This book is copyright. Apart from any fair dealing for the purposes of private study, research, criticism or review as permitted under the Copyright Act, no part may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission. Address all inquiries to the Director at the above address.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011.
Abstract
The old saying “you are what you eat” is becoming increasingly important in the field of neuroscience these days. There is mounting evidence that nutritional factors are beginning to play a major role in cognitive status, or cognitive wellbeing. One of these emerging factors is magnesium (Mg2+). Although the physiological investigation of Mg2+ has a long history, its role in cognitive function is just starting to emerge. The focus of this chapter is to review the available literature on the effects of Mg2+ on cognitive function in the healthy and diseased/injured brain. In addition, data from our laboratory will be presented that has investigated the effects of Mg2+ manipulation on learning and memory tasks in rodents, as well as the ability of Mg2+ therapy to improve cognitive performance in the damaged brain.
Introduction
The purpose of this chapter is to provide a review of the literature on the role of Mg2+ in cognitive function. Although the research on this topic is generally sparse, there is an accumulating body of evidence suggesting that Mg2+ is vitally important. The role that micronutrients play in maintaining and promoting cognitive ability and neural plasticity has started to receive a good deal of attention. A recent paper has provided an excellent review on how various nutrients promote cognitive performance and neural plasticity (Gómez-Pinilla, 2008). Although this review does not specifically discuss the role of Mg2+, it is clear that this is a rapidly evolving area of research. Another review does address the role of Mg2+ and its relationship with other micronutrients on cognitive function and performance. It especially highlights the important role that Mg2+ has in interacting with other micronutrients (Huskisson et al., 2007). Recent evidence also suggests that Mg2+ plays an important role in age-related deficits in neurotransmitter release, neuronal excitability and synaptic plasticity related to cognition (Billard, 2006), and these topics will be reviewed in Chapter 6.
Additionally, there is ample evidence that Mg2+ plays an important role in the pathophysiological processes following traumatic brain injury (TBI) and that Mg2+ therapy is effective in promoting functional recovery in a variety of animal models (Hoane and Barth, 2001; Hoane, 2004; Sen and Gulati, 2010; van den Heuvel and Vink, 2004; Vink et al., 2009). Specifically, this chapter will focus on reviewing the literature on Mg2+ and cognitive function and review a series of studies conducted in our laboratory that have investigated the ability of Mg2+ therapy to alter cognitive function and to improve cognitive recovery following focal and traumatic brain injuries in the rat.
Introduction to rodent cognitive assessment
The Morris water maze (MWM) is a standard task for measuring cognitive/spatial performance in rodents. This task uses a water-filled tank with a submerged escape platform and many different aspects of memory can be assessed (Hoane et al., 2003; Hoane, 2005; Hoane et al., 2009; Kaufman et al., 2010; Lindner, 1997; Lindner et al., 1998; Quigley et al., 2009). Briefly, a reference memory trial consists of placing the rat into the water at one of 4 randomly chosen start locations. A computer-assisted video tracking system is used to measure the swim latency and distance to the submerged escape platform. On trials designed to measure working memory the escape platform is relocated to a new position in the tank every day. The first trial (in the new location) is considered an information trial and the subsequent 3 trials are test trials and are averaged to form the dependent variable. Additional paradigms can be used that incorporate various mazes or dividers that are placed into the tank that can test other aspects of learning and memory in the rodent.
Additionally, the Barnes spatial maze, a dry version of the MWM, can also be used to assess cognitive/spatial performance (Vink et al., 2003). Rats are placed on an elevated 1.2 m circular tabletop with 19 holes cut around the periphery. The rat learns the location of the hole that leads to an escape tunnel. The latency to find the correct hole is then analysed. Operant chambers (Skinner boxes) can be used to assess appetitive conditioning of learning ratios under various conditions. In general, rats are shaped to bar- press for a reinforcer and then are shifted to more complicated reward paradigms. Thus, there are many ways in which rodent cognitive assessments can be made and many of these have been utilized to examine the role of Mg2+ in cognition.
