Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
CASRN: 55985-32-5
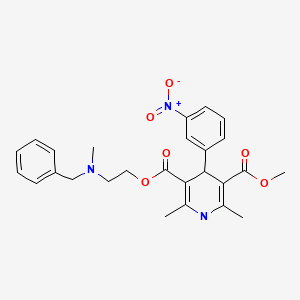
Drug Levels and Effects
Summary of Use during Lactation
Because of the low levels of nicardipine in breastmilk, amounts ingested by the infant are small and would not be expected to cause any adverse effects in breastfed infants. No special precautions are required.
Drug Levels
Maternal Levels. Ten women were treated with oral nicardipine 4 to 14 days postpartum. Four received an immediate-release product 40 to 80 mg daily, and 6 received a sustained-release product 100 to 150 mg daily. Another woman received intravenous nicardipine 120 mg daily. The authors calculated that the infants received an average of 0.073% of the maternal weight-adjusted dose with the oral dosage and 0.14% of the maternal weight-adjusted dose with the intravenous dosage. They estimated that the average dosage that an infant would receive is about 0.3% of the projected infant dosage.[1]
Seven women received intravenous infusions of nicardipine for an average of 1.9 days in the immediate postpartum period as therapy for pre-eclampsia. Thirty-four milk samples were obtained at unspecified times; nicardipine was undetectable (<5 mcg/L) in 82% of samples. Four women receiving 1 to 6.5 mg/hour of nicardipine had 6 milk samples with detectable nicardipine ranging from 5.1 to 18.5 mcg/L. The highest concentration of 18.5 mcg/L was found in a woman receiving 5.5 mg/hour of nicardipine. The authors estimated that the maximum dose that could be received by a breastfed infant was less than 0.3 mcg daily or between 0.015 to 0.004% of a therapeutic dose in a 1 kg infant.[2]
Eighteen women with severe preeclampsia were given continuous infusion of nicardipine to control their blood pressure during pregnancy and postpartum. Blood and breastmilk samples were taken between 2 and 7 days postpartum from 17 of the women. The median postpartum nicardipine dosage was 0.54 mg/kg daily (mean 0.26 0.54 mg/kg daily; range 0.43 to 1.63 mg/kg daily). The median breastmilk nicardipine concentration in milk was 4.7 mcg/L (mean 6.9 mcg/L; range 2.3 to 37.7 mcg/L). Milk nicardipine levels were proportional to maternal plasma levels. The median weight-adjusted percentage of the maternal dose was 0.045% (mean 0.08%; range 0.006 to 0.33%).[3][3]
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Alternate Drugs to Consider
References
- 1.
- Jarreau PH, Le Beller C, Guillonneau M, et al. Excretion of nicardipine in human milk. Paediatr Perinat Drug Ther. 2000;4:28–30.
- 2.
- Bartels PA, Mathot RAA, Steegers EAP, et al. Nicardipine in pre-eclamptic patients: Placental transfer and disposition in breast milk. BJOG. 2007;114:230–3. [PubMed: 17166219]
- 3.
- Matsumura H, Takagi K, Seki H, et al. Placental transfer of intravenous nicardipine and disposition into breast milk during the control of hypertension in women with pre-eclampsia. Hypertens Pregnancy. 2014;33:93–101. [PubMed: 24131296]
Substance Identification
Substance Name
Nicardipine
CAS Registry Number
55985-32-5
Drug Class
Breast Feeding
Lactation
Antihypertensive Agents
Calcium Channel Blockers
Vasodilator Agents
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Nitrendipine.[Drugs and Lactation Database (...]Review Nitrendipine.. Drugs and Lactation Database (LactMed®). 2006
- Review Nimodipine.[Drugs and Lactation Database (...]Review Nimodipine.. Drugs and Lactation Database (LactMed®). 2006
- Review Nisoldipine.[Drugs and Lactation Database (...]Review Nisoldipine.. Drugs and Lactation Database (LactMed®). 2006
- Review Felodipine.[Drugs and Lactation Database (...]Review Felodipine.. Drugs and Lactation Database (LactMed®). 2006
- Employing ionization behaviors to resolve a trace-level impurity: determination of 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5- pyridinedicarboxylic acid di-2-[methyl-(phenylmethyl)amino]ethyl ester in nicardipine drug substance.[Pharm Res. 1992]Employing ionization behaviors to resolve a trace-level impurity: determination of 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5- pyridinedicarboxylic acid di-2-[methyl-(phenylmethyl)amino]ethyl ester in nicardipine drug substance.Maurin MB, Vickery RD, Ma P, Manalo J, Hussain MA. Pharm Res. 1992 Nov; 9(11):1518-20.
- Nicardipine - Drugs and Lactation Database (LactMed®)Nicardipine - Drugs and Lactation Database (LactMed®)
- Pemirolast - Drugs and Lactation Database (LactMed®)Pemirolast - Drugs and Lactation Database (LactMed®)
- NS5B protein, partial [Hepacivirus hominis]NS5B protein, partial [Hepacivirus hominis]gi|1047390982|gb|ANW45899.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...
