This book is copyright. Apart from any fair dealing for the purposes of private study, research, criticism or review as permitted under the Copyright Act, no part may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission. Address all inquiries to the Director at the above address.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011.
Abstract
Schizophrenia and bipolar disorders are two of the most severe CNS conditions. Changes in plasma and intracellular magnesium concentration, as well as in other bivalent cations, have been found in both psychoses. Our data, as well as that of other authors, has shown that schizophrenic, paranoid patients admitted in the acute state and without previous treatment, have significantly decreased intracellular magnesium levels compared to healthy subjects. Therapy with haloperidol (a typical antipsychotic) or with risperidone (an atypical antipsychotic) both significantly raised the intracellular magnesium concentration without causing significant changes in plasma magnesium concentration. The increase in intracellular magnesium concentration was positively correlated with the improvement in clinical symptomatology. We consider that magnesium acts foremost by reducing glutamate release and by its action upon NMDA receptors, and results in an augmentation in the activity of the GABAergic systems. Unlike the hypothesis that only implicates zinc deficits in the pathogeny of schizophrenia, we consider that both intracellular magnesium and extracellular zinc deficits are equally involved in schizophrenia pathogeny. In patients with untreated bipolar disorder, our data showed a significant decrease in intracellular magnesium concentration and plasma zinc concentration during the manic episode. Therapy with mood modulators (carbamazepine and valproic acid) increased total intracellular magnesium and plasma zinc concentrations without having a significant effect on total plasma magnesium concentration. Other data showed that lithium also increases intracellular magnesium concentration. The fact that mood modulators with different mechanisms of action have in common the increase of intracellular magnesium concentration is an argument to consider this augmentation as an important element of their mechanism of action.
Introduction
Psychoses are amongst the most severe diseases of the CNS. They are diseases in thought processes and behaviour leading to a loss of contact with reality. Various neurological and psychiatric pathologies are associated with changes of plasma and intracellular magnesium and other bivalent cations. Such changes in magnesium concentrations have been observed in brain injury, stroke, headaches, epilepsy, major depression and others (Altura and Altura, 1999; Durlach and Bac, 1997). Schizophrenia and bipolar psychosis present an increasing incidence.
Schizophrenia
Schizophrenia is a chronic and severe brain disorder whose incidence is quite difficult to evaluate. Nonetheless, there are data that show that the prevalence of this disease could arrive at 0.5% of population (Kirkbride et al., 2008; Takei et al., 1992). It is a psychiatric illness that results in difficulty in telling the difference between real things and ideas and non-real ideas, which causes disturbed thinking and strong and inappropriate emotions. Four basic types of schizophrenia have been described:
- a.
catatonic schizophrenia;
- b.
disorganized schizophrenia (hebephrenic schizophrenia);
- c.
paranoid schizophrenia;
- d.
undifferentiated schizophrenia.
Identifying the changes in magnesium concen- tration in schizophrenic patients can be difficult, and sometimes contradictory, as a result of several factors including diversity of types of disease, unknown time of onset of the disease, and particularities of the disease evolution with periods of reduced intensity in occurring symptoms. In all circumstances, the association of other diseases can also modify intra and extracellular concentrations of magnesium and other bivalent cations.
Some authors have shown increased plasma Mg concentrations in schizophrenic patients. Specifically, Jabotinsky-Rubin et al., (1993) consider that schizophrenic patients present increased Mg plasma concentrations, but that haloperidol administration reduces Mg levels. These authors also show that decreased Mg concentration is involved in the generation of extrapyramidal side effects of haloperidol, and consider that in schizophrenia, hyper- magnesemia might be correlated with some failure of the catecholamine systems in the CNS. Gattaz et al., (1983) found a higher Mg level in the CSF of the schizophrenic patients. It was stated that schizophrenia appears to increase magnesium concentration in CSF, which was associated with decreased neuronal cGMP concentration.
In contrast, other authors didn’t find any differences in magnesium concentration between healthy subjects and schizophrenic patients. In the studies of Kornhuber et al., (1994), post-mortem determination of Mg concentrations in brain didn’t show any differences between the schizophrenic patient group and a control group. Athanassesnas (1983) indicated that there was no difference in plasma Mg2+ and Ca2+ concentrations in drug free schizophrenic patients.
