NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.
The mutated forms of small guanosinetriphosphatases (GTPases) may cause disease either through aberrant activation or through loss of function or diminished acitivity. For example, a dominant negative missense mutation in Rac2 was found to interfere with both Rac1 and Rac2 function and cause impaired resistance to infection, while reduced Cdc42 activity has been found associated with Fanconi anemia. Missense mutations in Rab GTPases or associated regulatory proteins has been associated with decreased normal activity, resulting in immune dysfunction, pigmentation or neurological disorders. Pathogens often evade immune surveillance or degradation by inactivating endosomal Rab5 or Rab7 GTPases. In Bardet Biedl Syndrome, an inherited cystic kidney disease, Rab8 activation is defective impairing exocytosis to cilia. Presently, the only known activation mechanism is via guanine nucleotide-exchange factors (GEFs), which are multidomain proteins that accelerate the exchange of guanosine diphosphate (GDP) by GTP by several orders of magnitude. GEFs catalyze the dissociation of the nucleotide from the GTPase by modifying the nucleotide-binding site such that the nucleotide affinity is decreased, and the GDP is released and replaced with GTP. Results from the current project revealed that the probe compounds ML099 (CID-888706), ML098 (CID-7345532), and ML097 (CID-2160985) function by increasing the affinity of the GTPases for guanine nucleotides, leading to the hypothesis that these activators interact with GTPases bind to an allosteric binding site localized between switch regions I and II. Based on these findings, the project further proposes that in biochemical systems, these novel small activators should prove useful towards: (1) elucidating the mechanism by which GEF regulates GTPase activity; (2) defining protein-protein interactions between GEFs and GTPases; (3) defining the sites of GTPase regulation in crystal structures and (4) platforms for the development of probes that can be used to activate GTPase function.
Assigned Assay Grant #: MH081231-01
Screening Center Name & PI: UNM Center for Molecular Discovery (UNMCMD) formerly NM Molecular Libraries Screening Center (NMMLSC)
Chemistry Center Name & PI: UNMCMD, Larry Sklar
Assay Submitter & Institution: Angela Wandinger-Ness, University of New Mexico
PubChem Summary Bioassay Identifier (AID): AID-1333, AID-1334, AID-1335, AID-1336, AID-1337, AID-1339, AID-1340, AID-1341
Probe (1) Structure & Characteristics
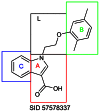
Compound ID: CID-888706
Substance ID: SID-57578335
Molecular Weight: 259.32352 [g/mol]
Molecular Formula: C14H13NO2SH-Bond Donor 1
H-Bond Acceptor: 3
Rotatable Bond Count: 5
Exact Mass: 259.066699
Topological Polar Surface Area: 50.2
Heavy Atom Count: 18
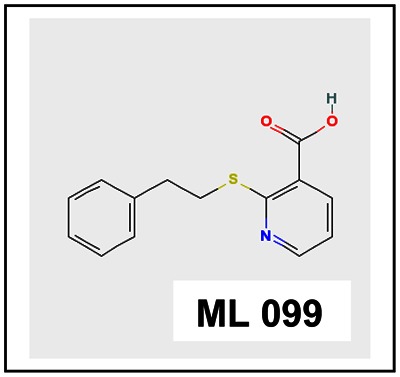
Chemical name: 2-(2-phenylethylsulfanyl)pyridine-3-carboxylic acid [ML 099]
| CID/ML # | Target Name | IC50/EC50 (nM) [SID, AID] | Anti-target Name(s) | IC50/EC50 (nM) [SID, AID] | Selectivity* | Secondary Assay(s) Name: IC50/EC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| CID-888706/ML099 | cell division cycle 42 (GTP binding protein, 25kDa) activated mutant | 58.88 [SID-57578335, AID-1333] | glutathione S-transferase | >100000 [SID-57578335, AID-1769] | >1000 | 12.5 [SID-57578335, AID-1762] |
| CID-888706/ML099 | cell division cycle 42 (GTP binding protein, 25kDa) wild type | 100.00 [SID-57578335, AID-1334] | glutathione S-transferase | >100000 [SID-57578335, AID-1769] | >1000 | 10.5 [SID-57578335, AID-1758] |
| CID-888706/ML099 | Ras protein wild type | 141.25 [SID-57578335, AID-1335] | glutathione S-transferase | >100000 [SID-57578335, AID-1769] | >500 | 10.8 [SID-57578335, AID-1759] |
| CID-888706/ML099 | GTP-binding protein (rab7) | 181.97 [SID-57578335, AID-1336] | glutathione S-transferase | >100000 [SID-57578335, AID-1769] | >500 | 5.8 [SID-57578335, AID-1760] |
| CID-888706/ML099 | Ras-related protein Rab-2A | 354.81 [SID-57578335, AID-1337] | glutathione S-transferase | >100000 [SID-57578335, AID-1769] | >200 | 6.2 [SID-57578335, AID-1763] |
| CID-888706/ML099 | Rac1 protein activated mutant | 25.42 [SID-57578335, AID-1339] | glutathione S-transferase | >100000 [SID-57578335, AID-1769] | >1000 | nd |
| CID-888706/ML099 | Rac1 protein wild type | 20.17 [SID-57578335, AID-1340] | glutathione S-transferase | >100000 [SID-57578335, AID-1769] | >1000 | nd |
| CID-888706/ML099 | Ras protein activated mutant | 95.50 [SID-57578335, AID-1341] | glutathione S-transferase | >100000 [SID-57578335, AID-1769] | >1000 | 16.6 [SID-57578335, AID-1761] |
Probe (2) Structure & Characteristics
Compound ID: CID-7345532
Substance ID: SID-57578337
Molecular Weight: 309.35906 [g/mol]
Molecular Formula: C19H19NO3
XLogP3-AA: 3.9
H-Bond Donor: 1
H-Bond Acceptor: 3
Rotatable Bond Count: 5
Topological Polar Surface Area: 51.5
Heavy Atom Count: 23
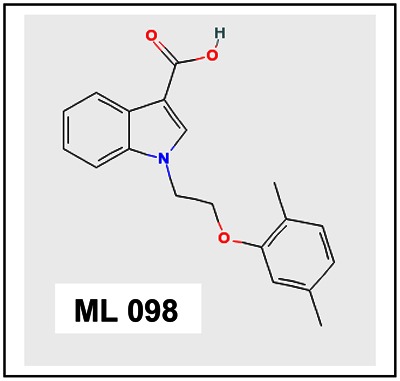
Chemical name: 1-[2-(2,5-dimethylphenoxy)ethyl]-1H-indole-3-carboxylic acid [ML 098]
| CID/ML | Target Name | IC50/EC50 (nM) [SID, AID] | Anti-target Name(s) | IC50/EC50 (nM) [SID, AID] | Selectivity* | Secondary Assay(s) Name: IC50/EC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| CID-7345532/ML098 | cell division cycle 42 (GTP binding protein, 25kDa) activated mutant | 354.81 [SID-57578337, AID-1333] | glutathione S-transferase | >100000 [SID-57578337, AID-1769] | >200 | nd |
| CID-7345532/ML098 | cell division cycle 42 (GTP binding protein, 25kDa) wild type | 588.84 [SID-57578337, AID-1334] | glutathione S-transferase | >100000 [SID-57578337, AID-1769] | >100 | nd |
| CID-7345532/ML098 | Ras protein wild type | 346.74 [SID-57578337, AID-1335] | glutathione S-transferase | >100000 [SID-57578337, AID-1769] | >200 | nd |
| CID-7345532/ML098 | GTP-binding protein (rab7) | 77.62 [SID-57578337, AID-1336] | glutathione S-transferase | >100000 [SID-57578337, AID-1769] | >1000 | nd |
| CID-7345532/ML098 | Ras-related protein Rab-2A | 158.48 [SID-57578337, AID-1337] | glutathione S-transferase | >100000 [SID-57578337, AID-1769] | >500 | nd |
| CID-7345532/ML098 | Rac1 protein activated mutant | 223.87 [SID-57578337, AID-1339] | glutathione S-transferase | >100000 [SID-57578337, AID-1769] | >200 | nd |
| CID-7345532/ML098 | Rac1 protein wild type | 794.32 [SID-57578337, AID-1340] | glutathione S-transferase | >100000 [SID-57578337, AID-1769] | >100 | nd |
| CID-7345532/ML098 | Ras protein activated mutant | 194.98 [SID-57578337, AID-1341] | glutathione S-transferase | >100000 [SID-57578337, AID-1769] | >500 | nd |
Probe (3) Structure & Characteristics
Compound ID: CID-2160985
Substance ID: SID-57578338
Molecular Weight: 307.13934 [g/mol]
Molecular Formula: C14H11BrO3
XLogP3-AA: 3.6
H-Bond Donor: 1
H-Bond Acceptor: 3
Rotatable Bond Count: 4
Topological Polar Surface Area: 46.5
Heavy Atom Count: 18
Chemical name: 2-[(2-bromophenyl)methoxy]benzoic acid [ML 097]

| CID/ML# | Target Name | IC50/EC50 (nM) [SID, AID] | Anti-target Name(s) | IC50/EC50 (nM) [SID, AID] | Selectivity | Secondary Assay(s) Name: IC50/EC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| CID-2160985/ML097 | cell division cycle 42 (GTP binding protein, 25kDa) activated mutant | 50.11 [57578338, AID-1333] | glutathione S-transferase | >100000 [SID-57578338, AID-1769] | >1000 | nd |
| CID-2160985/ML097 | cell division cycle 42 (GTP binding protein, 25kDa) wild type | 102.32 [57578338, AID-1334] | glutathione S-transferase | >100000 [SID-57578338, AID-1769] | >500 | nd |
| CID-2160985/ML097 | Ras protein wild type | 109.64 [57578338, AID-1335] | glutathione S-transferase | >100000 [SID-57578338, AID-1769] | >500 | nd |
| CID-2160985/ML097 | GTP-binding protein (rab7) | 20.41 [SID-57578338, AID-1336] | glutathione S-transferase | >100000 [SID-57578338, AID-1769] | >500 | nd |
| CID-2160985/ML097 | Ras-related protein Rab-2A | Inactive [SID-57578338, AID-1337] | glutathione S-transferase | >100000 [SID-57578338, AID-1769] | nd | |
| CID-2160985/ML097 | Rac1 protein activated mutant | 81.28 [SID-57578338, AID-1339] | glutathione S-transferase | >100000 [SID-57578338, AID-1769] | >1000 | nd |
| CID-2160985/ML097 | Rac1 protein wild type | 151.35 [SID-57578338, AID-1340] | glutathione S-transferase | >100000 [SID-57578338, AID-1769] | >500 | nd |
| CID-2160985/ML097 | Ras protein activated mutant | 93.32 [SID-57578338, AID-1341] | glutathione S-transferase | >100000 [SID-57578338, AID-1769] | >1000 | nd |
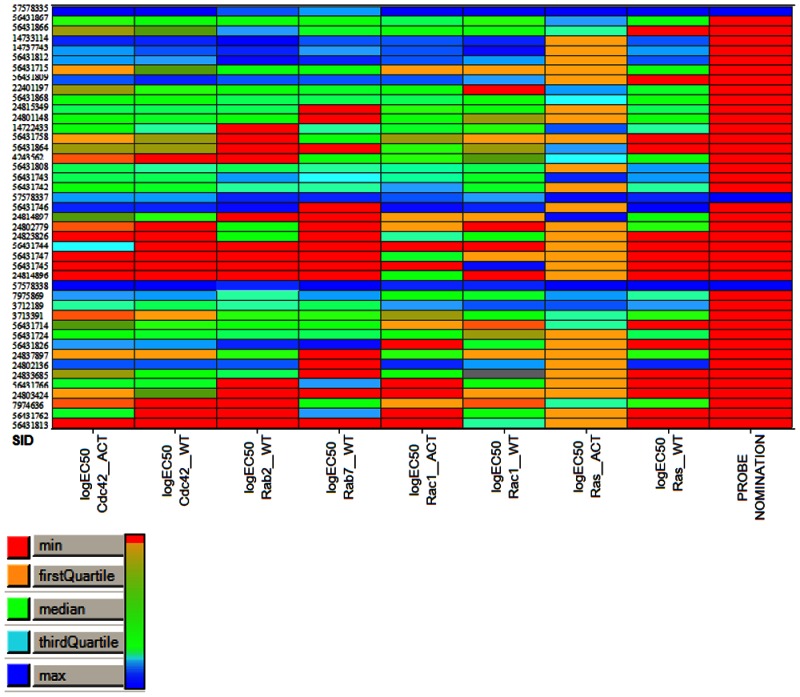
Biological activity (logEC50) heatmap of the 43 compounds across 8 assays. Shades of blue reflect better biological activity, whereas green-to-red reflects reduced activity. The right column highlights the 3 candidates for chemical probe nomination.
| Probe | Pub Chem Assays tested | PubChem Assays active | All assays other than GTPases | Confirmatory Assays |
|---|---|---|---|---|
| 1 (CID-888706) | 312 | 24 | 4 (nuclear factor kappa-B, subunit 1; Allosteric Modulators of D1 Receptors; β-glucocerebrosidase; small molecule inhibitors of MT1-MMP transcription) | 1 (MT1-MMP EC50 = 20 μM) |
| 2 (CID-7345532) | 152 | 12 | 0 | 0 |
| 3 (CID-2160985) | 151 | 10 | 1 (Beta lactamase) | 0 |
This table shows that these probe molecules, taken together, have only been found active in 1 PubChem confirmatory assay, and that the activity is 200 times lower than reported here. The first probe is reported in the patent literature only as a reactive intermediate.
