NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Steele D, Adam GP, Di M, et al. Tympanostomy Tubes in Children With Otitis Media [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2017 May. (Comparative Effectiveness Reviews, No. 185.)
Background and Objectives
Otitis media is often preceded by a viral upper respiratory tract infection that causes Eustachian tube obstruction, negative middle ear pressure, and accumulation of fluid in the normally air-filled space of the middle ear. Acute otitis media (AOM) is defined as the presence of fluid in the middle ear with signs and symptoms of an acute infection, such as fever and ear pain. Otitis media with effusion (OME) is defined as the presence of fluid in the middle ear behind an intact tympanic membrane without signs and symptoms of an acute infection. 1,2 OME is defined as chronic OME, if effusion persists for 3 months or longer.1 Acute otitis media and chronic OME have shared causes. Children with chronic OME are prone to recurrent AOM episodes, and after an AOM episode all children have OME for some time.3 Chronic OME can result in hearing deficits, which put a child at risk for speech and language delays, behavioral changes, and poor academic achievement. Recurrent AOM has been shown to impact quality of life for patients and their caregivers.4
Certain children, including those with Down syndrome and cleft palate, have a very high risk for middle ear disease. The American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) clinical practice guideline (CPG) identifies a subpopulation of children who may be at increased risk for speech, language, or learning problems from otitis media because of baseline sensory, physical, cognitive, or behavioral factors.1,5
Myringotomy with TT placement is the most common ambulatory surgery performed on children in the United States6, with 667,000 TT placed in children under the age of 15 in 2006.7 The proceedings of the National Summit on Overuse, convened in 2012, based on sample of continually enrolled children into a treatment pathways database and a Medicaid database, reported that 2.5 percent of all U.S. children 2 years old and older had TT inserted in 2010.8
The effectiveness of TT for chronic OME and recurrent AOM is likely influenced by the many factors that affect the prognosis for middle ear disease in children, including current age, age at first diagnosis, frequency of respiratory tract infections, and day care exposure.9
The AAO-HNS CPG recommends that clinicians offer TT to children with recurrent AOM who have middle ear effusion at the time of assessment for tube candidacy, and that clinicians do not perform TT insertion when middle ear effusion is not present.1
TT placement may result in acute otorrhea in some patients and conversely watchful waiting may result in continued episodes of recurrent AOM, which may include tympanic membrane perforation and otorrhea.
In children with TTs, episodes of otorrhea that reflect acute bacterial infection may be otherwise asymptomatic and less troublesome than AOM episodes in children with intact eardrums.10 However, otorrhea may be associated with a foul odor, fever, or pain, and it may negatively affect quality of life. Treatment is aimed at eradicating bacterial infection and reducing the duration and severity of symptoms.11
The objectives for this systematic review are to synthesize information on the effectiveness of TT in children with chronic otitis media with effusion and recurrent acute otitis media, summarize the frequency of adverse effects or complications associated with TT placement, synthesize information on the necessity for water precautions in children with TT, and assess the effectiveness of available treatments for otorrhea in children who have TT.
Key Questions
With input from clinical experts during Topic Refinement, and from the public, during a public review period, we developed the following Key Questions (KQs) and study eligibility criteria.
- Key Question 1.
For children with chronic otitis media with effusion, what is the effectiveness of TT, compared to watchful waiting, on resolution of middle ear effusion, hearing and vestibular outcomes, quality of life, and other patient-centered outcomes?
- What factors (such as age, age of onset, duration of effusion, comorbidities, and sociodemographic risk factors) predict which children are likely to benefit most from the intervention?
- Does obtaining a hearing test help identify which children are more likely to benefit from the intervention?
- Key Question 2.
For children with recurrent acute otitis media, what is the effectiveness of TT, compared to watchful waiting with episodic or prophylactic antibiotic therapy, on the frequency and severity of otitis media, quality of life, and other patient-centered outcomes?
- What factors (such as age, age of onset, number of recurrences, presence of persistent middle ear effusion, comorbidities, sociodemographic risk factors, history of complications of acute otitis media, antibiotic allergy or intolerance) predict which children are likely to benefit most from the intervention?
- Key Question 3.
What adverse events, surgical complications, and sequelae are associated with inserting TT in children with either chronic otitis media with effusion or recurrent acute otitis media?
- Key Question 4.
Do water precautions reduce the incidence of TT otorrhea or affect quality of life?
- Key Question 5.
In children with TT otorrhea, what is the comparative effectiveness of topical antibiotic drops versus systemic antibiotics or watchful waiting on duration of otorrhea, quality of life, or need for tube removal?
Analytic Frameworks
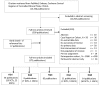
The analytic frameworks in Figures A through C describe the specific linkages associating the populations of interest, exposures, modifying factors, and outcomes of interest in the assessment of studies that examine the association between TT placement, intermediate and final health outcomes, and harms (KQs 1, 2 and 3; Figure A); need for water precautions (KQ 4; Figure B); and treatment of otorrhea (KQ 5; Figure C).