Role of Mg2+ in health and cognition
The importance of Mg2+ in normal cellular functioning has been well documented, as has its importance in the pathophysiology following injury. Previously, several reviews have been written that address these issues (Hoane and Barth, 2001; Hoane, 2004; van den Heuvel and Vink, 2004) so only a brief synopsis will be provided in this paper. Mg2+ is involved in many critical cellular processes including cellular respiration, protein synthesis, membrane stability and regulation of vascular tone. A more detailed discussion on the physiology of Mg2+ is presented in several chapters within Section 1 of this book.
Although the main focus of this chapter is on animal models, there are some interesting human studies that have examined the role of Mg2+ in cognitive ability. A recent study examined the correlation between levels of several trace minerals (iron, Mg2+, potassium and zinc) in the hair of adolescent girls and their academic record. Although care must be taken with the interpretation of correlational studies of this nature, there was evidence that some trace minerals correlated more highly with increased academic performance. Specifically, it was found that Mg2+ and zinc demonstrated a strong positive correlation with academic performance (Wang et al., 2008). Furthermore, a recent case report of a patient presenting with anorexia nervosa and Wernicke-Korsakoff syndrome was found to have a low serum Mg2+ level (Saad et al., 2010). Although the main cause of this condition was thiamine deficiency, a major adjunct factor was believed to be Mg2+ deficiency. The results from this case study are supported by a review article on micronutrients and cognitive performance that details the inter- relationships between Mg2+ and other micronutrients such as the B-group vitamins and how deficiencies in these nutrients interact to produce cognitive deficits (Huskisson et al., 2007). Thus, there is an expanding literature that suggests that Mg2+ status plays an important role in cognitive performance.
A unique condition in which Mg2+ has been implicated is TBI. McIntosh and colleagues have shown that Mg2+ homeostasis is disrupted following CNS injury (McIntosh et al., 1988; Vink et al., 1988). Fluid percussion injury (FPI) produced a rapid and severe decline in intra- and extracellular Mg2+ levels, which correlated significantly with the severity of the behavioural deficits observed following injury (McIntosh et al., 1988; Vink et al., 1988). Additional research on the role of Mg2+ and neurological diseases will be presented in Section 2 of this book, while Mg2+ in cognitive function following TBI is addressed later in this chapter. These findings suggest that Mg2+ plays an important role in normal physiology and in the pathophysiological events that occur following injury to the nervous system.
Recent laboratory data
Mg2+ therapy and learning
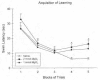
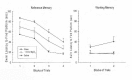
From a purely pharmacological standpoint, administration of Mg2+ immediately prior to the acquisition of a learning task should have a detrimental effect on that task given the non- competitive antagonistic properties of Mg2+ at the NMDA receptor. It has been shown that administration of other NMDA antagonists such as MK-801 and PCP have been shown to disrupt spatial learning in rodents (Kesner et al., 1993; McLamb et al., 1990; Murray and Ridley, 1997); however, in some cases a facilitative effect can be shown (Pussinen and Sirvio, 1999). In order to examine the biological activity of Mg2+ administration on the acquisition of learning, a MgCl2 solution was administered (1 mmol/kg or 2 mmol/kg, i.p.) prior to the acquisition of learning a reference memory task in the MWM (Hoane, 2007). Intact rats received daily injections of MgCl2, 30 min prior to the start of their MWM session and were tested for 5 consecutive days for 4 trials per day with an intertrial interval (ITI) of 15 min. The reference memory acquisition paradigm was used in the MWM. In this paradigm, the submerged escape platform stayed in the same location for every trial and the animals were released from 1 of 4 different starting points in the maze. As can be seen in Figure 1, the vehicle-treated (0.9% saline, i.p.) group showed steady acquisition of the task. In comparison, the MgCl2-treated animals showed a more varied response. The initial acquisition of the 2 mmol group was slightly lengthened on day 1 compared to the other groups. On day 4, both groups of MgCl2-treated animals started to show a lengthening of their escape latencies compared to vehicle-treated animals. On the last 2 test days the comparison of swim latencies between the 2 mmol and saline group was significantly different (p < 0.01) indicating a possible learning impairment on the task.
