This open access article is licenced under Creative Commons Attribution 4.0 International (CC BY 4.0). https://creativecommons.org/licenses/by-nc/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Ward WH, Farma JM, editors. Cutaneous Melanoma: Etiology and Therapy [Internet]. Brisbane (AU): Codon Publications; 2017 Dec 21. doi: 10.15586/codon.cutaneousmelanoma.2017.ch1
Abstract
Melanoma is a potentially lethal cancer that is most commonly cutaneous. The worldwide incidence of melanoma has risen rapidly over the course of the last 50 years. Its incidence is greatest among fair-skinned populations, and in regions of lower latitude. Incidence is greater among geriatric populations, but melanoma is also among the most common cancers found in adolescent and young adult populations. In fact, it is one of the leading cancers in average years of life lost per death from disease. Melanoma incidence varies by sex, which is also associated with differences in melanoma anatomic site. Similar differences by region, ethnicity, age, and sex are observed in mortality rates of melanoma. In the setting of rising incidence and mortality, melanoma bears a heavy health and economic burden. Attributable costs are several billion in nations with greater melanoma incidence. Preventative strategies have been implemented in multiple high-risk regions with variable success. It is imperative that research efforts achieve better understanding of the risk factors and etiology of disease, with the goal to halt and reverse the progressive trend of rising incidence and mortality from melanoma.
Key words:
Epidemiology; Incidence, Melanoma; Mortality; PreventionIntroduction
Melanoma is a malignant tumor that arises from uncontrolled proliferation of melanocytes—pigment-producing cells (1–4). While the most common form of melanoma is cutaneous, it can also arise in mucosal surfaces, the uveal tract, and leptomeninges. This chapter will focus on cutaneous melanoma.
Malignant melanoma is the most lethal form of skin cancer (5–7). Historically, melanoma was a rare cancer, but in the last 50 years its incidence has risen faster than almost any other cancer (8–11). In 2017, approximately 87,110 individuals are predicted to be diagnosed with melanoma in the United States alone (6, 9). While it still represents less than 5% of all cutaneous malignancies, melanoma accounts for the majority of skin cancer deaths (5, 6, 9). If melanoma is diagnosed in its early stages, resection of the lesion is associated with favorable survival rates. However, melanoma is an aggressive malignancy that tends to metastasize beyond its primary site (7, 12). Once melanoma is advanced, surgery is no longer sufficient and the disease becomes more difficult to treat (7, 12–14). Long-term prognosis after metastasis is grim; median survival with treatment, including treatment with immunotherapeutics like Ipilimumab, ranges from 8 to 12 months (6, 13, 14). However, more recently developed combined immunotherapeutic treatments with radiation can improve survival further to several years (13).
In addition to the considerable burden to public health, the annual costs of melanoma management are substantial (15). In the United States alone, the annual costs of melanoma treatment have risen by 288% in less than a decade. As new expensive pharmacologic treatments come to market, costs will likely rise at even greater rates. Melanoma comprises $3.3 billion of the total $8.1 billion in all direct skin cancer annual costs (16). Indirect costs associated with melanoma are estimated to be as high as over $3.5 billion annually (17). As incidence and mortality rises, costs for treatment and indirect care are projected to concurrently rise (15). However, as more preventative strategies are implemented to combat rising incidence, melanoma-related costs may improve with the potential of cutting economic burden by $2.1 billion a year (11).
To combat this cancer, population-based strategies have been implemented to reduce incidence through prevention (18). Epidemiological study has allowed investigators to better characterize which populations are most greatly affected, how they are affected, and what can be done to modify and improve upon prevention, treatment, and management strategies. Incidence, prevalence, and mortality studies reflect health and economic burden of disease. Incidence and prevalence includes all individuals that will and currently are receiving treatment, management, monitoring, and disability services as a result of their melanoma. These statistics underscore the demand and challenges of melanoma prevention and care, as well as the continued need for epidemiologic surveillance. The aim of this chapter is to highlight changing trends in melanoma occurrence and mortality, and how these rates have influenced or been influenced by prevention strategies.
Incidence
Worldwide incidence of melanoma has steadily increased over the last several decades (5, 9–11, 19, 20). Annual incidence has risen as rapidly as 4–6% in many fair-skinned populations that predominate regions like North America, Northern Europe, Australia, and New Zealand (10, 21–33). Increases in incidence rates vary considerably across populations of different ethnicity and geographical location, and even within populations across age and gender (6, 7, 9, 19, 34, 35). These differences are important to consider to avoid masking true trends in melanoma incidence.
ETHNICITY
Melanoma demonstrates greater variation in incidence rates across different ethnic groups than that of most cancers (9). Melanoma is disproportionally reported among fair-skinned Caucasian populations (6, 9, 36, 37). This variation is partly attributable to decreased photoprotection from reduced melanin (38). The increased melanin barrier in darker-pigmented individuals decreases both ultraviolet (UV) A and B radiation through the skin (38–40). UV radiation is known to induce both cell death and malignant transformation of skin cells; it is considered the paramount risk factor for melanoma (41–46). Compared to fairer-skinned people, UVB radiation through the epidermis is diminished by approximately 50% in darker-skinned people (38), and UVA transmission through the dermis decreases from 27 to 4% at 314 nm and 47 to 14% at 400 nm (39).
Within the United States, differences among melanoma incidence by race are well-illustrated (11). The United States has a comprehensive cancer database. The United States Cancer Statistics (USCS) provides official federal cancer incidence statistics in 49 states and the District of Columbia (99.1% of the US population) using data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results (SEER) program (6). Of the 65,647 melanomas reported in the United States from this database, the overall annual age- standardized rate (ASR) of melanoma incidence was 19.7:100,000 cases (11). Non-Hispanic Caucasians accounted for the greatest incidence of ASR at 24.6:100,000 cases, followed by American Indian/Alaska Natives at 4.3, then Hispanics at 4.2, Asian/Pacific Islanders at 1.3, and lastly African Americans at 1.0 (6, 11). Although melanoma does disproportionally affect Caucasian populations, the incidence of disease can vary considerably depending on the geographic location of the population (47).
GEOGRAPHY
Incidence of melanoma varies by geographic location among people of the same ethnicity (47–50). Differences in geography can translate to differences in atmospheric absorption, latitude, altitude, cloud cover, and season—all variables that influence incident UV radiation. In 1956, Lancaster found increasing melanoma mortality rates with increasing proximity to the equator, a phenomenon he termed the “latitude gradient” (47, 49, 51). Since then, similar trends of melanoma incidence have been reported around the world (Figure 1) (42, 48). In the lowest latitudes, melanoma annual incidence ASRs tend to be higher than that of ASRs in higher latitudes (Figure 1) (9). In 2012, of the 184 countries evaluated by the International Agency for Research on Cancer (IARC), the highest reported incidence rates for melanoma occurred in New Zealand (ASR = 35.8:100,000 cases per year), followed closely by Australia (ASR = 34.9:100,000 cases per year) (9). The following countries with elevated incidence ASRs were all of higher latitudes, including Switzerland (20.3:100,000 cases per year), the Netherlands (19.4:100,000 cases per year), Denmark (19.2:100,000 cases per year), Norway (18.8:100,000 cases per year), and Sweden (18.0:100,000 cases per year) (Table 1) (9). While these countries are at high latitudes, a north–south gradient of incidence rates has been observed, even among the northernmost Scandinavian nations (9, 52, 53). Similar observations have been made among Caucasian populations in the United States (54, 55), New Zealand (56), and other nations (48).