Finally, other research has shown low Mg2+ concentration in the CSF of acute schizophrenic patients, but that the levels increase in remitted schizophrenics (Levine et al., 1996). Other authors indicated that patients with chronic schizophrenia present magnesium deficiency (Kirov et al., 1990; Kanofsky and Sandyk, 1991). Magnesium deficiency was also noted in psychosis induced by cancer chemotherapy (Matzen and Martin, 1985).
Our studies (Nechifor et al., 2004) showed that intraerythrocyte total magnesium concentration is significantly lower in patients with paranoid schizophrenia before treatment, as compared to normal subjects. The level of intraerythrocyte magnesium was 4.82 ± 0.32 mg/dl in acute paranoid schizophrenia patients as compared to
5.82 ± 0.11 mg/dl in the normal control group (p<0.05). In this study, there were no differences between plasma magnesium concentrations of schizophrenic patients and the healthy subjects (18.2 ± 11 mg/L in schizophrenic patients; 18.7 ± 2.17 mg/L in the control group).
Kirov and Tsachev (1990) observed a rise in plasma magnesium level in schizophrenic patients in clinic remission, but other studies haven’t found any correlation between the decrease in plasma magnesium concentration and the symptoms of schizophrenic patients (Kirov et al., 1994).
The analysis of extracellular magnesium and other bivalent cation concentrations in post- mortem schizophrenic patients in a variety of cerebral areas (hippocampus, amygdala, caudate nucleus, cortex) showed significant differences between the drug-free schizophrenic group and treated schizophrenic patients. There weren’t significant differences regarding magnesium concentration, but drug-free schizophrenic patients presented an increased copper level and a reduced zinc level.
We tested the effects of two different anti- psychotic drugs (haloperidol and risperidone) in therapeutic doses on plasma and erythrocyte total magnesium concentrations in acute, paranoid, adult schizophrenic patients. Our data (Nechifor et al., 2004) showed that both typical antipsychotic drugs (haloperidol and risperidone) induced a rise in the intraerythrocyte magnesium concentration and also improved the clinical symptoms in paranoid schizophrenia patients. The data indicated a rise in erythrocyte magnesium concentration after haloperidol treatment from 4.82 ± 0.32mg/dl to 5.46 ± 0.19mg/dl (p<0.05) and after risperidone treatment to 5.28 ± 0.21 mg/dl (p<0.05). The antipsychotic drugs tested have very different mechanisms of action. Haloperidol is a strong blocker of D1, D2 and α1 receptors, and a weak blocker of 5-HT2A receptors. Risperidone is a strong blocker of D2 receptors and 5-HT2A receptors, but a very weak blocker of D1 receptors.
Renn et al., (2010) also found an increased magnesium level in schizophrenic patients treated with antipsychotics, which is in agreement with our data. These data favour the idea that an increase in magnesium concentration is not a peripheral phenomenon, but an essential element for the mechanism of action of the antipsychotic drugs utilized inschizophrenia treatment. However, there is also contrary information whereby treatment with classic neuroleptics decrease plasma concen- trations of magnesium, and chronic treatment with neuroleptics may even produce hypo- magnesemia in some schizophrenics (Alexander et al., 1979).
Regarding the involvement of variations in magnesium concentration in the etiopathogeny of psychosis, we should take into account that intracellular magnesium plays more roles than extracellular magnesium, and intracellular total magnesium and free Mg2+ are compart- mentalized between cell organelles and the cytosol (Gunther et al., 2006). In terms of the glutamatergic hypothesis of schizophrenia that involves the imbalance between glutamate and dopamine with an excess of glutamate (Krebs, 1992), an increase in magnesium concentration induced by typical and atypical neuroleptic administration is mainly involved in reduction of NMDA receptor activation by glutamate. In this circumstance, Mg2+ reduces the neuronal Ca2+ entrance through Ca2+ channels coupled with NMDA receptors. We suppose that Mg2+ acts in the same way in the experimental phencyclidine model of psychosis. We believe that the increase in cell Mg level is important for the antipsychotic action of haloperidol and risperidone. The effect of magnesium could be produced by decreasing the neuronal response to glutamate stimulation of NMDA receptors, decreasing the presynaptic release of glutamate, decreasing formation of peroxide radicals, and increasing GABAergic activity and glycine synthesis; glycine inhibits catecholamine release (Johnson et al., 1994).