Thus, it appears that daily dosing with the higher dose of MgCl2 produced a significant degree of amnesia after 3 days. Unfortunately, testing was terminated after 5 days so it is unknown if this effect would have persisted or worsened with additional testing. It should be noted that, in general, the NMDA antagonists that work at the PCP site on the NMDA receptor (i.e., MK-801 and PCP) seem to have a greater amnesic effect than MgCl2. This may raise some concerns for continued dosing regimens of Mg2+ therapy lasting more than a couple days. However, a longer window of time between administrations and testing may reveal different findings, because this effect is perceived to be state dependent. Thus, given these behavioural results it is clear that systemic injections of MgCl2 did indeed exert behavioural effects in uninjured animals with an intact blood-brain barrier (BBB), and therefore give support to the ability of MgCl2 to cross the BBB. This finding supports earlier research to this fact (Hallack et al., 1992).
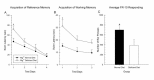
We have also recently investigated the effect of dietary Mg2+ deficiency on learning acquisition in the MWM. Rats were placed on either a standard laboratory diet or a commercially available Mg2+- deficient diet for 14 days. Peripheral blood collect- ions were performed for determination of serum Mg2+ levels and all animals were placed back on to the standard diet. The serum analysis indicated a significant loss in serum Mg2+ in the deficiency group (p < 0.05). One week later, animals were tested for the acquisition of a reference memory task over 4 days (4 trials per day, 15 min ITI). As can be seen in Figure 2A, the 14 days of Mg2+ deficiency significantly impaired the initial acquisition of the task on the first day (p<0.05). The animals were then switched to a more challenging working memory assessment in the MWM. As can be seen in Figure 2B, the animals that experienced Mg2+ deficiency showed impaired learning on the task (p<0.05). Additionally, animals were trained to acquire a fixed ration (FR-10) schedule of reinforcement in an operant chamber. The total number of bar presses is shown in Figure 2C. The higher number of responses results in a greater number of reinforcers being delivered and can be equated to the learning of the reinforcement schedule. Animals on the Mg2+-deficient diet responded at a significantly lower level (p<0.05) than those animals on the Mg2+-normal diet, suggesting a learning impairment. A somewhat similar finding has been shown in mice exposed to a Mg2+-deficient diet (Bardgett et al., 2005; Bardgett et al., 2007). In this study, 10 days of Mg2+ deficiency resulted in impaired learning of a conditioned fear task. However, maze performance in the MWM was not impaired. Thus, these data suggest that even temporary Mg2+ deficiency may interfere with learning.
A newly formulated form of Mg2+, magnesium-L- threonate (MgT) has been shown to increase cerebrospinal fluid levels of Mg2+ to a much greater degree than other Mg2+ preparations (e.g. MgCl2) (Slutsky et al., 2010). Administration of MgT has also been shown to result in an enhancement of various forms of cognitive/ learning ability in rodents, including working memory. This effect was also evident in aged rats following which cognitive abilities were generally reduced. The beneficial behavioural effects were thought to be caused by beneficial changes in synaptic plasticity within the hippocampus (Slutsky et al., 2010).
Effects of Mg2+ therapy on cognitive function following brain injury
Several studies have investigated the ability of Mg2+ to improve acute cognitive function in animal models of TBI. It has been shown that administration of MgCl2 following FPI improved cognitive outcome by reducing memory loss following injury (Smith et al., 1993). However, administration of MgCl2 failed to improve the acquisition of a reference memory task in the MWM following injury (Bareyre et al., 1999). It has also been recently shown that administration of MgSO4 (250 μmol/kg, i.v.), 30 min following diffuse axonal injury improved recovery of a spatial memory task on the Barnes maze (Vink et al., 2003). In addition, administration of an intravenous solution of MgCl2 (150 μmol) prior to FPI prevented the occurrence of injury-induced impairments on working and reference memory tasks in a radial maze (Enomoto et al., 2005).