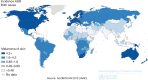
Figure 1
Worldwide melanoma age-standardized annual incidence rate by geography. Age-standardized rate (ASR) by world is expressed per 100,000 persons (9). Reproduced with permission.
TABLE 1
Melanoma Annual Incidence by Worldwide Region and Country (N > 1000 persons) in Descending Order of ASR.
In Australia, those who live closer to the equator, and thus have a higher degree of sun exposure, have higher incidences of melanoma (57). Queensland, a predominately tropical state in Australia (latitude 27°S) has higher melanoma rates than New South Wales (latitude 34°S) (24, 57, 58). An inverse latitude gradient is observed in Europe (59). Within Europe, melanoma incidence is 3- to 6-fold higher in northern countries in Scandinavia than in southern countries like Spain and Italy (Table 1) (9). The differing incidence rates between northern and southern Europe could be partly attributed to different pigmentation characteristics that predominate the populations of each region. The fairer-skinned populations in Scandinavia and darker olive-skinned populations in southern Europe reflect patterns of melanoma incidence discussed previously about ethnicity (9).
Other European populations, such as those in the United Kingdom, Germany, Austria, and France, report melanoma ASRs in the range of 14.6–9.9:100,000 cases per year (Table 1) (9). The predominantly non-Caucasian populations of Africa, Asia, and the Pacific, and the mixed populations of Central and South America, consistently report melanoma rates less than 4:100,000 per year (Table 1) (9). Despite the geographic location of many Asian nations being near the equator, the incidence among Asians has remained largely unchanged and minimal. An unchanged Asian incidence, as with the case of the incidence in countries in Southern Europe and Africa, is likely attributed to a homogeneous darker-pigmented population (9).
National figures allow for global comparisons of melanoma incidence. However, marked variances in melanoma incidence can be masked within countries that boast heterogeneous populations. These countries include the United States, New Zealand, Australia, Israel, and South Africa. Variances in melanoma incidence within a country can also be masked when nations span many degrees of latitude, like Australia. In all countries, melanoma rates are the highest among the fairest-skinned Caucasian residents (5, 9, 11). Conversely, lower incidence is seen among those with darker skin (6, 9, 11).
Differences in altitude have also been suggested to play a role in melanoma incidence. In countries with both high and low-latitude locations, regions of higher altitude have been associated with higher melanoma incidence (60–63). Similarly there are significantly higher incidence rates among individuals who regularly partake in high-altitude activities like mountaineering (64). UV irradiance is associated with higher altitude. However, with higher altitude there are also changes in ozone absorption, decreased cloud cover, and increased surface reflectance from snow cover which can all also increase UV radiation.
AGE
Worldwide melanoma incidence ASRs climb steadily and peak at the seventh and eighth decades of life (Figure 2) (9). This trend is seen among most high-risk populations, including individuals in Australia and New Zealand (23, 65), and Northern Europe (28, 30). Incidence in the United States, however, peaks at the sixth decade of life (6). Americans aged between 55 and 74 comprise 44.9% of all diagnosed melanomas in the United States (6). While melanoma incidence is lower among people <40 years of age, it is one of the most common cancers diagnosed among adolescent and young adults (66, 67). In the United States, melanoma is the second most common cancer among women aged between 20 and 29 (6). Similarly, melanoma is among the most commonly diagnosed cancers in young adults worldwide (9, 68).

Figure 2
Worldwide age-standardized annual incidence of melanoma by age. ASR = Age-standardized rate (world), expressed per 100,000 persons (9).
SEX
Melanoma affects women and men differently. This is in part reflected by differences in melanoma incidence by population (Figure 3) (9). When age is taken into account, adolescent and young adult women are more susceptible to melanoma than men (67–69). This may be in part due to the widespread use of indoor tanning among females, which is associated with increased melanoma risk (70–72). However, after the age of 40, rates reverse, and melanoma incidence among men is greater than that of women (6, 67–69). Overall, men are more susceptible to melanoma. Some posit that this increased susceptibility seen among men may be in part androgen driven (43, 72, 73). This difference in incidence by sex is exemplified in the United States with annual incidence ASRs of 29.2:100,000 cases in men compared to 17.3:100,000 cases in women (6). In fact, this increased incidence rate among men is observed across all ethnicities in the United States with ASRs (per 100,000 cases) of 33.1 for non-Hispanic Caucasian males and 19.9 for non-Hispanic Caucasian females, 5.0 for Hispanic males and 4.7 for Hispanic females, 1.6 for Asian/Pacific Islander males and 1.2 for Asian/Pacific Islander females, and 1.1 for African American males and 1.0 for African American females (6). The only exception is among American Indian/Alaska Natives in which the ASR is 4.3:100,000 cases for men and 4.9:100,000 cases for women (6).
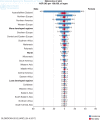
Figure 3
Melanoma age-standardized annual incidence and mortality rate by sex, world region, and development. ASR = Age-standardized rate (world), expressed per 100,000 persons (9). Reproduced with permission.
Historically, higher-latitude, lower-incidence populations in Scotland and Canada have reported substantially higher rates among females (27, 74). In Scotland, incidence of melanoma among females has been reported to be 2-fold higher than in males (74). Conversely, melanoma incidence is higher among men than women in most mid- to low-latitude populations like in the United States, Australia, and New Zealand (6, 9, 75). Overall, increases in melanoma incidence among men have since changed the lead women once had over men in high-latitude, low-incidence populations; men generally exceed women in these regions now (Figure 3) (9, 27, 28).
ANATOMIC DISTRIBUTION
Among Caucasian populations, melanoma is more frequently reported on the backs and shoulders of men and the lower limbs of women (76–80). For both sexes, given that these body site locations are associated with lower sunlight exposure, these findings have been used as supportive evidence for the intermittent UV exposure theory (81, 82). This theory posits that intermittent and intense sun exposure places individuals at increased risk for melanoma (81, 82). However, populations in low-latitude regions like Australia do not demonstrate similar patterns of distribution (83). Instead, Australians of both sexes most frequently report melanoma on high sun-exposed anatomic regions like their head and neck (83, 84). If risk of melanoma per unit area of skin is compared, the face is reported most frequently among both sexes (83). This calculation is made by adjusting for the surface areas of the body sites being compared (83). The next most frequently found sites for melanoma, when adjusting for surface area, are the shoulders, upper arms, and backs of women, and the shoulders and backs of men (83). The lowest rates of melanoma are found on the buttocks of both sexes, and the female scalp (76, 85).
