Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
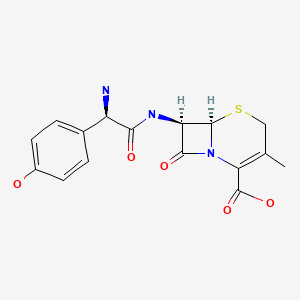
CASRN: 50370-12-2
Drug Levels and Effects
Summary of Use during Lactation
Limited information indicates that cefadroxil produces low levels in milk that are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Cefadroxil is acceptable in nursing mothers.
Drug Levels
Maternal Levels. After a single 500 mg oral dose of cefadroxil in 5 women, peak milk levels ranged from 0.6 to 0.9 mg/L. Peak levels occurred from 5 to 6 hours after the dose with an average of 0.65 mg/L at 5 hours and 0.68 mg/L at 6 hours after the dose.[1]
After a single 1 gram oral dose of cefadroxil in 6 women who were 2 days postpartum, peak levels occurred 6 to 7 hours after the dose and averaged 1.83 mg/L (range 1.2 to 2.4 mg/L).[2]
After a single 500 mg oral dose of cefadroxil in 2 or 3 women, peak milk levels of 0.4 mg/L occurred 4 hours after the dose.[3]
In all of the above studies, cefadroxil was undetectable in most women's milk for the first hour after the dose.
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
References
- 1.
- Takase Z, Shirafuji H, Uchida M. Experimental and clinical studies of cefadroxil in the treatment of infections in the field of obstetrics and gynecology. Chemotherapy (Tokyo). 1980;28 Suppl 2:424–31.
- 2.
- Kafetzis DA, Siafas CA, Georgakopoulos PA, et al. Passage of cephalosporins and amoxicillin into the breast milk. Acta Paediatr Scand. 1981;70:285–8. [PubMed: 7246123]
- 3.
- Matsuda S. Transfer of antibiotics into maternal milk. Biol Res Pregnancy. 1984;5:57-60.. [PubMed: 6743732]
Substance Identification
Substance Name
Cefadroxil
CAS Registry Number
50370-12-2
Drug Class
Breast Feeding
Lactation
Anti-Infective Agents
Antibacterial Agents
Cephalosporins
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Cefoperazone.[Drugs and Lactation Database (...]Review Cefoperazone.. Drugs and Lactation Database (LactMed®). 2006
- Review Cefazolin.[Drugs and Lactation Database (...]Review Cefazolin.. Drugs and Lactation Database (LactMed®). 2006
- Review Ceftizoxime.[Drugs and Lactation Database (...]Review Ceftizoxime.. Drugs and Lactation Database (LactMed®). 2006
- Review Cefotaxime.[Drugs and Lactation Database (...]Review Cefotaxime.. Drugs and Lactation Database (LactMed®). 2006
- Review Cefaclor.[Drugs and Lactation Database (...]Review Cefaclor.. Drugs and Lactation Database (LactMed®). 2006
- Cefadroxil - Drugs and Lactation Database (LactMed®)Cefadroxil - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
