Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
CASRN: 364-62-5
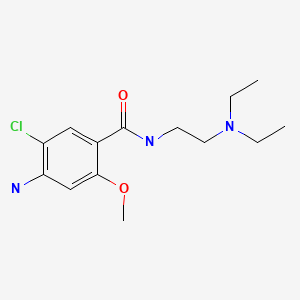
Drug Levels and Effects
Summary of Use during Lactation
Metoclopramide is excreted in variable amounts in breastmilk. After oral and intranasal administration, most infants would receive less than 10% of the maternal weight-adjusted dosage, but some receive doses that achieve pharmacologically active serum levels, elevated serum prolactin and possible gastrointestinal side effects. Although most studies have found no adverse effects in breastfed infants during maternal metoclopramide use, many did not adequately observe for side effects.
Metoclopramide increases serum prolactin and has been used as a galactogogue.[1,2] A meta-analysis of 5 placebo-controlled studies concluded that 2 weeks of metoclopramide caused no increase of serum prolactin over placebo, but 3 weeks of treatment did.[3] A more recent meta-analysis concluded that metoclopramide was of no benefit as a galactogogue in the mothers of preterm infants.[4] A third meta-analysis of 8 trials involving 342 lactating women with a preterm or full-term infant found that metoclopramide increased serum prolactin, but did not increase milk supply.[5] The clinical value of metoclopramide in increasing milk supply is questionable. Galactogogues should never replace evaluation and counseling on modifiable factors that affect milk production.[6,7] In well-designed studies that evaluated the effectiveness of metoclopramide as a galactogogue in women who continue to have difficulty producing milk after nursing techniques have been optimized, it was of no additional benefit. Prophylactic use in the mothers of preterm infants has also shown little or no benefit.
Metoclopramide has no officially established dosage for increasing milk supply. Most studies have used metoclopramide in a dosage of 10 mg 2 or 3 times daily for 7 to 14 days. Some studies used a tapering dosage for the last days few of the regimen to avoid an abrupt drop in milk supply after drug discontinuation. No published literature supports the efficacy or safety of higher dosages, longer treatment periods or repeated courses of therapy.
Postpartum mothers are at a relatively high risk for postpartum depression and metoclopramide can cause depression as a side effect. Therefore, metoclopramide should probably be avoided in women with a history of major depression and not used for prolonged periods in any mothers during this time of high susceptibility.[8,9] Long-term uses of metoclopramide also increases the risk of tardive dyskinesia.[10] Other reported side effects in nursing mothers include tiredness, nausea, headache, diarrhea, dry mouth, breast discomfort, vertigo, restless legs, intestinal gas, hair loss, irritability and anxiety.[9,11-13] In a survey of nursing mothers in the United States, 32 had used metoclopramide as a galactogogue and all reported having experienced an adverse reaction from the drug.[14] A larger survey of women taking metoclopramide for lactation enhancement found that 4.8% of women had either palpitations or racing heart rate, 12% reported depression, and 1 to 7% reported other central nervous system side effects ranging from dizziness and headache to involuntary grimacing and tremors. Diarrhea, irritability and fatigue were also relatively common.[15]
Drug Levels
Maternal Levels. Ten mothers who were 7 to 10 days postpartum were given a single oral dose of metoclopramide 10 mg. Two hours after the dose, the average milk level was 126 mcg/L. The authors estimated that a fully breastfed infant would receive a maximum daily dosage of 45 mcg/kg.[16]
Five mothers took metoclopramide 10 mg orally 3 times daily beginning on day 3 to 9 postpartum because of an insufficient milk supply. Metoclopramide in milk samples taken 1 to 2 hours after a 10 mg dose on days 4 and/or 14 ranged from 52 to 157 mcg/L. The authors estimated that these infants would receive maximum metoclopramide dosages of 6 to 24 mcg/kg daily. The same paper reported on 18 women who received metoclopramide 10 mg orally 3 times daily beginning in week 8 to 12 postpartum. Milk levels measured once on the morning of day 9 to 11 of therapy averaged 48 mcg/L (range 21 to 125 mcg/L). The authors estimated that these infants would receive maximum metoclopramide dosages averaging 5.2 mcg/kg (range 0.8 to 13.2 mcg/kg) daily.[17] This latter group of infants would receive about 1% of the maternal weight-adjusted dosage.