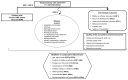
Figure A
TT in children with chronic OME or recurrent AOM (Key Questions 1, 2, and 3). OME=otitis media with effusion; AOM=acute otitis media; TT=tympanostomy tubes; KQ=Key Question
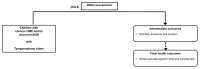
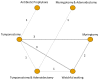
Figure B
Need for water precautions in children with TT (Key Question 4). OME=otitis media with effusion; AOM=acute otitis media; QoL= Quality of Life
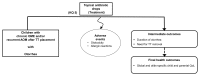
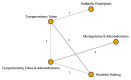
Figure C
Treatment of otorrhea in children with TT (Key Question 5). OME=otitis media with effusion; AOM=acute otitis media; QoL= Quality of Life; TT=tympanostomy tube
Methods
The Brown Evidence-based Practice Center (EPC) conducted this review based on a systematic review of the published scientific literature using established methodologies as outlined in the Agency for Healthcare Research and Quality’s (AHRQ) Methods Guide for Comparative Effectiveness Reviews.12 The PROSPERO protocol registration number is CRD42015029623.
Eligibility Criteria
We use the Population, Intervention, Comparator, Outcomes, and Designs (PICOD) formalism to define the characteristics of the eligible studies for this review.
Populations
For all KQs, studies of children and adolescents from 1 month to 18 years old were eligible. Subpopulations of interest included children at high risk of recurrent AOM or OME, such as children with Down syndrome, cleft palate, other craniofacial anomalies, and primary ciliary dyskinesia; and children at high risk of adverse clinical or developmental outcomes, such as those with preexisting hearing loss, speech and language problems, or developmental disorders. We were also interested in the population of children who have sociodemographic risk factors, such as day care exposure or low socioeconomic status.
For KQ 1, we included studies of children with chronic OME. We preferred the standard definition of effusion that persists for at least three months,1 but included results based on studies’ alternative definitions if our preferred one was not reported. We excluded children with chronic suppurative otitis media since it is usually associated with a persistently perforated tympanic membrane.
For KQ 2, we included children with recurrent AOM with or without middle ear effusion, defined as three or more well-documented and separate AOM episodes in the past 6 months or at least four well-documented and separate AOM episodes in the past 12 months with at least one in the past 6 months.1 For studies that did not report the preferred definition, we recorded the study specific definition.
For KQ 3 and 4, we included studies in children with TT placed for OME or AOM. For KQ 5, we included studies of symptomatic or asymptomatic children with acute TT otorrhea beyond the immediate postoperative period. We defined the immediate postoperative period as 30 days after surgery, but included studies reporting results near that period (e.g., 28 days, 4 weeks).
Interventions/Exposures
For KQs 1, 2 and 3, we considered all studies that included myringtomy with TT placement, with or without adenoidectomy. Tubes were classified as short-term tubes (generally in place for 10–18 months) and long-term tubes (which typically remain in place for several years).
In KQ 4, we distinguished three categories of interventions; avoidance of swimming or head immersion while bathing, canal occlusion methods (e.g. earplugs or headbands), and postexposure prophylaxis using ototopical antibiotics.
KQ 5 compares ototopical preparations, and includes products approved by the U.S. Food and Drug Administration (FDA) (i.e., ofloxacin otic 0.3%, ciprofloxacin 0.3% and dexamethasone 0.1%), and other non–FDA-approved agents, such as hydrocortisone, bacitracin, and colistin.
Comparators
For KQ 1, comparisons of primary interest were watchful waiting or adenoidectomy. Comparators for KQ 2 included watchful waiting, systemic or topical antibiotic therapy for recurrent episodes of AOM, prophylactic antibiotics, and adenoidectomy. KQ 3 did not address comparative harms. In KQ 4, comparators included no water precautions with or without avoidance of higher risk activities (e.g. diving or underwater swimming), and ear plugs or swimming caps. The primary comparators for KQ5 were watchful waiting and oral antibiotic therapy.
Outcomes
For KQs 1 and 2, which address the effectiveness of TT, we considered intermediate outcomes, including the prevalence of middle ear effusion, measures of hearing and vestibular function, such as improved hearing thresholds (audibility), tests of auditory perception and discrimination (clarity), and balance and coordination (vestibular function). For KQ 2, measures of recurrent AOM, including otorrhea were extracted.
Quality of life and patient-centered outcomes were considered, including global and otitis-specific child and parental quality of life, speech and language outcomes, educational achievement, behavioral outcomes such as disobedience, enuresis, or tantrums.
The following outcomes were extracted for KQ 3: Intraoperative and immediate postoperative anesthetic and surgical adverse events, otorrhea beyond the postoperative period, blockage of the tube lumen, granulation tissue, premature extrusion, TT displacement into the middle ear, persistent perforation of the tympanic membrane, myringosclerosis, tympanic membrane atrophy, atelectasis and retraction pockets, worsened hearing thresholds, and other reported (plausibly related to tubes).
Outcomes for KQ 4 included final health and patient-centered outcomes related to child and parental quality of life and intermediate outcomes related to the incidence and duration of otorrhea. Outcomes evaluated relating to KQ 5 (treatment of otorrhea) included global and otitis-specific child and parental quality of life, duration of otorrhea, and need for removal of TT.
