th left-side press. On this day, discriminative control would be assessed at 29% diazepam-appropriate responding (i.e., × 100). Alternatively, if the VI schedule of reinforcement is programmed, then discrimination performance is evaluated during a short period (e.g., 2.5 min) of non-reinforced responding (referred to as extinction) at the beginning of a session; extinction sessions usually occur once or twice per week. Each animal’s distribution of presses on the two levers is then evaluated in the same manner as it is under the FR schedule of reinforcement. As might be expected, the administration of drug or vehicle during initial training sessions under either FR or VI schedules of reinforcement usually results in the animals dividing their responses equally (e.g., 50% diazepam-appropriate responding after injection of drug or saline) between the two levers (Figure 3.1). However, as training sessions progress with drug and vehicle, the animals gradually learn to respond on the drug-designated lever (i.e., percent of responses on the drug-designated lever is high and percent of responses on the vehicle-designated lever is low) when given drug, and on the vehicle-designated lever (i.e., percent of responses on the drug-designated lever is low and percent of responses on the vehicle-designated lever is high) when given vehicle. In other words, the learning of a DD occurs gradually over time (Figure 3.1). A generally accepted guideline is that after 6 to 9 wk of training, animals (individually and, consequently, as a group mean) consistently make ≥ 80% of their responses on the drug-appropriate lever after administration of drug (e.g., 3 mg/kg of diazepam) and ≤ 20% of their responses on the same lever after administration of vehicle (Figure 3.1).

FIGURE 3.1
Learning curve results of rats trained to discriminate the stimulus effect of 3 mg/kg (IP) of diazepam (closed squares) from 1 mL/kg of vehicle (open squares). Ordinate: Mean (n = 12) percent (± SEM) of responses on the diazepam-appropriate lever (more...)
3.3.2.2. Response Rate
In addition to the animals’ distribution of responses on the two levers under FR or VI schedules of reinforcement, their response rate data (i.e., total number of responses on both levers expressed as responses per second or minute) also can be calculated. For example, animals’ (individual and/or group mean) response rate can be calculated under the FR schedule of reinforcement for a behavioral session (e.g., 15 min). Alternatively, under the VI schedule of reinforcement, the total number of responses made during the 2.5 min extinction session (or the entire session) can be recorded. The animals’ response rate can be viewed as another indicator of the effect(s) of a drug on behavior. In some cases, the animals’ response rate after the training dose of the training drug is suppressed when compared to that of the vehicle. In other instances, response rate data can assist the experimenter in the selection of (1) an appropriate training dose of the training drug, and/or (2) a range of doses to be examined for test drugs. Further, animals’ response rate can be an ancillary measure in cases where a test drug may, or may not, affect the main dependent variable (i.e., percent responding on the drug-designated lever). Finally, this parameter can be used in conjunction with an evaluation of the subjects’ general behavioral condition (e.g., sedated, incapacitated, overly excited).
3.4. APPLICATIONS
3.4.1. Stimulus Generalization
Stimulus generalization of the training dose of the training drug is said to occur to the test drug if administration of the test substance results in the animals responding on the drug-designated lever. It is noted, however, that the phrase “stimulus generalization of the vehicle to the test agent” is not used when the animals respond on the vehicle-designated lever after administration of the test treatment; typically, the latter result would be characterized as “ the test agent induced vehicle-like responding.” In the present example, maintenance of the diazepam/vehicle discrimination was ensured by continuation of training sessions that were intermingled between stimulus generalization test sessions. Discrimination training sessions were conducted with 3 mg/kg of diazepam or vehicle on the four days prior to a stimulus generalization test session. On at least one of those days, six of the animals received 3 mg/kg of diazepam and the other six rats received vehicle; percent diazepam-appropriate responding was then determined under the FR schedule of reinforcement as described above. Animals not meeting the above criteria (i.e., ≥ 80% drug-appropriate responding after drug administration and ≤ 20% drug-appropriate responding after vehicle injection) were not used in that week’s stimulus generalization test. During generalization investigations, test sessions were interposed between discrimination training sessions. In these test sessions, the rats were given a test treatment and then allowed to select one of the two levers in a 15-min session (FR procedure). The lever on which the animal first totaled 10 responses was regarded as the selected lever; percent diazepam-appropriate lever responding was calculated as described above. Subsequent reinforcement was delivered for responses on the selected lever according to the FR 10 schedule of reinforcement. Alternatively, if the VI schedule of reinforcement was programmed, then the animals would have been injected with test treatment, given a 2.5 min extinction session, and removed from the operant chambers; subsequently, percent diazepam-appropriate lever responding would have been calculated as described above.