The increase in magnesium concentration can reduce anxiety, hallucinations and agitation whereas hypomagnesemia could exacerbate anxiety and hallucinations. Ang et al., (1993) considered that hypomagnesemia is one of the causes of antipsychotic treatment resistance. The improvement of the clinical state and the increase in intracellular magnesium concentration leads to the idea that magnesium might be involved in the development of the antipsychotic drugs’ therapeutic effect. The positive effect of increased magnesium concentration could be explained by the decreased activity of NMDA receptors, using the same reasoning that the excessive activity of some glutamatergic brain systems are involved in some psychotic symptoms.
Some post-mortem studies in human subjects have also showed abnormalities of GABAergic systems in schizophrenia patients. There is evidence for a deficit in GABA-containing interneurons in the prefrontal cortex (Beasley et al., 2002; Reynolds et al., 2001). There are biochemical and morphological arguments for a deficit in GABAergic systems in schizophrenia and this deficit is limited to the parvum-albumin class of GABAergic interneurons (Blum and Mann, 2002). It is possible that by increasing neuronal magnesium concentration with antipsychotic drugs, GABAergic systems are activated and the partial deficit of the GABAergic cortical systems are corrected. Regarding the mechanisms by which magnesium can increase the activity of the GABAergic systems, there are at least two possibilities: by enhancing vesicular GABA-transporter activity (Gerstein et al., 2005) or by modulation of GABAA receptor activity (Stanley et al., 1995). There is evidence that magnesium potentiates the function of native and recombinant GABAA receptors and Moykkyven et al., (2001) suggested a putative Mg binding site on the GABAA receptor complex.
One of the most promising therapeutic prospects is the use of drugs acting at metabotropic glutamate receptors. Krystal et al., (2010) described the potential of mGluR2 and mGluR5 agonists and allosteric positive modulators in the treatment of schizophrenia. Magnesium has a positive modulator effect at the level of some mGlu receptors. We believe that the increase in intracellular magnesium concentration after antipsychotic drug treatment allows magnesium to exert this modulator role, especially at mGluR5 and mGluR2, and possibly at mGluR7 level. By these means, magnesium not only reduces the glutamate action at the level of NMDA receptors, but also can increase the GABAergic activity and reduce presynaptic glutamate release.
Autism is one of the main symptoms of schizophrenia but there are also patients with autism (especially children) who are not considered to be schizophrenic. In autistic children that are not schizophrenic, Strambi et al., (2006) showed that there are no differences in intracellular Mg in autistic children versus normal children. Unlike autistic children, patients with schizophrenia have significantly lower plasma and intracellular Mg concentrations compared to normal subjects. We consider this fact as an argument for a different pathogeny of autism compared with schizophrenia. Regarding the effect of treatment with vitamin-B6-magnesium in children with autism syndrome (non-schizophrenic), there are contradictory data. Nyl and Brice (2002) maintain that in these children (a small number of cases studied), a favourable effect of this treatment might exist. However, Findling (1997) showed no significant differences between the treated and placebo group.
Eating disorders and cognitive dysfunction has also been identified in schizophrenia (Wobrock et al., 2009). The serum levels of glutamate and glutamine in schizophrenic people with anorexia and cognitive impairment were significantly higher than those in the control group. Based on these findings, Nakazato et al., (2010) hypothesized that changes in the glutamate concentration are involved in the mechanism of cognitive impairment and anorexia of schizophrenic patients. We are in agreement with this point of view and we consider that magnesium, by decreasing the action of glutamate upon NMDA receptors, could contribute to the improvement of these symptoms in schizophrenic patients.
Although the etiopathogeny of schizophrenia is not sufficiently known, we consider that an increase in magnesium concentration by antipsychotic therapy is not just a secondary phenomenon, but is part of the mechanism of action of antipsychotics. We consider that the data above indicates that the changes of intracellular magnesium concentration are important for neuroleptic drug action in schizophrenic patients, and the fact that antipsychotic drugs with different chemical structures and mechanisms of action induce the increase in intracellular magnesium levels, further supports this idea. Contrary to Andrews (1992), who supported only the zinc deficiency theory of schizophrenia, we consider that intracellular magnesium and plasma zinc deficiency play an important role in the pathogenic mechanism of schizophrenia. The increase in intracellular magnesium concentration is important, and is not just a side effect of the therapeutic action of typical and atypical antipsychotics.