Timing
We included studies with any duration of followup.
Setting
We included studies performed in both primary and specialty care settings.
Study Design
We evaluated published, peer-reviewed studies only. For KQs 1, 2, 4, and 5, we included randomized comparative trials and nonrandomized comparative studies, prospective and retrospective where treatment was assigned on a per patient basis. Studies with per ear assignment were excluded (e.g. tubes placed by design in one ear only). For KQ 3, we included prospective surgical single group studies enrolling at least 50 subjects (including arms treated with TT that were part of randomized controlled trials [RCTs] or nonrandomized comparative studies [NRCSs]) and population based retrospective single group studies (registry studies) with at least 1000 subjects.
Searching for the Evidence
We conducted literature searches of all studies in MEDLINE®, the Cochrane Central Trials Registry and Cochrane Database of Systematic Reviews, Embase®, and CINAHL® databases (details in Appendix A of the full report). The last search was run on May 19, 2016. Additionally, we perused the reference lists of published clinical practice guidelines, relevant narrative and systematic reviews, and Scientific Information Packages from manufacturers. Citations were independently screened by two researchers in the open-source, online software Abstrackr (http://abstrackr.cebm.brown.edu/).
Data Extraction and Data Management
Each study was extracted by one methodologist and confirmed by at least one other methodologist. Data was extracted into customized forms in the Systematic Review Data Repository (SRDR) online system (http://srdr.ahrq.gov). Excluded studies are listed in Appendix B of the full report. Details of included studies are summarized in Appendix C, D and E of the full report.
Assessment of Risk of Bias of Individual Studies
We assessed the methodological quality of each study based on predefined criteria. For RCTs, we used the Cochrane risk of bias tool.13 For observational studies, we used relevant questions from the Newcastle Ottawa Scale.14
Data Synthesis
All included studies were summarized in narrative form and in summary tables that record the important features of the study populations, design, intervention, outcomes, and results.
We performed network meta-analysis of clinical outcomes to compare treatment alternatives across studies for KQs 1 and 5. We also conducted pairwise comparisons by means of random effects meta-analyses of comparative studies. Specific methods and metrics (summary measures) meta-analyzed were chosen based on available, reported study data. When available, these were summarized as odds ratios of categorical outcomes and net change of continuous outcomes (e.g., mean hearing thresholds). Statistical heterogeneity was explored qualitatively. We explored subgroup differences within across studies based on the list of comparisons described in the KQs.
Grading the Strength of Evidence
We graded the strength of evidence (SOE) as per the AHRQ Methods Guide on assessing the strength of evidence.15
Assessing Applicability
We assessed the direct applicability within and across studies with reference to children with specific comorbidities (Down syndrome, cleft palate, etc.), and whether interventions and comparators are used in current practice.
Results
The literature search yielded 10,129 citations (Figure D). We identified 481 of these as potentially relevant full-text studies, and retrieved them for further evaluation. Overall, 306 full text articles did not meet eligibility criteria (see Appendix B of the full report for a list of rejected articles along with reasons for rejection); thus 184 articles are included in this report.
A trial registry search did not turn up any completed trial that was not already identified through literature searches. As shown in Figure D, the majority of included publications (n=98) related to KQ3, with 50 related to KQ1. There is a relative paucity of studies available for the other KQs.
Key Question 1
We identified 54 publications Of these, there were 29 papers reporting results of 16 RCTs. There were 24 publications reporting 24 NRCSs that assessed the effectiveness of TT in pediatric patients with chronic middle ear effusion. These studies evaluated multiple interventions (TT, TT with adenoidectomy, myringotomy with adenoidectomy, myringtomy alone, adenoidectomy alone, oral antibiotic prophylaxis, and watchful waiting). Two studies included at least some patients with recurrent AOM with or without persistent middle ear effusion.
Randomized Comparative Studies
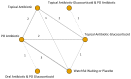
Hearing thresholds were measured in 16 RCTs. In 10 of these, mean hearing thresholds were reported by arm at various time points. For the network meta-analysis of these RCTs, we classified hearing thresholds obtained at one to three months as “early”. Similarly, hearing thresholds obtained between 12 and 24 months where were classified “late”. Not all studies had interventions at both “early” and “late” time points. Thus, the network of comparators differs for “early” and “late” comparisons. Figure E shows the topology of the network for early hearing thresholds at 1 to 3 months. Such network plots are a visual representation of the evidence base.
Figure F illustrates the effectiveness of various interventions at 1 to 3 months, compared with watchful waiting. Mean hearing thresholds improved (decreased) by average of 9.1 dB following TT, and by 10 dB following TT with adenoidectomy. Credible intervals for these effects exclude zero. The credible intervals for comparisons between watchful waiting and myringotomy alone, myringotomy with adenoidectomy, and oral antibiotic prophylaxis were wide.