3.4.2. Test Considerations
3.4.2.1. Dose Response
A very important consideration in tests of stimulus generalization is the necessity of a thorough dose-response investigation. An extensive literature review of DD tests of stimulus generalization reveals that certain agents produce saline-like effects at particular doses (usually relatively low doses), and disruption of behavior at some higher doses. While an initial conclusion to the results of such a study may be that there is a lack of stimulus generalization, it has been found in a number of instances that a careful evaluation of additional doses (i.e., doses between the highest dose that resulted in vehicle-like responding and the lowest dose that produced disruption of behavior) ultimately resulted in stimulus generalization. This has even been observed with agents where the difference in vehicle-like and disruptive doses has been quite small. Thus, several instances have been reported where doses of a challenge drug, administered in a logarithmic progression (e.g., 0.1, 0.3, 1, 3, 10 mg/kg), resulted in saline-like responding at the lower doses and disruption of behavior at the highest doses. However, an examination of doses between, for example, 3 mg/kg and 10 mg/kg resulted in stimulus generalization. As such, these types of situations appear to emphasize (1) that stimulus generalization to a test agent may occur within a “narrow window of doses,” and (2) the sensitive and specific nature of the drug-induced stimulus.
3.4.2.2. Comparison of Results of Test Agents
A preferred tactic is to evaluate doses of a challenge drug in drug-trained subjects until either stimulus generalization or disruption of behavior (i.e., no responding) occurs. If, for example, the highest test dose (e.g., dose X) of a challenge drug elicits 50% drug-appropriate responding, and, for some reason, the evaluation of higher doses is precluded, it is not appropriate to conclude that the challenge drug is half as potent as the training drug when, in fact, there has been no demonstration that the two agents can produce a common effect. In this situation, comparisons can only be made in a qualitative sense. That is, an appropriate conclusion that could be stated is that the challenge agent is less effective than the training drug in producing a training-drug–like effect (or, correspondingly, that it is less effective than some other challenge drug which, at a dose below dose X, produced training-drug–like effects). Likewise, if two challenge drugs produce partial generalization (e.g., 40% and 60% training-drug–appropriate responding) at dose X, it should not be stated with certainty that the second challenge drug is more potent than the first because the possibility exists that one (or both) agent(s) may not exert a stimulus effect that is common (i.e., complete generalization) to that of the training drug.
Data Analysis, Interpretation, Examples
In general, the phenomenon of generalization involves engaging in previously learned behaviors in response to new situations that resemble those in which the behavior was first learned. In the DD procedure, subjects respond to other drug stimuli that are more or less similar to those present during discrimination training. Stimulus generalization studies are used to determine whether a discriminative stimulus will generalize to (i.e., substitute for) other drugs. The rationale of this approach is that an animal trained to discriminate a dose of training drug will display stimulus generalization only to agents having a similar effect, though not necessarily an identical mechanism of action. Thus, in the present example, stimulus generalization is said to have occurred when the animals, after being administered a given dose of a challenge drug, make ≥ 80% of their responses on the diazepam-appropriate lever. Where stimulus generalization occurs, an effective dose 50% (ED50) value can be calculated, which reflects the dose at which the animals would be expected to make 50% of their responses on the diazepam-appropriate lever [47]. In addition to complete stimulus generalization, two other results might be encountered: partial generalization and vehicle-appropriate responding. Partial generalization is said to have occurred when the animals, after being administered a thorough dose-effect test, make approximately 40%–70% of their responses on the diazepam-appropriate lever. In this case, percent diazepam-appropriate lever responding is not fully appropriate for either training condition. Data of this type are very difficult to interpret. However, it has been posited that partial generalization may occur with a test compound because there are pharmacological effects that are common to both the training drug and the challenge drug. Complete stimulus generalization does not occur, however, because the overlap of effects is incomplete. For example, one explanation for a partial generalization result may be that low doses of a test compound are similar to low doses of the training drug. However, as the dose of challenge drug is increased, another kind of pharmacological effect emerges. A third type of test result is that the administration of various doses of a challenge drug may result in ≤ 20% diazepam-appropriate responding. Such a result does not necessarily mean that a challenge drug is inert (i.e., without a pharmacological effect), but does suggest that the effect of the challenge drug is different from that produced by the training drug. Finally, an important factor in the interpretation of any DD data is that results must be considered in the context of the training drug. That is, the DD paradigm is used to generate data that are only valid with respect to a particular dose of a given training drug. The sensitivity and duration of effect of a stimulus are related to the training drug and are time dependent. As such, dose response relationships represent relative, not absolute, relationships between challenge drugs and training drugs. Finally, it should be recognized that when challenge drugs are being examined, the data that are obtained relate to training-drug–like effects. For example, an investigation of the effect of a barbiturate in diazepam-trained animals does not provide data on barbiturate activity; rather, the data reflect the diazepam-like effect of the barbiturate (see example below). Thus, the result may or may not be the same as the effect of diazepam or a series of barbiturates in, for example, pentobarbital-trained animals.