Summary of Recommended Usage and State of the Art of G Protein Activators
Abnormal changes in the activation of small G proteins promote transformation of cells towards malignancy and other disease states. The mutated forms of small GTPases induce proliferation and transformation of a number of cell types as well as differentiation of neuronal cells. These findings have attracted the attention of scientists in cancer research as well as in variety of other fields, emphasizing importance of the identification of regulators of the activity of small GTPases. Recent studies have mainly been focused on the identification of GTPase inhibitors. To our knowledge, small molecule activators of low molecule weight GTPases, with the possible exception of aluminum tetrafluoride which activates the GDP bound form are unknown.
There is considerable data which suggests that many diseases are related to decreased activity of small GTPases (1-15). The only known activation mechanism is via guanine nucleotide-exchange factors (GEFs). These multidomain proteins accelerate the exchange of GDP by GTP by several orders of magnitude. GEFs catalyze the dissociation of the nucleotide from the G protein by modifying the nucleotide-binding site such that the nucleotide affinity is decreased and the GDP is released and replaced with GTP (since the cellular concentration of GTP is ten times higher compared to GDP). The molecules described in this report function by increasing the affinity of the GTPases for guanine nucleotides. We propose that in biochemical systems, these novel small activators should prove useful: 1) in defining the mechanism by which GEF regulates GTPase activity; 2) in defining protein-protein interactions between GEFs and GTPases; 3) defining the sites of GTPase regulation in crystal structures.
The activity of GTPases in cellular systems has been extensively characterized:
A. Rho GTPases
Rac2 is selectively expressed in immune cells and human Rac2 mutations result in immunodeficiency (8). Therefore, activators of Rac2 or other Rac family members might be of benefit in these diseases. Rac1 is important for maintaining hair follicle integrity and a rac1 deficiency in the skin causes nearly complete hair loss in an animal model (9). Could a topically applied Rac1 activator prevent hair loss? Cdc42 is crucial for maintenance of epithelial cell polarity (10). Activators of Cdc42 function might have a beneficial function in solid tumors and ameliorate faciogenital dysplasia where Cdc42 GEF is mutant. Rho GTPases affect cell proliferation and migration (11). In some cases underexpression is associated with cancer. Could an activator be beneficial in such cases?
B. Rab GTPases
Mutations in Rab7 cause neurologic disease Charcot-Marie-Tooth disease, an autosomal dominant disease (12). Thus, if the activity of the functional wild-type protein could be increased selectively, it might ameliorate disease pathogenesis. Mutations in the Rab8 effector optineurin cause open angle glaucoma (13). Activators of Rab8 might provide a bypass to the effector or enable an inefficient effector to function and improve patient outcomes. Point mutations in the Rab3a nucleotide binding domain with reduced nucleotide binding activity cause anomalies in circadian behavior and homeostatic response to sleep loss (14) Thus, activators can be used to test the impact of selectively increasing the GTP-bound pool of proteins bearing a point mutant on learning and memory and emotion control.
C. Ras GTPases
KRas is implicated in cardiac development and can be substituted for by HRas except in cardiovascular homeostasis (15). Activators of KRas may be useful in treatment of cardiomyopathy.
In cellular systems, some potential uses of the new activators could represent: 1) ameliorating mutations in GTPases and GEF proteins, thereby increasing the function of the wild-type or mutant GTPases; 2) however, because the activators described are not selective, one might imagine using the small molecules in conjunction with siRNA knockdowns of the target GTPase, evaluating cell function before and after activator administration.
References for Summary
- 1.
- Roberts AW, Kim C, Zhen L, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. [PubMed: 10072071]
- 2.
- Kim C, Williams DA, Dinauer MC. Rac2 is an essential regulator of specific signaling pathways which activate neutrophil NADPH oxidase. Blood. 1999;94:615a.
- 3.
- Ambruso DR, Knall C, Abell AN, et al. Human neutrophil immunodeficiecy syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci U S A. 2000;97:4654–4659. [PMC free article: PMC18288] [PubMed: 10758162]
- 4.
- Chrostek A, Wu X, Quondamatteo F, Hu R, Sanecka A, Niemann C, Langbein L, Haase I, Brakebusch C. Rac1 Is Crucial for Hair Follicle Integrity but Is Not Essential for Maintenance of the Epidermis. Mol Cell Biol . 2006 September;26:6957–6970. [PMC free article: PMC1592871] [PubMed: 16943436]
- 5.
- Hirano K, Ikegami C, Zhang Z. Contribution of Cdc42 to cholesterol efflux in fibroblasts from Tangier disease and Werner syndrome. Methods Enzymol. 2008;439:159–69. [PubMed: 18374163]
- 6.
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008 Sep;9(9):690–701. [PubMed: 18719708]
- 7.
- Spinosa MR, Progida C, De Luca A, Colucci AM, Alifano P, Bucci C. J Neurosci. 2008 Feb 13;28(7):1640–8. Functional characterization of Rab7 mutant proteins associated with Charcot-Marie-Tooth type 2B disease. [PMC free article: PMC6671532] [PubMed: 18272684]
- 8.
- Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, Levine JE, Petryniak B, Derrow CW, Harris C, Jia B, Zheng Y, Ambruso DR, Lowe JB, Atkinson SJ, Dinauer MC, Boxer L. Blood. 2000 Sep 1;96(5):1646–54. [PubMed: 10961859]
- 9.
- Chrostek A, Wu X, Quondamatteo F, Hu R, Sanecka A, Niemann C, Langbein L, Haase I, Brakebusch C. Mol Cell Biol. 2006 Sep;26(18):6957–70. [PMC free article: PMC1592871] [PubMed: 16943436]
- 10.
- Heasman SJ, Ridley AJ. Nat Rev Mol Cell Biol. 2008 Sep;9(9):690–701. [PubMed: 18719708]
- 11.
- Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Biochim Biophys Acta. 2009 [PubMed: 19327386]
- 12.
- Spinosa MR, Progida C, De Luca A, Colucci AM, Alifano P, Bucci C. J Neurosci. 2008 Feb 13;28(7):1640–8. [PMC free article: PMC6671532] [PubMed: 18272684]
- 13.
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Héon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Science. 2002 Feb 8;295(5557):1077–9. [PubMed: 11834836]
- 14.
- Yang S, Rarias M, Kapfhamer D, Tobias J, Grant G, Abel T, Bucan M. Genes Brain Behav . 2007;6:77–96. [PMC free article: PMC2914309] [PubMed: 16734774]
- 15.
- Potenza N, Vecchione C, Notte A, De Rienzo A, Rosica A, Bauer L, Affuso A, De Felice M, Russo T, Poulet R, Cifelli G, De Vita G, Lembo G, Di Lauro R. EMBO Rep. 2005 May;6(5):432–7. [PMC free article: PMC1299307] [PubMed: 15864294]
1. Scientific Rationale for Project
More than 150 small G proteins (small GTPases) have been identified as monomeric molecules with molecular masses of about 20 – 40 kDa that bind and hydrolyze guanine nucleotides. Functions for many of the small GTPases remain to be discovered, but it is obvious that they are very important intracellular signaling proteins that control diverse cellular functions including cell proliferation, survival and apoptosis, cell-to-cell and cell-to-extracellular matrix adhesion, cytoskeleton organization, transcription regulation, cell cycle progression, cell migration, cellular morphogenesis and polarization (1,2).
The mutated forms of small GTPases induce proliferation and transformation of the number of cell types, and differentiation of neuronal cells (3–5). Today, it is well established that deregulation or abnormal activation of these proteins are linked to disease processes (mostly promote malignant transformation of cell) (6,7). For this reason small GTPases represent a large class of potential drug targets which have been intensively pursued by the pharmaceutical industry (8,9). Currently, there are limited pharmacological tools targeting small GTPases, and most of the efforts have been focused on inhibiting the lipid modifications of GTPases, necessary for their plasma membrane localization and activation (10). Unfortunately, these inhibitors and drugs are not specific to GTPases and affect other cell signaling pathways, which complicate the interpretation of results.
According to molecular structures, small GTPases exhibit two inter-converting forms: GDP-bound inactive and GTP-bound active forms. GTP/GDP exchange studies usually require guanine nucleotide analogues, which behave similarly to the native species and have been modified such that they can be sensitively detected. The most common modification involves insertion of radiolabeled isotopes of phosphorous or sulfur to yield [γ-32 P] GTP and [γ-35S] GTPγS. While these analogs are very sensitive, their use has obvious drawbacks for HTS or other discovery approaches. For this reason, usage of fluorescently labeled nucleotides became more popular. Recently developed BODIPY dye-labeled nucleotides are excellent tools for characterization of nucleotide binding of proteins (11,12).
The fluorescence of BODIPY-guanine nucleotides are directly affected by protein binding. Unbound nucleotides exhibit quenched fluorescence, which is disrupted upon binding to proteins, resulting in a 2–10-fold fluorescence enhancement that allows real-time detection of protein-nucleotide interactions. For this reason, a sensitive fluorescence method is very useful for kinetic and high-throughput screening (HTS) studies. We had recently developed a bead-based flow cytometric, fluorescent GTP-binding assay which is highly sensitive and allows real-time measurements (13) that was applicable for HTS.
Here, for the first time we extended the novel bead-based format for a multiplexed target assay allowed us to perform a screen of the 200,000 compound MLSMR for identification of small molecules which alter GTP binding to small GTPases. The unique multiplexing capabilities of flow cytometry enabled the simultaneous quantitative analysis of the activation or inhibition of six or more GTPases immobilized on color-coded beads, leading to more than 1.2 million measurements. Three small molecule families with activating effects on GTP-binding activity were identified and reported here. High-throughput cytometry screening results were confirmed by biochemical and cell-based secondary screening data. Pull-down assay of active Rac1 showed that an activator increased Rac1activity in Swiss 3T3 cells. Live cell imaging and confocal microscopy studies revealed the activator-induced actin reorganization and cell morphology changes, characteristic to Rho GTPase family activation. Families of pan GTPase activators have the potential of being used broadly to determine when small GTPases are participating in any cellular signaling process of interest.
2. Project Description
a. Original goal for probe characteristics1
Overview
This target, which grow out of a collaboration between the Center and the target provider was assigned in MLSCN Cycle 5, prior to the time when a CPDP was required. As the first large multiplex performed by our Center on the complete MLSMR available at that time, it provided an extraordinarily rich data set which has continued to be evaluated through the so-called MLSCN Pilot Phase “Phase-out” period. A multiplex target has the potential to yield small molecules that are activators or inhibitors of individual targets, the entire set of targets, or a subset of targets. The primary screen and dose-response yielded apparently non-selective activators, and inhibitors with varying degrees of selectivity. Unfortunately, convenient cell-based secondary assays were not part of the R03 submission and the Center and the target provider worked hard to develop novel secondary assays with variable, but low throughput once the screen had been performed.
The characteristics of the novel small molecule activators of GTPases include the ability to modulate GTP-binding and protein-protein interaction activities of members of the GTPase families represented by the series of targets included in this multiplex, with a potency of at least 100 nM. While 3 families of probe scaffolds have been identified, we chose to characterize in extensive detail the probe identified for the first family in the extended project description below. (Figures, legends, and detailed methods are provided at the end of this section. The compound referred to in this section as MLS00088004 is Probe 1 also known by Compound ID 888706 or Substance ID 57578335.)
However, in the MLSCN Phase-out period, a CPDP for a selective GTPase inhibitor probe has also been developed with the Kansas University Chemistry Specialty Center. KUSCC is currently evaluating the results presented in this probe report for the possibility of synthetically creating selective activators. We have retained the lead probes from all three scaffold families to provide an optimal opportunity should we agree to proceed to selectivity.