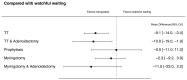
Figure F
Early (1 to 3 months) decrease (improvement) in mean hearing thresholds compared with watchful waiting. TT= tympanostomy tubes; CrI=Credible Interval
As shown in Table A, the strategies with the highest probability of being among the three most effective interventions with respect to early improvements in hearing thresholds were TT, TT with adenoidectomy, and myringotomy with adenoidectomy.
Table A
Probabilities (percent) that an intervention is among the three most effective with respect to early hearing thresholds.
Five RCTs reported hearing thresholds at 12 to 24 months. Figure G shows the topology of the network of comparisons at this time interval.
As shown in Figure H, by 12 to 24 months, the mean difference in hearing thresholds for TT alone, compared to watchful waiting was 0 dB (95% CrI [credible interval] −4 to 3). Compared to watchful waiting, myringotomy with adenoidectomy and TT with adenoidectomy have better hearing outcomes by about 4 dB, but the 95 percent credible intervals include zero.

Figure H
Late (12 to 24 months) decrease (improvement) in mean hearing thresholds compared with watchful waiting. TT= tympanostomy tubes; CrI=Credible Interval
As can be seen in Table B, TT with adenoidectomy and myringotomy with adenoidectomy were the two most effective strategies with respect to late hearing thresholds. TT alone, antibiotic prophylaxis, and watchful waiting were among the three least effective ones.
Table B
Probabilities (percent) that an intervention is among the two most effective with respect to late hearing thresholds.
The results for the studies that reported measuring hearing thresholds, but did not report mean hearing thresholds are summarized in the full report.
A network meta-analysis of the mean duration of middle ear effusion is presented in the full report.
Nonrandomized Comparative Studies
The nonrandomized comparative studies (NRCSs) are summarized in the full report. The NRCSs evaluated special populations and are summarized here. Six studies reported results in the populations with comorbidities of interest, including cleft palate/lip and primary ciliary dyskinesia. Three studies (two in cleft palate and one in primary ciliary dyskinesia) compared TT placement with nonsurgical treatment, while one study compared early versus delayed TT in different settings. Two studies assessed the effects of TT and cleft repairing versus cleft repairing alone. Hearing thresholds reported as pure tone averages were reported in four studies. In patients with cleft palate/lip and primary ciliary dyskinesia, respectively, average hearing threshold was lower in TT than non-surgical control, but the difference was not significant. TT in addition to cleft repair improved hearing thresholds with unknown significance. The improvement by early (mean age 3 months) compared to delayed (mean age 40.8 months or not at all in two subjects) TT procedures in patients with cleft palate was marginally significant (P=0.05 for ears with better hearing and P=0.10 for ear with worse hearing). The rate of normal hearing, defined as hearing threshold < 20 dB bilaterally, was significantly higher in TT than control (P <0.05).
Quality of Life and Patient-Centered Outcomes
Eight studies (five RCTs, three NRCSs, and one that combined both designs) in 12 papers reported on 119 quality of life and patient-centered outcomes (cognitive, language, and behavioral) in 1665 children over multiple time points and arms. These studies reported only 14 outcomes with significant results. In general, the results were not significant and varied in magnitude and direction, even across subscales of the same test.
Only two studies reported specifically on quality of life outcomes: Paradise reported on measures of parental stress at various ages, and Vlastos reported on pediatric health related quality of life. Neither found any significant differences. Full details for all outcomes are in Appendix G of the full report.
Key Question 2
We identified 8 publications, reporting 7 RCTs and 2 NRCSs. The Matilla 2003 paper reported two groups, an RCT which randomly allocated treatment in 137 patients, and a NRCS in which parental choice determined treatment in169 patients. Three RCTs compared TT placement with daily oral antibiotic prophylaxis. Two of these studies included a comparison with placebo and the third compared TT placement with no treatment. The effectiveness of TT alone versus TT with adenoidectomy was evaluable in three studies.
Randomized Comparative Studies
Frequency and Severity of Recurrent Acute Otitis Media
The majority of studies were done prior to widespread use of the conjugate pneumococcal vaccine, in an era where antibiotic resistance was less common, and prophylactic oral antibiotic therapy was more commonly used in clinical practice. Results are summarized by comparison below.
TT Versus Placebo or No Treatment
Gonzalez 1986 reported that in the placebo group three of 20 children had no further episodes of AOM, compared to 12 of 22 in the TT group (P = 0.01, an attack rate of 2.0 in the placebo group, compared to 0.86 in the TT group (P = 0.006). In a post-hoc subgroup comparison of children who had middle ear effusion upon entering the study, attack rate and number of patients who had no further bouts of AOM was significantly better (P < 0.05) in those children without middle ear effusion. However, this group consisted of only 22 patients.
Casselbrant 1992 reported the rate of new episodes per arm was 1.08 in the placebo group versus 1.02 in the TT group (P = 0.25). In the placebo group, 40 percent had no further episodes of AOM, compared to 35 percent in the TT group. In addition, TT placement significantly decreased the percentage of time with AOM compared to placebo (P < 0.001).