Seventeen women who were part of a study of metoclopramide for enhancing lactation in mothers of premature infants had complete 24-hour milk samples measured for metoclopramide. The dosage was 10 mg orally 3 times daily and milk samples were collected between day 6 and 14 of therapy. Three of the mothers had no measurable metoclopramide in milk. The other 14 mothers had metoclopramide milk levels averaging 45 mcg/L. The authors calculated that a fully breastfed infant would receive 11 mcg/kg daily.[18,19] This corresponds to about 2% of the maternal weight-adjusted dosage and 7 to 11% of a dose for preterm infants.[20]
Infant Levels. Five breastfed infants were studied whose mothers were taking metoclopramide 10 mg orally 3 times daily beginning on the day 3 to 9 postpartum because of an insufficient milk supply. Serum metoclopramide levels were undetectable (<2 mcg/L) in 4 infants; the fifth had serum levels of 20.9 and 18.6 mcg/L on days 4 and 14 postpartum, respectively. These levels averaged 8% of the infant's mother's metoclopramide serum levels which were very high.[17] A study of neonates who each received a single oral dose of 100 to 150 mcg/kg found peak serum levels averaging 17.7 mcg/L, which is similar to the levels achieved in the breastfed infant with metoclopramide detectable in serum.[20]
A 21-week-old breast-fed boy presented with an unexplained acute extrapyramidal syndrome that occurred 48 hours after his mother used a metoclopramide 10 mg rectal suppository. At 60 hours after the reaction, metoclopramide was found in the infant’s plasma at a concentration of 8 mcg/L. A hair sample taken 1.5 months after the reaction found metoclopramide in a concentration of 1058 pg/mg.[21]
Effects in Breastfed Infants
In an early report, 5 infants were nursed during 7 to 10 days of maternal metoclopramide therapy at a dosage of 10 mg orally 3 times daily. No adverse effects were noted.[22]
In a placebo-controlled study of the effect of metoclopramide on milk production in 37 women, an infant whose mother was taking oral metoclopramide 15 mg 3 times daily reportedly had intestinal discomfort. No infants whose mothers were taking a dosage of 5 or 10 mg 3 times daily or placebo had any adverse effects. Metoclopramide was possibly the cause of the adverse reaction.[11]
Seventeen mothers with poor lactation were treated with oral metoclopramide 10 mg 3 times daily for 3 weeks. One mother reported that she and her infant had increased intestinal gas formation during treatment. Metoclopramide was possibly the cause of the adverse reaction.[12]
Thirty-two mothers with complete or partial lactation failure were given oral metoclopramide 10 mg 3 times daily for 10 days and advised to nurse every 3 hours. None of the mothers reported adverse effects in their infants.[23]
Twenty-three premature infants whose mothers were having difficulty maintaining milk production had steady weight gain and no adverse effects related to feeding tolerance or stool frequency during maternal metoclopramide therapy. The mothers were taking oral metoclopramide 10 mg 3 times daily for 7 days, with a tapering dosage for 2 more days, beginning at an average of 32 days postpartum.[24]
Thirteen women with insufficient milk production who were 4 to 20 weeks postpartum were randomized to receive metoclopramide or placebo 10 mg orally 3 times daily. The average plasma prolactin levels before therapy and after 3 weeks of maternal therapy were no different in the infants of women who received metoclopramide or placebo.[25]
Eleven breastfed infants whose mothers were given oral metoclopramide 10 mg 3 times daily for 5 days beginning on day 1 postpartum were compared to the infants of 11 matched mothers who received no metoclopramide. No difference in average serum prolactin was found between the groups, indicating little transfer of the drug to the infants via breastmilk.[26]
Five breastfed infants were studied whose mothers were taking metoclopramide 10 mg orally 3 times daily beginning on the day 3 to 9 postpartum because of an insufficient milk supply. Before therapy, their plasma prolactin levels were similar to their mothers'. On day 4 of maternal therapy, 3 of the infants had plasma prolactin levels higher than the highest levels of control infants of the same age, and on day 14, one infant had a plasma prolactin level higher than the highest levels of control infants of the same age. Plasma levels in 3 other infants were in the normal range during therapy.[17]
A 21-week-old breast-fed boy presented with an unexplained acute extrapyramidal syndrome that occurred 48 hours after his mother used a metoclopramide 10 mg rectal suppository. Metoclopramide was found in the infant’s blood and a hair sample. The adverse reaction was probably caused by metoclopramide in milk.[27]
Effects on Lactation and Breastmilk