3.4.3.1. Statistical Analysis
The use of statistical analysis (e.g., analysis of variance, Fischer’s exact probability, t-tests, etc.) with DD data can be very problematic. One major concern is the failure of statistical procedures to account for behavioral disruption (i.e., no responding) into the analysis. In particular, an animal that fails to press a lever after being administered a dose of test agent in a stimulus generalization test cannot be assigned a percent score; 0% drug-appropriate responding cannot be assigned because it has a different meaning (i.e., the animal pressed the saline-appropriate lever). Some investigators argue that statistical analysis is robust enough to account for such missing data. However, the percent drug-appropriate lever responding data are not missing because the animals failed to appear for a test appointment. The data are lacking and unavailable because the drug interfered with the ability of the animal to respond. A more palpable description of such data is that the effect should be characterized as “disruption.” Statistically, one could (and some investigators do) ignore the disruptive effect of a dose of drug, use only the data from very few animals (sometimes n = 1 or 2 out of 6 or 8 subjects that were tested) that respond (usually those few subjects have responded to a high degree on the drug-designated lever), and statistically conclude the occurrence of stimulus generalization. However, in such cases, it would seem more appropriate and meaningful to characterize the effect as disruption rather than to promote a statistical conclusion that may be misleading. In any case, the most prudent approach to the presentation of stimulus generalization data in DD studies is to account fully, by description of the effects on subjects and/or statistically for the behavioral effect of the test agent in all subjects, individually and/or as a group.
3.4.3.2. Examples of Complete, Partial, and No Substitution
In rats trained to discriminate the benzodiazepine diazepam at 3 mg/kg from vehicle at 1 mL/kg, the administration of lower doses of diazepam (i.e., construction of a dose-response function for diazepam) led to progressively less responding on the diazepam-appropriate lever (Figure 3.2); furthermore, an ED50 value (ED50 = 1.2 mg/kg) was calculated. Moreover, several metabolites of diazepam were evaluated in tests of stimulus generalization. Figure 3.2 shows that the diazepam stimulus generalized in a dose-related manner to oxazepam (ED50 = 1.4 mg/kg), temazepam (ED50 = 1.4 mg/kg), and desmethyldiazepam (ED50 = 2.3 mg/kg). The latter results indicate that, in comparison with diazepam, the metabolites are relatively potent behaviorally, and indicate the distinct possibility that the metabolites may contribute to the stimulus effect of diazepam. Figure 3.2 also reveals that the diazepam stimulus generalized to the barbiturate anxiolytic/sedative pentobarbital (ED50 = 4.5 mg/kg), which illustrates the idea that animals trained to discriminate a dose of a training drug can display stimulus generalization to an agent that exerts a similar behavioral effect, although not necessarily through an identical mechanism of action; diazepam and pentobarbital do not share the same mechanisms of action (see antagonism tests below). In comparison, the administration of buspirone (0.3–3.0 mg/kg), a serotonin 5-HT1A receptor (partial) agonist anxiolytic agent that is structurally unrelated to diazepam, produced only partial diazepam-appropriate lever responding (i.e., maximal 43% diazepam-appropriate lever responding), while the administration of doses between 4 mg/kg and 10 mg/kg produced disruption of behavior (Figure 3.2; disruption data not shown). Thus, the diazepam stimulus may partially generalize to buspirone because there may be some degree of pharmacological effects that is common to both diazepam and buspirone at low doses. Complete stimulus generalization does not occur, however, because the overlap of effects is incomplete. Lastly, the administration of S(+)-amphetamine (0.1–1.5 mg/kg), a CNS stimulant, to the diazepam-trained animals produced vehicle-appropriate responding (i.e., maximal 18% diazepam-appropriate lever responding; data not shown in Figure 3.2), while the administration of doses of 2–3 mg/kg produced disruption of