Compound identification and verification using multiplex screen
For detection of GTP binding to small G-proteins we used a flow cytometry-based assay 13 that allowed us to measure a dose response of GTP binding to individual GST-tagged small G-proteins.
It was observed that different conditions were required for the optimal activity of different GTPases. BODIPY-GTPγNH was identified as a better substrate for some of the small GTPases such as RhoA wt, Rac1wt; Cdc42, while others (Rab proteins and Ras) prefer binding to BODIPY-FL-GTP. Mg2+ ions were crucial for the Rho enzyme activity, while Rab proteins and H-Ras more effectively bound fluorescent GTP in the presence of EDTA. Activity of H-Ras was completely inhibited in the presence of 0.01% dodecyl maltoside in the assay, while this detergent had negligible effect on GTP binding by other GTPases.
Based on these preliminary results, we had chosen specific conditions which are not optimal for all GTPases, but allow us to simultaneously measure the GTP binding of several small GTPases. This approach was used for multiplex analysis of GTPase activity and, therefore, HTS of the MLSMR. This is a low-cost, time saving and highly efficient method for the simultaneous measurements of the activity for the number of proteins. Moreover, it simplifies analysis, comparison and detection of the specific activators/inhibitors of individual GTPases.
In primary screening, each well contained beads of seven different red fluorescent intensities, coupled with individual GST-GTPases. This mixture was incubated for 45 min with 100 nM fluorescent GTP in the presence of 1μM compound, and protein binding fluorescence was measured using a flow cytometer with the beads delivered by a HyperCyt system20. Briefly, HyperCyt consists of an autosampler connected to a peristaltic pump: the autosampler sips ~2 μl of suspension from each of the wells of a multi-well plate, leaving an air gap between samples.
The primary screening of one plate of the MLSMR library using bead-based multiplex method is presented in Fig. 1. Fig. 1B demonstrates that only one compound out of 320, had an effect on BODIPY-FL-GTP binding. Analysis of the row data with IDLeQuery software, GraphPad Prism and Excel template revealed that this activator was MLS000088004 (2-[(2-phenylethyl)thio]nicotinic acid. IUPAC Name: 2-(2-phenylethylsulfanyl)pyridine-3-carboxylic acid) which non-specifically activated small G-proteins (Fig. 1D).
The MLSCN library of 194,738 compounds was screened in a multiplex for potential activators and inhibitors of fluorescent GTP binding to six different GTPase proteins: Cdc42, Rac1, Rac1 activated mutant, Rab2, Rab7, and Ras. The results from the multiplex screen were published on PubChem (http://pubchem.ncbi.nlm.nih.gov/) with PubChem Bioassay identification numbers of 761, 757, 764, 760, 758, 759, respectively. The quality control Z′ statistics were very good for each target. In particular, the average Z′ was 0.87 +/−0.04 for Cdc42, 0.85 +/− 0.04 for Rac1, and 0.90 +/− 0.03 for Rac1 activated mutant (Fig. 1C).
Dose response analysis
To verify the primary screen results, we used dose response assays with one multiplex (Rab7 wt, Rab2 wt, H-Ras wt, constitutively active mutant H-RasG12V, Cdc42 wt, and constitutively active mutant Cdc42Q61L) and 3 single-plexes (for Rac1 wt, constitutively active mutant Rac1Q61L and GST-GFP). GST-GFP single-plex was used to eliminate the possibility that compound affects GST-protein binding to beads.
Fig. 1 shows that MLS00088004 behaved as a non-selective, high affinity activator of eight different GTPases (Fig. 1E; EC50 1.6 x 10−8 – 1.4 x 10−7 M), though Rho-family GTPases (H-Rac1 and Cdc42) showed the greatest sensitivity to the compound. Table 1 shows that EC50, we have identified a novel compound with nanomolar affinity for the Ras-family GTPases and particular affinity for Rac1 and Cdc42 relative to members of the Ras and Rab subfamilies.
Small molecules modulate Rho-family GTP-binding kinetics and in vitro activation
When assays were performed at a fixed concentration of fluorescent GTP and optimal activator concentration, the magnitude of activation appeared to vary widely among the GTPase families, potentially because the Kd of the interaction between fluorescent GTP and/or the activator varies for individual GTPases. To explore this possibility, we varied the concentration of the fluorescent GTP as shown in Fig. 2A, B and quantified in the Table (Fig. 2C) where the apparent Bmax is shown to increase (1.2 to 1.8 fold) and the apparent Kd in the presence or absence of MLS000088004 were performed using wild-type Cdc42 and our established flow cytometry based assay 28 (Fig. 2D). The results are consistent with the activator increasing the affinity of nucleotide binding. All of the families in this report have been shown to exhibit an increase in affinity (data not shown). Real-time assays of GTP-binding kinetics of dissociation typically exhibits a corresponding decrease (1.2–3.1 fold).
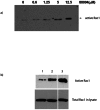
In vitro detection of active Rac 1. Two different approaches were used for detection of Rac 1 activation in vitro. Both of these methods are based on so-called pull down assay of Rac. This method is well known and widely used for detection of active Rac1 and Cdc42. It is based on the fact that only active, GTP bound form of Rac is capable of binding to protein binding domain of PBD of the serine/threonine kinase PAK, and activating this enzyme. To measure the in vitro activation of Rac1 by MLS000088004, His-Rac1 was incubated with increasing concentrations of the compound (0–12.5 μM) in the presence of GTPγS to prevent hydrolysis during the assay. The fraction of active His-Rac1 was identified by binding to GST-PAK-PBD immobilized on glutathione beads and immunoblot analysis of bound Rac1. The precipitated GTPase was then quantified by Western blotting, using specific antibodies 16. A dose dependent increase in Rac1 activation in the presence of increasing amounts of MLS000088004 was clearly evident (Fig. 3A). In second set of experiments, Swiss 3T3 cells were used to analyze the effect of MLS000088004 on Rac1 activation. For positive control, the cells were treated with 10 ng/ml EGF for 2 min. As shown in Fig. 3B, resting starved 3T3 cells treated with DMSO had low levels of active Rac1 (lane 1). Active Rac1 was dramatically elevated following treatment with MLS000088004 (lane 2).
Cell-based assay
GTPases play a key role in actin re-organization and morphological transformations of the cell. Rac1 promotes polymerization at the leading edge, orchestrating the formation of lamellipodia and membrane ruffles.21, while Cdc42 is responsible for filopodia formation22. As was mentioned above, MLS000088004 behaved as a non-selective activator, but Rho-family GTPases, Rac1 and Cdc42 showed the greatest sensitivity to the compound. For this reason, the cell-based assay was used to examine the effect of the compound on cell morphology and actin re-organization in RBL 2H3 cells. This cell line has been widely used as a model for studying of hypersensitivity diseases that include allergy, asthma, and anaphylaxis.
RBL cells can be activated via several pathways (IgE cross-linking or aggregation of GPI-anchored proteins.23. Cross-linking of the high affinity IgE receptor (FcɛRI) activates the main pathway, which initiates cascades of biochemical events leading to degranulation, membrane ruffling and other physiological responses 24. Early stages of activation are characterized by an increase of tyrosine phosphorylation of proteins (key enzymes are Syk and Lyn), while secretion of inflammatory mediators is observed at later stages of activation. In addition to secretion, activated cells begin to flatten (within 2min), spread on their substratum, and extend lamellipodia which show active ruffling 24, 25.
Recent studies indicate that the same GTPases are involved in activation of the RBL cells. Stable expression of dominant negative Rac and Cdc42 in RBL cells inhibits FcɛRI-mediated degranulation 26. The lethal toxin of Clostridium sordellii which inactivates Rac, and possibly Cdc42 (but not Rho) inhibits FcɛRI-mediated tyrosine phosphorylation of phospholipase C, inositol phosphate formation, calcium mobilization and secretion of hexosaminidase suggesting a crucial role of Rac/Cdc42 in RBL cell signaling27.
Treatment of resting RBL cells with MLS000088004 induces ruffling, spreading and flattening
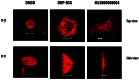
We examined the actin cytoskeleton structures of the resting, ligand stimulated and MLS000088004 treated RBL cells. Cells were grown overnight with or without 10μM MLS000088004. Immunofluorescence staining with rhodamine-phalloidin and confocal microscopy were used to detect the changes in cell shape, as well as reorganization of F-actin. Resting RBL cells were round and tall or modestly spread on the adherent growing surface (Fig. 4A). Activation of RBL cells by cross-linking of FcɛRI caused a remarkable transformation in cell surface topography, and the cell height decreased compared to DMSO controls. Cells lost their spherical shape and became spread and elongated on the substratum with actin becoming localized to the cell perimeter, leading edge and surface ruffles (Fig. 4). The same reorganization of actin and cell shape (dramatic increase in cell spreading and flattening) was observed after treatment of resting RBL cells with MLS000088004.
Live cell imaging
Live cell imaging studies revealed that whereas DMSO-treated control resting cells maintained a spherical shape with few protrusions (Fig. 5A), ligand-stimulated (cross-linking of IgE receptors) cells began flattening, ruffling and forming lamellipodia and filopodia within a minute after stimulation, and surface ridges were enhanced after 15–20 min (Fig. 5B). Addition of 10 μM activator to resting RBL cells induced formation of lamellipodia and microspikes within 10 min, and by 25 min cells were flattened and surface area was significantly increased (Fig. 5C).
Taken together, our observations demonstrate that small GTPase activator MLS000088004 induced similar morphological changes as cell activation via IgE receptor cross-linking. The magnitudes of those reorganizations are comparable. Live cell image figures show that formation of lamellipodia and fillopodia after addition of GTPase activator occurred rapidly and on a similar timescale as receptor-mediated activation. All of the probe families have been shown to induce morphological changes (data not shown) and quantitative dose-response analysis is currently being undertaken.
Signaling pathways upregulated by MLS000088004 are different from those induced by IgE receptor activation
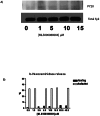
To determine if morphological changes induced by MLS000088004 involve the same signaling pathway as IgE receptor cross-linked stimulation, we examined the effect of this compound on Syk phosphorylation and β-hexosaminidase release (early and late phase activation markers, respectively). Syk tyrosine kinase is tyrosine-phosphorylated in the early stages of RBL cell activation via several pathways (IgE cross-linking, or aggregation of GPI-anchored proteins)18, 28, 29 and plays key role in activation of RBL cells. To determine the effect of MLS000088004 on Syk phosphorylation, resting RBL cells were treated with indicated concentration of the activator (Fig. 6A). Immunoprecipitation with anti-Syk polyclonal antibody and immunoblot with phosphotyrosine-specific antibody indicated that treatment of RBL cells with MLS000088004 did not initiate Syk tyrosine-phosphorylation (Fig. 6A).
Last, we examined the effect of MLS000088004 on RBL cell activation, characterized by β-hexosamindase secretion. Fig. 6B (black bars) shows that in the absence of ligand stimulation there was no β-hexosamindase release in MLS000088004-treated cells, indicating that compound is not toxic. In RBL cells activated via IgE-receptor cross-linking, the presence of MLS000088004 had no further stimulatory effect on secretion (Fig. 6B open bars). These data indicate that, MLS000088004 compound induces morphological changes of RBL cells via activation of small GTPases Rac and Cdc42. However, these changes do not involve traditional, FcɛRI -induced signaling pathway.
Summary
A multiplex flow cytometric assay was used for HTS of MLSCN library. Nearly 200,000 compounds were screened for identification of potential activators and inhibitors of GTP binding to six different small GTPases. This approach allowed us to collect and analyze more than 1.2 million data points in the primary screen. 100–500 positive compounds for each member of multiplex were identified by primary screening. (The results from this multiplex screen were published on PubChem http://pubchem.ncbi.nlm.nih.gov/, with PubChem Bioassay identification numbers of 761, 757, 764, 760, 758, 759, respectively). False positives such as autofluorescent compounds and compounds affecting GST-protein binding to glutathione-beads were eliminated, and ~1000 compounds were selected and tested in secondary dose-response assays. Dose-response results were reported in PubChem (AID-1333–AID-1337, AID-1339–AID-1341) with compounds categorized as having activating or inhibitory effect on one or more of the GTPases tested, including Ras (wild-type and activated), Rab2, Rab7, Rac1 (wild-type and activated), Cdc42 (wild-type and activated), and RhoA. Flow cytometry data of primary screening were confirmed with dose-response analysis and various biochemical and cell-based assays. Pull-down assay of active Rac1 shows that MLS88004 increases Rac1activity in Swiss 3T3 cells. Live cell imaging and confocal microscopy studies revealed that the activator-induced actin reorganization and cell morphology changes characteristic of Rho GTPase activation.