There have been very few attempts to examine the ability of Mg2+ therapy to resolve long-term cognitive dysfunction. A recent study has shown severe cognitive deficits in the acquisition of a reference memory task in the MWM when tested 8 months post-FPI (Browne et al., 2004). The administration of MgSO4 (125 μmol, i.v.) or NPS 1506 (an NMDA antagonist) failed to improve the acquisition of the reference memory task compared to vehicle controls. However, it was found that MgSO4 did reduce the amount of ipsilateral hippocampal cell loss. Thus, preserv- ation of the hippocampus failed to result in significant cognitive improvement (Browne et al., 2004). Given the limited number of studies and their mixed results, it is important to further investigate the effect of Mg2+ therapy on the recovery of cognitive function following injury.
Examination of Mg2+ therapy following focal injury
In our laboratory we have examined recovery of cognitive function in a bilateral focal cortical ablation model. Rats were given small (4 mm2) electrolytic lesions aimed at the bilateral anterior medial cortex (bAMC) of the frontal lobe (Hoane et al., 2003). Administration of Mg2+ therapy occurred 15 min following injury with rats receiving either injections of MgCl2 (1 or 2 mmol/kg, i.p.) or saline (1 ml/kg). This regimen was repeated again 24 and 72 hrs later, so that each rat received 3 injections within the first 72 hrs following injury. Behavioural testing began 5 days after injury and included the assessment of cognitive function. The MWM was used to investigate the acquisition of reference and working memory. In addition, the MWM tank was also used to examine spatial ability using a delayed matching-to-sample (DMTS) task, a very sensitive measure of spatial working memory.
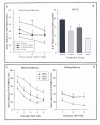
As can be seen in Figure 3A & 3B, the results of the behavioural testing indicated that bAMC lesions produced severe deficits in cognitive function on both the reference and working memory tasks in the MWM. Mg2+ therapy with either the 1 or 2 mmol dose did not significantly facilitate the acquisition of reference memory in the MWM. However, the mean swim latencies for both MgCl2 groups were greatly improved compared to saline in the last 2 blocks of trials. Although Mg2+ therapy did not demonstrate a statistically significant improvement on reference memory performance in the present study, it did improve working memory performance in the MWM. Administration of the 2 mmol dose of MgCl2 significantly reduced the working memory deficit compared to saline treatment on the first 2 days of testing. The 1 mmol dose of MgCl2 also reduced the working memory impairments on the first day of testing and significantly improved working memory performance on day 2 of testing. Severe cognitive impairments were also seen on the DMTS spatial memory test following injury. The 2 mmol dose of MgCl2 significantly reduced the number of trials needed to reach the criterion of 80% correct choices compared to saline- treated rats, while the 1 mmol dose of MgCl2 did not, although the number of trials was greatly reduced. Thus, Mg2+ therapy was effective in a task and dose-dependent manner in this study.
Mg2+ therapy in the traumatically injured brain
To examine the ability of Mg2+ therapy following TBI, groups of rats were prepared with a cortical contusion injury (CCI) or sham procedure and then assigned to either MgCl2 (1.0 mmol/kg, i.p.) or saline treatment conditions (Hoane, 2005). Mg2+ therapy was administered 15 min and 24 hr following injury. Rats were then examined for cognitive/spatial performance in the MWM, investigating the acquisition of reference and working memory. Administration of MgCl2 following CCI significantly reduced some of the behavioural impairments observed following injury (see Figure 3C & 3D). The acquisition of reference memory in the MWM was significantly improved compared to saline-treated rats. In contrast, MgCl2 did not improve working memory performance.