behavior (i.e., no responding; data not depicted in Figure 3.2). Since percent diazepam-appropriate lever responding is fairly low (i.e., S(+)-amphetamine–induced responding on the vehicle-designated lever), it can be stated that the stimulus effect produced by 3.0 mg/kg of diazepam is quite different from that produced by S(+)-amphetamine. However, the fact that S(+)-amphetamine, and for that matter buspirone, can serve as training drugs indicates that these are not inert substances. Thus, animals trained in a DD task respond on the drug-designated lever only when administered a test agent that produces some degree of effect that is similar to the training dose of the training drug. If the test agent produces an effect that is “inert” or unlike that of the training drug, then responding will occur on the vehicle-designated lever until doses of the test agent are administered that disrupt lever response behavior by the animal (i.e., little or no responding). In a final comment, it is noted that results from stimulus generalization studies of test drugs under particular training agents have been consistent across different species trained under different schedules of reinforcement.

FIGURE 3.2
Results of stimulus generalization tests with diazepam (closed squares), desmethyldiazepam (closed triangle), temazepam (closed inverted triangle), oxazepam (closed diamond), pentobarbital (closed circle), and buspirone (open square) in rats trained to (more...)
3.4.3.3. Time Course
Once a dose of a drug has been established as a discriminative stimulus, tests can be performed to determine its time course of actions. Such tests investigate the effects of changing the PSII of the training dose of drug and the beginning of a test session. In particular, the time course of any drug stimulus can be characterized by its latency of onset of action, peak activity, and duration of effect. The latency of onset of action refers to the time (or interval) between the administration of the training drug and the first indications of a marked effects on drug-appropriate responding. The peak activity of the drug refers to the time (or interval) that the drug exerts maximal percent drug-appropriate responding (i.e., ~80%–100% drug-appropriate responding). Lastly, the duration of action refers to the interval of time between onset of action and the point of time (or interval) that the drug no longer exerts percent drug-appropriate responding that is notable (i.e., ~20% drug-appropriate responding). In the present example, 3.0 mg/kg of diazepam was established as a discriminative stimulus with a 15 min PSII; PSII intervals of 5, 10, 30, 45, 90, 120, 180, and 240 min also were examined (Figure 3.3). The results indicated that the onset of effect of the diazepam stimulus occurred between the PSIIs of 5 min and 15 min; peak activity was exhibited from PSIIs of 10 min to approximately 90 min; and duration of action occurred between PSIIs of 10 min and ~180 min. In addition, Figure 3.3 illustrates the time course effects of the major metabolites of diazepam. These studies were conducted with the dose of each metabolite that produced stimulus generalization in the 3 mg/kg diazepam-trained animals: desmethyldiazepam (6 mg/kg), oxazepam (3 mg/kg), and temazepam (3 mg/kg). An important difference among benzodiazepines is their pharmacokinetic properties. For example, in humans, diazepam is considered to have a relatively rapid onset of action and a relatively long half-life. In comparison, oxazepam and temazepam are considered to have slower onsets of action and much shorter half-lives, relative to diazepam. The time course studies of the stimulus effects of these agents in the diazepam-trained animals are not inconsistent with the human data. In any case, it is clear that familiarity with the time-course of action of the training drug or challenge compounds in tests of stimulus generalization and/or stimulus antagonism is of great importance; drug responses should not be measured too long, or short, after drug administration.

FIGURE 3.3
Results of time course studies (i.e., stimulus generalization tests with various pre-session injection intervals) with diazepam (3 mg/kg; closed squares), desmethyldiazepam (6 mg/kg; open squares), temazepam (3 mg/kg; open triangle), and oxazepam (3 mg/kg; (more...)