Bipolar disorders
Bipolar disorder (BD), also called manic- depressive psychosis, is an affective psychosis. It is a recurrent and severe psychiatric disease characterized by episodes of mania followed by depression and by mood instability. Without adequate treatment, BD is associated with high rates of mortality. BD is considered a relatively frequent psychiatric disease. Vacheron-Trystan et al., (2004) consider that the prevalence of BD over a lifetime is around 1% in the general population. There are several forms of BD. According to DSM IV, the first type of BD (the most frequent one) has a dominance of manic symptoms, associated with periods of depression. According to DSM IV, there are two main subtypes of BD: type I BD with at least one manic episode or several manic and depressive episodes, and type II BD which is related to patients enduring recurrent depressive episodes.
There is some data, some contradictory, regarding magnesium and other bivalent cation concentrations in patients with bipolar disorder, although they haven't all been made in the same type of BD or at the same stage of this disease. Decreasing Mg level could exacerbate anxiety, weakness and other symptoms in BD patients (Kirov and Tsachev, 1990). Frazer et al., (1983) found an increased level of erythrocyte magnesium concentration in patients with bipolar disorder before hospitalization. Contrary to this, George et al., (1994) highlight significant changes of magnesium concentration in CSF of BD patients and cannot establish a correlation between the disease outcome and this cation concentration. Some authors have reported a decreased plasma magnesium concentration in BD patients compared to healthy control (Herzberg and Herzberg, 1977) while others found a disturbed ratio between plasma calcium and magnesium concentrations. An increased calcium/magnesium ratio was correlated with manic agitation (Carman et al., 1979). The current medication is mood modulators, themost commonly used drug from this category being lithium, and some anticonvulsive substances that have proven to be effective in BD, such as carbamazepine, sodium valproate, and lamotrigine (Arban et al., 2005). More rarely used in the treatment of BD are gabapentine and topiramate. Also, some atypical antipsychotics such as olanzapine, clozapine and risperidone have been administered in BD.
Lithium was and remains one of the most utilized mood modulators in BD therapy, especially in patients with the dominance of manic symptoms, although its therapeutic utility has a tendency to diminish (Gay and Olie, 2005). The data describing the influence of lithium administration upon serum magnesium concentrations are variable. Most studies show that repeated lithium salt administration increases magnesium concentration. Lithium increases the intracellular magnesium concen- tration by competing with magnesium for some intracellular binding sites (Leyden et al., 2000). Some data demonstrate Li+/Mg2+ competition at therapeutic intracellular Li+ concentration (after treatment) in human neuroblastoma SH-SY5Y cells (Abukhdeir et al., 2003). After experimental loading of neuroblastoma cells with 1-2 mM of extracellular Li+, the intracellular free magnesium concentration was significantly higher. With respect to Li+ and Mg+ competition at some intracellular sites, the existing data have identified the following molecules as sites of competition: G-protein transducin in the guanosine 5'-diphosphate bound conformation (Srinivasan et al., 2004); inositol mono- phosphatase; glycogen synthase kinase 3 (GSK- 3); fructose 1,6 biphosphatase; biphosphate nucleotidase (Gould et al., 2004); and finally, ATP and ADP phosphate binding sites. It is also possible that other intracellular binding sites for Li+/Mg2+ competition exist. Rybakowski and Szaynerman (1976), hypothesized that magnesium could increase the intracellular influx of lithium. This could be another means of magnesium involvement in the mechanism by which lithium reduces the clinical symptoms from bipolar disorders.
Our data (Nechifor et al., 2006; 2007) show that adult patients with bipolar disorder type I presenting acute manic attacks and with no previous treatment exhibit lower intracellular magnesium levels than the control group. Plasma zinc concentration was also significantly lower (0.72 ± 0.02 mg/l versus 89 ± 0.12 mg/l (p<0.05). There were no significant differences between patients with bipolar disorder type I and the control group with respect to plasma total magnesium concentration.