Also, when considering age, melanomas that develop on the trunk occur more often in the fifth to sixth decades of life, whereas melanomas that develop in high UV-exposed body regions, like the head and neck, occur more commonly in the eighth decade (75, 86–89).
PREDICTIONS AND TRENDS
For decades, melanoma incidence has progressively risen and is projected to continue to rise across the world (5, 9–11, 19, 20). Conversely, mortality rates have not always followed a similar trend (6, 9). The diverging trends between melanoma incidence and mortality has led some to question whether there is a true melanoma epidemic, or rather if increases in incidence represent improved screening techniques (5, 90).
Those who argue that increased incidence is largely attributable to increases in diagnosis cite the high percentage of diagnosed melanoma in situ (6, 22, 91). In the United States, the annual incidence of melanoma in situ is 9.5% (91). Some clinicians within the United States have suggested reclassifying nonmalignant diagnosis of melanoma to address, in part, inflated incidence (92). Increased number of biopsies has also been attributed to the increasing incidence of melanoma (92).
Some investigators suggest that increased screening and biopsy alone cannot account for the dramatic changes observed in incidence (5, 20). For example, in the United States, increases in melanoma incidence has been demonstrated across all melanoma thickness classifications, independent of socioeconomic status, which some argue is a surrogate marker for health care access and screening (5, 20). Similarly, others have found corresponding increases in incidence of aggressive melanoma subtypes, like nodular melanoma, and increases in later staged tumors (93). Taken together, these findings suggest that while increased diagnoses may play a role in increasing trends of melanoma incidence, there is also a true increase in incidence worldwide.
Mortality
Melanoma mortality trends are variable and, as with incidence, are influenced by geography, ethnicity, age, and sex (11, 19, 65, 67, 68, 94, 95). Melanoma mortality rates have marginally increased among fair-skinned populations (19, 32, 68, 96). Like with melanoma incidence, among fair-skinned populations, melanoma mortality rate is highest in low-latitude regions (Figures 3 and 4) (9). In high-risk regions like New Zealand, Australia, North America, and Europe, mortality rates historically increased until the 1980s (97, 98), peaked between 1988 and 1990, and then gradually maintained a slow increase (19, 32, 56, 68, 96, 99, 100). Over the last decade, mortality rate has steadily increased at 1.5% in the highest observed countries of New Zealand and Australia (19). In Scandinavia, mortality rate has also steadily increased over the last decade, with annual ASR in Norway at 6 × 10−5 per person and 4 × 10−5/ person in Sweden (19). In the United Kingdom, mortality rate has risen steadily at 1.59% per year (19). The US mortality rate has slowed to a 0.20% annual increase (19). Similar trends have also been reported in East Asian populations (101).

Figure 4
Worldwide melanoma age-standardized annual mortality rate by geography. Age-standardized rate (ASR) by world is expressed per 100,000 persons (9). Reproduced with permission.
Within ethnically heterogeneous countries like the United States, variations in melanoma mortality among population subgroups have been observed (94). Non-Hispanic African Americans have lower cause-specific mortality than non-Hispanic Caucasians (HR = 0.7, 95% CI = 0.6–0.8) (94). However, after controlling for stage and site at diagnosis, gender, and age and decade of diagnosis, non-Hispanic African Americans fare worse than non-Hispanic Caucasians in cause-specific mortality (94). Overall, 5-year survival is lower for African Americans than non-Hispanic Caucasians as well (22, 26). In fact, over the last decade, 5-year survival rate has decreased among African Americans, whereas it has increased among Caucasian populations (22). Some attribute this discrepancy in ethnicity in part to socioeconomic inequities (94).
Discrepancies in age and sex are also observed in melanoma mortality rates (Figure 3) (6, 9, 33). Worldwide, males have greater mortality rates than females (Figure 3) (9, 33). In the United States, mortality is greater among men than in women of all races with the annual ASR of deaths being 4.0:100,000 cases in men compared to 1.7:100,000 cases in women (6). This increased incidence of ASR is observed in 4.3:100,000 cases for non-Hispanic Caucasian males compared to 1.8:100,000 cases for non-Hispanic Caucasian females and 1.4:100,000 cases for American Indian/Alaska Native males and 0.5:100,000 for American Indian/Alaska Native females (6). ASR is 1.0:100,000 cases for American Hispanic males and 0.6:100,000 cases for American Hispanic females; 0.5:100,000 cases for African American males and 0.4:100,000 cases for African American females; and 0.4:100,000 cases for American Asian/Pacific Islander males and 0.3:100,000 cases for American Asian/Pacific Islander females (6). Similarly, with annual melanoma incidence, annual melanoma mortality is the greatest among individuals beyond their seventh decade worldwide (9, 33). Conversely in the United States, mortality peaks at between ages 75 and 84, and then declines when individuals are aged >84 (6).
Prevention
In the setting of rising global incidence of melanoma, health agencies across nations with substantial burden of disease have launched campaigns that aim to promote prevention. Prevention strategies that have been implemented range from primary prevention methods to reduce sun exposure and enforce stricter labeling protocol for sunscreens (18, 102), to secondary prevention methods like full-body visual skin exams (103).
PRIMARY PREVENTION
Nationwide efforts to reduce UV exposure have been attempted with variable success across high-incidence countries like the United States, the United Kingdom, Norway, Australia, and New Zealand (19, 102, 104, 105). In the United States, the indoor tanning industry accrues $3 billion per year in profit (106). Attempts have been made by the US Surgeon General to promote sun protection polices like mandatory sun protection factor (SPF) labeling on sunscreens, recommendations for use of broad spectrum sunscreen SPF 15+, and discouraging indoor tanning. Despite more rigorous attempts to regulate indoor tanning, 7.8 million women and 1.9 million men still engage in tanning device activity (70, 102). In the United Kingdom, a nationwide campaign, SunSmart, was launched in 2003 in an effort to reduce the rapid rise in melanoma incidence (107). As part of their campaign, SunSmart highlighted UV reducing methods like wearing sunscreen with SPF 15+, wearing protective clothing and hats, and staying indoors during high incident UV hours (107). Despite concerted efforts, melanoma incidence in the United Kingdom has continued to increase (9). Population-based investigations found that British residents continued to partake in high-risk behaviors and that men from lower socioeconomic groups were at the greatest risk for UV exposure (107). In Norway and Sweden, national campaigns have also been launched in an effort to address rapid rises in melanoma incidence (104, 105). Similar to the United Kingdom and the United States, there has been limited and varied success with their preventative measures (104, 105). Australia, however, has demonstrated one of the most successful responses to nationwide melanoma prevention efforts (19, 108). Historically, Australia has had the highest melanoma incidence rates in the world (9). In the 1980s, the Australian government launched a massive melanoma education campaign, SunSmart (what the British later adopted for themselves). Sunsmart was integrated into primary school curriculums and permeated community forums and workplace training (108). In the decades since launching the SunSmart campaign, melanoma incidence rates in Australia have slowed, and within younger birth cohorts, incidence has even decreased (9, 19, 108). Australia is no longer the global leader in melanoma incidence (9).