Kujala 2012 reported failure rates (defined as at least two episodes of AOM in 2 months, three in 6 months or persistent effusion lasting at least 2 months), percent of children with no recurrent AOM, cumulative number of AOM episodes, and one-year incidence rates. There was an absolute difference in the proportion of failures of −13 percent (95% CI −25 to −01) between the TT and control groups, favoring TT. The one year incidence rate (infections per child per year) was 0.55 (95% CI 0.93 to 0.17) lower in the TT group compared to the control group.
TT Versus Antibiotic Prophylaxis
In the Gonzalez 1986 RCT, 54.5 percent of children in the TT group and 24 percent in the sulfisoxazole prophylaxis group had no recurrent AOM (P = 0.02). The attack rate was 0.86 infections per child in the TT group and 1.4 in prophylaxis group (P = 0.08).
Casselbrant 1992 reported a rate of 0.6 episodes of recurrent AOM per child-year children treated with Amoxicillin and a rate of 1.02 in their TT group (P = 0.001).
El-Sayed found no difference in the treatment outcomes of children treated with trimethoprim/sulfamethoxazole compared to children treated with TT (P = 0.37).
TT Versus TT and Adenoidectomy
An RCT by Mattila 2003 found no difference in cumulative hazard of recurrent AOM or in efficacy, defined as one minus the adjusted relative risk in randomized and nonrandomized comparisons of children who underwent TT with adenoidectomy compared with TT alone.
In the Kujala 2012 study, there was no significant difference in the TT with adenoidectomy group compared to the TT only group in the number of failures (absolute difference −5%, 95% CI −16 to 6, P = 0.37), in the time to failure (P = 0.29) or to the first AOM (P = 0.6), or in the proportion of children with no AOM episodes (absolute difference 1%, CI −13 to 15, P =1.0).
A subsequent 2005 RCT, which enrolled 217 children, 162 of whom had recurrent AOM, again found no differences in the mean number of otitis media episodes overall or in the subgroup of children with recurrent AOM at enrollment.
Quality of Life Outcomes
Although Kujala 2014 found that insertion of TT tubes, with or without adenoidectomy, significantly reduced the risk of recurrent AOM, a subsequent publication from the same trial examining quality of life outcomes at study entry, 4 months and 12 months found no differences in overall ear-related quality of life (Otitis Media-6 questionnaire [OM-6]), or for the subscales of caregiver concern, emotional distress, physical suffering, activity limitations, hearing loss, or speech impairment between surgically treated and no surgery groups.
Nonrandomized Comparative Studies
In a cross-sectional study, Grindler 2014 reported both disease-specific quality of life outcomes, utilizing OM-6 score, and health related quality of life, using the PedsWL Infant Impact Module, in 1208 patients. The OM-6 score was higher (reflecting worse otitis specific quality of life) in children in otolaryngology practices who had been recommended to undergo TT placement than in children with prior TT placement.
Key Question 2a
There are no prospective planned comparisons evaluating whether the presence of middle ear effusion (at time of surgical evaluation) modifies the effectiveness of TT placement for recurrent AOM. Gonzalez 1986 report a retrospective subgroup comparison based on the presence or absence of middle ear effusion at initial evaluation and conclude that the attack rate, as well as the number of patients who had no further bouts of AOM, was significantly better (p < 0.05) in those children without middle ear effusion. However, this group consisted of only 22 patients. Two studies specifically excluded patients with middle ear effusion at time of surgical evaluation.
Casselbrant 1992 analyzed data with a multivariable Poisson model, and concluded that TT reduced the number of episodes of AOM/otorrhea only in those subjects whose episodes of AOM occurred year round. In their model, younger age and white race were significantly associated with higher rates of recurrent AOM, but there treatment by age and treatment by race interactions were not found.
Key Question 3
We extracted data on the occurrence of 11 adverse events from 85 cohorts and from RCTs and NRCSs included in KQs 1 and 2. The adverse events considered were: perioperative complications, otorrhea, tube blockage, granulation tissue formation, premature extrusion, displacement of the TT into the middle ear space, persistent perforation, myringosclerosis (tympanosclerosis), presence of atrophy, atelectasis or retraction, cholesteatoma and long term hearing loss. We did not consider other adverse events, such as antibiotic resistance, gastrointestinal side effects of antibiotics or pain related to ear drops. The number of publications reporting each event, and the median (with 25th and 75th percentiles) percent of patients and ears are summarized in Table C.
Table C
Median percentage of patients and ears with adverse events associated with TT placement.
See Appendix G of the full report for adverse event details by study, and for study specific details, including design, recruitment period, tube type(s) used, age, proportion with recurrent AOM, followup time, and study specific definitions. In general, the study specific definitions of adverse events are poorly reported and/or highly variable between studies.
Key Question 4
We identified 11 publications which reported 2 RCTs and 9 NRCSs, which evaluate a range of interventions, from complete water restriction (e.g., no swimming or head immersion while bathing), physical protection while swimming (utilizing ear plugs or bathing caps), postexposure prophylactic ear drops, avoidance of high risk activities (e.g., diving), to completely unrestricted exposure to water. All studies compared either no-swimming or ear plugs with a second group of swimmers with or without post-exposure antibiotic ear drops.