Carbamazepine treatment (600mg/day per os for 4 weeks) and sodium valproate (900mg/day for 4 weeks) significantly increased intracellular magnesium levels from 45.0 ± 1.87 mg/l before treatment to 52.02 ± 0.9 mg/l after sodium valproate, and 53.72 ± 2.18 mg/l after carbamazepine (p<0.05). Plasma magnesium concentration wasn’t significantly modified but there was a significant rise in plasma zinc concentration (Nechifor et al., 2005; 2006). Walden et al., (1993) showed that magnesium deficiency decreased the beneficial effect of carbamazepine in the treatment of epilepsies but we think that hypomagnesemia also decreases the effect carbamazepine in the treatment of affective disorders. Magnesium oxide increases the verapamil maintenance therapy in mania (Giannini et al., 2000). This fact favours the idea that an increase in magnesium concentration is important, maybe essential for the therapeutic effect of some drugs used in BD treatment. Magnesium valproate reduces the hyperactivity in an animal model of mania. This effect of magnesium valproate could be abolished by bicuculline, suggesting that actions on postsynaptic GABA may also be involved in the antimanic action of magnesium valproate (Cao and Peng, 1993).
We consider that the increase in intracellular magnesium concentration is an important feature of carbamazepine, valproic acid and other mood modulators used in BD treatment. We also consider that transmembrane calcium influx plays an important role in the development of some psychiatric disorders. The calcium channel antagonists (verapamil and others) increase the carbamazepine effect. Magnesium, acting like a natural calcium antagonist on some ionic channels, is a factor that contributes at the pharmacodynamic effect of some mood modulators. In contrast, calcium ions have an antagonistic effect on carbamazepine action. Carbamazepine reduces the neuronal excitability and glutamate release and we consider that this effect is due, at least in part, by increasing magnesium concentration. The increase in intracellular magnesium concentration at the neuronal level is important for all the mood modulator drugs utilized in BD therapy, and not only for lithium (Nechifor et al., 2008). Some of the possible mechanisms describing magnesium’s involvement in mood modulator action are presented in Figure 1. Gobbi and Janiri (2006) showed that magnesium valproate significantly modulated the response induced by NMDA-receptor stimulation. Chuinard et al., (1990) showed that magnesium aspartate administration was effective in stabilizing the mood of rapid-cycling BD, which favours the idea that the increase in magnesium concentration is an important factor of lithium and other mood modulators mechanism of action. Heiden et al., (1999) administered magnesium sulphate iv as supplementary treatment in severe acute mania.
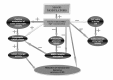
Figure 1.
The mechanism of magnesium’s involvement in mood modulator action. + = stimulation; - = inhibition
In conclusion, we suggest that the increase in plasma zinc level and the erythrocyte magnesium level is not a peripheral phenomenon but is involved in the mechanism by which some mood modulators can reduce clinical symptoms of bipolar disorders, not only the manic episodes of BD but also the depressive periods. The changes in intra and extracellular plasma magnesium concentrations and other bivalent cations are an important part of the mechanism of action of some mood modulators and other psychotropic drugs.
References
- Abukhdeir AM, Layden BT, Minadeo N, Bryant FB, Stubbs EB Jr, Mota De Freitas D. Effect of chronic Li+ treatment on free intracellular Mg2+ on human neuroblastoma SH-SY5Y cells. Bipolar Disord. 2003;5:6–13. [PubMed: 12656932]
- Alexander DR, Deeb M, Bitar F, Antun F. Sodium-potassium, magnesium and calcium ATPase activities in erythrocyte membranes from maniac- depressive patients responding to lithium. Biol Psychiatry. 1986;21:997–1007. [PubMed: 3017463]
- Altura BM, Altura BT. Association of alcohol in brain injury, headaches and stroke with brain-tissue and serum levels of ionized magnesium. Alcohol. 1999;19:119–30. [PubMed: 10548155]
- Andrews RC. An update of the zinc deficiency theory of schizophrenia. Identification of the sex determininig system as the site of action of reproductive zinc deficiency. Med Hypotheses. 1992;38:284–91. [PubMed: 1491625]
- Ang AN, Ko SM, Tan CH. Calcium, magnesium and psychotic symptoms in a girl with idiopathic hypoparathyroidism. Psychoso. Med. 1995;57:299–302. [PubMed: 7652132]
- Athanassenas G, Papadopoulos E, Kourkoubas A, Tsitourides S, Gabriel Hoidas E, Frangos E. Serum calcium and magnesium levels in chronic schizophrenics. J Clin Psychopharmacol. 1983;3:212–216. [PubMed: 6886033]
- Arban R, Marian G, Brackenborough K, Winyard L, Wilson A, Gerard P, Large C. Evaluation of the effects of lamotrigine, valproate and carbamazepine in a rodent model of mania. Behav Brain Res. 2005;158:123–32. [PubMed: 15680200]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol. Psychiatry. 2002;52:708–15. [PubMed: 12372661]
- Blum BP, Mann JJ. The GABAergic system in schizophrenia. Int J Neuropsychopharmacol. 2002;5:159–79. [PubMed: 12135541]
- Cao BJ, Peng NA. Magnesium valproate attenuates hyperactivity induced by dexamphetamine- chlordiazepoxide mixture in rodents. Eur J Pharmacol. 1993;237:177–81. [PubMed: 8103460]
- Carman JS, Post R, Runkle DC, Bunney WE Jr, Wyatt RJ. Increased serum calcium and phosphorus with the switch into maniac or excited psychotic state. Br J Psychiatry. 1979;135:55–61. [PubMed: 497626]
- Chouinard G, Beauclai I, Geiser R, Etienne P. A pilot study of magnesium aspartate hydrochloride (Magnesiocard) as a mood stabilized for a rapid cycling bipolar affective disorder patients. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:171–80. [PubMed: 2309035]
- Durlach J, Bac P (1997) Mechanisms of action in the nervous system in Magnesium deficiency and dementia, In Mineral and Neurotoxicity (Yasui M, Strong MJ, Ota K, Verit MA, eds) CRR Press, Boca Raton, 27-38.