3.4.3.4. Stimulus Antagonism
An effective strategy to determine the mechanisms of action of psychoactive agents is to study drugs that block their effects. In DD studies, the rationale of such an approach is that the training dose of the training agent will only be blocked by receptor antagonists that interfere with the mechanism of action of the drug. The results of antagonism tests, as with generalization tests, typically fall into one of three categories: (1) complete antagonism (i.e., saline-appropriate responding); (2) partial antagonism (i.e., ~ 40%–70% drug-appropriate responding); and (3) no antagonism (i.e., ≥ 80% drug-appropriate responding). Three strategies to study stimulus antagonism can be employed. One approach can determine whether the stimulus effect of the training dose of the training drug can be attenuated when various doses of an appropriate receptor antagonist are combined with the training dose of the training drug. Thus, the rats trained to 3.0 mg/kg of diazepam were administered various doses of the benzodiazepine receptor antagonist flumazenil prior to the administration of their training dose of training drug. If a drug is an effective antagonist, then a dose-related antagonism of the animals’ percent drug-appropriate responding should occur. Figure 3.4 shows that the administration of various doses of flumazenil (3–12 mg/kg) prior to the injection of the 3 mg/kg training dose of diazepam was sufficient to produce antagonism (i.e., responding ultimately occurred on the vehicle-designated lever). In contrast, the administration of various doses of flumazenil prior to the injection of the dose of pentobarbital (10 mg/kg) that produced complete stimulus generalization in these animals (see above) failed to produce antagonism (i.e., responding occurred on the diazepam-designated lever). Lastly, when the animals were administered doses of flumazenil (3–40 mg/kg) in control tests, they failed to respond on the diazepam-designated lever. Taken together, these data support the idea that diazepam and pentobarbital can induce a similar stimulus effect but the mechanism of action can be differentiated by flumazenil, a benzodiazepine receptor antagonist.

FIGURE 3.4
The effect of flumazenil administered alone (open squares), in combination with 3 mg/kg of diazepam (DZP; open triangles), or in combination with 10 mg/kg of pentobarbital (PB; open circles). The administration of various doses of flumazenil prior to (more...)
In a second approach, the dose response of the training drug is determined in both the presence and absence of a constant dose of the antagonist. If the antagonism is competitive, then the dose response of the training drug (in the presence of the constant dose of the antagonist) should shift in a rightward and parallel manner. Figure 3.5 (top figure) shows the dose-response effect of diazepam in the absence (i.e., left dose-response function) and the presence (right dose-response effect) of flumazenil (5 mg/kg). As can be seen, pretreatment of the animals with flumazenil induced a rightward shift of the dose-response function of diazepam. In a third approach, various doses of the training drug can be combined with various doses of the receptor antagonist. This approach will generate a series of training-drug/antagonist dose-response curves and probably provide the most comprehensive or detailed picture of the interaction between the agents. Figure 3.5 (bottom figure) shows the dose-response effect of diazepam in the absence (i.e., left dose-response function) and the presence (middle and far right dose-response effects) of flumazenil (5 mg/kg and 12 mg/kg, respectively). Clearly, the dose-response functions of the discriminative stimulus effect of diazepam were shifted rightward and these data strongly indicate the presence of competitive antagonism.
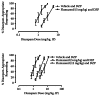
FIGURE 3.5
The effect of various doses of diazepam alone (DZP; closed squares) or in combination with 5 mg/kg of flumazenil (open squares) in rats trained to discriminate 3 mg/kg of diazepam from vehicle (top figure). The bottom figure depicts the effects of various (more...)
3.5. SUMMARY
The DD assay is a behavioral procedure whereby an organism must recognize a particular drug state, choose a correct response, and receive reinforcement. Most often, subjects are presented with the choice of two levers: one response (i.e., press of a left- or right-side lever) should be emitted in the presence of the training dose of a drug and a similar response (i.e., press of the alternate lever) should be emitted in the absence of the training drug. All other environmental conditions are held constant. Overall, DD studies have involved different species (including humans), learning paradigms (typically two-lever operant choice tasks that employ FR or VI schedules of reinforcement), and either solid or liquid reinforcement. Most often, studies have used rats that are trained to press levers on FR schedules of reinforcement for food pellets. Many agents from different psychoactive drug or chemical classes have been shown to serve as discriminative stimuli. Once trained, subjects can be “asked” whether they recognize a novel agent as producing a stimulus effect similar to that produced by the training dose of the training drug. Several factors can influence the results of such studies. For example, an important factor to be considered is the choice of doses to be examined for a particular challenge compound under investigation; the need for thorough dose-response investigations cannot be overemphasized because stimulus generalization can occur within a narrow window of doses. Moreover, studies have shown that drugs that generalize (substitute, transfer) to one another in tests of stimulus generalization in animals often produce similar effects in humans. Lastly, antagonism studies have evaluated the effects of purported receptor antagonists in combination with the training dose (or other doses) of the training drug. Such studies can elucidate a neurochemical mechanism involved in the discriminative stimulus properties of a drug. Taken together, the DD paradigm can be characterized as a highly sensitive and relatively specific “drug detection” assay that provides qualitative, quantitative, and mechanistic results of psychoactive agents.
