NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Coffee, Tea, Mate, Methylxanthines and Methylglyoxal. Lyon (FR): International Agency for Research on Cancer; 1991. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 51.)
1. Chemical and Physical Data
1.1. Synonyms
Chem. Abstr. Services Reg. No.: 83–67–0
Chem. Abstr. Name: 3,7-Dihydro-3,7-dimethyl-1H-purine-2,6-dione
Synonym: 3,7-Dimethylxanthine
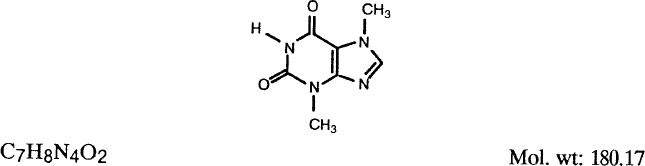
1.2. Structural and molecular formulae and molecular weight
1.3. Chemical and physical properties of the pure substance
- Description: White crystalline powder (Moffat, 1986)
- Sublimation-point: 290–295°C (Windholz, 1983)
- Melting-point: 357°C (Windholz, 1983)
- Spectroscopy data: Ultraviolet spectra: aqueous acid-272 nm; aqueous alkali-274 nm; infrared spectra: principal peaks at wave numbers 1690, 1665, 1221, 1550, 1595, 680 nm (potassium bromide disc); mass spectra: principal peaks at m/z 180, 55, 67, 109, 82, 42, 137, 70; 3-methylxanthine, 166, 68, 95, 41, 53, 123; 7-methylxanthine, 166, 68, 123, 53, 42, 41, 95 (Moffat, 1986)
- Solubility: Soluble in water (1.0 g/2l), boiling water (1.0 g/0.15l) and 95% ethanol (1.0 g/2.2l) (Windholz, 1983); slightly soluble in chloroform (1.0 g/6l; Moffat, 1986); almost insoluble in benzene, diethyl ether and carbon tetrachloride (Windholz, 1983)
- Equilibrium constants: acidic (Ka) 0.9 × 10−10 and basic (Kb) 1.3 × 10−14 at 18°C (Windholz, 1983)
- Reactivity: Forms salts, which are decomposed by water, and compounds with bases, which are more stable (Windholz, 1983)
- Octanol/water partition coefficient (P): log P -0.8 (Moffat, 1986)
1.4. Technical products and impurities
Theobromine should contain not less than 99.0% and not more than 101.0% of the product calculated on a dry basis (Anon., 1988).
Trade names: Riddospas; Riddovydrin; Santheose; Seominal; Theobrominum; Theoguardenal; Theominal; Théoxalvose
2. Production, Use, Occurrence and Analysis
2.1. Production and use
(a) Production
Theobromine is the principal alkaloid (1.5–3%) of the cacao bean (Theobroma cacao); it is usually extracted from the husks of cacao beans, which contain 0.7–1.2% theobromine. It has been synthesized from 3-methyluric acid (Windholz, 1983), but is not known to be produced commercially. Annual production of theobromine in the mid-1970s was over 33 000 tonnes (Cordell, 1978). In 1980, 607 million pounds (276 million kg) of cocoa were imported into the USA, which represents 9.11 million pounds (4.1 million kg) of theobromine (Hirsh, 1984).
(b) Use
Theobromine is used principally to make caffeine (McCutheon, 1969). Formerly, theobromine and its derivatives were used in diuretics, myocardial stimulants, vasodilators and smooth muscle relaxants (Windholz, 1983). Theobromine salts (calcium salicylate, sodium salicylate and sodium acetate) were used previously to dilate coronary arteries (Tarka, 1982; Gennaro, 1985) at doses of 300 to 600 mg per day (Moffat, 1986). There is no current therapeutic use of theobromine (Tarka, 1982).
2.2. Occurrence
(a) Natural occurrence
Theobromine is found in chocolate, tea and cocoa products (Graham, 1984a; Shively & Tarka, 1984; Stavric, 1988). Cacao is the major natural source of theobromine; the concentration in whole cacao beans and nibs (cotyledon) increases during the first day of fermentation and that in the shells increases subsequently (Timbie et al., 1978; Shively & Tarka, 1984).
Theobromine has been reported in cacao husks and beans at 0.7–1.2% and 1.5–3% (15–30 g/kg) (Windholz, 1983). Levels have been reported to be 20 mg/kg in green coffee beans (Spiller, 1984), 0.15–0.20% in manufactured tea (Jalal & Collin, 1976; Graham, 1984a) and 0.3% in dried mate (Michl & Haberler, 1954; Graham, 1984b).
(b) Occupational exposure
No data were available to the Working Group.
(c) Water and sediments
Theobromine has not been found in US industrial effluents (Perry et al., 1979) or drinking-water (National Research Council, 1977).
(d) Foods and beverages (see also the monograph on caffeine, pp. 296 et seq.)
Theobromine is a component of the cocoa solids, or nonlipid portion, of chocolate liquor (Shively & Tarka, 1984). An average theobromine level of 1.89% was found in eight commercial brands of cocoa powder (not calculated on a dry basis; Zoumas et al., 1980; Shively & Tarka, 1984). A level of 2% was reported in one cocoa powder (Sontag & Kral, 1980) and 2.5–3.3% in bulk unsweetened cocoa (Martinek & Wolman, 1955; Shively & Tarka, 1984). Hot chocolate beverages had average levels of 65 mg/5-oz serving (Zoumas et al., 1980); chocolate milk samples prepared from instant, cold, sweetened cocoa powders had an average level of 58 mg theobromine per serving (Zoumas et al., 1980; Shively & Tarka, 1984), and hot cocoa prepared from nine commercial instant mixes had an average of 62 mg theobromine per serving (Blauch & Tarka, 1983; Shively & Tarka, 1984). The mean concentration of theobromine in 12 varieties of cocoa powder was 0.26% (Craig & Nguyen, 1984).
Dark chocolate contains the largest amount of theobromine per serving of any type of eating chocolate; concentrations vary widely (0.36–0.63%) owing to the initial large difference in the theobromine content in chocolate liquors, but one 1-oz bar of dark chocolate contained 130 mg theobromine, and one 1-oz bar of milk chocolate contained 44 mg theobromine. The theobromine content of chocolate foods prepared from home recipes using standard chocolate sources (i.e., cocoa and baking chocolate) varies widely (24 mg per serving in chocolate brownies to 724 mg in chocolate frostings); chocolate frostings have relatively higher theobromine levels (0.055–0.213%) than chocolate cakes. The methylxanthine (theobromine and caffeine) content of manufactured chocolate foods and beverages varies according to food source and within different brands of the same item (Shively & Tarka, 1984).