In the two RCTs, Goldstein 2005 reported a slightly higher average rate of otorrhea per month in children who did not wear ear plugs (mean 0.10 episodes/month, compared to a mean of 0.07; P = 0.05) in a Poisson regression model adjusting for compliance. Parker 1994 reported identical mean otorrhea rates in nonswimmers and swimmers. Table D summarizes the occurrence of one or more episodes of otorrhea in the RCTs.
Table D
RCTs: Water precautions—one or more episodes of otorrhea.
The forest plot in Figure I, summarizes the results of a random effects meta-analysis from the NRCSs only with separate summary estimates for ear plugs and avoidance of swimming. The summary odds ratio comparing ear plugs versus no precautions of having one or more episodes of otorrhea was 1.70 (95% CI 0.95 to 3.06). The odds ratio for nonswimming compared to no precautions was 1.52 (95% CI 0.71 to 3.25). It is notable that events rates in the RCTs are systematically higher in both control and intervention arms in the RCTs compared with event rates in NRCSs. A possible explanation is more complete ascertainment of outcomes in RCTs.
There appears to be a statistically nonsignificant trend in the NRCSs, which favor no ear plugs and no precautions. This trend may reflect a possible bias (e.g. patients who chose to swim may be less likely to report minor degrees of otorrhea).
Overall, aside from the small reduction in mean number of episodes of otorrhea found in the Goldstein RCT, the available evidence does not support the conclusion that either ear plugs or avoidance of swimming reduces the risk of otorrhea related to swimming.
Key Question 5
We identified 12 papers, representing 11 studies, reporting 10 RCTs and 1 NRCS, with a total of 1811 patients analyzed (1405 in RCTs and 406 in NRCSs) that assessed the effectiveness of various interventions to treat TT otorrhea. The studies reported multiple comparisons, including oral antibiotics (amoxicillin and amoxicillin/clavulanate), various antibiotic drops and antibiotic-glucocorticoid drops, oral corticosteroids, and combinations. Several studies had a watchful waiting or placebo arm.
Two studies were excluded from our meta-analysis, the NRCS by Dohar where specific treatments used in the historical practice group and concurrent practice group were not reported and a study which compared an antibiotic-glucocorticoid topical drop containing neomycin sulfate, polymyxin B sulfate and hydrocortisone with a topical spray formulation containing neomycin sulfate and dexamethasone. The network of available comparisons is shown in Figure J.
Outcomes
Clinical Cure
Eleven studies reported the number of clinically cured patients in each arm, often at multiple time points. All studies reported additional intermediate outcomes (e.g., cessation, improvement or duration of otorrhea).
After excluding 4 studies, 7 studies were included in the network meta-analysis. We chose the time designated by each study as the test of cure (range 7 to 20 days after initiation of treatment). As shown in Table E, treatment strategies that include topical antibiotic drops predominate over both oral antibiotics and watchful waiting or placebo.
Table E
Probabilities (percent) that an intervention is among the three most effective with respect to clinical resolution of otorrhea.
The plots show that topical antibiotic-glucocorticoid and antibiotic-only drops are superior to watchful waiting (Figure K).

Figure K
Relative effectiveness of interventions compared to watchful waiting or placebo therapy. OR=Odds Ratio, CrI=Credible Interval
When compared to oral antibiotics, topical antibiotic-glucocorticoid drops are superior to oral antibiotics and there is a suggestion that topical antibiotic drops are also superior, although the credible interval overlaps the null effect (Figure L).
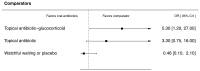
Figure L
Relative effectiveness of interventions compared to treatment with oral antibiotics. OR=Odds Ratio, CrI=Credible Interval
Quality of Life
Van Dongen 2014 was the only study to report quality of life outcomes. They evaluated quality of life in 230 children with otorrhea who received watchful waiting, oral antibiotics, or antibiotic-glucocorticoid drops for 7 days. At baseline, the generic and disease-specific health-related quality-of-life scores indicated good quality of life and were similar across the groups. At 2 weeks of follow-up, the change in the generic health-related quality-of-life scores did not differ significantly among the study groups. The changes in the disease-specific health-related quality-of-life scores at 2 weeks were small but consistently favored eardrops. Confidence intervals were relatively wide.
Discussion
Overall Summary and Strength of Evidence
Our systematic review of 172 publications focused on five Key Questions (KQ), which evaluate the evidence for effectiveness of TT in children with chronic middle ear effusion and recurrent acute otitis media, the adverse events (harms) associated with this procedure, the need for water precautions in children with TT, and the treatment of TT otorrhea. Table E summarizes our dispositions about the strength of the evidence.
Key Question 1
In children with chronic otitis media with effusion, 54 publications reported results of 29 RCTs.
Risk of bias for evaluation of hearing and middle ear effusion outcomes was rated as moderate to high. Limited information on quality of life and other patient-centered outcomes (cognitive, language, and behavioral) suggests that effects for these outcomes varied in magnitude and direction, even across subscales of the same test, and were not significantly different across the compared interventions. Risk of bias for quality of life outcomes as rated as low to moderate. Risk of bias for various outcomes in high risk populations was rated as high.