In a second study, the ability of Mg2+ therapy to improve cognitive/spatial performance following unilateral CCI was examined. Groups of rats were given unilateral CCIs or sham surgeries of the left sensorimotor motor/frontal cortex. One hr following injury, rats were administered 1 mmol/kg MgCl2 or saline. They were then tested for their ability to acquire a reference memory task in the MWM on 4 consecutive days (4 trials/day) starting 11 days after CCI. Their working memory performance was measured on days 16 and 17. It was found that the single 1 mmol/kg dose of MgCl2 effectively facilitated the acquisition of the reference memory task compared to treatment with saline (see Figure 4). In a similar manner, the working memory performance was greatly enhanced following CCI in the Mg2+-treated rats compared to the saline-treated rats. In fact, the working memory performance of the Mg2+- treated rats could not be distinguished from the sham controls on either day of working memory testing (see working memory graph in Figure 4). Mg2+ treatment appeared to have prevented the occurrence of the working memory deficit following unilateral frontal injury.
Discussion
The results of these studies have demonstrated a wide range of conditions in which Mg2+ therapy regulates cognitive function. It was first shown that daily injections of MgCl2 administered 30 mins prior to training on the task worsened acquisition of the reference memory task. It was also demonstrated that a two-week regimen of dietary Mg2+ deficiency impaired learning on 3 different cognitive tasks. This finding is especially interesting because the animals had been placed back onto a normal laboratory diet prior to the assessment phase of the study.
Mg2+ therapy in several models of cortical ablation and TBI have demonstrated positive effects on cognitive recovery. However, these effects occurred in a task and dose-dependent manner. Following focal ablation of the bAMC, MgCl2 improved working memory performance on several measures and slightly improved reference memory performance. The CCI studies performed in our laboratory have shown that MgCl2 administration following injury improved cognitive performance in a task-dependent manner. Previous studies that have examined the ability of Mg2+ therapy to improve cognitive performance following injury have shown mixed results. For example, administration of MgCl2 following FPI has been shown to improve cognitive outcome by reducing memory loss in the MWM (Smith et al., 1993), but administration of MgCl2 failed to improve the acquisition of a reference memory task in the MWM following injury (Bareyre et al., 1999). Thus, in a similar manner the current series of studies has shown similar mixed results. That is, significant effects were seen in some cases and non-significant effects were seen in others. However, in general we saw significant improvements in cognitive function by MgCl2 in each of our studies. The discrepant results mainly varied based on dose and task-dependent properties of the studies.
From a mechanistic standpoint, Mg2+ therapy has multiple routes by which it can disrupt the pathophysiological processes that occur following injury and enhance cognitive recovery. In addition to offsetting injury-induced Mg2+ depletion (McIntosh et al., 1988; Vink et al., 1988) and preventing excitotoxic neuronal death (Nowak et al., 1984) mediated by the NMDA receptor, Mg2+ has been shown to have several other effects. For instance, administration of MgSO4 has been shown to limit the generation of injury-induced edema following closed-head injury (Feldman et al., 1996) and it has been more recently shown that MgSO4 (30 mg/kg) reduced aquaporin-4 immunoreactivity, thus contributing to edema reduction following injury (Ghabriel et al., 2006). Administration of MgCl2 has also been shown to reduce the expression of p53 mRNA, a gene associated with the induction of cell death, following lateral FPI (Muir et al., 1999). In this study it was found that 750 μmol/kg of MgCl2 reduced the expression of p53 mRNA in the injured cortex compared to saline-treated controls (Muir et al., 1999). High concentrations of Mg2+ (3 mM) have been shown to inhibit lipid peroxidation (Regan et al., 1998). Regardless of the mechanisms of action, the data presented in this review has shown that MgCl2 has strong biological activity, appears to cross the BBB, and can improve cognitive performance following cortical ablation or TBI.
Conclusion
The studies presented in this book chapter have demonstrated a wide range of activities for Mg2+ therapy in relationship to cognitive function in the rodent. Daily injections of MgCl2 prior to the acquisition of a learning task blocked the acquisition of a reference memory task and dietary deficiency of Mg2+ impaired learning on a number of different tasks. Using the damaged brain as a model to examine the ability of Mg2+ therapy to improve cognitive performance also demonstrated significant advantages with the therapy. Thus, it does appear that Mg2+ status and therapy have significant effects on cognitive performance in the brain and that further research is warranted. In addition, an accumulating body of research suggests that Mg2+ also plays an important role in human cognitive performance. Given the reported rates of magnesium deficiency in humans it is likely that this could impair cognitive performance (Barbagallo and Dominguez, 2010; Elin, 2010). In addition, the most intriguing factor related to Mg2+ therapy for cognitive wellbeing may reside in the use of Mg2+ as a co-therapy with other vital nutrients, which may produce the strongest effects.