Theobromine has been found in blended black tea beverage at a level of 0.69% of dry extractable solids present (Graham, 1984a).
In the USA in 1980, the daily per-caput intake of theobromine from food and beverages was estimated to be 39.05 mg; daily per-caput consumption of theobromine from cocoa was calculated to be 38.3 mg on the basis of the 276 million kg of cocoa imported (Hirsh, 1984).
2.3. Analysis
Analytical procedures for the determination of methylxanthines (including theobromine) in biological fluids (Schack & Waxier, 1949; Christensen & Whitsett, 1979; Tang-Liu & Riegelman, 1982; Klassen & Stavric, 1983; Christensen & Neims, 1984; Hurst et al., 1984) and in foods (Hurst et al., 1984) include ultraviolet spectroscopy, thin-layer chromatography, gas chromatography and high-performance liquid chromatography.
Modern methods for the determination of theobromine in foods and beverages (cocoa beans, cocoa and chocolate products) usually rely on high-performance liquid chromatography (Wildanger, 1975; Kreiser & Martin, 1978; Timbie et al., 1978; Hatfull et al., 1980; Horwitz, 1980; Kreiser & Martin, 1980; Sontag & Kral, 1980; Zoumas et al., 1980; De Vries et al., 1981; Reid & Good, 1982; Woollard, 1982; Blauch & Tarka, 1983; Craig & Nguyen, 1984; Vergnes & Alary, 1986) and, to a lesser extent, on thin-layer chromatography (Senanayake & Wijesekera, 1968a,b, 1971). These methods replaced the traditional titrimetric procedure using silver nitrate (Gerritsma & Koers, 1953), paper chromatography (Jalal & Collin, 1976), gravimetry and ultraviolet spectroscopy (Hurst et al., 1984).
3. Biological Data Relevant to the Evaluation of Carcinogenic Risk to Humans
3.1. Carcinogenicity studies in animals
No data were available from studies on the carcinogenicity of theobromine.
Modifying effects on the activity of known carcinogens
Urethane
Groups of female ICR/Jcl mice [initial numbers unspecified], 25 days of age, received a single subcutaneous injection of 0.1 mg/g bw urethane followed immediately by seven intraperitoneal injections (0.05 µmol/g bw) of theobromine [purity unspecified] at 6-h intervals up to 36 h after urethane treatment, to give a total dose of 63 µg/g theobromine. Mice were killed five months after urethane treatment. The number of mice with lung tumours was significantly reduced in groups that received post-treatment with theobromine (11/56 versus 31/59; p < 0.001). The number of tumours/lung was also reduced (0.28 versus 1.07 in controls) (Nomura, 1983). [The Working Group noted that the effective numbers of mice varied considerably among the different groups.]
3.2. Other relevant data
(a) Experimental systems
(i) Absorption, distribution, metabolism and excretion
The pharmacokinetics and toxicity of theobromine have been reviewed extensively (Tarka, 1982; Arnaud, 1984, 1987; Tarka & Shively, 1987). As a metabolite of caffeine, theobromine has been detected in variable amounts in plasma and urine of humans and different animal species (Arnaud, 1984).
When theobromine was given as a single oral dose of 15–50 mg/kg bw to male dogs, peak plasma concentrations, with considerable individual variations, were observed within 3 h. With a higher dose (150 mg/kg bw), the peak plasma concentrations were attained 14–16 h later, showing delayed intestinal absorption (Gans et al., 1980). In rats, plasma protein binding was very low (8–17%) after oral administration of 1–100 mg/kg bw theobromine (Bonati et al., 1984).
Similar kinetic parameters were observed in male and female rabbits when theobromine was administered intravenously or orally at doses of 1 and 5 mg/kg bw, with complete gastrointestinal absorption. A reduction in the absorption rate constant was seen in rabbits when the dose was increased from 10 to 100 mg/kg bw. In spite of delayed gastrointestinal absorption at high doses, probably due to the low solubility of the compound, the absolute bioavailability of theobromine approached 100% (Latini et al., 1984). Labelled theobromine was almost completely absorbed after oral administration (1–6 mg/kg; Arnaud & Welsch, 1979); the peak blood level tended to appear later with larger doses (100 mg/kg; Shively & Tarka, 1983).
Theobromine is absorbed and distributed rapidly after oral administration to rats (Shively & Tarka, 1983) and equilibrates freely between plasma and testicular fluid (Shively et al., 1984).
The ratio of brain:blood theobromine concentrations decreased continuously from 0.96 at birth to 0.60 in 30-day-old rats (Arnaud & Getaz, 1982). After 24 h, no organ accumulation of theobromine or its metabolites could be seen in adult animals (Arnaud & Welsch, 1979).
In dogs, an average plasma half-time of 17.5 h was reported after single oral doses of theobromine ranging from 15 to 150 mg/kg bw (Gans et al., 1980). In rabbits, the mean elimination half-time was 4.3–5.6 h for doses ranging from 1 to 100 mg/kg bw (Latini et al., 1984). From these data, it was concluded that the pharmacokinetics of theobromine in rabbits are linear and not dose-dependent up to 100 mg/kg (Traina & Bonati, 1985). Linear pharmacokinetics were also observed in rats up to a dose of 100 mg/kg. No significant first-pass effect or sex difference were observed (Shively & Tarka, 1983; Bonati et al., 1984). Repeated administration of theobromine to dogs, rabbits or rats did not alter its kinetics or metabolism (Gans et al., 1980; Bonati et al., 1984; Latini et al., 1984). Pretreatment of rats with 3-methylcholanthrene in vivo markedly increased theobromine elimination, while phenobarbital had no effect (Shively & Vesell, 1987).
The kinetic parameters in rats on day 19 of gestation were similar to those of non-pregnant rats at doses ranging from 5 to 100 mg/kg bw (Shively & Tarka, 1983). A decrease in the elimination rate constant was observed in pregnant rabbits at a dose of 50 mg/kg bw, suggesting saturation (Latini et al., 1984).
The metabolic pathway of theobromine reported in rats and several other animal species is shown in Figure 1. 6-Amino-5-[N-methylformylamino]-1-methyl-uracil is quantitatively the most important theobromine metabolite in rats, accounting for 20–35% of urinary metabolites (Arnaud & Welsch, 1979; Shively & Tarka, 1983; Bonati et al., 1984). The majority of theobromine-derived radioactivity in the faeces of rats could be accounted for by 3,7-dimethyluric acid (Shively & Vesell, 1987). The most extensive metabolism of theobromine was observed in rabbits and mice; male mice converted theobromine more extensively into this metabolite than did female mice. In contrast, oxidation of theobromine to 3,7-dimethyluric acid was significantly greater in female than in male rats. Rabbits and dogs metabolized theobromine primarily to 7-methylxanthine and 3-methylxanthine, respectively, and dogs excreted small quantities of an unidentified metabolite (Miller et al., 1984).