SECONDARY PREVENTION
The predominant method of secondary prevention of melanoma is visual skin examination. Among the largest efforts to promote standardized screening, Germany launched the Skin Cancer Research to Provide Evidence for Effectiveness of Screening (SCREEN) in Northern Germany (109). After a year of implementing the program, a 48% reduction in melanoma mortality was found in SCREEN regions compared to neighboring communities (109). There was an overall decrease in mortality from 1.7 deaths per 100,000 cases to 0.9 deaths per 100,000 cases (109). This decrease in mortality was not observed in regions that did not implement SCREEN (109, 110). There are few other nationwide campaigns that promote secondary prevention. Australia has integrated bolstered screening measures as part of their overall screening campaign (103). In the United States, the national Preventative Services Task Force (USPSTF) deemed that there was insufficient data available to recommend visual skin exams (111). In a systematic evidence review, the Agency for Healthcare Research and Quality on the behalf of the USPSTF examined >12,500 scientific abstracts and 450 articles and reported that they were unable to find any evidence on the potential harms or benefits of screening to conclusively make a recommendation (111). Continued efforts must be made to better understand prevention methods and their effects on melanoma incidence and mortality.
Conclusion
Global melanoma incidence and mortality continues to rise (9). While its incidence is over 10-fold lower than that of other skin cancers (6), its capacity to rapidly metastasize and affect younger patients makes melanoma a significant health and economic burden on society (5, 6, 21). In the United States alone, the estimated attributable health care cost in 2020 to melanoma is $4.58 billion (112, 113). Among high-risk populations, melanoma incidence will likely continue to rise in the geriatric subpopulation (6). However, incidence trends among younger individuals are hopeful, with some trends stagnating and some even decreasing in high-risk populations (19, 58, 103). In high melanoma incidence nations like Australia, melanoma rates have largely stabilized (9, 19). This is also true of mortality rates (9, 19). Hopefully, as these trends continue to be monitored, rates will decrease further. Although melanoma incidence has not slowed in other high-incidence regions like Northern Europe, a similar decrease should be expected to follow if continued concerted efforts are launched toward melanoma prevention campaigns. Nevertheless, continued surveillance is necessary. As the average age of nationwide populations is projected to increase in the United States, the United Kingdom, and Northern Europe, there may be a continued increase in melanoma incidence. It is imperative that primary and secondary methods of prevention are implemented and studied. National health campaigns can look to countries like Australia for examples of successful skin cancer prevention. Ultimately, preventative measures must take the cornerstone in melanoma control.
Conflict of interest: The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
Copyright and Permission statement: To the best of our knowledge, the materials included in this chapter do not violate copyright laws. All original sources have been appropriately acknowledged and/or referenced. Where relevant, appropriate permissions have been obtained from the original copyright holder(s).
References
- 1.
- Lerner AB, McGuire JS. Melanocyte-stimulating hormone and adrenocorticotrophic hormone. Their relation to pigmentation. N Engl J Med. 1964;270:539–46. [PubMed: 14091524] [CrossRef]
- 2.
- Abdel-Malek Z, Suzuki I, Tada A, Im S, Akcali C. The melanocortin-1 receptor and human pigmentation. Ann N Y Acad Sci. 1999;885:117–33. [PubMed: 10816645] [CrossRef]
- 3.
- Abdel-Malek Z, Swope VB, Suzuki I, Akcali C, Harriger MD, Boyce ST, et al. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci U S A. 1995;92(5):1789–93. [PMC free article: PMC42605] [PubMed: 7878059] [CrossRef]
- 4.
- Tsatmali M, Ancans J, Thody AJ. Melanocyte function and its control by melanocortin peptides. J Histochem Cytochem. 2002;50(2):125–33. [PubMed: 11799132] [CrossRef]
- 5.
- Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Investig Dermatol. 2009;129(7):1666–74. [PMC free article: PMC2866180] [PubMed: 19131946] [CrossRef]
- 6.
- Surveillance, Epidemiology, and End Results (SEER). Program Cancer Statistics Review, 1975–2013, National Cancer Institute [Internet] Nov, 2015. SEER data submission [cited posted to the SEER web site, 2016 Apr]. Available from: http://seer
.cancer.gov/csr/1975_2013/ - 7.
- Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Exp Rev Anticancer Ther. 2010;10(11):1811–23. [PMC free article: PMC3074354] [PubMed: 21080806] [CrossRef]
- 8.
- Rigel DS, Carucci JA. Malignant melanoma: Prevention, early detection, and treatment in the 21st century. CA Cancer J Clin. 2000;50(4):215–36. quiz 37–40. [PubMed: 10986965] [CrossRef]
- 9.
- GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] 2013. [cited 2017 Apr 6]. Available from: http://globocan
.iarc.fr. - 10.
- Kosary CL, Altekruse SF, Ruhl J, Lee R, Dickie L. Clinical and prognostic factors for melanoma of the skin using SEER registries: Collaborative stage data collection system, version 1 and version 2. Cancer. 2014;120 Suppl 23:3807–14. [PubMed: 25412392] [CrossRef]
- 11.
- Guy GP Jr., Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: Melanoma incidence and mortality trends and projections – United States, 1982–2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591–6. [PMC free article: PMC4584771] [PubMed: 26042651]
- 12.
- Califano J, Nance M. Malignant melanoma. Facial Plast Surg Clin North Am. 2009;17(3):337–48. [PubMed: 19698915] [CrossRef]
- 13.
- Filippi AR, Fava P, Badellino S, Astrua C, Ricardi U, Quaglino P. Radiotherapy and immune checkpoints inhibitors for advanced melanoma. Radiother Oncol. 2016;120(1):1–12. [PubMed: 27345592] [CrossRef]
- 14.
- Goodson AG, Grossman D. Strategies for early melanoma detection: Approaches to the patient with nevi. J Am Acad Dermatol. 2009;60(5):719–35. quiz 36–8. [PMC free article: PMC2690513] [PubMed: 19389517] [CrossRef]
- 15.
- Tripp MK, Watson M, Balk SJ, Swetter SM, Gershenwald JE. State of the science on prevention and screening to reduce melanoma incidence and mortality: The time is now. CA Cancer J Clin. 2016 [PMC free article: PMC5124531] [PubMed: 27232110] [CrossRef]
- 16.