REFERENCES
- 1.
- Beecher HK. Measurement of subjective responses: quantitative effects of drugs. New York: Oxford University Press; 1959. pp. 193–424.
- 2.
- Kilbey MM, Ellinwood EH. Discriminative stimulus properties of psychomotor stimulants in the cat. Psychopharmacology. 1979;63:151–153. [PubMed: 38476]
- 3.
- Cook L, Davidson A, Davies DJ, Kelleher RG. Epinephrine, nor-epinephrine, and acetylcholine as conditioned stimuli for avoidance behavior. Science. 1960;131:990–991. [PubMed: 13811708]
- 4.
- Hudzik TJ, Yanek M, Porrey T, et al. Behavioral pharmacology of AR-A000002, a novel, selective 5-hydroxytryptamine1B antagonist. J Pharmacol Exp Ther. 2003;304:1072–1084. [PubMed: 12604684]
- 5.
- Jarbe TUC, Johansson JO, Henriksson BG. Delta-9-tetrahydrocannabinol and pentobarbital as discriminative cues in the mongolian gerbil (Meriones unguiculatus). Pharmacol Biochem Behav. 1975;3:403–410. [PubMed: 1153442]
- 6.
- Altman JL, Albert J, Milstein SL, Greenberg I. Drugs as discriminative events in humans. Psychopharmacol Commun. 1976;2:327–330. [PubMed: 792962]
- 7.
- Snoddy AM, Tessel RE. Nisoxetine and amphetamine share discriminative stimulus properties in mice. Pharmacol Biochem Behav. 1983;19:205–210. [PubMed: 6605539]
- 8.
- Schuster CR, Brady JV. The discriminative control of a food-reinforced operant by interoceptive stimulation. In: Thompson T, Pickens R, editors. Stimulus properties of drugs. Vol. 1. New York: Appleton-Century Crofts; 1971. pp. 133–148.
- 9.
- Carey MP, Fry JP. A behavioral and pharmacological evaluation of the discriminative stimulus induced by pentylenetetrazole in the pig. Psychopharmacology. 1991;111:244–250. [PubMed: 7870959]
- 10.
- Henriksson BG, Johansson JO, Jarbe TUC. Delta-9-tetrahydrocannabinol produced discrimination in pigeons. Pharmacol Biochem Behav. 1975;3:771–774. [PubMed: 1208619]
- 11.
- Barry H. III, 1968. Prolonged measurements of discrimination between alcohol and nondrug states. J Comp Psychol Psychol. 65:349–352. [PubMed: 5668323]
- 12.
- Chait LD, Uhlenhuth EH, Johanson CE. An experimental paradigm for studying the discriminative stimulus properties of drugs in humans. Psychopharmacology. 1984;82:272–274. [PubMed: 6425913]
- 13.
- Kamien JB, Bickel WK, Hughes JR, Higgins ST, Smith BJ. Drug discrimination by humans compared to nonhumans: Current status and future directions. Psychopharmacology. 1993;111:259–270. [PubMed: 7870962]
- 14.
- Schechter MD, Rosecrans JA. D-amphetamine as a discriminative cue: Drugs with similar stimulus properties. Eur J Pharmacol. 1973;21:212–216. [PubMed: 4696103]
- 15.
- Colpaert FC, Niemegeers CJE, Kuyps JJMD, Janssen PAJ. Apomorphine as a discriminative stimulus, and its antagonism by haloperidol. Eur J Pharmacol. 1975;32:383–386. [PubMed: 1149818]
- 16.
- Barry H III, Kubena RK. Discriminative stimulus characteristics of alcohol, marihuana and atropine. In: Singh JM, Miller L, Lal H, editors. Drug addiction 1: Experimental pharmacology. New York: Futura; 1972. pp. 3–16.