Figure 1.
Metabolism of theobrominea
The compounds identified in bile of phenobarbital-treated rats were 3,7-dimethyluric acid (64–76% of biliary radioactivity), dimethylallantoin (5–8%), 6-amino-5-[N-methylformylamino]-1-methyluracil (10–17%) and theobromine (8–10%). In 3-methylcholanthrene-treated rats, urinary elimination of unchanged theobromine was reduced from 23–27% to only 2%, while excretion of 6-amino-5-[N-methylformylamino]-1-methyluracil was significantly increased. Only 3,7-dimethyluric acid was produced by liver microsomal incubation in control rats while phenobarbital and 3-methylcholanthrene pretreatment enhanced the biotransformation resulting in the production of all metabolites found in vivo as well as unknown polar compounds (Shively & Vesell, 1987).
Pregnancy and increased doses of theobromine were shown to modify theobromine metabolism. At a dose of 50 mg/kg bw, pregnant rabbits excreted more unchanged theobromine in the urine (51% versus 35%; Latini et al., 1984). Pregnant rats excreted a higher percentage of a 5 mg/kg dose as unchanged theobromine (53%) than non-pregnant rats (39%); this difference disappeared at the saturation dose (100 mg/kg), when unchanged theobromine corresponded to about 60% of the dose in the urine of both pregnant and non-pregnant animals (Shively & Tarka, 1983). Rats given 100 mg/kg excreted more unchanged theobromine than those given 1 mg/kg (73% versus 51%), and showed a corresponding relative decrease in excretion of its uracil metabolite, 6-amino-5-[N-methylformylamino]-1-methyluracil (16% versus 28%) (Bonati et al., 1984).
About 60% of orally administered labelled theobromine was recovered unchanged in rat urine; 94–106% total urine radioactivity was recovered (Shively & Tarka, 1983). Large variations in faecal excretion (2–38% of the dose) were reported in metabolic experiments performed in rats, mice, hamsters, rabbits and dogs (Miller et al., 1984). Theobromine was excreted into the bile of dogs fed theobromine (Gans et al., 1980). Biliary secretion accounted for 5–10% of the administered [8−14C]theobromine dose in phenobarbital-induced rats (Shively & Vesell, 1987).
Urinary excretion of theobromine metabolites and theobromine clearance were increased in rats on a commercial diet compared to those on a semipurified diet (Shively et al., 1986xref>).
(ii) Toxic effects
The toxicity of theobromine as compared to other methylxanthines has been reviewed by Tarka (1982). The acute oral LD50 in rats was 950 mg/kg bw, whereas in mice it was 1356 mg/kg (for the sodium acetate). While the toxicity of theobromine in domestic animals has been indirectly attributed to excessive consumption of cocoa and chocolate products, there are few direct studies where theobromine was evaluated,because of its extremely low solubility in aqueous media. The oral LD50 of theobromine in dogs appears to be about 300 mg/kg bw (Gans et al., 1980).
The effects of theobromine on rodent and dog testis are reviewed below; the other target organ identified in rodents is the thymus gland. High doses — 250–300 mg/kg bw (mature animals) and 500 mg/kg bw (immature animals) — have been shown to cause complete thymic atrophy in male and female rats. This effect was seen in hamsters only at a level of 850 mg/kg bw and in mice at levels of 1840–1880 mg/kg bw (Tarka et al., 1979).
The only study of non-rodent species is that of Gans et al. (1980), who fed male dogs 100–150 mg/kg bw theobromine for periods of 21–28 days as well as various doses over a one-year period. They reported a degenerative and fibrotic lesion in the right atrial appendage of the heart. This finding appears to be unique to the dog since no such appendage exists in man. The study is further confounded by administration of varying doses in early treatment groups, with adjustments at several points in the one-year study.
A subchronic toxicity study performed in male and female Sprague-Dawley rats was reported in an abstract (Tarka & Zoumas, 1983). Theobromine was fed at levels of 0, 0.02, 0.1 and 0.2% of a chow diet for 90 days [corresponding to 25, 125 and 250 mg/kg bw/day]. The only changes noted were a reduction in body weight gain and testicular weight in males at the high dose. No pathological lesion was observed and there was no haematological change.
(iii) Effects on reproduction and prenatal toxicity
Reproductive toxicity: Feeding theobromine to male Osborne-Mendel rats at a dietary level of 0.5% for 64 weeks resulted in severe testicular atrophy in 94% of animals, with aspermatogenesis in 82% (Friedman et al., 1979). The results were confirmed in another strain of rats (Holtzman); following 19 weeks of feeding theobromine, all rats showed atrophy, and 79% had aspermatogenesis.
Tarka et al. (1979) found that feeding theobromine at levels of 0.2–1.0% in the diet (90–140 to 500–600 mg/kg bw per day) for a period of 28 days to rats produced severe testicular atrophy at the 0.8% level and seminiferous tubular-cell degeneration at the 0.6% level. Rats were found to be most sensitive, while mice (doses, 0.2–1.2%; 300–1850 mg/kg bw per day) were more resistant, and testicular changes were seen only at concentrations that caused considerable mortality. Hamsters (doses, 0.2–1.0%; 182–1027 mg/kg bw per day) were almost totally resistant to testicular changes. These authors also studied the potential reversibility of this phenomenon by feeding proven breeder male Sprague-Dawley rats 0.2, 0.6 or 0.8% theobromine (88, 244 or 334 mg/kg bw per day, respectively) for 49 days, performing unilateral orchiectomy at that time and allowing rats to recover on a theobromine-free diet for an additional 49 days. Histologically, the effects at the two highest dose levels were largely irreversible (Tarka et al., 1981). Daily administration of 500 mg/kg bw theobromine to Fü albino rats for three or five days interfered with germ cell kinetics but did not cause significant damage to spermatogonia. The release of late spermatids into the tubular lumen was retarded and generally occurred two weeks after treatment (Ettlin et al., 1986).
Subsequent studies (Gans, 1982, 1984) substantiated these observations. Significantly higher serum theobromine concentrations were achieved with a semisynthetic diet, resulting in more advanced morphological changes in the testes in rats, than with a chow diet. Shively et al. (1986) demonstrated that rats fed 0.6% theobromine in a certified chow diet for 28 days did not develop the testicular atrophy induced by addition of theobromine to a semisynthetic diet, due to induction of theobromine metabolism in animals on the chow diet.
Gans et al. (1980) studied the effects of short-term and long-term theobromine administration to male dogs; no testicular atrophy was seen at doses of 25, 50, 100 or 150 mg/kg per day over a one-year period.