TT placement (compared to watchful waiting) resulted in improved average hearing thresholds 1 to 3 months after surgery (a period when the majority of tubes are functioning). Mean hearing thresholds after TT placement with or without adenoidectomy improved by approximately 10 dB when assessed at 1 to 3 months.
By 1 to 2 years, when most tubes have extruded, hearing thresholds are no longer different, reflecting the favorable natural history of spontaneous resolution of middle ear effusion in most children. There was a trend suggesting improved thresholds in children undergoing adenoidectomy, but credible intervals (CrI) are wide and include the null effect. The individual patient data meta-analysis (IPD) by Boonacker et. al., which relied on a composite outcome, concluded that adenoidectomy is most beneficial in children four years or older with persistent otitis media with effusion. In this group at 12 months, 51 percent of those who had adenoidectomy failed whereas 70 percent of those who did not have adenoidectomy failed (Risk Difference 19%: 95% Crl 12% – 26%; NNT (Number Needed to Treat) = 6). No significant benefit of adenoidectomy was found in children less than 4 years old.3
Data were very sparse with respect to which factors might predict those children more likely to benefit from TT. The individual patient data (IPD) meta-analysis reported by Rovers et al. focused on interactions between treatment and baseline characteristics. They found significant interactions between daycare attendance in children 3 years or younger, and in children over 4 years of age with a hearing level of 25 dB or greater in both ears, and concluded that TT might be used in young children attending day-care; or in older children with a hearing level of 25 dB or baseline hearing level persisting for at least 12 weeks. They noted that average hearing level at baseline did not obviously modify the effect estimate.16
There is limited evidence regarding quality of life outcomes, but neither of the two studies that evaluated parental stress and health related quality of life found significant improvements in surgically treated children. Evidence for improved cognitive, language, or behavioral outcomes after TT, compared to watchful waiting, is similarly lacking.
Key Question 2
In children with recurrent acute otitis media, seven publications reported results of six RCTs. We were unable to provide pooled results due to the small number of studies, multiple interventions, and heterogeneity in reported outcomes. The limited available evidence suggests that TT placement decreases the number of further episodes and the overall number of episodes of recurrent AOM. Three RCTs consistently found no difference in recurrent episodes of AOM when comparing TT versus TT and adenoidectomy.
Very little evidence from RCTs is available to evaluate factors that identify children most likely to benefit from TT placement. Only one study addressed any predisposing factors. A post hoc subgroup (n=22) comparison in one study concluded that patients with middle ear effusion at the time of surgical evaluation had improved outcomes.17 Risk of bias across outcomes ranged from moderate to high.
Key Question 3
In general, the study specific definitions of adverse events were poorly reported and/or highly variable between studies. Not all cohorts followed all patients until extrusion of the tube, nor was followup complete in all studies. Several adverse event categories have very wide interquartile ranges (e.g. otorrhea, premature extrusion, and myringosclerosis). This is likely due to highly variable definitions. For example, in some studies counts of patients with at least one episode of otorrhea were included, while other studies included only patients with purulent ear discharge. Otorrhea is particularly complex to characterize, as it may with respect to frequency, duration, volume, character, and associated symptoms. Other adverse events, such as hearing loss and cholesteatoma, are likely confounded by the severity of preexisting and ongoing middle ear disease.
Key Question 4
We identified nine studies, two RCTs and seven NRCSs that evaluated physical ear protection (e.g. ear plugs) or water restriction (e.g. no swimming or head immersion while bathing) in children after TT placement. One RCT reported a slightly higher average rate of otorrhea (after adjusting for compliance) in children who did not wear ear plugs.18 A second RCT, with high risk of bias, found a statistically nonsignificant difference in the odds ratio in nonswimmers versus swimmers.19 A meta-analysis of NRCSs with evaluated ear protection and nonswimming tended to favor no precautions and swimming, but these RCTs are subject to high risk of bias and the analysis did not exclude a null effect. For the comparison of ear plugs vs. no precautions, risk of bias was rated as moderate. For those comparisons and outcomes where the evidence consists of nonrandomized comparative studies only, risk of bias was rated as high.
Key Question 5
Seven RCTs were included in a network meta-analysis of the comparative effectiveness of various treatments for TT otorrhea.
Seven studies were included in a network meta-analysis of the comparative effectiveness of various treatments for TT otorrhea. The common outcome evaluated was clinical cure, defined as absence of otorrhea after completion of treatment.
The odds of clinical cure were 12-fold (95% CrI: 1.9 – 82) higher [NNT 2.2 (assuming a baseline rate 0.45)]a for antibiotic-glucocorticoid drops, compared to watchful waiting/placebo. Similarly, the odds of clinical cure were 7.3-fold (95% CrI: 1.2 – 51) higher [NNT 2.5 (assuming a baseline rate of 0.45)]b for topical antibiotic drops (compared to watchful waiting/placebo).