Metoclopramide increases serum prolactin in lactating and nonlactating women.[3,28,29] This effect is thought to be caused by the drug's antidopaminergic effect. Galactorrhea has been reported after long-term use of metoclopramide for nausea associated with migraine. The patient was taking 10 to 40 mg 1 to 4 times weekly for about 4 months.[30] Another case of galactorrhea was reported in a woman after 5 days of treatment and having a slightly low serum prolactin level.[31]
Numerous papers have reported studies that used metoclopramide to increase milk production. All studies were small with 40 or fewer patients. Most of the studies have designs that would not be considered valid using today's standards of evidence-based medicine. Many of the studies had no placebo control;[12,17,23,24,26,32-34] only 7 studies employed randomization;[11,16,19,25,35-37] and only 2 studies were clearly and adequately blinded.[19,35] The studies that meet or come close to meeting current evidence-based medicine standards are described in more detail below.
In one early double-blind study, 20 women who had undergone delivery by emergency or elective cesarean section were randomized to take oral metoclopramide 10 mg 3 times daily (n=10) or placebo for 7 days (n=10) beginning on the first day after cesarean section.[16] All mothers expressed a desire to breastfeed their infants for at least 3 months and received daily visits by an investigator to discuss breastfeeding problems and were given advice and encouragement to breastfeed. The mothers in the 2 groups were closely matched except that 3 preterm infants in the metoclopramide group were separated from their mothers in the intensive care unit and were nursed there initially and fed expressed milk until discharge. At 10 days postpartum, there were no differences in the number of infants being breastfed in each group; at 6 weeks postpartum, 9 women were breastfeeding in the metoclopramide group and 8 in the placebo group; and 3 months postpartum 4 were breastfeeding in each group. Although this was a small study, it was well designed and executed. It provided preliminary evidence of the benefit of patient counseling and encouragement on breastfeeding success.
Thirteen primiparous nursing mothers without breastfeeding difficulties and normal infants were given either oral metoclopramide 10 mg 3 times daily (n=7) or placebo (n=6) for 8 days beginning on the first day postpartum in a randomized, double-blind study.[35] No attempt was made to improve nursing technique, but mothers nursed on a 3-hour schedule beginning at 6:30 am on the day following delivery. No mention was made of the type of feeding, or the number of feedings that the infants received between birth and the initiation of breastfeeding or any differences in the two groups of infants in this regard. All women completed the trial. No differences were found in serum prolactin of treated and control women throughout 28 days of observation. Milk intake as measured by infant weight change before and after the second daily feeding on days 3 through 8 was greater by an average of 24.3 mL (51.1 mL vs 75.4 mL) in the infants of treated mothers; however, statistically significant differences in milk production did not occur until day 5 postpartum. This paper has several serious flaws related to its analysis. One is that the paper does not state the number of feedings per day, so the fraction of the infant's daily feedings that this one feeding represented is unknown. The study also failed to report serial infant weight gain during the study period. These serious problems invalidate the study results.
Fifty mothers who had complete or partial lactation failure received extensive instruction on how to increase their milk supply.[36] Their infants were hospitalized for various illnesses and ranged from 29 to 100 day of age. Maternal lactation history was comparable in the two groups. Mothers were randomized to either receive or not receive metoclopramide 10 mg 3 times daily for 10 days. Although no specific metoclopramide placebo was given to control mothers, all mothers received multivitamins, iron, and folic acid daily which the authors used to obscure to the mother whether she was receiving an active drug or not. No statistically significant differences were found between the groups in the time to initiation of milk secretion, to partial restoration of breastfeeding, or to complete breastfeeding or in the weight gain of the infants during the study period of 96 days. The authors concluded that successful relactation can be accomplished without galactogogues such as metoclopramide. This study lacked a true placebo control; however, it employed excellent breastfeeding instruction and infant evaluation techniques and had the best overall design of any of the studies on mothers who had older infants and well documented insufficient milk supplies at the start of the study.