Male and female Sprague-Dawley rats were given cocoa powder containing 2.50–2.58% theobromine and 0.19% caffeine in the diet at concentrations of 0, 1.5, 3.5 and 5.0% for three generations. Males and females were given diets containing cocoa powder for 12 and 2 weeks, respectively, prior to mating. The average methylxanthine doses for males/females were 30/36, 72/86 and 104/126 mg/kg bw per day in the 1.5, 3.5 and 5.0% cocoa powder groups, respectively. No consistent dose-related effect was observed in any reproductive index; nonreproductive toxicity was observed at the two highest dose levels (Hostetler et al., 1990).
Developmental toxicity: ICR-JCL mice received a single intraperitoneal injection of 500 or 600 mg/kg bw theobromine on day 12 of gestation. Maternal deaths occurred in 40% of the higher-dose group but not in the lower-dose group. The incidence of resorptions was significantly increased with the higher dose; at both dose levels, fetal body weight was decreased and the incidence of malformations and subcutaneous haematomas was increased (Fujii & Nishimura, 1969).
In Sprague-Dawley rats fed diets containing theobromine (daily doses, 53 or 99 mg/kg bw) on gestation days 6–19, no maternal toxicity was observed. Although no malformation occurred, slight decreases in fetal body weight were observed with the high dose, and a significant increase was seen in the frequency of skeletal variations. Serum concentrations of theobromine in the high-dose group were 15–20 µl/ml (Tarka et al., 1986a).
New Zealand white rabbits were administered up to 200 mg/kg bw theobromine by gavage on gestation days 6–29. Maternal deaths occurred in 40% of the group receiving the highest dose level, but little or no maternal toxicity was observed with 25, 75 or 125 mg/kg bw per day. Decreased fetal body weight and malformations were seen at doses of 125 or 200 mg/kg; the incidence of skeletal variations was increased with 75 mg/kg and over. With 75 mg/kg per day — the lowest dose at which developmental toxicity was observed — serum concentrations of theobromine were 24–86 µl/ml. In other groups of New Zealand rabbits fed diets containing theobromine (daily doses, 21, 41 or 63 mg/kg bw), little or no maternal toxicity was observed at any dose level. Fetal body weight was decreased at 41 and 63 mg/kg bw, and there were significant increases in the frequency of skeletal variations. Average serum concentrations at the lowest effective concentrations were 12–15 µl/ml (Tarka et al., 1986b). [The Working Group noted that serum concentrations of theobromine following administration of the lowest effective dose were proportionally higher in rabbits than in rats.]
(iv) Genetic and related effects
The genetic effects of theobromine have been reviewed (Timson, 1975, 1977; Tarka, 1982; Grice, 1987; Rosenkranz & Ennever, 1987a,b).
The results described below are listed in Table 1 on p. 433, with the evaluation of the Working Group, as positive, negative or inconclusive, as defined in the footnotes. The results are tabulated separately for the presence and absence of an exogenous metabolic system. The lowest effective dose (LED), in the case of positive results, or the highest ineffective dose (HID), in the case of negative results, are shown, together with the appropriate reference. The studies are summarized briefly below.
Table 1.
Genetic and related effects of theobromine.
Theobromine has only a very weak capacity to displace acridine orange from DNA in vitro (Richardson et al., 1981). In extracts of Escherichia coli, it selectively inhibited some purine nucleoside phosphorylases (Koch & Lamont, 1956). Theobromine was not incorporated to ‘any great extent’ into the DNA of E. coli, which possibly cannot demethylate this substance (Koch, 1956). The effects of theobromine on relevant targets other than DNA are discussed in the monograph on caffeine (p. 332).
Theobromine was mutagenic to E. coli under conditions in which a constant growth rate and cell population density were maintained, but it was not mutagenic to Salmonella typhimurium. Theobromine induced mutations in a lower eukaryote, Euglena gracilis.
Theobromine did not induce chromosomal aberrations in plants (Vicia faba). It was reported in an abstract that chromosomal aberrations were not observed in Drosophila melanogaster treated with 0.45% theobromine in feeding experiments (Mittler & Mittler, 1968).
Theobromine increased the frequency of mutant tk colonies in mouse lymphoma cells, but only at extremely cytotoxic doses. Significant increases in the frequency of sister chromatid exchange were induced in Chinese hamster CHO cells in the absence of an exogenous metabolic system; in the presence of an exogenous metabolic system the results were equivocal and not dose-related (Brusick et al., 1986). Chromosomal aberrations were not induced by theobromine in Chinese hamster cells. BALB/c 3T3 cells were not morphologically transformed by treatment with theobromine, and, unlike theophylline but like caffeine, theobromine did not reduce the expression of parameters associated with morphological transformation (Rajaraman & Faulkner, 1984).
In human lymphocyte cultures, theobromine did not significantly increase the number of sister chromatid exchanges per cell, but, in another experiment using higher doses, the numbers of sister chromatid exchanges per cell were increased in the absence of an exogenous metabolic system. The induction of sister chromatid exchange is not necessarily due to a directly damaging effect upon DNA, since theobromine can have indirect effects (Levi et al., 1978) which are associated with the induction of sister chromatid exchange (Morgan & Cleaver, 1982) and may even give rise to false-positive effects, the primary effect upon DNA being due to bromodeoxyuridine (Natarajan et al., 1981). Theobromine did, however, induce breaks in human lymphocytes in culture, contrary to the results with rodent cells (see above).
Theobromine induced sister chromatid exchange and micronuclei, but not chromosomal aberrations, in the bone marrow of Chinese hamsters treated in vivo. No dominant lethal effect (increases in either preimplantation loss or dead implants) was observed in either CD-1 mice or in male Sprague-Dawley rats. The negative result in rats was not due to pharmacokinetic limitations, as demonstrated above.
(b) Humans
(i) Absorption, distribution, excretion and metabolism
Theobromine is readily absorbed from food and evenly distributed in body fluids; the half-times in plasma and saliva are highly correlated (Drouillard et al., 1978). Theobromine has been reported to pass into the breast milk of nursing mothers (Resman et al., 1977).
The mean half-time of theobromine in human serum ranged from 6.1 to 10 h (Drouillard et al., 1978; Tarka et al., 1983; Shively et al., 1985); the apparent volumes of distribution and clearance were estimated to be 0.761/kg bw and 0.88 ml/min/kg bw, respectively (Shively et al., 1985).
The major metabolite of theobromine in human urine is 7-methylxanthine (34–48%), followed by 3-methylxanthine (20%) and 7-methyluric acid (7–12%), 6-amino-5-[N-methylformylamino]-1-methyluracil (6–9%) and 3,7-dimethyluric acid (1%). Of the dose, 1–18% is recovered in the urine as unchanged theobromine (Tarka et al., 1983; Birkett et al., 1985). Theobromine metabolites were not found in the plasma of human subjects (Tarka et al., 19833). Theobromine has a low protein binding capacity in both serum (15–21%) and breast milk (12%) (Resman et al., 1977; Birkett et al., 1985).