- Guy GP Jr., Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med. 2015;48(2):183–7. [PMC free article: PMC4603424] [PubMed: 25442229] [CrossRef]
- 17.
- Pollack LA, Li J, Berkowitz Z, Weir HK, Wu XC, Ajani UA, et al. Melanoma survival in the United States, 1992 to 2005. J Am Acad Dermatol. 2011;65(5) Suppl 1:S78–86. [PMC free article: PMC4890628] [PubMed: 22018071] [CrossRef]
- 18.
- Seite S, Del Marmol V, Moyal D, Friedman AJ. Public primary and secondary skin cancer prevention, perceptions and knowledge: An international cross-sectional survey. J Eur Acad Dermatol Venereol. 2017;31(5):815–20. [PMC free article: PMC6084324] [PubMed: 28045207] [CrossRef]
- 19.
- Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: Projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Investig Dermatol. 2016;136(6):1161–71. [PubMed: 26902923] [CrossRef]
- 20.
- Erdmann F, Lortet-Tieulent J, Schuz J, Zeeb H, Greinert R, Breitbart EW, et al. International trends in the incidence of malignant melanoma 1953–2008—Are recent generations at higher or lower risk? Int J Cancer. 2013;132(2):385–400. [PubMed: 22532371] [CrossRef]
- 21.
- Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170(1):11–19. [PubMed: 23815297] [CrossRef]
- 22.
- American Cancer Society. Atlanta, GA: American Cancer Society; 2017. Cancer facts & figures 2017 [Internet] [cited 2017 Apr]. Available from: https://www
.cancer.org /content/dam/cancer-org /research/cancer-facts-and-statistics /annual-cancer-facts-and-figures /2017/cancer-facts-and-figures-2017.pdf. - 23.
- Coory M, Baade P, Aitken J, Smithers M, McLeod GR, Ring I. Trends for in situ and invasive melanoma in Queensland, Australia, 1982–2002. Cancer Causes Control. 2006;17(1):21–7. [PubMed: 16411049] [CrossRef]
- 24.
- Marrett LD, Nguyen HL, Armstrong BK. Trends in the incidence of cutaneous malignant melanoma in New South Wales, 1983–1996. Int J Cancer. 2001;92(3):457–62. [PubMed: 11291086] [CrossRef]
- 25.
- Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93(9):678–83. [PubMed: 11333289] [CrossRef]
- 26.
- Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65(5) Suppl 1:S17–25. e1–3. [PubMed: 22018063]
- 27.
- Ulmer MJ, Tonita JM, Hull PR. Trends in invasive cutaneous melanoma in Saskatchewan 1970–1999. J Cutan Med Surg. 2003;7(6):433–42. [PubMed: 15926213] [CrossRef]
- 28.
- MacKie RM, Bray CA, Hole DJ, Morris A, Nicolson M, Evans A, et al. Incidence of and survival from malignant melanoma in Scotland: An epidemiological study. Lancet (London, England). 2002;360(9333):587–91. [PubMed: 12241928] [CrossRef]
- 29.
- Lasithiotakis KG, Leiter U, Gorkievicz R, Eigentler T, Breuninger H, Metzler G, et al. The incidence and mortality of cutaneous melanoma in Southern Germany: Trends by anatomic site and pathologic characteristics, 1976 to 2003. Cancer. 2006;107(6):1331–9. [PubMed: 16909413] [CrossRef]
- 30.
- Stang A, Pukkala E, Sankila R, Soderman B, Hakulinen T. Time trend analysis of the skin melanoma incidence of Finland from 1953 through 2003 including 16,414 cases. Int J Cancer. 2006;119(2):380–4. [PubMed: 16477634] [CrossRef]
- 31.
- Lipsker D, Engel F, Cribier B, Velten M, Hedelin G. Trends in melanoma epidemiology suggest three different types of melanoma. Br J Dermatol. 2007;157(2):338–43. [PubMed: 17596175] [CrossRef]
- 32.
- de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: Rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer. 2003;107(1):119–26. [PubMed: 12925966] [CrossRef]
- 33.
- Shen W, Sakamoto N, Yang L. Melanoma-specific mortality and competing mortality in patients with non-metastatic malignant melanoma: A population-based analysis. BMC Cancer. 2016;16:413. [PMC free article: PMC4936003] [PubMed: 27389173] [CrossRef]
- 34.
- Olsen CM, Neale RE, Green AC, Webb PM, Whiteman DC. Independent validation of six melanoma risk prediction models. J Investig Dermatol. 2015;135(5):1377–84. [PubMed: 25548858] [CrossRef]
- 35.
- Apalla Z, Nashan D, Weller RB, Castellsague X. Skin cancer: Epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther. 2017;7 Suppl 1:5–19. [PMC free article: PMC5289116] [PubMed: 28150105] [CrossRef]
- 36.
- Padovese V, Franco G, Valenzano M, Pecoraro L, Cammilli M, Petrelli A. Skin cancer risk assessment in dark skinned immigrants: The role of social determinants and ethnicity. Ethn Health. 2017:1–10. [PubMed: 28277022] [CrossRef]
- 37.
- Chao LX, Patterson SS, Rademaker AW, Liu D, Kundu RV. Melanoma perception in people of color: A targeted educational intervention. Am J Clin Dermatol. 2017;18(3):419–27. [PubMed: 28035649] [CrossRef]
- 38.
- Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84(3):539–49. [PMC free article: PMC2671032] [PubMed: 18435612] [CrossRef]
- 39.
- Everett MA, Yeargers E, Sayre RM, Olson RL. Penetration of epidermis by ultraviolet rays. Photochem Photobiol. 1966;5(7):533–42. [PubMed: 5981960] [CrossRef]
- 40.
- Grigalavicius M, Moan J, Dahlback A, Juzeniene A. Daily, seasonal, and latitudinal variations in solar ultraviolet A and B radiation in relation to vitamin D production and risk for skin cancer. Int J Dermatol. 2016;55(1):e23–8. [PubMed: 26547141] [CrossRef]
- 41.
- Seebode C, Lehmann J, Emmert S. Photocarcinogenesis and skin cancer prevention strategies. Anticancer Res. 2016;36(3):1371–8. [PubMed: 26977038]
- 42.
- Moan J, Grigalavicius M, Baturaite Z, Dahlback A, Juzeniene A. The relationship between UV exposure and incidence of skin cancer. Photodermatol Photoimmunol Photomed. 2015;31(1):26–35. [PubMed: 25213656] [CrossRef]
- 43.
- Li WQ, Cho E, Weinstock MA, Mashfiq H, Qureshi AA. Epidemiological assessments of skin outcomes in the nurses’ health studies. Am J Public Health. 2016;106(9):1677–83. [PMC free article: PMC4981523] [PubMed: 27459457] [CrossRef]
- 44.