On the following pages are Figures, Legends, and detailed Materials and Methods for Section 2a.
Materials and Methods for Section 2b
Reagents and Cell Lines
(BODIPY FL GTP 2′-(or-3′)-O-(N-(2-aminoethyl) urethane, G-12411 from Invitrogen Molecular Probes (http://probes.invitrogen.com). GST-GTPase chimeras were purchased from commercial vendors (Cytoskeleton Inc. Denver, CO) or purified from E. coli (GST-Rab2, GST-Rab7) as described 13. Bead sets for multiplex assays were from Duke Scientific Corp., Fremont, CA. Anti-Syk polyclonal antibody was obtained from Dr. P. Draber (Institute of Molecular Genetics, Prague). All other reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. RBL and Swiss 3T3 cells were generously provided by Dr. B. Wilson (University New Mexico).
Multiplexed Primary Screens
For multiplex analysis of small GTPases, we used 4 μm size glutathione-beads distinguished by seven different intensities of red color (various magnitude of emission at 665 +/−10 nm with excitation at 635 nm). Bead sets were coated with individual GST-GTPase proteins, blocked with 0.1% BSA in Buffer NP-HPS (0.01% (vol/vol) NP-40; 30 mM HEPES pH 7.5; 100 mM KCl; 20 mM NaCl) containing 1mM EDTA (NP-HPSE) and incubated overnight at 4°C. Individual GTPase coupled beads were washed in NP-HPSE buffer (NP-HPS supplemented with 0.1% BSA and 1mM DTT) and polled together and this mixture used for multiplex analyzes of GTP binding. The assay was conducted in 384-well microplates in a total well volume of 10.1 μl (5 μl protein-coated beads in NP-HPSE, 0.1 μl of test compound, and 5 μl 200 nM BODIPY-FL-GTP (in NP-HPSE for a final concentration of GTP of 100 nM). Positive controls, containing bead mixture and fluorescent GTP but no test compound, were located in columns 1 and 2 on each plate. Negative controls, containing bead mixture with fluorescent GTP and 0.5 mM unlabeled GTP, were assayed separately. Plates were placed on rotators and incubated for 40–45 min at 4°C. Sample analysis was conducted with the HyperCyt® high throughput flow cytometry platform as described previously 14, 15. Flow cytometric light scatter and fluorescence emission at 530 +/− 20 nm (FL1) and 665 +/− 10 nm (FL8) were collected on a Cyan ADP flow cytometer (Beckman Coulter, Fullerton, CA). The resulting time-dependent data (single file per plate) were analyzed using IDLQuery software to determine the compound activity in each well. Gating based on FL8 emission distinguishes the beads coated with different proteins, and the median fluorescence per bead population was calculated.
Dose Response Measurements
Test compounds identified for further analysis after the primary screen were cherry-picked from compound storage plates, then serially diluted 1:3 eight times from a starting concentration of 10 mM for a total of 9-point dilution series in DMSO. The final concentrations in the assay ranged from 10 nM to 100 μM. Beads were coated with proteins as described under Multiplex Primary Screens. For dose–response analysis, we used one multiplex (Rab7 wt, Rab2 wt, H-Ras wt, constitutively active mutant H-RasG12V, Cdc42 wt, and constitutively active mutant Cdc42Q61L) and 3 single-plexes (for Rac1 wt, constitutively active mutant Rac1Q61L and GST-GFP).
Kinetic Assays
Wild-type GST-Cdc42 (4 μM) was bound to glutathione beads overnight at 4°C. Cdc42 on GSH-beads was depleted of nucleotide by incubating with 10 mM EDTA containing buffer for 20 min at 30°C, washing twice with 0.01% NP-40 containing HPS buffer, then resuspending in the same buffer containing 1 mM EDTA, 1 mM DTT and 0.1% BSA. Kinetic assays were performed by incubating 50 μl of GST-Cdc42-GSH-bead suspension for 2 min with either DMSO, or 10 μM MLS000088004 and subsequently adding 50 μl of various concentration ice cold BODIPY-GTP. Association of the fluorescent nucleotide was measured using a FacSCAN flow cytometer in the kinetic mode. Data were converted to ASCII format using IDLQuery (software available free from the authors). Raw data were exported and plotted using GraphPad Prism software (www.graphpad.com).
In vitro and In vivo Rac1 Activation
For in vitro Rac1 activation measurements, DMSO or an equal volume of MLS000088004 in DMSO was incubated for 10 min at 30°C with 80 ng His-Rac1 protein and 125 nM GTPγS in 50 μl of 10 mM EDTA-containing buffer (NP-HPS with 1mM DTT). Further nucleotide exchange was inhibited with the addition of MgCl2 to a final concentration of 60 mM and transferring tubes on ice. Activated Rac1 protein was isolated from solution by adding 5 μg GST-PAK-PBD and glutathione beads. The suspension was rotated at 4°C for 1 h. The beads bearing activated Rac1 bound to GST-PAK-PDB were collected by centrifugation at 14,000 x g (2 min, 4°C), washed with NP-HPS buffer, mixed, boiled with Laemmli buffer, and subjected to SDS-PAGE (12% gels), followed by transfer of proteins onto nitrocellulose membranes. Immunoblot analysis was performed using a mAb directed against Rac1 (Cytoskeleton, Inc.). Protein bands were visualized with an ECL reagent (Pierce Biotechnology, Rockford, IL). Swiss 3T3 cells were used to monitor in vivo Rac1 activation by MLS000088004. Cells were serum starved overnight and treated with DMSO (1%, negative control) or 10 μM compound in DMSO for 20–30 min. As a positive control cells were treated with 10 ng/ml EGF for 2 min. Lysis, immunoprecipitation of active Rac1 with GST-PAK-PBD immobilized on GSH beads, SDS-PAGE and immunoblotting was performed as in 16.
Live Cell Microscopy
Live cell microscopy was carried out on a rat basophil leukemia cell line (RBL-2H3). For experiments RBL-2H3 cells were grown on cover slips overnight, washed and overlaid with Tyrode’s buffer (10mM Hepes, pH7.4, 130mM NaCl, 5mM KCl, 1.4mM CaCl2, 1mM MgCl2, 5.6mM Glucose and 0.1% BSA). Time lapse images were taken after addition of 10 μM MLS000088004 (final concentration) at intervals of 60 s for up to 100 min. For ligand stimulation IgE primed RBL cells were treated with 1μg/ml DNP-BSA. Imaging was performed using a Bio-Rad confocal microscope equipped with a 60x 1.4 NA oil immersion objective equipped with Lasersharp3000 software.
Immunofluorescence Staining and Microscopy
RBL-2H3 cells were grown on cover slips and cultured overnight in the absence or presence of 10 μM MLS000088004. As a positive control, cells were stimulated with 1μg/ml DNP-BSA for 30 min as previously described 17. Cells were washed with phosphate buffered saline, fixed with 3% paraformaldehyde, permeabilized for 5 min with 0.1% Triton X-100 in Tyrode’s buffer, blocked for 1 h with 1% BSA in Tyrode’s buffer, and stained for 1 h ea with rhodamine phalloidin (Cytoskeleton Inc.) according to manufacturer’s instructions. All incubations were performed at room temperature. For imaging, samples were mounted on glass slides using ProLong® Gold antifade reagent (Invitrogen). A Zeiss LSM 510 microscope, 40x objective was used to collect images.
Syk immunoprecipitation
IP of Syk and Western Blot analysis performed as described in 18.
β-Hexosaminidase Secretion Measurements
For β-hexosaminidase release, cells were seeded in 24-well culture plates and incubated for 1 h, with or without indicated concentrations of activators. Hexosaminidase release was determined as described 19. The values were expressed as percent of total amount of β-hexosaminidase, determined using 1% Triton X-100 in Tyrode’s buffer.
b. For each assay implemented and screening run please provide
i. PubChem Bioassay Name(s), AID(s), Assay-Type (Primary, DR, Counterscreen, Secondary)
| AID | Bioassay name | Assay Type |
|---|---|---|
| 757 | HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac wildtype | Primary Screen |
| 758 | HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rab7 | Primary Screen |
| 759 | HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | Primary Screen |
| 760 | HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rab2 wildtype | Primary Screen |
| 761 | HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Cdc wildtype | Primary Screen |
| 764 | HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac activated mutant | Primary Screen |
| 1333 | Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Cdc42 activated mutant | Confirmatory Dose Response |
| 1334 | Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Cdc42 wild type | Confirmatory Dose Response |
| 1335 | Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wild type | Confirmatory Dose Response |
| 1336 | Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rab 7 wild type | Confirmatory Dose Response |
| 1337 | Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rab2 wild type | Confirmatory Dose Response |
| 1339 | Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac activated mutant | Confirmatory Dose Response |
| 1340 | Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac wild type | Confirmatory Dose Response |
| 1341 | Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras activated mutant | Confirmatory Dose Response |
| 1758 | Activator binding kinetics on Ras and Ras-related GTPases, specifically Cdc42 wild type | Secondary |
| 1759 | Activator binding kinetics on Ras and Ras-related GTPases, specifically Ras wild type | Secondary |
| 1760 | Activator binding kinetics on Ras and Ras-related GTPases, specifically Rab 7 wild type | Secondary |
| 1761 | Activator binding kinetics on Ras and Ras-related GTPases, specifically Ras activated mutant | Secondary |
| 1762 | Activator binding kinetics on Ras and Ras-related GTPases, specifically Cdc42 activated mutant | Secondary |
| 1763 | Activator binding kinetics on Ras and Ras-related GTPases, specifically Rab2 wild type | Secondary |
| 1769 | Profiling assay to determine GST-GSH interactions in multiplex bead-based assays | Counterscreen |
| Submitted, AID to be assigned | Summary Report for Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases | Summary |
ii. Assay rationale and descriptions, table of reagents and source
| Bioassay name | Assay Rationale/Description | Reagents/source |
|---|---|---|
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases: all 6 targets (AID-757–AID-761, and AID-764) | This is a no-wash fluorescent GTP-binding assay adapted to multiplexed, high-throughput measurements whereby multiple GTPases are simultaneously screened against the MLSCN library. The specificity is based on the observation that individual GTPases including wt and activated forms exhibit measurably distinct affinities for Bodipy-FI-GTP vs GTP. The multiplex assay involves the binding of fluorescent GTP to G protein-GST fusion proteins on GSH beads. A set of six G proteins (Rac 1 wt, Rab7 wt, Rac 1 activated, Ras wt, Rab 2 wt., CDC wt) are arrayed under conditions of divalent molecule depletion. The average Z’ for the screen was 0.85 +/− 0.04. | Bodipy Fl GTP: Invitrogen, USA GST-GTPase chimeric proteins: Cytoskeleton, USA or purified from E. coli (GST-Rab2, GST-Rab7) Bead sets: Duke Scientific, USA |
| Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras-related GTPases: all 6 targets (AID-1333–AID-1337, and AID-1339–AID-1341) | The assay is the same as the primary HTS against the MLSCN library compounds in a 9-point dose response curve over an assay concentration range of 10 nM to 100 μM. | Same reagents as the HTS |
| Activator binding kinetics on Ras and Ras-related GTPases: all 6 targets (AID-1758–AID-1763) | Real-time binding kinetics between GTP and each of the protein targets were characterized in a multiplex assay. The resulting binding of fluorescent GTP after 3 minutes was utilized for determining Bmax and Kd for each target in the presence of 10 microM small molecule activator compound MLS000088004 versus DMSO as control. | Same reagents as the HTS |
| Profiling assay to determine GST-GSH interactions in multiplex bead-based assays (AID-1769) | Compounds were evaluated for the ability to interfere with the binding of the GST fusion proteins to the GSH beads (thus producing potential false positive results). Dose response experiments were conducted to test an assay concentration range of 10 nanoM to 100 microM. | GST-green fluorescent protein: purified at UNMCMD GSH beads: Duke Scientific, USA |
iii. Summary of Results
| Bioassay name | Compounds Tested/Actives |
|---|---|
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases: all 6 targets (AID-757–AID-761, and AID-764) | 194,635 tested/1,877 active |
| Multiplex dose response to identify specific small molecule inhibitors of Ras and Ras- related GTPases: all 6 targets (AID-1333–AID-1337, and AID-1339–AID-1341) | 1,275 tested/964 active |
| Activator binding kinetics on Ras and Ras- related GTPases: all 6 targets (AID-1758–AID-1763) | 1 compound tested (activator probe candidate binding equilibrium kinetics against all targets) |
| Profiling assay to determine GST-GSH interactions in multiplex bead-based assays (AID-1769) | 1117 tested/397 active |
c. Probe Optimization
i. Description of SAR & chemistry strategy (including structure and data) that led to the probe
This information is provided for each probe scaffold in Section 3.