- Frazer A, Ramsey TA, Swann A. Plasma and erythrocyte in affective disorders. J Affect Disorder. 1983;5:103–13. [PubMed: 6222090]
- Findling RL, Maxwell K, Scotese-Wojtila L, Huang J, Yamashita T, Wiznitzer M. High-dose pyridoxine and magnesium administration in children with autistic disorder: an absence of salutary effect sin a double-blind placebo-controlled study. J Autism Dev Disord. 1997;27:467–78. [PubMed: 9261669]
- Gattaz WF, Kattermann R, Gattaz D, Beckmann H. Magnesium and calcium in the CSF of schizophrenic patients and healthy controls: correlations with cyclic GMP. Biol Psychiatry. 1983;18:935–9. [PubMed: 6137251]
- Gay C, Olie JP. Management of bipolar disorders. Rev Prat. 2005;55:513–22. [PubMed: 15895954]
- George MS, Rosenstein D, Rubinow DR, Kling MA, Post RM. CSF magnesium in affective disorders lack correlation with clinical course of treatment. Psychiatry Res. 1994;5:139–46. [PubMed: 8022948]
- Giannini AJ, MacKenzie AM, Melemis SM, Ventresco J, Condon M. Magnesium oxide augmentation of verapamil maintenance therapy in mania. Psychiatry Res. 2000;93:83–7. [PubMed: 10699232]
- Gerstein M, Huleihel M, Mane R, Stilman M, Kashtuzki I., Hallak M, Golan H. Remodelling of hippocampal GABAergic system in adult offspring after maternal hypoxia and magnesium sulphate load: immunohistochemical study. Exp Neurol. 2005;196:18–29. [PubMed: 16081066]
- Gobbi G., Janiri L. Sodium and magnesium valproate in vivo modulate glutamatergic and GABAergic synapses in the medial prefrontal cortex. Psychopharmacol. 2006;185:255–262. [PubMed: 16496131]
- Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry. 2004;9:734–55. [PubMed: 15136794]
- Gunther T. Concentration, compartmentation and metabolic function of intracellular free Mg2+. Magnes Res. 2006;19:225–36. [PubMed: 17402290]
- Heiden A, Frey R, Presslich O, Blasbichle T, Smetana R, Kasper S. Treatment of severe mania with intravenous magnesium sulphate as a supplementary therapy. Psychiatry Res. 1999;3:236–46. [PubMed: 10708270]
- Herzberg L, Herzberg B. Mood change and magnesium. A possible interaction between magnesium and lithium. J New Ment Dis. 1977;163:423–26. [PubMed: 591941]
- Jabotisky-Rubin K, Durst R, Levitin LA. Effects of haloperidol on human plasma magnesium. J Psychiatry Res. 1993;27:155–9. [PubMed: 8366466]
- Johnson D, Blandina P, Goldfarb J. Glycine inhibition of glutamate-evoked release of norepinephrine in hypothalamus is strycnine- insensitive. Brain Res. 1994;650:70–4. [PubMed: 7953679]
- Kanofsky JD, Sandyk R. Magnesium deficiency in chronic schizophrenia. Int J Neurosci. 1991;61:87–90. [PubMed: 1809739]
- Kirkbride JB, Boydell J, Ploubidis GB, Morgan C, Dazzan P, McKenzie K, Murray RM, Jones PB. Testing the association between the incidence of schizophrenia and social capital in an urban area. Psychol Med. 2008;38:1083–94. [PubMed: 17988420]
- Kirov GK, Birch NJ, Steadman P, Ramsey RG. Plasma magnesium levels in a population of psychiatric patients: correlation with symptoms. Neuropsychobiology. 1994;30:73–8. [PubMed: 7800167]