- Qureshi AA, Laden F, Colditz GA, Hunter DJ. Geographic variation and risk of skin cancer in US women. Differences between melanoma, squamous cell carcinoma, and basal cell carcinoma. Arch Intern Med. 2008;168(5):501–7. [PubMed: 18332296] [CrossRef]
- 45.
- Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: A cohort study. Cancer Epidemiol Biomark Prev. 2014;23(6):1080–9. [PMC free article: PMC4151553] [PubMed: 24876226] [CrossRef]
- 46.
- Wu S, Han J, Vleugels RA, Puett R, Laden F, Hunter DJ, et al. Cumulative ultraviolet radiation flux in adulthood and risk of incident skin cancers in women. Br J Cancer. 2014;110(7):1855–61. [PMC free article: PMC3974077] [PubMed: 24595003] [CrossRef]
- 47.
- Lancaster HO. Some geographical aspects of the mortality from melanoma in Europeans. Med J Aust. 1956;43(26):1082–7. [PubMed: 13347440]
- 48.
- Crombie IK. Variation of melanoma incidence with latitude in North America and Europe. Br J Cancer. 1979;40(5):774–81. [PMC free article: PMC2010099] [PubMed: 508580] [CrossRef]
- 49.
- Lancaster HO, Nelson J. Sunlight as a cause of melanoma; a clinical survey. Med J Aust. 1957;44(14):452–6. [PubMed: 13430035]
- 50.
- Weinstock MA, Colditz GA, Willett WC, Stampfer MJ, Bronstein BR, Mihm MC Jr., et al. Nonfamilial cutaneous melanoma incidence in women associated with sun exposure before 20 years of age. Pediatrics. 1989;84(2):199–204. [PubMed: 2748244]
- 51.
- Elwood JM, Lee JA, Walter SD, Mo T, Green AE. Relationship of melanoma and other skin cancer mortality to latitude and ultraviolet radiation in the United States and Canada. Int J Epidemiol. 1974;3(4):325–32. [PubMed: 4435983] [CrossRef]
- 52.
- Moan J, Dahlback A. The relationship between skin cancers, solar radiation and ozone depletion. Br J Cancer. 1992;65(6):916–21. [PMC free article: PMC1977777] [PubMed: 1616864] [CrossRef]
- 53.
- Magnus K. Incidence of malignant melanoma of the skin in the five Nordic countries: Significance of solar radiation. Int J Cancer. 1977;20(4):477–85. [PubMed: 914393] [CrossRef]
- 54.
- Lee JA, Scotto J. Melanoma: Linked temporal and latitude changes in the United States. Cancer Causes Control. 1993;4(5):413–18. [PubMed: 8218872] [CrossRef]
- 55.
- Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations—US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch Dermatol. 2005;141(4):477–81. [PubMed: 15837865] [CrossRef]
- 56.
- Bulliard JL, Cox B, Elwood JM. Latitude gradients in melanoma incidence and mortality in the non-Maori population of New Zealand. Cancer Causes Control. 1994;5(3):234–40. [PubMed: 8061171] [CrossRef]
- 57.
- Baade P, Meng X, Youlden D, Aitken J, Youl P. Time trends and latitudinal differences in melanoma thickness distribution in Australia, 1990–2006. Int J Cancer. 2012;130(1):170–8. [PubMed: 21344376] [CrossRef]
- 58.
- Iannacone MR, Youlden DR, Baade PD, Aitken JF, Green AC. Melanoma incidence trends and survival in adolescents and young adults in Queensland, Australia. Int J Cancer. 2015;136(3):603–9. [PMC free article: PMC4277328] [PubMed: 24806428]
- 59.
- Armstrong BK. Epidemiology of malignant melanoma: Intermittent or total accumulated exposure to the sun? J Dermatol Surg Oncol. 1988;14(8):835–49. [PubMed: 3397443] [CrossRef]
- 60.
- Krishnamurthy S. The geography of non-ocular malignant melanoma in India: Its association with latitude, ozone levels and UV light exposure. Int J Cancer. 1992;51(2):169–72. [PubMed: 1568786] [CrossRef]
- 61.
- Gerbaud L, Lejeune ML, Abou-Samra T, Doz M, Mathey MF, D’Incan M, et al. Epidemiological survey of melanoma in the Auvergne region (France): Is there an increased incidence in Auvergne? Eur J Epidemiol. 2003;18(4):331–5. [PubMed: 12803373] [CrossRef]
- 62.
- Aceituno-Madera P, Buendia-Eisman A, Olmo FJ, Jimenez-Moleon JJ, Serrano-Ortega S. [Melanoma, altitude, and UV-B radiation] Actas Dermosifiliogr. 2011;102(3):199–205. [PubMed: 21334587] [CrossRef]
- 63.
- Haluza D, Simic S, Moshammer H. Temporal and spatial melanoma trends in Austria: An ecological study. Int J Environ Res Public Health. 2014;11(1):734–48. [PMC free article: PMC3924471] [PubMed: 24398911] [CrossRef]
- 64.
- Moehrle M, Garbe C. Does mountaineering increase the incidence of cutaneous melanoma? A hypothesis based on cancer registry data. Dermatology (Basel, Switzerland). 1999;199(3):201–3. [PubMed: 10592397] [CrossRef]
- 65.
- Sneyd MJ, Cox B. A comparison of trends in melanoma mortality in New Zealand and Australia: The two countries with the highest melanoma incidence and mortality in the world. BMC Cancer. 2013;13:372. [PMC free article: PMC3750694] [PubMed: 23915380] [CrossRef]
- 66.
- Ballantine KR, Watson H, Macfarlane S, Winstanley M, Corbett RP, Spearing R, et al. Small numbers, big challenges: Adolescent and young adult cancer incidence and survival in New Zealand. J Adolesc Young Adult Oncol. 2017;6(2):277–85. [PubMed: 28207291] [CrossRef]
- 67.
- Watson M, Geller AC, Tucker MA, Guy GP Jr., Weinstock MA. Melanoma burden and recent trends among non-Hispanic whites aged 15–49 years, United States. Prev Med. 2016;91:294–8. [PMC free article: PMC5146952] [PubMed: 27565055] [CrossRef]
- 68.
- Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27(1):3–9. [PubMed: 19095149] [CrossRef]
- 69.
- Weir HK, Marrett LD, Cokkinides V, Barnholtz-Sloan J, Patel P, Tai E, et al. Melanoma in adolescents and young adults (ages 15–39 years): United States, 1999–2006. J Am Acad Dermatol. 2011;65(5) Suppl 1:S38–49. [PMC free article: PMC3254089] [PubMed: 22018066] [CrossRef]
- 70.