Acknowledgements
A special thanks is given to Alicia Swan for critical reading of an earlier draft of this chapter. Partial support provided by ARRA funds from NINDS grant NS045647-04
References
- Barbagallo M, Dominguez LJ. Magnesium and aging. Curr Pharm Des. 2010;16:832–9. [PubMed: 20388094]
- Bardgett ME, Schultheis PJ, McGill DL, Richmond RE, Wagge JR. Magnesium deficiency impairs fear conditioning in mice. Brain Res. 2005;1038:100–6. [PubMed: 15748878]
- Bardgett ME, Schultheis PJ, Muzny A, Riddle MD, Wagge JR. Magnesium deficiency reduces fear- induced conditional lick suppression in mice. Magnes Res. 2007;20:58–65. [PubMed: 17536490]
- Bareyre FM, Saatman KE, Helfaer MA, Sinson GP, Weisser JD, Brown AL, McIntosh TK. Alterations in ionized and total blood magnesium after experimental traumatic brain injury: Relationship to neurobehavioral outcome and neuroprotective efficacy of magnesium chloride. J Neurochem. 1999;73:271–80. [PubMed: 10386980]
- Billard JM. Ageing, hippocampal synaptic activity and magnesium. Magnes Res. 2006;19:199–215. [PubMed: 17172010]
- Browne KD, Leoni MJ, Iwata A, Chen XH, Smith DH. Acute treatment with MgSO4 attenuates long- term hippocampal tissue loss after brain trauma in the rat. J Neurosci Res. 2004;77:878–83. [PubMed: 15334605]
- Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010;23:194–8. [PubMed: 20736141]
- Enomoto T, Osugi T, Satoh H, McIntosh TK, Nabeshima T. Pre-Injury magnesium treatment prevents traumatic brain injury-induced hippocampal ERK activation, neuronal loss, and cognitive dysfunction in the radial-arm maze test. J Neurotrauma. 2005;22:783–92. [PubMed: 16004581]
- Feldman Z, Gurevitch B, Artru AA, Oppenheim A, Shohami E, Reichenthal E, Shapira Y. Effect of magnesium given 1 hour after head trauma on brain edema and neurological outcome. J Neurosurg. 1996;85:131–7. [PubMed: 8683262]
- Ghabriel MN, Thomas A, Vink R. Magnesium restores altered aquaporin-4 immunoreactivity following traumatic brain injury to a pre-injury state. Acta Neurochir Suppl. 2006;96:402–6. [PubMed: 16671494]
- Gómez-Pinilla F. Brain foods: The effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–78. [PMC free article: PMC2805706] [PubMed: 18568016]
- Hallack M, Berman RF, Ortemkauf SM, Evans MI, Cotton DB. Peripheral magnesium sulfate enters the brain and increases the threshold for hippocampal seizures in rats. Am J Obstet Gynecol. 1992;167:1605–10. [PubMed: 1471674]
- Hoane MR. Magnesium therapy and recovery of function in experimental models of brain injury and neurodegenerative disease. Clin Calcium. 2004;14:65–70. [PubMed: 15577099]
- Hoane MR. Treatment with magnesium improves reference memory but not working memory while reducing GFAP expression following traumatic brain injury. Restor Neurol Neurosci. 2005;23:67–77. [PubMed: 15990413]
- Hoane MR. Assessment of cognitive function following magnesium therapy in the traumatically injured brain. Magnes Res. 2007;20:229–36. [PubMed: 18271492]
- Hoane MR, Barth TM. The behavioral and anatomical effects of MgCl2 therapy in an electrolytic lesion model of cortical injury in the rat. Magnes Res. 2001;14:51–63. [PubMed: 11300622]
- Hoane MR, Kaufman NA, Vitek MP, McKenna SE. COG1410 improves cognitive performance and reduces cortical neuronal loss in the traumatically injured brain. J Neurotrauma. 2009;26:1–10. [PMC free article: PMC2749004] [PubMed: 19119914]