In a well-controlled and analyzed, randomized, double-blinded study of the mothers of premature (23 to 34 weeks) infants, mothers received either metoclopramide 10 mg 3 times daily (n=31) or placebo (n=29) for 10 days beginning within 96 hours of delivery.[19] The groups were well matched and all mothers received standardized instructions from a lactation consultant and provided access to breastfeeding support. No selection was made for mothers who were having difficulties producing milk. Six subjects each in the drug and placebo groups dropped out for a relatively high dropout rate of 17.4%. Although by far the best designed and executed study to date on any galactogogue, the study enrolled all mothers of preterm infants without any evaluation of their ability to produce milk. This population may in general need lactation support, but the possible inclusion of women in both the active drug and placebo groups who would have had little difficulty in milk production may have minimized differences between the groups.
A study of 20 primiparous mothers whose infant were not gaining weight adequately compared metoclopramide (n = 10) to placebo (n = 10) for increasing milk supply. All mothers passed a brief training course on improving breastfeeding technique and the benefits of breastfeeding before entering the study. All infants gained weight over the next 2 weeks. The increase in weight in the metoclopramide group was not different from the placebo group.[37]
Two women whose infants were born via a surrogate pregnancy. One woman stopped metoclopramide 1 week prior to the expected delivery date and the other continued metoclopramide postpartum. They also underwent postpartum nipple stimulation with an electric breast pump. Lactation was established and they were each able to partially breastfeed their infants for 3 months.[38,39]
A double-blind randomized study compared the effect on milk production of metoclopramide 10 mg to placebo 3 times daily for 10 days in the mothers of preterm infants born at 28 to 34 weeks of gestation. At the beginning of investigation, all participating women were taught a standard breastfeeding method. Mothers used a breast pump for 10 to 15 minutes every 2 hours and the volume of milk was measured and recorded at each pumping for 10 days. No difference in daily milk volumes between the two groups occurred until day 7 postpartum, when treated mothers pumped an average of 373 mL compared with 352 mL in the mothers receiving placebo. This difference of about 20 mL per day persisted until the end of the study on day 10 when the treated group pumped 446 mL and the placebo group pumped 422 mL.[40] The clinical importance of the extra 20 mL of milk per day is questionable.
A randomized, double-blind study of 26 mothers who delivered at 34 weeks of gestation or less, compared metoclopramide 10 mg 3 times daily to placebo. The drug or placebo was taken for 8 days beginning within 36 hours of delivery. All women were provided breast pumps and support from a lactation consultant. The total volume of milk pumped per day was recorded by the women, 19 of whom completed the entire protocol. Although milk production increased over the 8-day period, metoclopramide was no better than placebo and the standard care provided. No difference in reported side effects was found.[41]
Mothers who were expressing milk for their infants in a neonatal intensive care unit (mean gestational age 28 weeks) were given instructions on methods for increasing milk supply. If they were producing less than 160 mL of milk per kg of infant weight daily after several days, mothers were randomized to receive either domperidone or metoclopramide 10 mg by mouth 3 times daily for 10 days in a double-blinded fashion. Thirty-one mothers who received domperidone and 34 who received metoclopramide provided data on daily milk volumes during the 10 days. Milk volumes increased over the 10-day period by 96% with domperidone and 94% with metoclopramide, which was not statistically different between the groups. Some mothers continued to measure milk output after the end of the medication period. Results were similar between the 2 groups. Side effects in the domperidone group (3 women) included headache, diarrhea, mood swings and dizziness. Side effects in the metoclopramide group (7 women) included headache (3 women), diarrhea, mood swings, changed appetite, dry mouth and discomfort in the breasts. Of 29 women who took metoclopramide after the trial ended, 8 reported side effects including diarrhea, mood swings, depression (2 women), itchy skin, tiredness, restless legs and less effective milk stimulation.[9] The lack of a placebo group and the projection of milk volumes to impute missing data from some mothers detract from the findings of this study.