(ii) Toxic effects
It has been stated that ‘in large doses’ theobromine may cause nausea and anorexia (Reynolds, 1982) and that daily intake of 50–100 g cocoa (0.8–1.5 g theobromine) by humans has been associated with sweating, trembling and severe headache (Czok, 1974). In a study of 13 volunteers who consumed 200 mg theobromine orally three times during a 24-h period, no clinical symptom or other pharmacological activity was observed (Birkett et al., 1985). Ingestion of theobromine in sweet chocolate at a dose of 6 mg/kg bw per day had no effect on clinical parameters in 12 human subjects (Shively et al., 1985).
Studies on a possible association between consumption of methylxanthines and benign breast disease are summarized on pp. 347–350.
(iii) Effects on reproduction and prenatal toxicity
No data were available to the Working Group.
(iv) Genetic and related effects
No data were available to the Working Group.
3.3. Epidemiological studies of carcinogenicity to humans
Studies on methylxanthines are summarized in the monograph on caffeine.
4. Summary of Data Reported and Evaluation
4.1. Exposure data
Theobromine is the principal alkaloid of the cacao bean. It is extracted from the bean husks and used in the synthesis of caffeine. It has been used in various pharmaceutical products. Theobromine is consumed in cocoa and chocolate beverages and in various forms of chocolate-based foods. Theobromine is also present in small amounts in green coffee beans, tea and mate.
Daily per-caput consumption of theobromine in the USA in 1980 from food and beverages was estimated to be 39 mg.
4.2. Experimental carcinogenicity data
No data on the carcinogenicity of theobromine were available.
4.3. Human carcinogenicity data
No data were available to the Working Group to evaluate the carcinogenicity of theobromine per se.
For descriptions of studies on methylxanthines, see the monograph on caffeine.
4.4. Other relevant data
Oral administration of high doses of theobromine to rats caused severe testicular atrophy, which was largely irreversible. Administration of lower levels for prolonged periods had no significant adverse effect on the testis. Mice, hamsters and dogs were less sensitive than rats or were resistant to the effect of theobromine in causing testicular changes. No adverse reproductive effect was observed in a three-generation study in rats given cocoa powder containing theobromine in their diet. Teratogenic effects were observed in rabbits after gavage but not after dietary administration of theobromine. The signs of developmental toxicity observed at the lowest dose level included decreased fetal body weight and increased skeletal variations in rabbits. No teratogenic effect was seen in rats.
In vivo, theobromine did not induce dominant lethal effects in mice or rats. It induced sister chromatid exchange and micronuclei but not chromosomal aberrations in the bone marrow of Chinese hamsters. In human cells in vitro, theobromine induced sister chromatid exchange and chromosomal breaks. In cultured mammalian cells, it induced gene mutations and sister chromatid exchange but not chromosomal aberrations or cell transformation. In plants, theobromine did not induce chromosomal aberrations. It induced gene mutations in lower eukaryotes and bacteria but gave negative results in the Salmonella/mammalian microsome assay.
4.5. Evaluation1
There is inadequate evidence for the carcinogenicity in humans of theobromine. There are no data on the carcinogenicity of theobromine in experimental animals.
Overall evaluation
Theobromine is not classifiable as to its carcinogenicity to humans (Group 3).
5. References
- Anon. (1988) British Pharmacopoeia, Vol. 1, London, Her Majesty’s Stationery Office, p. 564.
- Arnaud, M.J. (1984) Products of metabolism of caffeine. In: Dews, P.B., ed., Caffeine. Perspectives from Recent Research, Berlin, Springer, pp. 3–38.
- Arnaud M.J. The pharmacology of caffeine. Progr. Drug Res. 1987;31:273–313. [PubMed: 3326033]
- Arnaud M.J., Getaz F. Postnatal establishment of a blood-brain barrier for theobromine in the rat (Abstract). Experientia. 1982;38:752.
- Arnaud M.J., Welsch C. Metabolic pathway of theobromine in the rat and identification of two new metabolites in human urine. J. agric. Food Chem. 1979;27:524–527. [PubMed: 447925]
- Birkett D.J., Dahlqvist R., Miners J.O., Lelo A., Billing B. Comparison of theophylline and theobromine metabolism in man. Drug Metab. Disposition. 1985;13:725–728. [PubMed: 2867879]
- Blauch J.L., Tarka S.M. Jr. HPLC determination of caffeine and theobromine in coffee, tea and instant hot cocoa mixes. J. Food Sci. 1983;48:745–750.
- Bonati M., Latini R., Sadurska B., Riva E., Galletti F., Borzelleca J.F., Tarka S.M., Arnaud M.J., Garattini S. I. Kinetics and metabolism of theobromine in male rats. Toxicology. 1984;30:327–341. [PubMed: 6729831]
- Brusick D., Myhr B., Galloway S., Rundell J., Jagannath D.R., Tarka S. Genotoxicity of theobromine in a series of short-term assays. Mutat. Res. 1986;169:105–114. [PubMed: 3512993]
- Christensen, H.D. & Neims, A.H. (1984) Measurement of caffeine and its metabolites in biological fluids. In: Dews, PB., ed., Caffeine. Perspectives from Recent Research, Berlin, Springer, pp. 39–47.
- Christensen, H.D. & Whitsett, T.L. (1979) Measurement of xanthines and their metabolites by means of high pressure liquid chromatography. In: Hawk, G.L., ed., Biological/Biomedical Applications of Liquid Chromatography (Chromatogr. Ser. 10), New York, Marcel Dekker, pp. 507–537.
- Come T.V., Travis D.M. Induction of auxotrophic mutations in Euglena gracilis. J. Hered. 1969;60:39–41. [PubMed: 5798142]
- Cordell, G.A. (1978) Alkaloids. In: Mark, H.F., Othmer, D.F., Overberger, C.G., Seaborg, G.T. & Grayson, M., eds, Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed., Vol. 1, New York, John Wiley & Sons, pp. 923–925.
- Craig W.J., Nguyen T.T. Caffeine and theobromine levels in cocoa and carob products. J. Food Sci. 1984;49:302–305.
- Czok G. Concerning the question of the biological effectiveness of methylxanthines in cocoa products (Ger.). Z. Ernahrungswiss. 1974;13:165–171. [PubMed: 4450589]
- De Vries J.W., Johnson K.D., Heroff J.C. HPLC determination of caffeine and theobromine content of various natural and red dutched cocoas. J. Food Sci. 1981;46:1968–1969.