- Hoane MR, Knotts AA, Akstulewicz SL, Aquilano M, Means LW. The behavioral effects of magnesium therapy on recovery of function following bilateral anterior medial cortex lesions in the rat. Brain Res Bull. 2003;60:105–14. [PubMed: 12725898]
- Hoane MR, Wolyniak J, Akstulewicz SL. Administration of riboflavin improves behavioral outcome and reduces edema formation and GFAP expression following traumatic brain injury. J Neurotrauma. 2005;22:1112–22. [PubMed: 16238487]
- Huskisson E, Maggini S, Ruf M. The influence of micronutrients on cognitive function and performance. J Int Med Res. 2007;35:1–19. [PubMed: 17408051]
- Kaufman NA, Beare JE, Tan AA, Vitek MP, McKenna SE, Hoane MR. COG1410, an apolipoprotein E- based peptide, improves cognitive performance and reduces cortical loss following moderate fluid percussion injury in the rat. Behav Brain Res. 2010;214:395–401. [PMC free article: PMC2936242] [PubMed: 20600347]
- Kesner RP, Dakis M, Bolland BL. Phencyclidine disrupts long- but not short-term memory within a spatial learning task. Psychopharmacology. 1993;111:85–90. [PubMed: 7870938]
- Lindner MD. Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol Learn Mem. 1997;68:203–20. [PubMed: 9398584]
- Lindner MD, Plone MA, Cain CK, Frydel BR, Francis JM, Emerich DF, Sutton RL. Dissociable long-term cognitive deficits after frontal versus sensorimotor cortical contusions. J Neurotrauma. 1998;15:199–216. [PubMed: 9528920]
- McIntosh TK, Faden AI, Yamakami I, Vink R. Magnesium deficiency exacerbates and pretreatment improves outcome following traumatic brain injury in rats: 31P magnetic resonance spectroscopy and behavioral studies. J Neurotrauma. 1988;5:17–31. [PubMed: 3193462]
- McLamb RL, Williams LR, Nanry KP, Wilson WA, Tilson HA. MK-801 impedes the acquisition of a spatial memory task in rats. Pharmacol Biochem Behav. 1990;37:41–5. [PubMed: 2148213]
- Muir JK, Raghupathi R, Emery DL, Bareyre FM, McIntosh TK. Postinjury magnesium treatment attenuates traumatic brain injury-induced cortical induction of p53 mRNA in rats. Exp Neurol. 1999;159:584–93. [PubMed: 10506531]
- Murray TK, Ridley RM. The effect of dizocilpine (MK-801) on conditional discrimination learning in the rat. Behav Pharmacol. 1997;8:383–8. [PubMed: 9832977]
- Nowak L, Bregestovski P, Ascher P, Herbert A, Prochiantz A. Magnesium gates glutamate activated channels in mouse central neurones. Nature. 1984;307:462–5. [PubMed: 6320006]
- Pussinen R, Sirvio J. Effects of D-cycloserine, a positive modulator of N-methyl-D-aspartate receptors, and ST 587, a putative alpha-1 adrenergic agonist, individually and in combination, on the non-delayed and delayed foraging behaviour of rats assessed in the radial arm maze. J Psychopharmacol. 1999;13:171–9. [PubMed: 10475724]
- Quigley A, Tan AA, Hoane MR. The effects of hypertonic saline and nicotinamide on sensorimotor and cognitive function following cortical contusion injury in the rat. Brain Res. 2009;1304:138–48. [PMC free article: PMC2784246] [PubMed: 19781534]
- Regan RF, Jasper E, Guo Y, Panter SS. The effect of magnesium on oxidative neuronal injury in vitro. J Neurochem. 1998;70:77–85. [PubMed: 9422349]
