Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
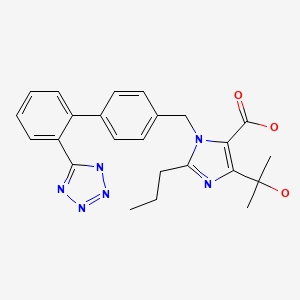
CASRN: 144689-24-7
Drug Levels and Effects
Summary of Use during Lactation
With the exception of one adverse reaction in a breastfed infant, no information is available on the use of olmesartan during breastfeeding. An alternate drug may be preferred, especially while nursing a newborn or preterm infant.
Drug Levels
Maternal Levels. Relevant published information was not found as of the revision date.
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
A 6-day-old healthy full-term newborn was exposed to olmesartan in breastmilk. The maternal dose of olmesartan and extent of nursing were not reported. Weight gain was normal during the first two weeks of life, but at the pediatric checkup on the 17th day postpartum, an abrupt decrease in body weight was recorded. He was hospitalized on the 21st day, and mixed feeding with milk and formula was started. Biochemical examinations showed aspartate-aminotransferase (AST) 250 mg/dL, and regular urinalysis. Virologic and metabolic causes of elevated transaminase were ruled out. The baby started to regain weight and his AST gradually normalized to 50 mg/dL by the 24th day. Olmesartan intake was stopped and the child was discharged on the 24th day of life.[1]
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Alternate Drugs to Consider
References
- 1.
- Salimova M, Maggi M, Crevani M, et al. Olmesartan-induced reversible transaminase elevation in a breastfed newborn. A case report. Neurotoxicol Teratol 2023;98:24. doi:10.1016/j.ntt.2023.107236 [CrossRef]
Substance Identification
Substance Name
Olmesartan
CAS Registry Number
144689-24-7
Drug Class
Breast Feeding
Milk, Human
Antihypertensive Agents
Angiotensin II Type 1 Receptor Blockers
Angiotensin Receptor Blockers
ARBs
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PubChem SubstanceRelated PubChem Substances
- Review Losartan.[Drugs and Lactation Database (...]Review Losartan.. Drugs and Lactation Database (LactMed®). 2006
- Review Valsartan.[Drugs and Lactation Database (...]Review Valsartan.. Drugs and Lactation Database (LactMed®). 2006
- Review Azilsartan.[Drugs and Lactation Database (...]Review Azilsartan.. Drugs and Lactation Database (LactMed®). 2006
- Review Candesartan.[Drugs and Lactation Database (...]Review Candesartan.. Drugs and Lactation Database (LactMed®). 2006
- Review Cefazolin.[Drugs and Lactation Database (...]Review Cefazolin.. Drugs and Lactation Database (LactMed®). 2006
- Olmesartan - Drugs and Lactation Database (LactMed®)Olmesartan - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
