Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
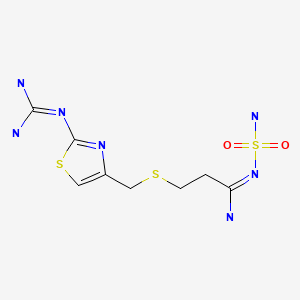
CASRN: 76824-35-6
Drug Levels and Effects
Summary of Use during Lactation
Famotidine doses in breastmilk result in infant dosages that are lower than those used in newborn infants. Famotidine would not be expected to cause any adverse effects in breastfed infants. No special precautions are required.
Drug Levels
Maternal Levels. Eight women who had "recently given birth" (not defined, but apparently within a few days postpartum) were given famotidine 40 mg orally. An average peak breastmilk level of 72 mcg/L occurred 6 hours after the dose.[1] Using the peak milk level data from this study, an exclusively breastfed infant would receive an estimated maximum of 0.01 mg/kg daily with this maternal dosage regimen or less than 2% of the maternal weight-adjusted dosage.
Seven women were given oral famotidine 40 mg daily in 2 or 4 divided doses for 3 days at 12 to 16 weeks postpartum. Average concentrations of famotidine in breastmilk were 53 and 55 mcg/L at 3 and 6 hours after a dose, respectively.[2]
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
Histamine H2-receptor blockade is known to stimulate prolactin secretion.[3] Oral famotidine usually does not affect serum prolactin levels, but rare cases of hyperprolactinemia and galactorrhea have been reported.[4,5] The prolactin level in a mother with established lactation may not affect her ability to breastfeed.
Alternate Drugs to Consider
References
- 1.
- Courtney TP, Shaw RW, Cedar E, et al. Excretion of famotidine in breast milk. Br J Clin Pharmacol 1988;26:615p-78p.
- 2.
- Wang X, Zhan Y, Hankins GD, et al. Pharmacokinetics of famotidine in pregnant women. Am J Obstet Gynecol 2011;204 (1 Suppl) :S72-3.
- 3.
- Knigge UP. Histaminergic regulation of prolactin secretion. Dan Med Bull 1990;37:109-24. [PubMed: 2188799]
- 4.
- Delpre G, Lapidot M, Lipchitz A, et al. Hyperprolactinaemia during famotidine therapy. Lancet 1993;342:868. [PubMed: 8104296]
- 5.
- Güven K, Kelestimur F. Hyperprolactinemia and galactorrhea with standard-dose famotidine therapy. Ann Pharmacother 1995;29:788. [PubMed: 8520102]
Substance Identification
Substance Name
Famotidine
CAS Registry Number
76824-35-6
Drug Class
Breast Feeding
Lactation
Milk, Human
Anti-Ulcer Agents
Histamine H2 Antagonists
Gastrointestinal Agents
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Comparison of an intravenous bolus of famotidine and Mylanta II for the control of gastric pH in critically ill patients.[Am J Surg. 1993]Comparison of an intravenous bolus of famotidine and Mylanta II for the control of gastric pH in critically ill patients.Wilson P, Clark GW, Anselmino M, Welch NT, Singh S, Perdikis G, Hinder RA. Am J Surg. 1993 Dec; 166(6):621-4; discussion 624-5.
- Famotidine for infant gastro-oesophageal reflux: a multi-centre, randomized, placebo-controlled, withdrawal trial.[Aliment Pharmacol Ther. 2003]Famotidine for infant gastro-oesophageal reflux: a multi-centre, randomized, placebo-controlled, withdrawal trial.Orenstein SR, Shalaby TM, Devandry SN, Liacouras CA, Czinn SJ, Dice JE, Simon TJ, Ahrens SP, Stauffer LA. Aliment Pharmacol Ther. 2003 May 1; 17(9):1097-107.
- Quantum mechanical, spectroscopic studies (FT-IR, FT-Raman, NMR, UV) and normal coordinates analysis on 3-([2-(diaminomethyleneamino) thiazol-4-yl] methylthio)-N'-sulfamoylpropanimidamide.[Spectrochim Acta A Mol Biomol ...]Quantum mechanical, spectroscopic studies (FT-IR, FT-Raman, NMR, UV) and normal coordinates analysis on 3-([2-(diaminomethyleneamino) thiazol-4-yl] methylthio)-N'-sulfamoylpropanimidamide.Muthu S, Uma Maheswari J, Sundius T. Spectrochim Acta A Mol Biomol Spectrosc. 2013 May; 108:307-18. Epub 2013 Feb 21.
- Review Nizatidine.[Drugs and Lactation Database (...]Review Nizatidine.. Drugs and Lactation Database (LactMed®). 2006
- Review Valacyclovir.[Drugs and Lactation Database (...]Review Valacyclovir.. Drugs and Lactation Database (LactMed®). 2006
- Famotidine - Drugs and Lactation Database (LactMed®)Famotidine - Drugs and Lactation Database (LactMed®)
- Homo sapiens mitogen-activated protein kinase 8 interacting protein 3, mRNA (cDN...Homo sapiens mitogen-activated protein kinase 8 interacting protein 3, mRNA (cDNA clone MGC:166852 IMAGE:9007222), complete cdsgi|152013049|gb|BC150266.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...