- Kirov GK, Tsachev KN. Magnesium, schizophrenia and maniac-depressive disease. Neuropsychobiology. 1990;23:79–81. [PubMed: 2077436]
- Kornhuber J, Lange KW, Kruzik P, Rausch WD, Gabriel E, Jellinger K, Riederer P. Iron, copper, zinc, magnesium and calcium in post-mortem brain tissue from schizophrenic patients. Biol Psychiatry. 1994;36:31–4. [PubMed: 8080900]
- Krebs MO. Glutamatergic hypothesis of schizophrenia: psychoses induced by phencyclidine and cortical-subcortical imbalance. Encephale. 1995;21:581–8. [PubMed: 8529568]
- Krystal JH, Mathew SJ, D’souza DC., Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24:669–3. [PubMed: 20658799]
- Layden B, Diven C, Minadeo N, Bryant FB, Mota de Freitas D. Li+/Mg2+ competition at therapeutic intracellular Li+ levels in human neuroblastoma SH- SY5Y cells. Bipolar Dis. 2000;2:2000–4. [PubMed: 11256688]
- Levine J, Rapoport A, Mashiah M, Dolev E. Serum and cerebrospinal levels of calcium and magnesium in acute versus remitted schizophrenic patients. Neuropsychobiology. 1996;33:169–172. [PubMed: 8840338]
- Matzen TA, Martin RL. Magnesium deficiency in psychosis induced by cancer chemotherapy. Biol Psychiatry. 1985;20:788–94. [PubMed: 4039951]
- McLean MJ, McDonald RL. Sodium valproate but not ethosuximide produces use and voltage dependent limitation of high-frequency repetitive firing of action-potentials of mouse central neurons in cell culture. J Pharmacol Exp Ther. 1986;238:727–38. [PubMed: 2874218]
- Moykkyen T, Uusi-Oukari M, Heikkla J, Lovinger DM, Luddens H, Korpi ER. Magnesium potentiation of the function of native and recombinant GABA-A receptors. Neuroreport. 2001;12:2175–79. [PubMed: 11447329]
- Murck H. Magnesium and affective disorders. Nutr Neurosci. 2002;5:375–89. [PubMed: 12509067]
- Nakazato M, Hashimoto K, Schmidt U, Tchanturia K, Campbell IC, Collier DA, Iyo M, Treasure J. Serum glutamine, set-shifting ability and anorexia nervosa. Ann Gen Psychiatry. 2010;9:29–43. [PMC free article: PMC2902473] [PubMed: 20576166]
- Nechifor M (2007) Magnesium in Psychoses, in New Perspectives in Magnesium Research – Nutrition and Health, (Nishizawa Y, Mori H, Durlach J, eds) Springer- Verlag, London, pp 369-77.
- Nechifor M. Interaction between magnesium and psychotropic drugs. Magnes Res. 2008;21:97–100. [PubMed: 18705537]
- Nechifor M, Vaideanu C, Mindreci I, Borza C (2006) Variation of magnesium concentrations in psychoses, In: Advances in Magnesium Research-New Data (Porr P J, Nechifor M, Durlach J, eds) John Libbey Eurotext, Paris, pp 25-30.
- Nechifor M, Vaideanu C, Palamaru I, Borza C, Mindreci I. The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid schizophrenia. J Am Coll Nutr. 2004;23:5493–519. [PubMed: 15466963]
- Nechifor M, Vaideanu C, Mandreci I, Palamaru I, Boisteanu P (2005) Research on plasma and erythrocyte concentration of some bivalent cations in patients with bipolar disorders, In (Ermidu-Pollet S., Pollet S. eds.) Proceedings Book of 3rd International Symposium on Trace Elements in Humans - New Perspectives, Athens, pp 150-159.