- Drouillard D.D., Vesell E.S., Dvorchik B.H. Studies on theobromine disposition in normal subjects. Alterations induced by dietary abstention or exposure to methylxanthines. Clin. pharmacol. Ther. 1978;23:296–302. [PubMed: 627135]
- Epstein S.S., Shafner H. Chemical mutagens in the human environment. Nature. 1968;219:385–387. [PubMed: 5662155]
- Epstein S.S., Arnold E., Andrea J., Bass W., Bishop Y. Detection of chemical mutagens by the dominant lethal assay in the mouse. Toxicol. appl. Pharmacol. 1972;23:288–325. [PubMed: 5074577]
- Ettlin R.A., Armstrong J.M., Buser S., Hennes U. Retardation of spermiation following short-term treatment of rats with theobromine. Arch. Toxicol. 1986;(9):441–446. [PubMed: 3468927]
- Friedman L., Weinberger M.A., Farber T.M., Moreland F.M., Peters E.L., Gilmore C.E., Khan M.A. Testicular atrophy and impaired spermatogenesis in rats fed high levels of the methylxanthines caffeine, theobromine, or theophylline. J. environ. Pathol. Toxicol. 1979;2:687–706. [PubMed: 422930]
- Fujii T., Nishimura H. Teratogenic actions of some methylated xanthines in mice. Okajimas Folia anat. jpn. 1969;46:167–175. [PubMed: 5394617]
- Gans J.H. Dietary influences on theobromine-induced toxicity in rats. Toxicol. appl. Pharmacol. 1982;63:312–320. [PubMed: 6283693]
- Gans J.H. Comparative toxicities of dietary caffeine and theobromine in the rat. Food chem. Toxicol. 1984;22:365–369. [PubMed: 6539285]
- Gans J.H., Korson R., Cater M.R., Ackerly C.C. Effects of short-term and long-term theobromine administration to male dogs. Toxicol. appl. Pharmacol. 1980;53:481–496. [PubMed: 6446176]
- Gennaro, A.R., ed. (1985) Remington’s Pharmaceutical Sciences, 17th ed., Easton, PA, Mack Publishing, pp. 944, 1070, 1135.
- Gerritsma K.W., Koers J. Determination of theobromine in cocoa residues. Analyst. 1953;78:201–205.
- Graham, H.N. (1984a) Tea: the plant and its manufacture: chemistry and conception of the beverage. In: Spiller, G.A., ed., The Methylxanthine Beverages and Foods: Chemistry, Consumption, and Health Effects, New York, Alan R. Liss, pp. 29–74.
- Graham, H.N. (1984b) Mate. In: Spiller, G.A., ed., The Methylxanthine Beverages and Foods: Chemistry, Consumption, and Health Effects, New York, Alan R. Liss, pp. 179–183.
- Grice H.C. Genotoxicity and carcinogenicity assessment of caffeine and theobromine (Letter to the Editor). Food chem. Toxicol. 1987;25:295–296.
- Hatfull R.S., Milner I., Stanway V. Determination of theobromine in animal feeding stuffs. J. Assoc. public Analysts. 1980;18:19–22.
- Hirsh, K. (1984) Central nervous system pharmacology of the dietary methylxanthines. In: Spiller, G.A., ed., The Methylxanthine Beverages and Foods: Chemistry, Consumption, and Health Effects, New York, Alan R. Liss, pp. 235–301.
- Horwitz, W., ed. (1980) Official Methods of Analysis of the Association of Official Analytical Chemists, 13th ed., Washington DC, Association of Official Analytical Chemists.
- Hostetler K.A., Morrissey R.B., Tarka S.M. Jr, Apgar J.L., Shively C.A. Three generations reproductive study of cocoa powder in rats. Food chem. Toxicol. 1990;28:483–490. [PubMed: 2210520]
- Hurst, W.J., Martin, R.A. & Tarka, S.M., Jr (1984) Analytical methods for quantitation of methylxanthines. In: Spiller, G.A., ed., The Methylxanthine Beverages and Foods: Chemistry, Consumption and Health Effects, New York, Alan R. Liss, pp. 17–28.
- Jalal M.A.F., Collin H.A. Estimation of caffeine, theophylline and theobromine in plant material. New Phytol. 1976;76:277–281.
- Klassen R., Stavric B. HPLC separation of theophylline, paraxanthine, theobromine, caffeine and other metabolites in biological fluids. J. liquid Chromatogr. 1983;6:895–906.
- Koch A.L. The metabolism of methylpurines by Escherichia coli. I. Tracer studies. J. biol. Chem. 1956;219:181–188. [PubMed: 13295270]
- Koch A.L., Lamont W.A. The metabolism of methylpurines by Escherichia coli. IL Enzymatic studies. J. biol. Chem. 1956;219:189–201. [PubMed: 13295271]
- Kreiser W.R., Martin R.A. Jr. High pressure liquid chromatographic determination of theobromine and caffeine in cocoa and chocolate products. J. Assoc. off. anal. Chem. 1978;61:1424–1427. [PubMed: 730647]
- Kreiser W.R., Martin R.A. Jr. High pressure liquid chromatographic determination of theobromine and caffeine in cocoa and chocolate products: collaborative study. J. Assoc. off. anal. Chem. 1980;63:591–594. [PubMed: 7430044]
- Latini R., Bonati M., Gaspari F., Traina G.L., Jiritano L., Bortolotti A, Borzelleca J.F., Tarka S.M., Arnaud M.J., Garattini S. II. Kinetics and metabolism of theobromine in male and female non-pregnant and pregnant rabbits. Toxicology. 1984;30:343–354. [PubMed: 6729832]
- Levi V., Jacobson E.L., Jacobson M.K. Inhibition of poly(ADP-ribose)polymerase by methylated xanthines and cytokinins. FEBS Lett. 1978;88:144–146. [PubMed: 205432]
- Martinek R.G., Wolman W. Xanthines, tannins and sodium in coffee, tea and cocoa. J. Am. med. Assoc. 1955;158:1030–1031. [PubMed: 14392051]
- McCutheon, G.F. (1969) Caffeine. In: Snell, F.D. & Ettre, L.S., eds, Encyclopedia of Industrial Chemical Analysis, Vol. 8, New York, Interscience, pp. 55–71.
- Michl H., Haberler F. Determination of purines in caffeine-containing drugs (Ger.). Monatshefte Chem. 1954;85:779–795.
- Miller G.E., Radulovic L.L., Dewit R.H., Brabec M.J., Tarka S.M., Cornish H.H. Comparative theobromine metabolism in five mammalian species. Drug Metab. Disposition. 1984;12:154–160. [PubMed: 6144479]
- Mittler S., Mittler J.E. Theobromine and theophylline and chromosome aberrations in Drosophila melanogaster (Abstract). Genetics. 1968;60:205.