3. Probes
3.1. Probe 1
a. Chemical name of probe compound
2-(2-phenylethylsulfanyl)pyridine-3-carboxylic acid [ML099]
b. Probe chemical structure including stereochemistry
Molecular Weight 259.32352 [g/mol]
Molecular Formula C14H13NO2S
XLogP3-AA 3.2
H-Bond Donor 1
H-Bond Acceptor 3
Rotatable Bond Count 5
Exact Mass 259.066699
Topological Polar Surface Area 50.2
Heavy Atom Count 18
Solubility Sparingly Soluble (0.080 g/L calc in water RT)
Molar Volume 200.6±5.0 cm3/mol
pKa 3.01±0.36

c. Structural Verification Information of probe SID

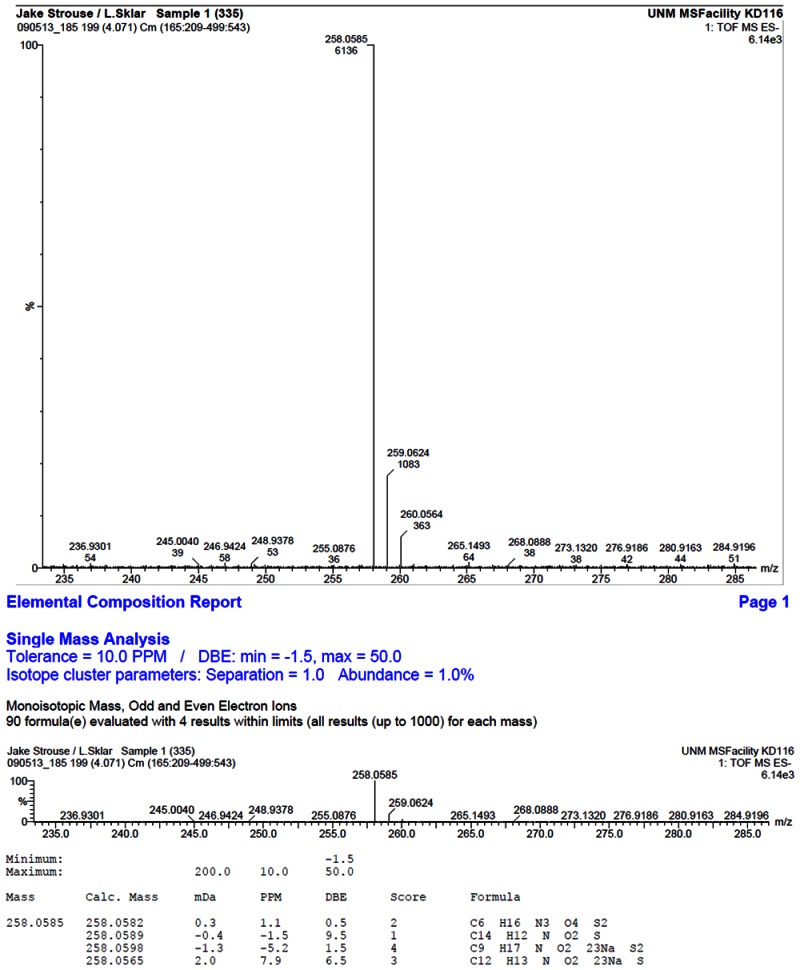
HRMS analysis of 57578335. The results are consistent with the presence of the [M-H]- of C14H13NO2S with a -1.5 ppm difference from the expected m/z of 258.0589 (observed mass = 258.0585)
d. PubChem CID (corresponding to the SID)
Compound ID CID-888706; Substance ID SID-57578335
e. Availability from a vendor
ChemBridge ID 7360080
f. MLS#'s of probe molecule and five related samples that were submitted to the SMR collection
Compound is in the MLSMR; not submitted
g. Mode of action for biological activity of probe
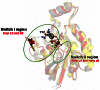
As shown in Fig. 2 above, the activators appear to increase the affinity of binding of the fluorescent GTP analogs to the GTPases. To understand the action and binding mode of these small molecule activators against the GTPase proteins, docking simulations were performed on Rac1 and Cdc42 using the Surflex-dock program, as implemented in Sybyl 8.0. On the basis of the mechanism of recruitment and activation of GTPases by GEFs (guanine nucleotide exchange factors are GTPase binding regulatosr) a working hypothesis has been developed. The assumption is that activators interact with the GTPases in their inactive form (GDP-bound state) and bind to an allosteric binding site localized between switch regions I and II (Fig 7).
The sequence identity and similarity between Rac1 and Cdc42 is about 70% and 81% respectively, and the sequence alignment indicated only one difference in the binding site of these proteins. At position 56 there is a Trp in the binding site of Rac1 and a Phe for the corresponding position from Cdc42 (Figure 8). This similarity between the binding sites could explain the lack of selectivity of these activators with respect to Rac1 and Cdc42.

Figure 8
Sequence alignment of human Rac1 and Cdc42. The cyan boxes emphasize residues involved in the interaction with GEF regulator while grey boxes show the amino acids selected to define the binding cavity in docking calculations
This binding mode of these compounds involves favorable hydrophobic interactions with surrounding amino acids, specifically the residues from position 56, Trp for Rac1 and Phe for Cdc42. The views obtained from the docking of MLS00088004 at Rac1 and Cdc42 are depicted in Fig. 9. The binding mode of this compound in Rac1 binding cavity (Fig. 9A) shows the carboxylic group oriented towards the NH group of Asn39 backbone and involves in H-bond interactions. Additional hydrophobic contacts are favored by the close contact between the pyridine moiety of the compound and the indole side chain of Trp56, while the peripheral phenyl group is oriented towards the hydrophobic region delimited by the side chain of Leu67. In the Cdc42 active site (Fig. (B), the binding mode is different. The terminal phenyl group of the compound is oriented towards a hydrophobic favourable region delimited by the benzyl ring of Phe37, while the carboxylic group is exposed to the solvent and the H-bond interactions are lost. The presence of Phe at position 56 instead of Trp triggers a different orientation of the amino acids in the binding site which result in a shallower cavity compared with the binding cavity of Rac1. The consequent loss of the favorable interactions with Asn39 and Leu67 could explain the decrease of biological activity of MLS00088004 for Cdc42 in comparison with Rac1.
h. Detailed synthetic pathway for making probe
The original hit compound (SID-57578335) was identified during the primary HTS campaign and confirmed in dose response screen to have a logEC50=7.7 on Rac1 wild type, logEC50=7.59 on Rac1 activated mutant, logEC50=7.23 on cell division cycle 42 (GTP binding protein, 25kDa) activated mutant, logEC50=7.00 on cell division cycle 42 (GTP binding protein, 25kDa) wild type, logEC50=6.45 on Ras-related protein Rab-2A, logEC50=6.74 on GTP-binding protein (rab7), logEC50=7.02 on Ras protein activated mutant, logEC50=6.85 on Ras protein wild type. The activity was confirmed by purchasing the compound from a commercial source (ChemBridge ID 7360080). The chemical optimization strategy involved modification of section A, B, and L (Fig. 10). Analogs of nicotinic acid were available commercially and were purchased. A chemical synthesis project was also done by ChemDiv, The following variations were investigated: linker section sulfur atom was substituted with nitrogen or oxygen atoms, linker length was varied from 1 to 3 atoms; section A ring small substituents were added and the carboxylic group was preserved in all variations because primary data in close analogs to SID-57578335 indicated that it is essential for biological activity; ring B section was varied by introduction of small substituents on phenyl ring. Based on these modifications, 9 compounds are synthetic derivatives, 8 compounds were commercially purchased, and 8 compounds were cherry picked from MLSMR. The synthetic analogs around SID-57578335 were synthesized by ChemDiv in an attempt to improve activity (Schema 1).
Fig. 10
SID-57578335.
Probe 1
Representative synthetic scheme. Reagents and conditions.
SAR analysis led to the following conclusions:
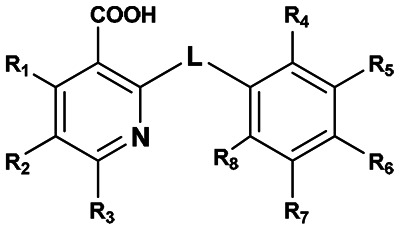
- Pyridine ring (section A): for active compounds at position 2 is carboxyl group, while R1 through R3 are hydrogen i.e. unsubstituted.
- Phenyl ring (section B): active compounds can have small non-polar substituents like: methyl, methoxy, halogen. Polar substituents like nitro or aldehyde groups render the compound inactive.
- Linker (section L): is between 1 and 3 atoms the first linker atom attached to pyridine ring can be sulfur, oxygen or nitrogen.
The detailed SAR table and biological activities are presented in the following:
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Supplier ID | PubChem SID | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Linker |
| MLS000088004 | 57578335 | 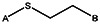 | ||||||||
| Z494-0019 | 56431867 | CH3 | 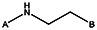 | |||||||
| Z494-0017 | 56431866 | CH3 | CH3 | 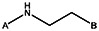 | ||||||
| MLS000522071 | 14733114 | CH3 | 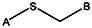 | |||||||
| MLS000522069 | 14737743 | CH3 | CH3 |  | ||||||
| K783-0843 | 56431812 | OCH3 |  | |||||||
| 0412-0036 | 56431715 | 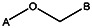 | ||||||||
| K781-5215 | 56431809 | F | 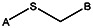 | |||||||
| MLS000394214 | 22401197 | CH3 | CH3 | 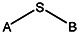 | ||||||
| Z494-0020 | 56431868 | CH3 |  | |||||||
| MLS000673633 | 24815349 | Cl | 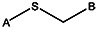 | |||||||
| MLS000715411 | 24801148 | Cl | 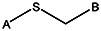 | |||||||
| MLS000122880 | 14722433 | CH3 | 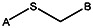 | |||||||
| 8012-6709 | 56431758 | Cl | 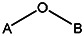 | |||||||
| Z494-0015 | 56431864 | CH3 | CH3 |  | ||||||
| MLS000046237 | 4243562 | 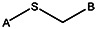 | ||||||||
| K781-0014 | 56431808 | CH3 | 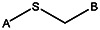 | |||||||
| Z494-0011 | 56431862 | CH3 | CH3 | 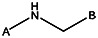 | ||||||
| R052-0595 | 56431824 | OCH3 | CHO | 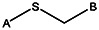 | ||||||
| 8017-3981 | 56431763 | CH3 | 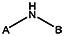 | |||||||
| Z494-0007 | 56431859 | CH3 | CH3 | CH3 |  | |||||
| Z494-0006 | 56431858 | CH3 | CH3 |  | ||||||
| 2189-1384 | 56431719 | NO2 |  | |||||||
| Z494-0016 | 56431865 | CH3 | CH3 | CH3 |  | |||||
| Z494-0010 | 56431861 | CH3 |  | |||||||
Active Inactive Inconclusive
| Supplier ID | PubChem SID | logEC50 Cdc42_ACT | logEC50 Cdc42_WT | logEC50 Rab2_WT | logEC50 Rab7_WT | logEC50 Rac1_ACT | logEC50 Rac1_WT | logEC50 Ras_ACT | logEC50 Ras_WT |
|---|---|---|---|---|---|---|---|---|---|
| MLS000088004 | 57578335 | 7.23 | 7.00 | 6.45 | 6.74 | 7.59 | 7.70 | 7.02 | 6.85 |
| Z494-0019 | 56431867 | 5.79 | 5.66 | 5.65 | 5.76 | 6.12 | 5.66 | 5.75 | 5.61 |
| Z494-0017 | 56431866 | 5.51 | 5.30 | 6.23 | 5.87 | 5.88 | 5.72 | 5.45 | |
| MLS000522071 | 14733114 | 6.72 | 6.52 | 7.13 | 6.97 | 6.76 | 7.14 | 6.37 | |
| MLS000522069 | 14737743 | 6.44 | 6.39 | 6.68 | 6.54 | 6.77 | 7.29 | 6.13 | |
| K783-0843 | 56431812 | 6.39 | 6.18 | 6.90 | 7.05 | 6.75 | 6.34 | 6.41 | |
| 0412-0036 | 56431715 | 5.38 | 5.29 | 5.49 | 5.62 | 5.33 | 5.04 | 5.30 | |
| K781-5215 | 56431809 | 6.62 | 6.63 | 6.58 | 6.88 | 6.79 | 6.15 | ||
| MLS000394214 | 22401197 | 5.50 | 5.48 | 5.17 | 5.97 | 5.95 | 5.96 | 5.73 | |
| Z494-0020 | 56431868 | 5.94 | 5.72 | 5.85 | 6.00 | 6.14 | 5.80 | 5.63 | 5.64 |
| MLS000673633 | 24815349 | 6.06 | 5.86 | 5.89 | 5.82 | 5.71 | 5.76 | ||
| MLS000715411 | 24801148 | 5.91 | 5.75 | 5.61 | 5.85 | 5.41 | 5.62 | ||
| MLS000122880 | 14722433 | 5.95 | 6.07 | 6.33 | 5.98 | 5.70 | 6.07 | 5.96 | |
| 8012-6709 | 56431758 | 5.27 | 5.17 | 5.39 | 5.41 | 5.00 | |||
| Z494-0015 | 56431864 | 5.54 | 5.08 | 5.78 | 5.39 | 5.82 | |||
| MLS000046237 | 4243562 | 4.85 | 5.31 | 5.74 | 5.51 | 5.70 | 5.64 | ||
| K781-0014 | 56431808 | 6.10 | 6.03 | 5.99 | 6.27 | 6.22 | 5.92 | 6.18 |
i. Summary of probe properties (solubility, absorbance/fluorescence, reactivity, toxicity, etc.)