A triple-blind, randomized, controlled study in Iran compared maternal serum prolactin and milk volume and infant bilirubin in mothers who received placebo (n = 51) or metoclopramide 10 mg (n = 50) three times daily for 5 days beginning 2 to 10 hours postpartum. In the per-protocol analysis, serum prolactin increased on day 6 postpartum from 271.5 mcg/L to 287.5 mcg/L in the domperidone group, but decreased from 273.3 mcg/L to 250.7 mcg/L in the placebo group, which was a statistically significant difference. There were no statistically significant differences in the volume of manually expressed milk 2 hours after a feeding in the morning or infant serum bilirubin between the groups on day 6 postpartum.[2]
In a randomized study in Singapore reported as a preprint, 105 mothers with preterm and term infants were randomized to placebo (n = 49) or metoclopramide (n = 56) 30 mg daily (exact regimen not stated) starting within 12 hours postpartum. Metoclopramide achieved 25% augmentation in lactogenesis II onset, but the difference was not statistically significant, with greater expressed human milk volumes in mothers of preterm infants. Daily expressed human milk volumes were higher among preterm mothers on metoclopramide compared to term placebo mothers who served as controls. The difference was significant on day 2 (19.9 mL vs 2.4 mL) and day 3 (32.6 mL vs 8.8 mL). Total expressed human milk volumes had increased by 8.2 fold by the end of week one. Most mothers reported first initiation of lactogenesis II by day 6, with 95-100% of term mothers confirmed by day 5, with no significant difference among groups.[42]
In a survey of nursing mothers in Australia, 21 mothers were taking metoclopramide as a galactogogue. On average, mothers rated metoclopramide as being between “slightly effective” and “moderately effective” on a Likert scale. Twenty-nine percent of mothers taking metoclopramide reported experiencing adverse reactions, most commonly weight gain, nausea, headache, dry mouth, fatigue, irritability, depression and involuntary movements.[13]
References
- 1.
- Winterfeld U, Meyer Y, Panchaud A, Einarson A. Management of deficient lactation in Switzerland and Canada: A survey of midwives' current practices. Breastfeed Med 2012;7:317-8. [PubMed: 22224508]
- 2.
- Tabrizi SO, Mirghafourvand M, Dost AJ, et al. Effect of metoclopramide administration to mothers on neonatal bilirubin and maternal prolactin: A randomized, controlled, clinical trial. World J Pediatr 2019;15:135-42. [PubMed: 30519818]
- 3.
- Tabrizi SO, Mirghafourvand M, Seyedi R. The effect of metoclopramide on prolactin levels in breastfeeding mothers: A systematic review and meta-analysis. International Journal of Pediatrics (Mashhad) 2017;5:5827-38. doi:10.22038/ijp.2017.24678.2083 [CrossRef]
- 4.
- Shen Q, Khan KS, Du MC, et al. Efficacy and safety of domperidone and metoclopramide in breastfeeding: A systematic review and meta-analysis. Breastfeed Med 2021;16:516-29. [PubMed: 33769844]
- 5.
- Hussain NHN, Noor NM, Ismail SB, et al. Metoclopramide for milk production in lactating women: A systematic review and meta-analysis. Korean J Fam Med 2021;42:453-63. [PMC free article: PMC8648493] [PubMed: 34871486]
- 6.
- Brodribb W. ABM Clinical Protocol #9: Use of galactogogues in initiating or augmenting maternal milk production, second revision 2018. Breastfeed Med 2018;13:307-14. [PubMed: 29902083]
- 7.
- Breastfeeding challenges: ACOG Committee Opinion, Number 820. Obstet Gynecol 2021;137:e42-e53. [PubMed: 33481531]
- 8.
- Balikci A, Balibey H. [Postpartum depression due to use of metoclopramide: A case report]. Anatolian J Clin Invest 2012;6:258-60.
- 9.
- Ingram J, Taylor H, Churchill C, et al. Metoclopramide or domperidone for increasing maternal breast milk output: A randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2012;97:F241-5. [PubMed: 22147287]
- 10.