- Moffat, A.C., ed. (1986) Clarke’s Isolation and Identification of Drugs, 2nd ed., London, The Pharmaceutical Press, pp. 1010–1011.
- Morgan W.F., Cleaver J.E. 3-Aminobenzamide synergistically increases sister-chromatid exchanges in cells exposed to methyl methanesulfonate but not to ultraviolet light. Mutat. Res. 1982;104:361–366. [PubMed: 6287250]
- Mourelatos D., Dozi-Vassiliades J., Granitsas A. Anti-tumour alkylating agents act synergistically with methylxanthines on induction of sister-chromatid exchange in human lymphocytes. Mutat. Res. 1982;104:243–247. [PubMed: 7202117]
- Mourelatos D., Dozi-Vassiliades J., Tsigalidou-Balla, Granitsas A. Enhancement by methylxanthines of sister-chromatid exchange frequency induced by cytostatics in normal and leukemic human lymphocytes. Mutat. Res. 1983;121:147–152. [PubMed: 6410232]
- Natarajan A.T., Csukás I., van Zeeland A.A. Contribution of incorporated 5-bromodeoxyuridine in DNA to the frequencies of sister-chromatid exchanges induced by inhibitors of poly-(ADP-ribose)-polymerase. Mutat. Res. 1981;84:125–132. [PubMed: 7199115]
- National Research Council (1977) Drinking Water and Health, Washington DC, National Academy of Sciences.
- Nomura T. Comparative inhibiting effects of methylxanthines on urethan-induced tumors, malformations, and presumed somatic mutations in mice. Cancer Res. 1983;43:1342–1346. [PubMed: 6825104]
- Novick A. Mutagens and antimutagens. Brookhaven Symp. Biol. 1956;8:201–214. [PubMed: 13293429]
- Novick A., Szilard L. Experiments in spontaneous and chemically induced mutations of bacteria growing in the chemostat. Cold Spring Harbor Symp. quant. Biol. 1951;16:337–343. [PubMed: 14942748]
- Novick A., Szilard L. Anti-mutagens. Nature. 1952;170:926–927. [PubMed: 13013261]
- Perry, D.L., Chuang, C.C., Jungclaus, G.A. & Warner, J.S. (1979) Identification of Organic Compounds in Industrial Effluent Discharges (EPA-600/4-79-016), Athens, GA, US Environmental Protection Agency.
- Rajaraman R., Faulkner G. Reverse transformation of Chinese hamster ovary cells by methyl xanthines. Exp. Cell Res. 1984;154:342–356. [PubMed: 6090184]
- Reid S.J., Good T.J. Use of chromatographic mode sequencing for sample preparation in the analysis of caffeine and theobromine from beverages. J. agric. Food Chem. 1982;30:775–778.
- Renner H.W. Sister chromatid exchanges induced by methylxanthines contained in coffee, tea and cocoa. Experientia. 1982;38:600. [PubMed: 7095098]
- Renner H.W, Münzner R. Genotoxicity of cocoa examined by microbial and mammalian systems. Mutat. Res. 1982;103:275–281. [PubMed: 7045646]
- Resman B.H., Blumenthal H.P., Jusko W.J. Breast milk distribution of theobromine from chocolate. J. Pediatr. 1977;91:477–480. [PubMed: 894424]
- Reynolds, J.E.F., ed. (1982) Martindale. The Extra Pharmacopoeia, 28th ed., London, The Pharmaceutical Press, pp. 348–349.
- Richardson C.L., Grant A.D., Schulman G.E. The interaction of caffeine and other xanthine analogs with DNA as measured by competitive flurorescence polarization (Abstract No. Ac-5). Environ. Mutagenesis. 1981;3:343.
- Rosenkranz H.S., Ennever F.K. Evaluation of the genotoxicity of theobromine and caffeine. Food chem. Toxicol. 1987a;25:247–251. [PubMed: 3106176]
- Rosenkranz H.S., Ennever F.K. Genotoxicity and carcinogenicity assessment of caffeine and theobromine (Letter to the Editor). Food chem. Toxicol. 1987b;25:795–796. [PubMed: 3679025]
- Schack J.A., Waxier S.H. An ultraviolet spectrophotometric method for the determination of theophylline and theobromine in blood and tissues. J. Pharmacol., exp. Ther. 1949;97:283–291. [PubMed: 15392550]
- Senanayake U.M., Wijesekera R.O.B. Determination of the fat-free cocoa mass in chocolate products based on the theobromine and caffeine content. Int. Chocolate Rev. 1968a;23:214–217.
- Senanayake U.M., Wijesekera R.O.B. A rapid micro-method for the separation, identification and estimation of the purine bases: caffeine, theobromine and theophylline. J. Chromatogr. 1968b;32:75–86. [PubMed: 4297182]
- Senanayake U.M., Wijesekera R.O.B. Theobromine and caffeine content of the cocoa bean during its growth. J. Sci. Food Agric. 1971;22:262–263.
- Shively C.A., Tarka S.M. Jr. Theobromine metabolism and pharmacokinetics in pregnant and nonpregnant Sprague-Dawley rats. Toxicol. appl. Pharmacol. 1983;67:376–382. [PubMed: 6845367]
- Shively, C.A. & Tarka, S.M., Jr (1984) Methylxanthine composition and consumption patterns of cocoa and chocolate products. In: Spiller, G.A., ed., The Methylxanthine Beverages and Foods: Chemistry, Consumption, and Health Effects, New York, Alan R. Liss, pp. 149–178.
- Shively C.A., Vesell E.S. In vivo and in vitro biotransformation of theobromine by phenobarbital- and 3-methylcholanthrene-inducible cytochrome P-450 mono-oxygeneses in rat liver Role of thiol compounds. Drug Metab. Disposition. 1987;15:217–224. [PubMed: 2882982]
- Shively C.A., White D.M., Blauch J.L., Tarka S.M. Jr. Dominant lethal testing of theobromine in rats. Toxicol. Lett. 1984;20:325–329. [PubMed: 6701919]
- Shively C.A., Tarka S.M. Jr, Arnaud M.J., Dvorchik B.H., Passananti G.T., Vesell E.S. High levels of methylxanthines in chocolate do not alter theobromine disposition. Clin. pharmacol. Ther. 1985;37:415–424. [PubMed: 3979003]
- Shively C.A., White D.M., Tarka S.M. Jr. Diet-induced alterations in theobromine disposition and toxicity in the rat. Toxicol. appl. Pharmacol. 1986;84:593–598. [PubMed: 3726878]
- Sontag, G. & Kral, K. (1980) Determination of caffeine, theobromine and theophylline in tea, coffee, cocoa and beverages by HPLC with electrochemical detector (Ger.). Mikrochim. Acta (Wien), II, 39–52.
