Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
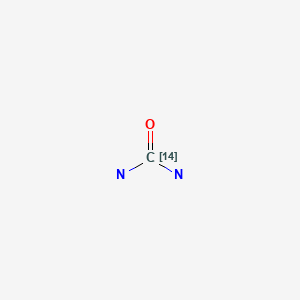
CASRN: 594-05-8
Drug Levels and Effects
Summary of Use during Lactation
Information in this record refers to the use of urea C 14 as a diagnostic agent. No information is available on the use of urea C 14 during breastfeeding. However, administration of urea C 14 directly to pediatric patients is considered to be safe.[1] International guidelines state that breastfeeding need not be interrupted after administration of urea C 14.[2]
Drug Levels
Carbon 14 is a low-energy beta emitter with a physical half-life of about 5730 years.[3]
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Alternate Drugs to Consider
References
- 1.
- Pathak CM, Kaur B, Khanduja KL. 14C-urea breath test is safe for pediatric patients. Nucl Med Commun. 2010;31:830–5. [PubMed: 20864821]
- 2.
- Mattsson S, Johansson L, Leide Svegborn S, et al. Radiation dose to patients from radiopharmaceuticals: A compendium of current information related to frequently used substances. ICRP Publication 128. Annex D. Recommendations on breast-feeding interruptions. Ann ICRP. 2015;44(2) Suppl:319–21. [PubMed: 26069086]
- 3.
- Leide-Svegborn S, Ahlgren L, Johansson L, et al. Excretion of radionuclides in human breast milk after nuclear medicine examinations. Biokinetic and dosimetric data and recommendations on breastfeeding interruption. Eur J Nucl Med Mol Imaging. 2016;43:808–21. [PubMed: 26732471]
Substance Identification
Substance Name
Urea C 14
CAS Registry Number
594-05-8
Drug Class
Breast Feeding
Lactation
Radiopharmaceuticals
Carbon Radioisotopes
Urea
Diagnostic Agents
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Water O 15.[Drugs and Lactation Database (...]Review Water O 15.. Drugs and Lactation Database (LactMed®). 2006
- Review Xenon Xe 133.[Drugs and Lactation Database (...]Review Xenon Xe 133.. Drugs and Lactation Database (LactMed®). 2006
- Review Iobenguane I 131.[Drugs and Lactation Database (...]Review Iobenguane I 131.. Drugs and Lactation Database (LactMed®). 2006
- Review Urea C 13.[Drugs and Lactation Database (...]Review Urea C 13.. Drugs and Lactation Database (LactMed®). 2006
- Review Iodohippurate Sodium I 123.[Drugs and Lactation Database (...]Review Iodohippurate Sodium I 123.. Drugs and Lactation Database (LactMed®). 2006
- Urea C 14 - Drugs and Lactation Database (LactMed®)Urea C 14 - Drugs and Lactation Database (LactMed®)
- 15 [Bacillus phage phi29]15 [Bacillus phage phi29]Gene ID:6446520Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