- 17.
- Holtzman SG. Discriminative stimulus effects of buprenorphine in the rat. Psychopharmacology. 1997;130:292–299. [PubMed: 9151365]
- 18.
- Hendry JS, Balster RL, Rosecrans JA. Discriminative stimulus properties of buspirone compared to central nervous system depressants in rats. Pharmacol Biochem Behav. 1983;19:97–101. [PubMed: 6137837]
- 19.
- Carney JM, Christensen HD. Discriminative stimulus properties of caffeine: Studies using pure and natural products. Pharmacol. Biochem. Behav. 1980;13:313.
- 20.
- De Witte PH, Swanet E, Gewiss M, Goldman S, Roques B, Vanderhaeghen J. Psychopharmacological profile of cholecystokinin using the self- stimulation and drug discrimination paradigms. Ann NY Acad Sci. 1985;448:470–487. [PubMed: 3861129]
- 21.
- Goas JA, Boston JE. Discriminative stimulus properties of clozapine and chlorpromazine. Pharmacol Biochem Behav. 1978;8:235–241. [PubMed: 652832]
- 22.
- Browne RG, Koe BK. Clozapine and agents with similar behavioral and biochemical properties. In: Colpaert FC, Slangen JL, editors. Drug discrimination: Applications in CNS pharmacology. Amsterdam: Elsevier; 1982. pp. 241–254.
- 23.
- Jarbe TUC. Cocaine as a discriminative cue in rats: Interactions with neuroleptics and other drugs. Psychopharmacology. 1978;59:183–187. [PubMed: 31651]
- 24.
- Shearman GT, Miksic S, Lal H. Discriminative stimulus properties of desipramine. Neuropharmacology. 1978;17:1045–1048. [PubMed: 745690]
- 25.
- Holtzman SG. Discriminative stimulus effects of dextromethorphan in the rat. Psychopharmacology. 1994;116:249–254. [PubMed: 7892413]
- 26.
- Young R, Glennon RA, Brasse DA, Dewey WL. Potencies of diazepam metabolites in rats trained to discriminate diazepam. Life Sci. 1986;39:17–20. [PubMed: 3088348]
- 27.
- Winter JC. Sedation and the stimulus properties of antihistamines. Pharmacol Biochem Behav. 1985;22:15–17. [PubMed: 2858107]
- 28.
- Young R, Glennon RA, Rosecrans JA. Discriminative stimulus properties of the hallucinogenic agent DOM. Commun Psychopharmacol. 1980;4:501–506. [PubMed: 7297054]
- 29.
- Young R, Glennon RA. Discriminative stimulus properties of (-)ephedrine. Pharmacol Biochem Behav. 1998;60:771–775. [PubMed: 9678664]
- 30.
- Schechter MD. Effect of propranolol, d-amphetamine and caffeine on ethanol as a discriminative cue. Eur J Pharmacol. 1974;29:52–57. [PubMed: 4435044]
- 31.
- Goudie AJ. Discriminative stimulus properties of fenfluramine in an operant task: An analysis of its cue function. Psychopharmacology. 1977;53:97–102. [PubMed: 407619]
- 32.
- Colpaert FC, Niemegeers CJE. On the narcotic cuing action of fentanyl and other narcotic analgesic drugs. Arch Int Pharmacodyn Ther. 1975;217:170–172. [PubMed: 242284]
- 33.
- Hirschhorn ID, Winter JC. Mescaline and lysergic acid diethylamide (LSD) as discriminative stimuli. Psychopharmacologia. 1971;22:64–71. [PubMed: 5119576]
- 34.
- Glennon RA, Young R. MDA: A psychoactive agent with dual stimulus effects. Life Sci. 1984;34:379–383. [PubMed: 6694526]
- 35.
- Glennon RA, Misenheimer BR. Stimulus effects of N-monoethyl-1-(3,4-methylendioxyphenyl)-2-aminopropane (MDE) and N-hydroxy-1-(3,4-methylene-dioxyphenyl)-2-aminopropane (N-OH MDA) in rats trained to discriminate MDMA from saline. Pharmacol Biochem Behav. 1989;33:909–912. [PubMed: 2575759]
- 36.
- Hirschhorn ID, Rosecrans JA. A comparison of the stimulus effects of morphine and lysergic acid diethylamide (LSD). Pharmacol Biochem Behav. 1974;2:361–366. [PubMed: 4837907]
- 37.