- Guy GP Jr., Zhang Y, Ekwueme DU, Rim SH, Watson M. The potential impact of reducing indoor tanning on melanoma prevention and treatment costs in the United States: An economic analysis. J Am Acad Dermatol. 2017;76(2):226–33. [PMC free article: PMC5737631] [PubMed: 27939556] [CrossRef]
- 71.
- Colantonio S, Bracken MB, Beecker J. The association of indoor tanning and melanoma in adults: Systematic review and meta-analysis. J Am Acad Dermatol. 2014;70(5):847–57. e1–18. [PubMed: 24629998]
- 72.
- Zhang M, Qureshi AA, Geller AC, Frazier L, Hunter DJ, Han J. Use of tanning beds and incidence of skin cancer. J Clin Oncol. 2012;30(14):1588–93. [PMC free article: PMC3383111] [PubMed: 22370316] [CrossRef]
- 73.
- Li WQ, Qureshi AA, Ma J, Goldstein AM, Giovannucci EL, Stampfer MJ, et al. Personal history of prostate cancer and increased risk of incident melanoma in the United States. J Clin Oncol. 2013;31(35):4394–9. [PMC free article: PMC3842907] [PubMed: 24190118] [CrossRef]
- 74.
- MacKie R, Hunter JA, Aitchison TC, Hole D, McLaren K, Rankin R, et al. Cutaneous malignant melanoma, Scotland, 1979–89. Lancet (London, England). 1992;339(8799):971–5. [PubMed: 1348807] [CrossRef]
- 75.
- Bulliard JL, Cox B. Cutaneous malignant melanoma in New Zealand: Trends by anatomical site, 1969–1993. Int J Epidemiol. 2000;29(3):416–23. [PubMed: 10869312]
- 76.
- Osterlind A, Hou-Jensen K, Moller Jensen O. Incidence of cutaneous malignant melanoma in Denmark 1978–1982. Anatomic site distribution, histologic types, and comparison with non-melanoma skin cancer. Br J Cancer. 1988;58(3):385–91. [PMC free article: PMC2246607] [PubMed: 3179192] [CrossRef]
- 77.
- Magnus K. Habits of sun exposure and risk of malignant melanoma: An analysis of incidence rates in Norway 1955–1977 by cohort, sex, age, and primary tumor site. Cancer. 1981;48(10):2329–35. [PubMed: 7296483] [CrossRef]
- 78.
- Popescu NA, Beard CM, Treacy PJ, Winkelmann RK, O’Brien PC, Kurland LT. Cutaneous malignant melanoma in Rochester, Minnesota: Trends in incidence and survivorship, 1950 through 1985. Mayo Clinic Proc. 1990;65(10):1293–302. [PubMed: 2214878] [CrossRef]
- 79.
- Masback A, Westerdahl J, Ingvar C, Olsson H, Jonsson N. Cutaneous malignant melanoma in south Sweden 1965, 1975, and 1985. A histopathologic review. Cancer. 1994;73(6):1625–30. [PubMed: 8156490] [CrossRef]
- 80.
- Cho E, Rosner BA, Colditz GA. Risk factors for melanoma by body site. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1241–4. [PubMed: 15894679] [CrossRef]
- 81.
- Elder DE. Human melanocytic neoplasms and their etiologic relationship with sunlight. J Investig Dermatol. 1989;92(5) Suppl:297s–303s. [PubMed: 2654303] [CrossRef]
- 82.
- Stierner U, Augustsson A, Rosdahl I, Suurkula M. Regional distribution of common and dysplastic naevi in relation to melanoma site and sun exposure. A case-control study. Melanoma Res. 1992;1(5–6):367–75. [PubMed: 1422192] [CrossRef]
- 83.
- Green A, MacLennan R, Youl P, Martin N. Site distribution of cutaneous melanoma in Queensland. Int J Cancer. 1993;53(2):232–6. [PubMed: 8425760] [CrossRef]
- 84.
- Green A. A theory of site distribution of melanomas: Queensland, Australia. Cancer Causes Control. 1992;3(6):513–16. [PubMed: 1420853] [CrossRef]
- 85.
- Chen YT, Zheng T, Holford TR, Berwick M, Dubrow R. Malignant melanoma incidence in Connecticut (United States): Time trends and age-period-cohort modeling by anatomic site. Cancer Causes Control. 1994;5(4):341–50. [PubMed: 8080946] [CrossRef]
- 86.
- Lachiewicz AM, Berwick M, Wiggins CL, Thomas NE. Epidemiologic support for melanoma heterogeneity using the surveillance, epidemiology, and end results program. J Investig Dermatol. 2008;128(5):1340–2. [PubMed: 18408748] [CrossRef]
- 87.
- Stang A, Stabenow R, Eisinger B, Jockel KH. Site- and gender-specific time trend analyses of the incidence of skin melanomas in the former German Democratic Republic (GDR) including 19351 cases. Eur J Cancer (Oxford, England : 1990). 2003;39(11):1610–18. [PubMed: 12855269] [CrossRef]
- 88.
- Elwood JM, Gallagher RP. Body site distribution of cutaneous malignant melanoma in relationship to patterns of sun exposure. Int J Cancer. 1998;78(3):276–80. [PubMed: 9766557] [CrossRef]
- 89.
- Perez-Gomez B, Aragones N, Gustavsson P, Lope V, Lopez-Abente G, Pollan M. Do sex and site matter? Different age distribution in melanoma of the trunk among Swedish men and women. Br J Dermatol. 2008;158(4):766–72. [PubMed: 18241261] [CrossRef]
- 90.
- Dennis LK. Analysis of the melanoma epidemic, both apparent and real: Data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135(3):275–80. [PubMed: 10086448] [CrossRef]
- 91.
- American Cancer Society. Atlanta, GA: American Cancer Society; 2016. Cancer facts & figures 2016. [cited 2017 Apr]. Available from: https://old
.cancer.org /acs/groups/content /@research/documents /document/acspc-047079.pdf. - 92.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: Population based ecological study. BMJ (Clinical research ed). 2005;331(7515):481. [PMC free article: PMC1199022] [PubMed: 16081427] [CrossRef]
- 93.
- Shaikh WR, Dusza SW, Weinstock MA, Oliveria SA, Geller AC, Halpern AC. Melanoma Thickness and Survival Trends in the United States, 1989 to 2009. J Natl Cancer Inst. 2016;108(1):djv294. [PMC free article: PMC4857148] [PubMed: 26563354] [CrossRef]
- 94.
- Ward-Peterson M, Acuna JM, Alkhalifah MK, Nasiri AM, Al-Akeel ES, Alkhaldi TM, et al. Association between race/ethnicity and survival of melanoma patients in the United States over 3 decades: A secondary analysis of SEER data. Medicine. 2016;95(17):e3315. [PMC free article: PMC4998683] [PubMed: 27124020] [CrossRef]
- 95.