- Spiller, M.A. (1984) The chemical components of coffee. In: Spiller, G.A., ed., The Methylxanthine Beverages and Foods: Chemistry, Consumption, and Health Effects, New York, Alan R. Liss, pp. 91–147.
- Stavric B. Methyl xanthines: toxicity to humans. 3. Theobromine, paraxanthine and the combined effects of methylxanthines. Food chem. Toxicol. 1988;26:725–733. [PubMed: 3058562]
- Sturelid S., Kihlman B.A. Enhancement by methylated oxypurines of the frequency of induced chromosomal aberrations. I. The dependence of the effect on the molecular structure of the potentiating agent. Hereditas. 1975;79:29–42. [PubMed: 809386]
- Tang-Liu D.D.-S., Riegelman S. An automated HPLC assay for simultaneous quantitation of methylated xanthines and uric acids in urine. J. chromatogr. Sci. 1982;20:155–159. [PubMed: 7096523]
- Tarka, S.M., Jr (1982) The toxicology of cocoa and methylxanthines: a review of the literature. Crit. Rev. Toxicol. 9, 275–312. [PubMed: 6765610]
- Tarka, S.M., Jr & Shively, C.A. (1987) Methylxanthines. In: Miller, K., ed., Toxicological Aspects of Food, Amsterdam, Elsevier, pp. 373–423.
- Tarka S.M. Jr, Zoumas B.L. Subchronic and oral toxicity evaluation of cocoa powder and theobromine in Sprague-Dawley rat (Abstract). Toxicologist. 1983;3:4.
- Tarka S.M. Jr, Zoumas B.L., Gans J.H. Short-term effects of graded levels of theobromine in laboratory rodents. Toxicol. appl. Pharmacol. 1979;49:127–149. [PubMed: 473198]
- Tarka S.M. Jr, Zoumas B.L., Gans J.H. Effects of continuous administration of dietary theobromine on rat testicular weight and morphology. Toxicol. appl. Pharmacol. 1981;58:76–82. [PubMed: 7233440]
- Tarka S.M. Jr, Arnaud M.J., Dvorchik B.H., Vesell E.S. Theobromine kinetics and metabolic disposition. Clin. pharmacol. Ther. 1983;34:546–555. [PubMed: 6617078]
- Tarka S.M. Jr, Applebaum R.S., Borzelleca J.F. Evaluation of the perinatal, postnatal and teratogenic effects of cocoa powder and theobromine in Sprague-Dawley/C rats. Food chem. Toxicol. 1986a;24:375–382. [PubMed: 3744190]
- Tarka S.M. Jr, Applebaum R.S., Borzelleca J.F. Evaluation of the teratogenic potential of cocoa powder and theobromine in New Zealand white rabbits. Food chem. Toxicol. 1986b;24:363–374. [PubMed: 3744189]
- Timbie D.J., Sechrist L., Keeney P.G. Application of high-pressure liquid chromatography to the study of variables affecting theobromine and caffeine concentrations in cocoa beans. J. Food Sci. 1978;43:560–565.
- Timson J. Theobromine and theophylline. Mutat. Res. 1975;32:169–178. [PubMed: 765793]
- Timson J. Caffeine. Mutat. Res. 1977;47:1–52. [PubMed: 342928]
- Traina G.L., Bonati M. Pharmacokinetics of theobromine and its metabolites in rabbits. J. Pharmacokinet. Biopharmacol. 1985;13:41–53. [PubMed: 4020621]
- Vergnes M.F., Alary J. Determination of natural xanthines by HPLC (Fr.). Talanta. 1986;33:997–1000. [PubMed: 18964243]
- Weinstein D., Mauer I., Katz M.L., Kazmer S. The effect of caffeine on chromosomes of human lymphocytes: a search for the mechanism of action. Mutat. Res. 1973;20:115–125. [PubMed: 4357386]
- Weinstein D., Mauer I., Katz M.L., Kazmer S. The effect of methylxanthines on chromosomes of human lymphocytes in culture. Mutat. Res. 1975;31:57–61. [PubMed: 1128545]
- Wildanger W. Separation of caffeine, theophylline and theobromine using high-pressure liquid chromatography (Ger.). J. Chromatogr. 1975;114:480–482. [PubMed: 1202052]
- Windholz, M., ed. (1983) The Merck Index, 10th ed., Rahway, NJ, Merck & Co., p. 1327.
- Woollard D.C. The determination of cocoa solids in milkpowder products using high performance liquid chromatography. N.Z. J. Dairy Sci. Technol. 1982;17:63–68.
- Zoumas B.L., Kreiser W.R., Martin R.A. Theobromine and caffeine content of chocolate products. J. Food Sci. 1980;45:314–316.
Footnotes
- 1
For descriptions of the italicized terms, see Preamble, pp. 27–31.
- PubMedLinks to PubMed
- Review Recent advances in caffeine and theobromine toxicities: a review.[Plant Foods Hum Nutr. 1997]Review Recent advances in caffeine and theobromine toxicities: a review.Eteng MU, Eyong EU, Akpanyung EO, Agiang MA, Aremu CY. Plant Foods Hum Nutr. 1997; 51(3):231-43.
- Smoking, alcohol, coffee, tea, caffeine, and theobromine: risk of prostate cancer in Utah (United States).[Cancer Causes Control. 1993]Smoking, alcohol, coffee, tea, caffeine, and theobromine: risk of prostate cancer in Utah (United States).Slattery ML, West DW. Cancer Causes Control. 1993 Nov; 4(6):559-63.
- [Quantitative determination of theobromine in theobromine-sodium salicylate].[Acta Pol Pharm. 1952][Quantitative determination of theobromine in theobromine-sodium salicylate].KULIGA T, BOBRANSKI B. Acta Pol Pharm. 1952; 9(1):1-11.
- Behavioral effects of cocoa and its main active compound theobromine: evaluation by ambulatory activity and discrete avoidance in mice.[Arukoru Kenkyuto Yakubutsu Iso...]Behavioral effects of cocoa and its main active compound theobromine: evaluation by ambulatory activity and discrete avoidance in mice.Kuribara H, Tadokoro S. Arukoru Kenkyuto Yakubutsu Ison. 1992 Apr; 27(2):168-79.
- Review Theobromine and the pharmacology of cocoa.[Handb Exp Pharmacol. 2011]Review Theobromine and the pharmacology of cocoa.Smit HJ. Handb Exp Pharmacol. 2011; (200):201-34.
- Theobromine - Coffee, Tea, Mate, Methylxanthines and MethylglyoxalTheobromine - Coffee, Tea, Mate, Methylxanthines and Methylglyoxal
Your browsing activity is empty.
Activity recording is turned off.
See more...