- Anon. FDA requires boxed warning and risk mitigation strategy for metoclopramide-containing drugs. FDA News February 26, 2009.
- 11.
- Kauppila A, Kivinen S, Ylikorkala O. A dose response relation between improved lactation and metoclopramide. Lancet 1981;1:1175-7. [PubMed: 6112526]
- 12.
- Kauppila A, Kivinen S, Ylikorkala O. Metoclopramide increases prolactin release and milk secretion in puerperium without stimulating the secretion of thyrotropin and thyroid hormones. J Clin Endocrinol Metab 1981;52:436-9. [PubMed: 6780593]
- 13.
- McBride GM, Stevenson R, Zizzo G, et al. Use and experiences of galactagogues while breastfeeding among Australian women. PLoS One 2021;16:e0254049. [PMC free article: PMC8248610] [PubMed: 34197558]
- 14.
- Bazzano A, Thibeau S, Brandt A, Theall KP. Mothers' experiences with using galactagogues for lactation: An exploratory cross-sectional study. Ochsner J 2016;16:388.
- 15.
- Hale TW, Kendall-Tackett K, Cong Z. Domperidone versus metoclopramide: Self-reported side effects in a large sample of breastfeeding mothers who used these medications to increase milk production. Clin Lact (Amarillo) 2018;9:10-7. doi:10.1891/2158-0782.9.1.10 [CrossRef]
- 16.
- Lewis PJ, Devenish C, Kahn C. Controlled trial of metoclopramide in the initiation of breast feeding. Br J Clin Pharmacol 1980;9:217-9. [PMC free article: PMC1429870] [PubMed: 6986894]
- 17.
- Kauppila A, Arvela P, Koivisto M, et al. Metoclopramide and breast feeding: Transfer into milk and the newborn. Eur J Clin Pharmacol 1983;25:819-23. [PubMed: 6662181]
- 18.
- Hansen W, Hunter S, McAndrew S, et al. Metoclopramide concentration in breast milk of women delivering between 23-34 weeks gestation. Am J Obstet Gynecol 2001;185 (6 Suppl):S116-127. doi:10.1016/S0002-9378(01)80162-9 [CrossRef]
- 19.
- Hansen WF, McAndrew S, Harris K, Zimmerman MB. Metoclopramide effect on breastfeeding the preterm infant: A randomized trial. Obstet Gynecol 2005;105:383-9. [PubMed: 15684169]
- 20.
- Kearns GL, van den Anker JN, Reed MD, Blumer JL. Pharmacokinetics of metoclopramide in neonates. J Clin Pharmacol 1998;38:122-8. [PubMed: 9549642]
- 21.
- Bellouard M, Abe E, Etting I, et al. Metoclopramide intoxication through breast milk: Relevance of blood and hair analyses. Indian J Pediatr 2023;90:314. [PubMed: 36694076]
- 22.
- Sousa PLR. Metoclopramide and breast-feeding. Br Med J 1975;1:512. [PMC free article: PMC1672597] [PubMed: 1173219]
- 23.
- Gupta AP, Gupta PK. Metoclopramide as a lactogogue. Clin Pediatr (Phila) 1985;24:269-72. [PubMed: 3987166]
- 24.
- Ehrenkranz RA, Ackerman BA. Metoclopramide effect on faltering milk production by mothers of premature infants. Pediatrics 1986;78:614-20. [PubMed: 3763270]
- 25.
- Kauppila A, Anunti P, Kivinen S, et al. Metoclopramide and breast feeding: Efficacy and anterior pituitary responses of the mother and the child. Eur J Obstet Gynecol Reprod Biol 1985;19:19-22. [PubMed: 3884406]
- 26.
- Ertl T, Sulyok E, Ezer E, et al. The influence of metoclopramide on the composition of human breast milk. Acta Paediatr Hung 1991;31:415-22. [PubMed: 1790024]
- 27.
- Bellouard M, Abe E, Etting I, et al. Metoclopramide intoxication through breast milk: Relevance of blood and hair analyses. Indian J Pediatr 2023. [PubMed: 36694076]
- 28.