See Section 3.1b above
j. A tabular presentation summarizing known probe properties
Section 3.1c above
3.2. Probe 2
a. Chemical name of probe compound
1-[2-(2,5-dimethylphenoxy)ethyl]-1H-indole-3-carboxylic acid [ML098]
b. Probe chemical structure including stereochemistry
Molecular Weight 309.35906 [g/mol]
Molecular Formula C19H19NO3
XLogP3-AA 3.9
H-Bond Donor 1
H-Bond Acceptor 3
Rotatable Bond Count 5
Topological Polar Surface Area 51.5
Heavy Atom Count 23
Solubility Sparingly Soluble (3.7E-3 g/L calc in water RT)
Molar Volume 263.0±7.0 cm3/mol
pKa 4.07±0.10

c. Structural Verification Information of probe SID
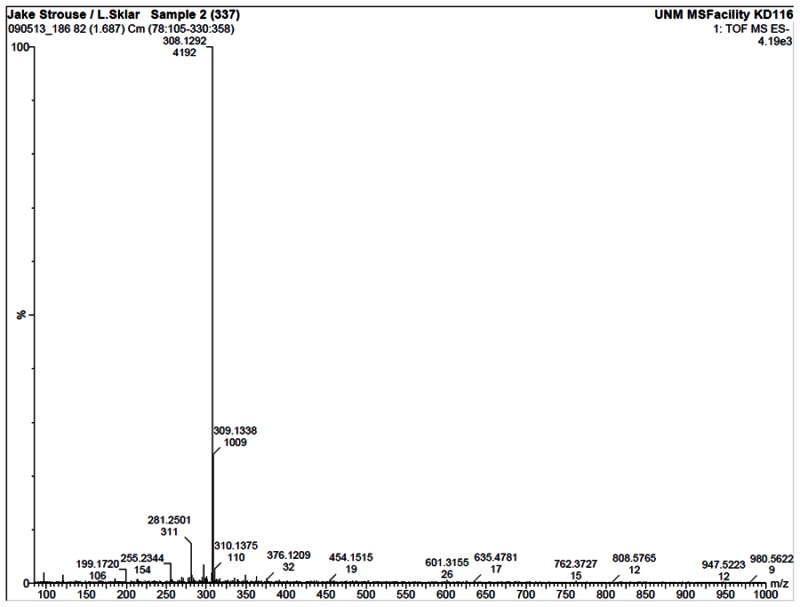
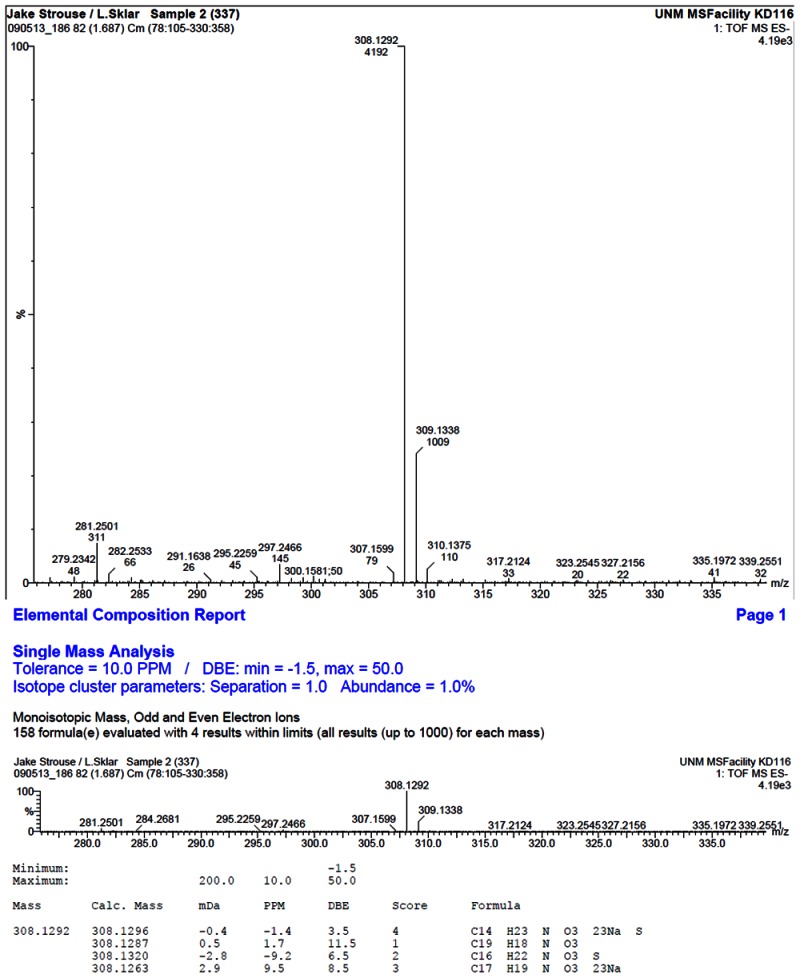
HRMS analysis of 57578337. The results are consistent with the presence of the [M-H]- of C19H19NO3 with a 1.7 ppm difference from the expected m/z of 308.1287 (observed mass = 308.1292).
d. Pub chem. CID (corresponding to the SID)
Compound ID 7345532; Substance ID 57578337
e. Availability from a vendor
ChemDiv ID 7815-0263
f. Provide MLS#'s of probe molecule and five related samples that were submitted to the SMR collection
Compound is in the MLSMR, not submitted.
g. Mode of action for biological activity of probe
Section 3.1g above.
h. Detailed synthetic pathway for making probe
The original hit compound (SID-57578337) was identified during the primary HTS campaign and confirmed ina dose response screen to have a logEC50=6.82 on Rac1 wild type, logEC50=7.09 on Rac1 activated mutant, logEC50=7.30 on cell division cycle 42 (GTP binding protein, 25kDa) activated mutant, logEC50=6.99 on cell division cycle 42 (GTP binding protein, 25kDa) wild type, logEC50=6.80 on Ras-related protein Rab-2A, logEC50=6.74 on GTP-binding protein (rab7), logEC50=6.71 on Ras protein activated mutant, logEC50=6.46 on Ras protein wild type. The activity was confirmed by purchasing the compound from a commercial source (ChemDiv ID 7815-0263). The chemical optimization strategy involved modification of section A, B, C, and L (Figure 11). Analogs of substituted indole scaffold were available commercially and were purchased and chemical synthesis project was also done by ChemDiv. The following variations were investigated: linker section oxygen atom was substituted with carbon atom, linker length was varied from 1 to 4 atoms; section A ring small substituents were varied by introduction of methyl group; ring B section was varied by introduction of methyl, methoxy, halogen, tert-butyl, and iso-propyl substituents at various positions; section C ring was varied by introduction of methyl, methoxy, and halogen substituents at various positions. Based on these modifications a total of 14 compounds are synthetic derivatives and 14 compounds were commercially purchased, 4 compounds were cherry picked from MLSMR. The synthetic analogs around SID-57578337 were synthesized by ChemDiv in an attempt to improve activity (Schema 2).
Probe 2
Representative synthetic scheme. Reagents and conditions.
SAR analysis led to the following conclusions:
- Ring B: small substituents like methyl, methoxy, chlorine, bromine, fluorine are allowed. Larger hydrophobic substituents like tert-butyl, iso-propyl lead to inactive compounds.
- Ring A: no substituents allowed
- Ring C: no substituents allowed
- Linker: size between 1 to 3 atoms; one atom can be heteroatom like oxygen. Linker size larger than 3 leads to inactive compounds
Some compounds like 7815-0268, 7815-0267, 7815-0265 have noisy data or low response and were marked as inconclusive. The best active compound MLS000689786 has ring B substituted with methyl in position R1 and R4 and linker size 3
 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Supplier ID | PubChem SID | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | Linker |
| 7815-0253 | 56431743 | F |  | |||||||||
| 7815-0252 | 56431742 | F |  | |||||||||
| MLS000689786 | 57578337 | CH3 | CH3 |  | ||||||||
| 7815-0259 | 56431746 | Br |  | |||||||||
| MLS000689788 | 24814897 | Cl |  | |||||||||
| MLS000716591 | 24802779 | OCH3 |  | |||||||||
| MLS000689785 | 24823826 | CH3 |  | |||||||||
| 7815-0255 | 56431744 | Cl |  | |||||||||
| 7815-0260 | 56431747 | 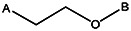 | ||||||||||
| 7815-0256 | 56431745 | Cl |  | |||||||||
| MLS000689787 | 24814896 | CH3 |  | |||||||||
| Z494-0049 | 56431894 | CH3 | 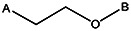 | |||||||||
| Z494-0047 | 56431892 | OCH3 | Cl | 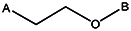 | ||||||||
| Z494-0051 | 56431896 | CH3 | CH3 | 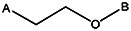 | ||||||||
| Z494-0050 | 56431895 | CH3 | CH3 | 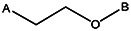 | ||||||||
| Z494-0048 | 56431893 | OCH3 | Cl |  | ||||||||
| Z494-0046 | 56431891 | OCH3 | Cl |  | ||||||||
| Z494-0045 | 56431890 | OCH3 | Cl | 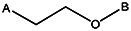 | ||||||||
| Z494-0044 | 56431889 | OCH3 | CH3 | 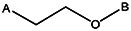 | ||||||||
| Z494-0043 | 56431888 | OCH3 | CH3 | 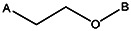 | ||||||||
| Z494-0042 | 56431887 | OCH3 | CH3 | 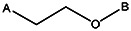 | ||||||||
| Z494-0041 | 56431886 | OCH3 | CH3 | 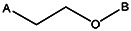 | ||||||||
| Z494-0039 | 56431885 | CH3 | OCH3 | 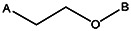 | ||||||||
| Z494-0038 | 56431884 | CH3 | OCH3 | 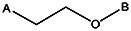 | ||||||||
| Z494-0037 | 56431883 | CH3 | OCH3 | 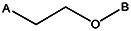 | ||||||||
| 7815-0272 | 56431752 | t-Butyl | 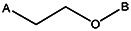 | |||||||||
| 7815-0276 | 56431753 | CH3 |
 | |||||||||
| 7815-0277 | 56431754 | CH3 | CH3 |
 | ||||||||
| 7815-0265 | 56431748 | CH3 | CH3 |
 | ||||||||
| 7815-0267 | 56431749 | Cl |
 | |||||||||
| 7815-0278 | 56431755 | CH3 | Cl |
 | ||||||||
| 7815-0271 | 56431751 | i-Propyl | CH3 | 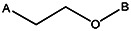 | ||||||||
| 7815-0268 | 56431750 | CH3 | Cl |
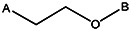 | ||||||||
Active Inactive Inconclusive
| Supplier ID | PubChem SID | logEC50 Cdc42_ACT | logEC50 Cdc42_WT | logEC50 Rab2_WT | logEC50 Rab7_WT | logEC50 Rac1_ACT | logEC50 Rac1_WT | logEC50 Ras_ACT | logEC50 Ras_WT |
|---|---|---|---|---|---|---|---|---|---|
| 7815-0253 | 56431743 | 6.13 | 5.87 | 6.30 | 6.46 | 6.22 | 5.89 | 6.33 | 6.09 |
| 7815-0252 | 56431742 | 5.90 | 5.84 | 6.11 | 6.29 | 6.36 | 5.88 | 5.96 | |
| MLS000689786 | 57578337 | 6.45 | 6.23 | 6.80 | 7.11 | 6.65 | 6.10 | 6.71 | 6.46 |
| 7815-0259 | 56431746 | 6.78 | 6.53 | 6.84 | 7.16 | 6.91 | 6.83 | ||
| MLS000689788 | 24814897 | 5.56 | 5.52 | 5.26 | 5.09 | 6.60 | 5.57 | ||
| MLS000716591 | 24802779 | 4.32 | 5.15 | 5.06 | 4.55 | ||||
| MLS000689785 | 24823826 | 5.48 | 6.29 | 5.77 | |||||
| 7815-0255 | 56431744 | 6.24 | |||||||
| 7815-0260 | 56431747 | 6.07 | 5.26 | ||||||
| 7815-0256 | 56431745 | 7.35 | |||||||
| MLS000689787 | 24814896 | 5.94 |
i. Summary of probe properties (solubility, absorbance/fluorescence, reactivity, toxicity, etc.)