- Carter RB, Leander JD. Discriminative stimulus properties of naloxone. Psychopharmacology. 1982;77:305–308. [PubMed: 6291084]
- 38.
- Schechter MD, Rosecrans JA. Nicotine as a discriminative cue in rats: Inability of related drugs to produce a nicotine-like cueing effect. Psychopharmacologia. 1972;27:379–387. [PubMed: 4648621]
- 39.
- Willetts J, Balster RL. Effects of competitive and non competitive N-methyl-D-aspartate (NMDA) antagonists in rats trained to discriminate NMDA from saline. J Pharmacol Exp Ther. 1989;251:627–633. [PubMed: 2553930]
- 40.
- Kuhn DM, Greenberg I, Appel JB. Stimulus properties of the narcotic antagonist pentazocine similarity to morphine and antagonism by naloxone. J Pharmacol Exp Ther. 1976;196:121–127. [PubMed: 1246006]
- 41.
- Herling S, Valentino RJ, Winger GD. Discriminative stimulus effects of pentobarbital in pigeons. Psychopharmacology. 1980;71:21–28. [PubMed: 6779321]
- 42.
- Brady KT, Balster RL. Discriminative stimulus properties of phencyclidine and five analogues in the squirrel monkey. Pharmacol Biochem Behav. 1981;14:213–218. [PubMed: 7208560]
- 43.
- Vanover KE. Discriminative stimulus effects of the endogenous neuroactive steroid pregnanolone. Eur J Pharmacol. 1997;327:97–101. [PubMed: 9200546]
- 44.
- Jarbe TUC, Henriksson BG, Ohlin GC. Delta-9-THC as a discriminative cue in pigeons: Effects of delta-8-THC, CBD and CBN. Arch Int Pharmacodyn Ther. 1977;228:68–72. [PubMed: 921403]
- 45.
- Rees DC, Knisely JS, Jordan S, Balster RL. Discriminative stimulus properties of toluene in the mouse. Toxicol Appl Pharmacol. 1987;88:97–104. [PubMed: 3564034]
- 46.
- Extance K, Goudie AJ. Inter-animal olfactory cues in operant drug discrimination procedures in rats. Psychopharmacology. 1981;73:363–371. [PubMed: 6789359]
- 47.
- Finney D. Probit analysis. London: Cambridge University Press; 1952. pp. 183–197.
- PubMedLinks to PubMed
- Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats.[Nat Protoc. 2006]Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats.Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Nat Protoc. 2006; 1(3):1194-206.
- In a low-versus high-dose drug discrimination task, random reinforcement in one drug state alters discriminative control only in that state.[Behav Pharmacol. 1993]In a low-versus high-dose drug discrimination task, random reinforcement in one drug state alters discriminative control only in that state.Rijnders HJ, Järbe TU, Slangen JL. Behav Pharmacol. 1993 Feb; 4(1):37-45.
- Discriminative stimulus effects of zolpidem in pentobarbital-trained subjects: II. Comparison with triazolam and caffeine in humans.[J Pharmacol Exp Ther. 1997]Discriminative stimulus effects of zolpidem in pentobarbital-trained subjects: II. Comparison with triazolam and caffeine in humans.Rush CR, Madakasira S, Goldman NH, Woolverton WL, Rowlett JK. J Pharmacol Exp Ther. 1997 Jan; 280(1):174-88.
- Review Drug Discrimination: Historical Origins, Important Concepts, and Principles.[Curr Top Behav Neurosci. 2018]Review Drug Discrimination: Historical Origins, Important Concepts, and Principles.Porter JH, Prus AJ, Overton DA. Curr Top Behav Neurosci. 2018; 39:3-26.
- Review Drug discrimination: A versatile tool for characterization of CNS safety pharmacology and potential for drug abuse.[J Pharmacol Toxicol Methods. 2...]Review Drug discrimination: A versatile tool for characterization of CNS safety pharmacology and potential for drug abuse.Swedberg MD. J Pharmacol Toxicol Methods. 2016 Sep-Oct; 81:295-305. Epub 2016 May 25.
- Drug Discrimination - Methods of Behavior Analysis in NeuroscienceDrug Discrimination - Methods of Behavior Analysis in Neuroscience
Your browsing activity is empty.
Activity recording is turned off.
See more...