- Saad L, Silva L, Banzato C, Dantas C, Garcia C. Anorexia nervosa and Wernicke-Korsakoff syndrome: a case report. J Med Case Reports. 2010;4:1–5. [PMC free article: PMC2917440] [PubMed: 20646296]
- Sen AP, Gulati A. Use of magnesium in traumatic brain injury. Neurotherapeutics. 2010;7:91–9. [PMC free article: PMC5084116] [PubMed: 20129501]
- Slutsky I, Abumaria N, Wu LJ, Huang C, Zhang L, Li B, Zhao X, Govindarajan A, Zhao MG, Zhuo M, Tonegawa S, Liu G. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65:165–77. [PubMed: 20152124]
- Smith DH, Okiyama K, Gennarelli TA, McIntosh TK. Magnesium and ketamine attenuate cognitive dysfunction following experimental brain injury. Neurosci Lett. 1993;157:211–4. [PubMed: 8233056]
- Van Den Heuvel C, Vink R. The role of magnesium in traumatic brain injury. Clin Calcium. 2004;14:9–14. [PubMed: 15577090]
- Vink R, Cook NL, van de Heuvel C. Magnesium in acute and chronic brain injury: an update. Magnes Res. 2009;22:158S–62S. [PubMed: 19780402]
- Vink R, McIntosh TK, Demediuk P, Weiner MW, Faden AI. Decline in intracellular free Mg2+ is associated with irreversible tissue injury after brain trauma. J Biol Chem. 1988;263:757–61. [PubMed: 3335524]
- Vink R, O'Connor CA, Nimmo AJ, Heath DL. Magnesium attenuates persistent functional deficits following diffuse traumatic brain injury in rats. Neurosci Lett. 2003;336:41–4. [PubMed: 12493598]
- Wang C-T, Li Y, Wang F-J, Shi Y-M, Lee B-T. Correlation between iron, magnesium, potassium and zinc content in adolescent girl's hair and their academic records. Chang Gung Med J. 2008;31:358–62. [PubMed: 18935793]
- Magnesium efflux from Drosophila Kenyon cells is critical for normal and diet-enhanced long-term memory.[Elife. 2020]Magnesium efflux from Drosophila Kenyon cells is critical for normal and diet-enhanced long-term memory.Wu Y, Funato Y, Meschi E, Jovanoski KD, Miki H, Waddell S. Elife. 2020 Nov 26; 9. Epub 2020 Nov 26.
- Enrichment Effects on Adult Cognitive Development: Can the Functional Capacity of Older Adults Be Preserved and Enhanced?[Psychol Sci Public Interest. 2...]Enrichment Effects on Adult Cognitive Development: Can the Functional Capacity of Older Adults Be Preserved and Enhanced?Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Psychol Sci Public Interest. 2008 Oct; 9(1):1-65. Epub 2008 Oct 1.
- Review Spatial Navigation (Water Maze) Tasks.[Methods of Behavior Analysis i...]Review Spatial Navigation (Water Maze) Tasks.Terry AV Jr. Methods of Behavior Analysis in Neuroscience. 2009
- Review Assessment of cognitive function following magnesium therapy in the traumatically injured brain.[Magnes Res. 2007]Review Assessment of cognitive function following magnesium therapy in the traumatically injured brain.Hoane MR. Magnes Res. 2007 Dec; 20(4):229-36.
- Review Nutritional Cognitive Neuroscience: Innovations for Healthy Brain Aging.[Front Neurosci. 2016]Review Nutritional Cognitive Neuroscience: Innovations for Healthy Brain Aging.Zamroziewicz MK, Barbey AK. Front Neurosci. 2016; 10:240. Epub 2016 Jun 6.
- The role of magnesium therapy in learning and memory - Magnesium in the Central ...The role of magnesium therapy in learning and memory - Magnesium in the Central Nervous System
Your browsing activity is empty.
Activity recording is turned off.
See more...