See Section 3.2b above.
j. A tabular presentation summarizing known probe properties
See Section 3.2b above
3.3. Probe 3
a. Chemical name of probe compound
2-[(2-bromophenyl)methoxy]benzoic acid [ML097]
b. Probe chemical structure including stereochemistry
Molecular Weight 307.13934 [g/mol]
Molecular Formula C14H11BrO3
XLogP3-AA: 3.6
H-Bond Donor: 1
H-Bond Acceptor: 3
Rotatable Bond Count: 4
Topological Polar Surface Area: 46.5
Heavy Atom Count: 18
Solubility Sparingly Soluble (0.046 g/L calculated in water RT)
Molar Volume: 202.8±3.0 cm3/mol
pKa: 3.38±0.36

c. Structural Verification Information of probe SID
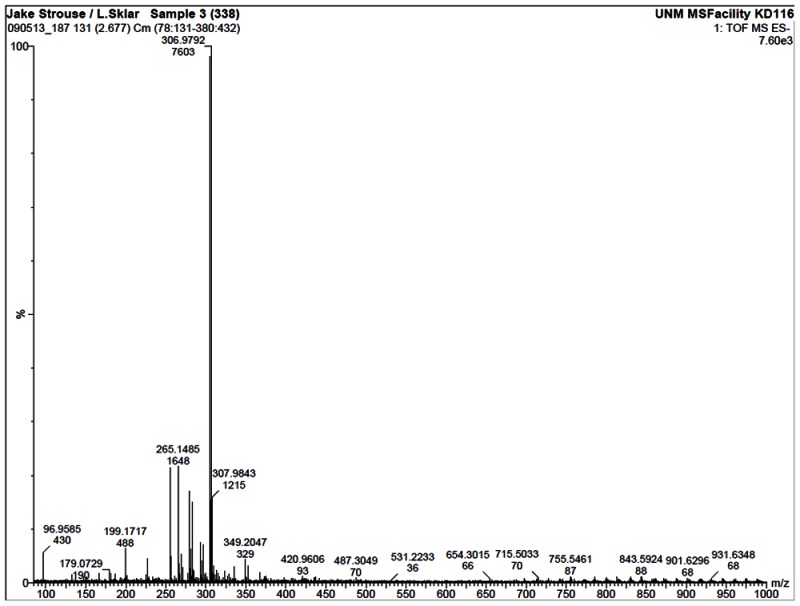
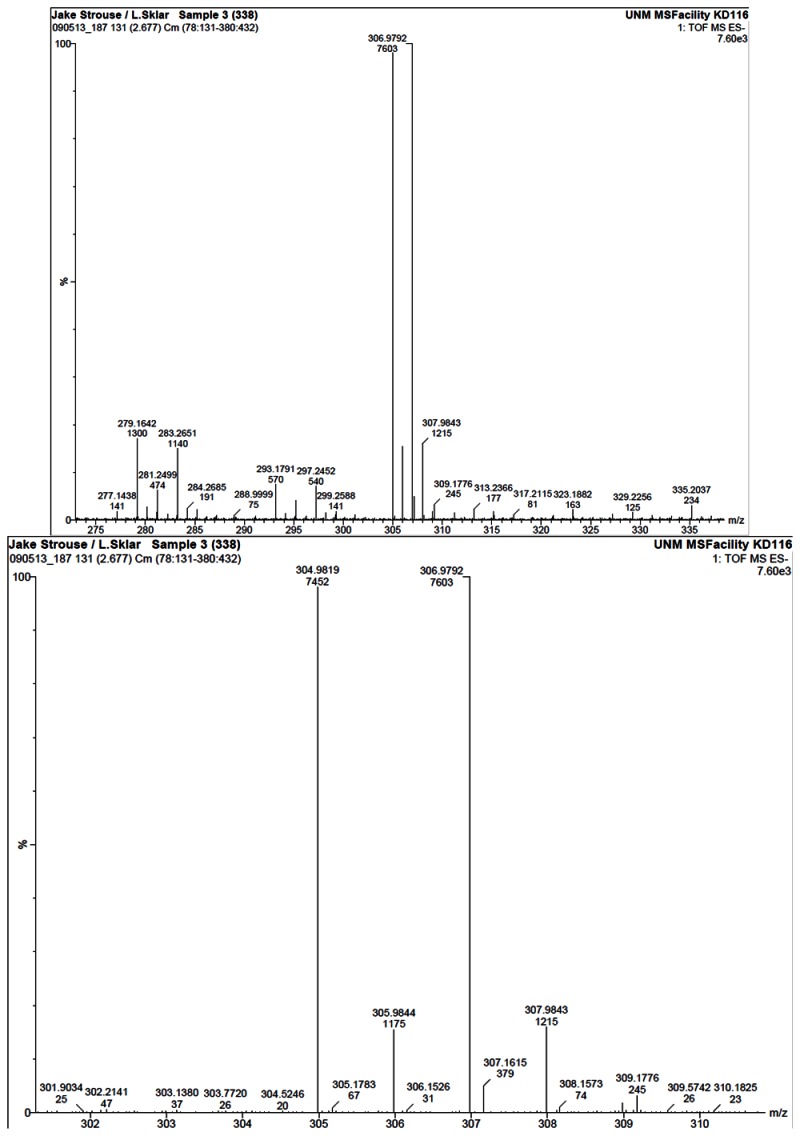
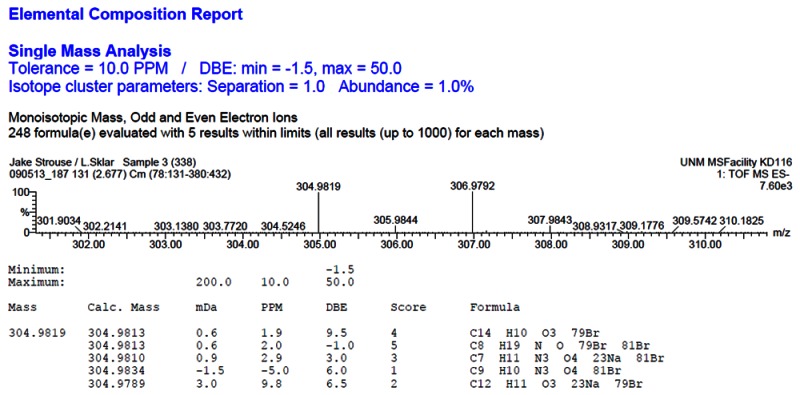
HRMS analysis of 57578338. The results are consistent with the presence of the [M-H]- of C14H11BrO3 with a 1.9 ppm difference from the expected m/z of 304.9813 for the 79Br isotope (observed mass = 304.9819).
d. PubChem CID (corresponding to the SID)
Compound ID CID-2160985; Substance ID SID-57578338
e. Availability from a vendor
Ryan Scientific ID T5225985
f. Provide MLS#'s of probe molecule and five related samples that were submitted to the SMR collection
Compound is in the MLSMR; not submitted.
g. Mode of action for biological activity of probe
See Section 3.1g above.
h. Detailed synthetic pathway for making probe
The original hit compound (SID-57578338) was identified during the primary HTS campaign and confirmed in dose response screen to have a logEC50=6.82 on Rac1 wild type, logEC50=7.09 on Rac1 activated mutant, logEC50=6.60 on cell division cycle 42 (GTP binding protein, 25kDa) activated mutant, logEC50=6.84 on cell division cycle 42 (GTP binding protein, 25kDa) wild type, logEC50=6.84 on GTP-binding protein (rab7), logEC50=6.53 on Ras protein activated mutant, logEC50=6.47 on Ras protein wild type. The activity was confirmed by purchasing the compound from a commercial source (Ryan Scientific ID T5225985). The chemical optimization strategy involved modification of section A, B, and L (Fig. 12). Analogs of salicylic acid were available commercially and were purchased, chemical synthesis project was also done by ChemDiv, the following variations were investigated: linker section ether oxygen moiety was substituted with carboxylic, carbonyl amine, amide, and thioether moieties, linker length was varied from 1 to 4 atoms; section A ring small substituents were varied by introduction of methyl, methoxy, halogen, nitro, heteroalkyl, and amine substituents at various positions, group; ring B section was varied by introduction of methyl, methoxy, halogen, alkyl, and nitro substituents at various positions. Based on these modifications a total of 15 compounds are synthetic derivatives and 9 compounds were commercially purchased. 17 compounds were cherry picked from MLSMR. The synthetic analogs around SID-57578338 were synthesized by ChemDiv in an attempt to improve activity (Schema 3).
Figure 12
SID-57578338.

Probe 3
Representative synthetic scheme. Reagents and conditions.
SAR analysis led to the following conclusions:
- Ring A: No substitutions R1–R4 are allowed, any substitutions will render the compound inactive.
- Ring B: Small hydrophobic substituents are allowed like: methyl, methoxy, halogen, isopropyl.
- Linker: Length is between 1–4 atoms, with the following functional groups: amine, ether, carbonyl, carboxyl.
- Steric effects seems to play a role in the case of compounds: MLS000760373, MLS000714513 and R092-0019, the last compound (R092-0019) is not substituted at positions R5 and R9 whereas MLS000760373 and MLS000714513 are substituted with methyl. The R5 and R9 methyl induce a preferential out of ring B plane conformation which can explain the observed bioactivity for MLS000760373 and MLS000714513 and no activity for R092-0019.