- Khosrotehrani K, Dasgupta P, Byrom L, Youlden DR, Baade PD, Green AC. Melanoma survival is superior in females across all tumour stages but is influenced by age. Arch Dermatol Res. 2015;307(8):731–40. [PubMed: 26103951] [CrossRef]
- 96.
- Jemal A, Devesa SS, Fears TR, Hartge P. Cancer surveillance series: Changing patterns of cutaneous malignant melanoma mortality rates among whites in the United States. J Natl Cancer Inst. 2000;92(10):811–18. [PubMed: 10814676] [CrossRef]
- 97.
- Stang A, Stang K, Stegmaier C, Hakulinen T, Jockel KH. Skin melanoma in Saarland: Incidence, survival and mortality 1970–1996. Eur J Cancer Prev. 2001;10(5):407–15. [PubMed: 11711755] [CrossRef]
- 98.
- Balzi D, Carli P, Geddes M. Malignant melanoma in Europe: Changes in mortality rates (1970–90) in European Community countries. Cancer Causes Control. 1997;8(1):85–92. [PubMed: 9051327] [CrossRef]
- 99.
- Gaudette LA, Altmayer CA, Wysocki M, Gao RN. Cancer incidence and mortality across Canada. Health Rep. 1998;10(1):51–66. 55–72. (eng) (fre) [PubMed: 9836886]
- 100.
- de Vries E, Bray FI, Eggermont AM, Coebergh JW. Monitoring stage-specific trends in melanoma incidence across Europe reveals the need for more complete information on diagnostic characteristics. Eur J Cancer Prev. 2004;13(5):387–95. [PubMed: 15452451] [CrossRef]
- 101.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. PeerJ. 2016;4:e2809. [PMC free article: PMC5182992] [PubMed: 28028475] [CrossRef]
- 102.
- Lazovich D, Choi K, Vogel RI. Time to get serious about skin cancer prevention. Cancer Epidemiol Biomarkers Prev. 2012;21(11):1893–901. [PubMed: 22962407] [CrossRef]
- 103.
- Brunssen A, Waldmann A, Eisemann N, Katalinic A. Impact of skin cancer screening and secondary prevention campaigns on skin cancer incidence and mortality: A systematic review. J Am Acad Dermatol. 2017;76(1):129–39.e10. [PubMed: 27707591] [CrossRef]
- 104.
- Nilsen LT, Aalerud TN, Hannevik M, Veierod MB. UVB and UVA irradiances from indoor tanning devices. Photochem Photobiol Sci. 2011;10(7):1129–36. [PubMed: 21445424] [CrossRef]
- 105.
- Nilsen LT, Hannevik M, Aalerud TN, Johnsen B, Friberg EG, Veierod MB. Trends in UV irradiance of tanning devices in Norway: 1983–2005. Photochem Photobiol. 2008;84(5):1100–8. [PubMed: 18399922] [CrossRef]
- 106.
- Le Clair MZ, Cockburn MG. Tanning bed use and melanoma: Establishing risk and improving prevention interventions. Prev Med Rep. 2016;3:139–44. [PMC free article: PMC4929140] [PubMed: 27419006] [CrossRef]
- 107.
- Miles A, Waller J, Hiom S, Swanston D. SunSmart? Skin cancer knowledge and preventive behaviour in a British population representative sample. Health Educ Res. 2005;20(5):579–85. [PMC free article: PMC3943395] [PubMed: 15644381] [CrossRef]
- 108.
- Sinclair C, Foley P. Skin cancer prevention in Australia. Br J Dermatol. 2009;161 Suppl 3:116–23. [PubMed: 19775367] [CrossRef]
- 109.
- Breitbart EW, Waldmann A, Nolte S, Capellaro M, Greinert R, Volkmer B, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66(2):201–11. [PubMed: 22074699] [CrossRef]
- 110.
- Leiter U, Buettner PG, Eigentler TK, Garbe C. Prognostic factors of thin cutaneous melanoma: An analysis of the central malignant melanoma registry of the german dermatological society. J Clin Oncol. 2004;22(18):3660–7. [PubMed: 15302905] [CrossRef]
- 111.
- Wernli KJ, Henrikson NB, Morrison CC, Nguyen M, Pocobelli G, Whitlock EP. U.S. Preventive services task force evidence syntheses, formerly systematic evidence reviews. Screening for skin cancer in adults: An updated systematic evidence review for the US preventive services task force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2016. [PubMed: 27583318]
- 112.
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28. [PMC free article: PMC3107566] [PubMed: 21228314] [CrossRef]
- 113.
- Tsao H, Rogers GS, Sober AJ. An estimate of the annual direct cost of treating cutaneous melanoma. J Am Acad Dermatol. 1998;38(5) Pt 1:669–80. [PubMed: 9591809] [CrossRef]
- The health burden and economic costs of cutaneous melanoma mortality by race/ethnicity-United States, 2000 to 2006.[J Am Acad Dermatol. 2011]The health burden and economic costs of cutaneous melanoma mortality by race/ethnicity-United States, 2000 to 2006.Ekwueme DU, Guy GP Jr, Li C, Rim SH, Parelkar P, Chen SC. J Am Acad Dermatol. 2011 Nov; 65(5 Suppl 1):S133-43.
- International trends in the incidence of malignant melanoma 1953-2008--are recent generations at higher or lower risk?[Int J Cancer. 2013]International trends in the incidence of malignant melanoma 1953-2008--are recent generations at higher or lower risk?Erdmann F, Lortet-Tieulent J, Schüz J, Zeeb H, Greinert R, Breitbart EW, Bray F. Int J Cancer. 2013 Jan 15; 132(2):385-400. Epub 2012 May 21.
- Review Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force[ 2016]Review Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the U.S. Preventive Services Task ForceWernli KJ, Henrikson NB, Morrison CC, Nguyen M, Pocobelli G, Whitlock EP. 2016 Jul
- Review Optical Technologies for the Improvement of Skin Cancer Diagnosis: A Review.[Sensors (Basel). 2021]Review Optical Technologies for the Improvement of Skin Cancer Diagnosis: A Review.Rey-Barroso L, Peña-Gutiérrez S, Yáñez C, Burgos-Fernández FJ, Vilaseca M, Royo S. Sensors (Basel). 2021 Jan 2; 21(1). Epub 2021 Jan 2.
- Latitude gradient for melanoma incidence by anatomic site and gender in Norway 1966-2007.[J Photochem Photobiol B. 2010]Latitude gradient for melanoma incidence by anatomic site and gender in Norway 1966-2007.Cicarma E, Juzeniene A, Porojnicu AC, Bruland ØS, Moan J. J Photochem Photobiol B. 2010 Nov 3; 101(2):174-8. Epub 2010 Apr 6.
- Epidemiology of Melanoma - Cutaneous MelanomaEpidemiology of Melanoma - Cutaneous Melanoma
Your browsing activity is empty.
Activity recording is turned off.
See more...

 1
1