Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
CASRN: 85622-93-1
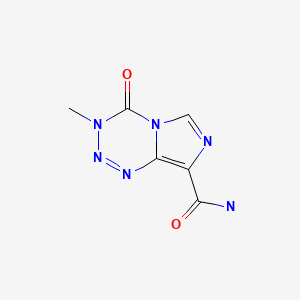
Drug Levels and Effects
Summary of Use during Lactation
Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy, especially alkylating agents such as temozolamide.[1] It might be possible to breastfeed safely during intermittent therapy with an appropriate period of breastfeeding abstinence. The manufacturer recommends withholding breastfeeding for 1 week after the last dose. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk.
Drug Levels
Temozolamide is metabolized to the same active metabolite as dacarbazine.
Maternal Levels. Relevant published information was not found as of the revision date.
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
A woman diagnosed with Hodgkin's lymphoma during the second trimester of pregnancy received 3 rounds of chemotherapy during the third trimester of pregnancy and resumed chemotherapy 4 weeks postpartum. Milk samples were collected 15 to 30 minutes before and after chemotherapy for 16 weeks after restarting. The regimen consisted of doxorubicin 40 mg, bleomycin 16 units, vinblastine 9.6 mg and dacarbazine 600 mg, all given over a 2-hour period every 2 weeks. The microbial population and metabolic profile of her milk were compared to those of 8 healthy women who were not receiving chemotherapy. The breastmilk microbial population in the patient was markedly different from that of the healthy women, with increases in Acinetobacter sp., Xanthomonadacae and Stenotrophomonas sp. and decreases in Bifidobacterium sp. and Eubacterium sp. Marked differences were also found among numerous chemical components in the breastmilk of the treated woman, most notably DHA and inositol were decreased.[2]
References
- 1.
- Pistilli B, Bellettini G, Giovannetti E, et al. Chemotherapy, targeted agents, antiemetics and growth-factors in human milk: How should we counsel cancer patients about breastfeeding? Cancer Treat Rev. 2013;39:207–11. [PubMed: 23199900]
- 2.
- Urbaniak C, McMillan A, Angelini M, et al. Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome. 2014;2:24. [PMC free article: PMC4109383] [PubMed: 25061513]
Substance Identification
Substance Name
Temozolamide
CAS Registry Number
85622-93-1
Drug Class
Breast Feeding
Lactation
Antineoplastic Agents
Antineoplastic Agents, Alkylating
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Temozolamide - Drugs and Lactation Database (LactMed®)Temozolamide - Drugs and Lactation Database (LactMed®)
- ST8SIA3 [Macaca fascicularis]ST8SIA3 [Macaca fascicularis]Gene ID:101866240Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