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Supplier ID | PubChem SID | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | Linker |
| MLS000761515 | 57578338 | Br |  | ||||||||
| MLS000104449 | 7975869 |  | |||||||||
| MLS000081810 | 3712189 |  | |||||||||
| MLS000081844 | 3713391 | OCH3 |  | ||||||||
| 0384-0034 | 56431714 | 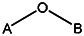 | |||||||||
| 4478-5409 | 56431724 | F | F |  | |||||||
| R152-1213 | 56431826 | Cl | 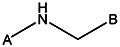 | ||||||||
| MLS000760373 | 24837897 | CH3 | CH3 |  | |||||||
| MLS000715316 | 24802136 | OCH3 | 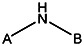 | ||||||||
| MLS000775662 | 24833685 | OCH3 | 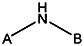 | ||||||||
| 8524-6914 | 56431766 | Cl | 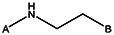 | ||||||||
| MLS000714513 | 24803424 | CH3 | CH3 | CH3 | CH3 | 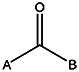 | |||||
| MLS000111718 | 7974636 | OCH3 | 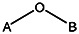 | ||||||||
| 8016-5752 | 56431762 | C(CH3)2 | CH3 |  | |||||||
| K783-0963 | 56431813 | Cl |  | ||||||||
| MLS000043585 | 4243978 | F | F | F | F | OCH3 |  | ||||
| K783-0702 | 56431811 | Cl | Cl |  | |||||||
| MLS000689738 | 24814013 | F | F | F | F | CH3 | CH3 |  | |||
| MLS000689734 | 24814024 | F | F | F | F | CH3 |  | ||||
| MLS000775808 | 24831309 | NO2 | Cl |  | |||||||
| MLS000693348 | 24795918 | Cl | NO2 | 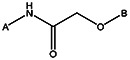 | |||||||
| MLS000663391 | 24797131 | Cl | OCH3 | 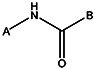 | |||||||
| Z494-0034 | 56431880 | OCH3 | CH3 | CH3 | 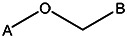 | ||||||
| Z494-0023 | 56431870 | OCH3 | Br |  | |||||||
| Z494-0022 | 56431869 | CF3 | 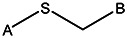 | ||||||||
| Z494-0025 | 56431872 | OCH3 | Br |  | |||||||
| Z494-0028 | 56431875 | CH3 | CH3 | 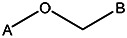 | |||||||
| 8017-4247 | 56431764 | NH2 | Cl |  | |||||||
| Z494-0033 | 56431879 | OCH3 | CH3 | CH3 | 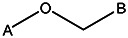 | ||||||
| Z494-0029 | 56431876 | CH3 | CH3 |  | |||||||
| Z494-0031 | 56431878 | OCH3 | CH3 |  | |||||||
| Z494-0024 | 56431871 | OCH3 | Br |  | |||||||
| MLS000760107 | 24835967 | NO2 |  | ||||||||
| Z494-0036 | 56431882 | OCH3 | CH3 | CH3 |  | ||||||
| Z494-0027 | 56431874 | CH3 |  | ||||||||
| Z494-0026 | 56431873 | OCH3 | Br | 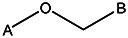 | |||||||
| Z494-0035 | 56431881 | OCH3 | CH3 | CH3 |  | ||||||
| R092-0019 | 56431825 | CH3 |  | ||||||||
| Z494-0030 | 56431877 | CH3 | CH3 | 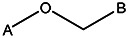 | |||||||
| Z494-0002 | 56431857 | CF3 | H | 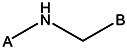 | |||||||
| MLS000621480 | 24794372 | NO2 | 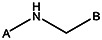 | ||||||||
| MLS000621480 | 24794372 | NO2 | 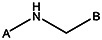 | ||||||||
Active Inactive Inconclusive
| Supplier ID | PubChem SID | logEC50 Cdc42_ACT | logEC50 Cdc42_WT | logEC50 Rab2_WT | logEC50 Rab7_WT | logEC50 Rac1_ACT | logEC50 Rac1_WT | logEC50 Ras_ACT | logEC50 Ras_WT |
|---|---|---|---|---|---|---|---|---|---|
| MLS000761515 | 57578338 | 6.60 | 6.84 | 6.61 | 6.76 | 7.09 | 6.82 | 6.53 | 6.47 |
| MLS000104449 | 7975869 | 6.26 | 6.23 | 6.18 | 6.59 | 5.90 | 5.86 | 5.86 | 5.89 |
| MLS000081810 | 3712189 | 6.17 | 5.97 | 6.14 | 6.01 | 6.41 | 6.73 | 6.09 | 5.99 |
| MLS000081844 | 3713391 | 4.86 | 4.87 | 4.46 | 4.63 | 5.37 | 5.77 | 4.84 | 4.90 |
| 0384-0034 | 56431714 | 5.65 | 5.47 | 5.55 | 5.46 | 5.11 | 4.84 | 5.47 | |
| 4478-5409 | 56431724 | 5.97 | 5.79 | 5.94 | 6.07 | 5.97 | 5.40 | 5.78 | |
| R152-1213 | 56431826 | 6.25 | 6.30 | 6.75 | 7.36 | 5.84 | |||
| MLS000760373 | 24837897 | 5.32 | 4.97 | 4.93 | 5.76 | 5.21 | 4.91 | ||
| MLS000715316 | 24802136 | 6.56 | 6.44 | 6.55 | 6.95 | 6.34 | 6.46 | ||
| MLS000775662 | 24833685 | 5.55 | 5.58 | 5.97 | 5.86 | ||||
| 8524-6914 | 56431766 | 6.05 | 5.81 | 6.47 | 5.82 | ||||
| MLS000714513 | 24803424 | 5.31 | 5.23 | 5.21 | |||||
| MLS000111718 | 7974636 | 5.05 | 5.57 | 5.08 | 4.80 | 5.20 | 4.95 | ||
| 8016-5752 | 56431762 | 5.99 | 6.50 | 5.81 | |||||
| K783-0963 | 56431813 | 6.05 |
i. Summary of probe properties (solubility, absorbance/fluorescence, reactivity, toxicity, etc.)
See Section 3.3c above.
j. A tabular presentation summarizing known probe properties
See Section 3.3c above.
4. Appendices
Data for each probe in sections 3.1, 3.2, 3.3
- Comparative data on (1) probe, (2) similar compound structures (establishing SAR) and (3) compounds representing “best in class” probes prior to this probe project (to demonstrate that the probe molecule is an “improvement on the current state of the art” in the same assay under the same conditions).
- Comparative data showing probe specificity for target
5. Bibliography
- 1.
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. [PubMed: 12478284]
- 2.
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. [PubMed: 16212495]
- 3.
- Capon DJ, Seeburg PH, McGrath JP, Hayflick JS, Edman U, Levinson AD, Goeddel DV. Activation of Ki-ras2 gene in human colon and lung carcinomas by two different point mutations. Nature. 1983;304:507–513. [PubMed: 6308467]
- 4.
- Noda M, Ko M, Ogura A, Liu DG, Amano T, Takano T, Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985;318:73–75. [PubMed: 4058592]
- 5.
- Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. [PubMed: 2996779]
- 6.
- Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. [PMC free article: PMC3915522] [PubMed: 18568040]
- 7.
- Ayllon V, Rebollo A. Ras-induced cellular events (review). Mol Membr Biol. 2000;17:65–73. [PubMed: 10989457]
- 8.
- Cox AD, Der CJ. Ras family signaling: therapeutic targeting. Cancer Biol Ther. 2002;1:599–606. [PubMed: 12642680]
- 9.
- Loirand G, Scalbert E, Bril A, Pacaud P. Rho exchange factors in the cardiovascular system. Curr Opin Pharmacol. 2008;8:174–180. [PubMed: 18222728]
- 10.
- Sebti SM, Hamilton AD. Farnesyltransferase and geranylgeranyltransferase I inhibitors and cancer therapy: lessons from mechanism and bench-to-bedside translational studies. Oncogene. 2000;19:6584–6593. [PubMed: 11426643]
- 11.
- McEwen DP, Gee KR, Kang HC, Neubig RR. Fluorescent BODIPY-GTP analogs: real-time measurement of nucleotide binding to G proteins. Anal Biochem. 2001;291:109–117. [PubMed: 11262163]
- 12.
- Jameson EE, Roof RA, Whorton MR, Mosberg HI, Sunahara RK, Neubig RR, Kennedy RT. Real-time detection of basal and stimulated G protein GTPase activity using fluorescent GTP analogues. J Biol Chem. 2005;280:7712–7719. [PubMed: 15613467]
- 13.
- Schwartz SL, Tessema M, Buranda T, Pylypenko O, Rak A, Simons PC, Surviladze Z, Sklar LA, Wandinger-Ness A. Flow cytometry for real-time measurement of guanine nucleotide binding and exchange by Ras-like GTPases. Anal Biochem. 2008;381:258–266. [PMC free article: PMC2633595] [PubMed: 18638444]
- 14.
- Kuckuck FW, Edwards BS, Sklar LA. High throughput flow cytometry. Cytometry. 2001;44:83–90. [PubMed: 11309812]
- 15.
- Sklar LA, Carter MB, Edwards BS. Flow cytometry for drug discovery, receptor pharmacology and high-throughput screening. Curr Opin Pharmacol. 2007;7:527–534. [PMC free article: PMC2230635] [PubMed: 17652026]
- 16.
- Benard V, Bokoch GM. Assay of Cdc42, Rac, and Rho GTPase activation by affinity methods. Methods Enzymol. 2002;345:349–359. [PubMed: 11665618]
- 17.
- Wilson BS, Seagrave J, Oliver JM. Impaired secretion and increased insolubilization of IgE-receptor complexes in mycophenolic acid-treated (guanine nucleotide-depleted) RBL-2H3 mast cells. J Cell Physiol. 1991;149:403–407. [PubMed: 1835980]
- 18.
- Tolar P, Draberova L, Draber P. Protein tyrosine kinase Syk is involved in Thy-1 signaling in rat basophilic leukemia cells. Eur J Immunol. 1997;27:3389–3397. [PubMed: 9464827]
- 19.
- Ortega E, Hazan B, Zor U, Pecht I. Mast cell stimulation by monoclonal antibodies specific for the Fc epsilon receptor yields distinct responses of arachidonic acid and leukotriene C4 secretion. Eur J Immunol. 1989;19:2251–2256. [PubMed: 2532599]
- 20.
- Edwards BS, Young SM, Oprea TI, Bologa CG, Prossnitz ER, Sklar LA. Biomolecular screening of formylpeptide receptor ligands with a sensitive, quantitative, high-throughput flow cytometry platform. Nat Protoc. 2006;1:59–66. [PubMed: 17406212]
- 21.
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. [PubMed: 1643658]
- 22.
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. [PubMed: 7536630]
- 23.
- Draberova L, Draber P. Cross-linking of Thy-1 glycoproteins or high-affinity IgE receptors induces mast cell activation via different mechanisms. Immunology. 1993;80:103–109. [PMC free article: PMC1422128] [PubMed: 7902332]
- 24.
- Pfeiffer JR, Seagrave JC, Davis BH, Deanin GG, Oliver JM. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J Cell Biol. 1985;101:2145–2155. [PMC free article: PMC2113986] [PubMed: 2933414]
- 25.
- Metzger H, Alcaraz G, Hohman R, Kinet JP, Pribluda V, Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. [PubMed: 3011032]
- 26.
- Guillemot JC, Montcourrier P, Vivier E, Davoust J, Chavrier P. Selective control of membrane ruffling and actin plaque assembly by the Rho GTPases Rac1 and CDC42 in FcepsilonRI-activated rat basophilic leukemia (RBL-2H3) cells. J Cell Sci. 1997;110(Pt 18):2215–2225. [PubMed: 9378771]
- 27.
- Djouder N, Schmidt G, Frings M, Cavalie A, Thelen M, Aktories K. Rac and phosphatidylinositol 3-kinase regulate the protein kinase B in FcɛRI signaling in RBL 2H3 mast cells. J Immunol. 2001;166:1627–1634. [PubMed: 11160204]
- 28.
- Kihara H, Siraganian RP. Src homology 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J Biol Chem. 1994;269:22427–22432. [PubMed: 8071371]
- 29.
- Scharenberg AM, Kinet JP. Initial events in FcɛRI signal transduction. J Allergy Clin Immunol. 1994;94:1142–1146. [PubMed: 7798551]
- PMCPubMed Central citations
- PubChem BioAssay for Chemical ProbePubChem BioAssay records reporting screening data for the development of the chemical probe(s) described in this book chapter
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Mechanism of the guanine nucleotide exchange reaction of Ras GTPase--evidence for a GTP/GDP displacement model.[Biochemistry. 2005]Mechanism of the guanine nucleotide exchange reaction of Ras GTPase--evidence for a GTP/GDP displacement model.Zhang B, Zhang Y, Shacter E, Zheng Y. Biochemistry. 2005 Feb 22; 44(7):2566-76.
- Review Regulation of small GTPases by GEFs, GAPs, and GDIs.[Physiol Rev. 2013]Review Regulation of small GTPases by GEFs, GAPs, and GDIs.Cherfils J, Zeghouf M. Physiol Rev. 2013 Jan; 93(1):269-309.
- Review The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.[Cell Signal. 2014]Review The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Boissier P, Huynh-Do U. Cell Signal. 2014 Mar; 26(3):483-91. Epub 2013 Dec 2.
- A competitive nucleotide binding inhibitor: in vitro characterization of Rab7 GTPase inhibition.[ACS Chem Biol. 2012]A competitive nucleotide binding inhibitor: in vitro characterization of Rab7 GTPase inhibition.Agola JO, Hong L, Surviladze Z, Ursu O, Waller A, Strouse JJ, Simpson DS, Schroeder CE, Oprea TI, Golden JE, et al. ACS Chem Biol. 2012 Jun 15; 7(6):1095-108. Epub 2012 Apr 23.
- Oncogenic Dbl, Cdc42, and p21-activated kinase form a ternary signaling intermediate through the minimum interactive domains.[Biochemistry. 2004]Oncogenic Dbl, Cdc42, and p21-activated kinase form a ternary signaling intermediate through the minimum interactive domains.Wang L, Zhu K, Zheng Y. Biochemistry. 2004 Nov 23; 43(46):14584-93.
- Three small molecule pan activator families of Ras-related GTPases - Probe Repor...Three small molecule pan activator families of Ras-related GTPases - Probe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...