- Andersen AN, Tabor A. Prl, TSH, GH and LH responses to metoclopramide and breast-feeding in normal and hyperprolactinaemic women. Acta Endocrinol (Copenh) 1982;100:177-83. [PubMed: 7202316]
- 29.
- Bohnet HG, Kato K. Prolactin secretion during pregnancy and puerperium: Response to metoclopramide and interactions with placental hormones. Obstet Gynecol 1985;65:789-92. [PubMed: 3839061]
- 30.
- Finnis WA, Bird CE, Wilson DL. Metoclopramide hydrochloride and galactorrhea. Can Med Assoc J 1976;115:845. [PMC free article: PMC1879081] [PubMed: 1033026]
- 31.
- Menon P, Thunga G, Nambiar S, Khera K. Atypical presentation of metoclopramide-induced galactorrhea. J Pharm Pract Res 2015;45:437-9. doi:10.1002/jppr.1123 [CrossRef]
- 32.
- Nemba K. Induced lactation: A study of 37 non-puerperal mothers. J Trop Pediatr 1994;40:240-2. [PubMed: 7932939]
- 33.
- Tolino A, Tedeschi A, Farace R, Granata P. The relationship between metoclopramide and milk secretion in puerperium. Clin Exp Obstet Gynecol 1981;8:93-5. [PubMed: 6804132]
- 34.
- Toppare MF, Laleli Y, Senses DA, et al. Metoclopramide for breast milk production. Nutr Res 1994;14:1019-29. doi:10.1016/S0271-5317(05)80256-8 [CrossRef]
- 35.
- de Gezelle H, Ooghe W, Thiery M, Dhont M. Metoclopramide and breast milk. Eur J Obstet Gynecol Reprod Biol 1983;15:31-6. [PubMed: 6350073]
- 36.
- Seema, Patwari AK, Satyanarayana L. Relactation: An effective intervention to promote exclusive breastfeeding. J Trop Pediatr 1997;43:213-6. [PubMed: 9283123]
- 37.
- Sakha K, Behbahan AG. Training for perfect breastfeeding or metoclopramide: Which one can promote lactation in nursing mothers? Breastfeed Med 2008;3:120-3. [PubMed: 18564001]
- 38.
- Biervliet FP, Maguiness SD, Hay DM, et al. Induction of lactation in the intended mother of a surrogate pregnancy. Hum Reprod 2001;16:581-3. [PubMed: 11228232]
- 39.
- Shiva M, Frotan M, Arabipoor A, Mirzaaga E. A successful induction of lactation in surrogate pregnancy with metoclopramide and review of lactation induction. Int J Fertil Steril 2010;3:191-4.
- 40.
- Dastgerdi E, Shirazi M, Mohammadzadeh A, et al. Effect of metoclopramide on increased milk production in mothers of preterm infants. Iran J Obstet Gynecol Infertil 2011;14:32-6.
- 41.
- Fife S, Gill P, Hopkins M, et al. Metoclopramide to augment lactation, does it work? A randomized trial. J Matern Fetal Neonatal Med 2011;24:1317-20. [PubMed: 21410420]
- 42.
- Fok D, Chan YH, Ho J, et al. Oral metoclopramide boosts lactogenesis II in mothers with preterm infants: a randomized placebo-controlled trial. Preprint 2020. doi:10.21203/rs.3.rs-16243/v1 [CrossRef]
Substance Identification
Substance Name
Metoclopramide
CAS Registry Number
364-62-5
Drug Class
Breast Feeding
Milk, Human
Antiemetics
Dopamine Antagonists
Galactogogues
Gastrointestinal Agents
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Domperidone.[Drugs and Lactation Database (...]Review Domperidone.. Drugs and Lactation Database (LactMed®). 2006
- Review Sulpiride.[Drugs and Lactation Database (...]Review Sulpiride.. Drugs and Lactation Database (LactMed®). 2006
- Review Fenugreek.[Drugs and Lactation Database (...]Review Fenugreek.. Drugs and Lactation Database (LactMed®). 2006
- Review Marine Oils.[Drugs and Lactation Database (...]Review Marine Oils.. Drugs and Lactation Database (LactMed®). 2006
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Metoclopramide - Drugs and Lactation Database (LactMed®)Metoclopramide - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