- Nye C, Brice A. Combined vitamin B6- magnesium treatment in autism spectrum disorder. Cochrane Database Syst Rev. 2005;19:CD003497. [PMC free article: PMC7003675] [PubMed: 16235322]
- Renn JH, Yang NP, Choup P. Effects of plasma magnesium and prolactin in quantitative ultrasound measurements of heel bone among schizophrenic patients. Muscoloskeletal Disord. 2010;I:35–52. [PMC free article: PMC2834603] [PubMed: 20163720]
- Reynolds GP, Zhang ZJ, Beasley CL. Neurochemical correlates of cortical GABAergic deficits in schizophrenia: selective losses of calcium binding protein immunoreactivity. Brain Res Bull. 2001;55:579–84. [PubMed: 11576754]
- Rybakowski J, Szaynerman Z. Lithium- magnesium relationship in red blood cells during lithium prophylaxis. Pharmakopsychiatr Neuropsychopharmacol. 1974;9:242–6. [PubMed: 996088]
- Srinivasan C, Toon J, Amari L, Abukhdeir AM, Hamm H, Geraldes CF, Ho YK, Mota De Freitas D. Competition between lithium and magnesium ions for the G-protein transducin in the guanosine 5’-diphosphate bound conformation. J Inorg Biochem. 2004;98:691–701. [PubMed: 15134914]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–81. [PubMed: 7638623]
- Stambi M, Longini M, Hayek J, Berni S, Macucci F, Scalacci E, Vezzosi P. Magnesium profile in autism. Biol Trace Elem Res. 2006;109:97–104. [PubMed: 16443999]
- Takei N, Lewis G, Sham PC, Murray RM. Age- period-cohort analysis of the incidence of schizophrenia. Br J Psychiatry. 1992;26:963–73. [PubMed: 8878329]
- Vacheron-Trystram MN, Braitman A, Cheref S, Affray L. Antipsychotics in Bipolar disorders. Encephale. 2004;30:417–24. [PubMed: 15627046]
- Warden J, Grunze H, Mayer A. Calcium antagonistic effects of carbamazepine in epileptics and affective psychoses. Neuropsychobiology. 1993;27:171–5. Dusing ., Schirrmacher K, Liu Z, Bingmann D. [PubMed: 8232834]
- Wobrock T, Ecker UK, Scheerk H, Schneider-Axmann T, Falkdi P, Gruber O. Cognitive impairment of executive function as a core symptom of schizophrenia. World J Biol Psychiatry. 2009;10:442–51. [PubMed: 18609418]
- Review [Antipsychotics in bipolar disorders].[Encephale. 2004]Review [Antipsychotics in bipolar disorders].Vacheron-Trystram MN, Braitman A, Cheref S, Auffray L. Encephale. 2004 Sep-Oct; 30(5):417-24.
- The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid schizophrenia.[J Am Coll Nutr. 2004]The influence of some antipsychotics on erythrocyte magnesium and plasma magnesium, calcium, copper and zinc in patients with paranoid schizophrenia.Nechifor M, Vaideanu C, Palamaru I, Borza C, Mindreci I. J Am Coll Nutr. 2004 Oct; 23(5):549S-551S.
- Review Interactions between magnesium and psychotropic drugs.[Magnes Res. 2008]Review Interactions between magnesium and psychotropic drugs.Nechifor M. Magnes Res. 2008 Jun; 21(2):97-100.
- Review [Cost-effectiveness analysis of schizophrenic patient care settings: impact of an atypical antipsychotic under long-acting injection formulation].[Encephale. 2005]Review [Cost-effectiveness analysis of schizophrenic patient care settings: impact of an atypical antipsychotic under long-acting injection formulation].Llorca PM, Miadi-Fargier H, Lançon C, Jasso Mosqueda G, Casadebaig F, Philippe A, Guillon P, Mehnert A, Omnès LF, Chicoye A, et al. Encephale. 2005 Mar-Apr; 31(2):235-46.
- Review Magnesium in neuroses and neuroticism.[Magnesium in the Central Nervo...]Review Magnesium in neuroses and neuroticism.Papadopol V, Nechifor M. Magnesium in the Central Nervous System. 2011
- Magnesium in psychoses (schizophrenia and bipolar disorders) - Magnesium in the ...Magnesium in psychoses (schizophrenia and bipolar disorders) - Magnesium in the Central Nervous System
Your browsing activity is empty.
Activity recording is turned off.
See more...
 1.
1.