Compared to oral antibiotics, treatment with topical-glucocorticoid drops demonstrated higher effectiveness, odds ratio 5.3 (95% CrI: 1.2 to 27) [NNT 3.2 (assuming a baseline rate 0.56)].c The odds ratio for topical antibiotic drops was 3.3 (95% CrI: 0.74 – 16)[NNT 5 (assuming a baseline rate of 0.69)]d, although the credible interval includes 1. Risk of bias was low for random sequence generation and allocation concealment. However, 8 of 10 studies had high risk of bias due to open label design, which precluded blinding of personnel and care providers. Risk of bias was rated moderate overall.
An overall summary of main conclusions with an assessment of the strength of evidence is summarized in Table F.
Table F
Summary of conclusions and associated strength of evidence dispositions.
Limitations
The available evidence base is composed of studies that evaluate multiple interventions. Several of these (e.g. myringotomy alone and oral antibiotic prophylaxis) are rarely used in current practice. Thus, the direct evidence relating to the comparisons of interest relies on a smaller subset of studies or must be augmented with indirect evidence from network meta-analysis. Many of these trials were performed prior to widespread use of conjugate pneumococcal vaccines and in an era where antibiotic resistance was less common. It is unclear whether these or other factors affect the relative (current vs. historical) benefits of TT placement for recurrent AOM.
The majority of trials utilized similar inclusion criteria and subgroup analysis of higher or lower risk groups is sparse. The generalizability of results to infants and young toddlers and to school age children is also uncertain, given that children in these age groups are underrepresented in available trials. With the exception of two older trials that included children with chronic middle ear effusion (MEE) and/or recurrent AOM, most enrolled predominately children with chronic MEE. The degree to which patients in clinical practice may have both chronic MEE and recurrent AOM is unclear.
Reporting of possible sociodemographic risk factors is sparse and inconsistent, which limits the ability to draw conclusion about which of these factors might influence the relative effectiveness of TT.
With the exception of a few NRCSs, patients with cleft palate and Down syndrome have been systematically excluded from comparative trials, limiting the applicability of the evidence to these and other small subgroups, who experience a higher burden of middle ear disease. Similarly, patients at increased risk of developmental or behavioral sequelae from middle ear disease are not included (or at least identified) in trials to date.
Across RCTs relative to KQs 1 and 2, there was universal lack of blinding of participants and, in many cases, of outcome assessors. Given the intervention in question, placement of a tube in a visible anatomic structure, blinding of participants is not easily accomplished. In addition, many studies are at risk for attrition bias due to dropouts and incomplete followup.
Our meta-analysis of hearing levels used average pure tone hearing levels (typically reported as an average over frequencies of 500, 1000, 2000 and 4000 Hz). This simple measurement is likely insufficient to fully elucidate the complex relationships between hearing and speech perception and development in children.
Assessment of the effectiveness of TT in children with recurrent acute otitis media is particularly challenging, since an episode of AOM in control children (with intact tympanic membrane) results in otalgia and inflammatory changes, whereas children with a functioning TT may present with varying degrees of otorrhea. Bacterial cultures performed in the setting of research may assist in differentiating infections due to organisms associated with AOM from superinfections or colonization with other organisms (e.g. Staphylococcus or Pseudomonas species). Intermediate outcomes, which rely on simple counts or rates of otorrhea, fail to account for the variable nature of otorrhea with respect to duration, character, and patient impact.
Our network meta-analysis of the effectiveness of treatments for otorrhea combines trials of fluoroquinolones with other non FDA approved preparations. This presumes equivalent effectiveness and does not consider variable side effects such as ototoxicity, which may be associated with some agents.
Future Research Recommendations
Current indications for TT placement largely reflect the inclusion criteria used in clinical trials. Prognostic models are urgently needed to stratify children with regard to their risk of persistence of middle ear effusion or recurrent AOM.
Pragmatic trials are needed, particularly in children with recurrent AOM, but also in children with chronic MEE and children with risk factors, such as cleft palate or Down syndrome. If possible, trials should be powered with planned subgroup analyses in groups at higher versus lower risk of outcomes.
Since TT are no longer effective after extrusion, future trials should record per-ear and per-patient outcomes that are conditional on whether the TT has extruded. Trialists should explore methods to control for high rates of potential cross-over from watchful waiting to surgical intervention.
Outcome assessment in children with recurrent acute otitis media is challenging, since an episode of AOM in children with an intact tympanic membrane results in otalgia and inflammatory changes, whereas children with a functioning TT exhibit otorrhea. Reliance on outcomes based on simple counts or rates of otorrhea fail to account for the variable character of otorrhea, which can be transient (of little to no concern), recurrent (of more concern, but usually readily managed), or chronic (of considerable concern and difficult to manage). Future trials would benefit from standardization and consistent definition of adverse events. In some cases, e.g. premature extrusion, one author’s premature extrusion may be another’s time extrusion, depending on the duration of anticipated need.20
Bacteriologic evaluations performed in the research setting may assist in differentiating otorrhea resulting from infection with organisms associated with AOM (e.g. Streptoccus pneumoniae, nontypable Haemophilus influenza)from superinfections with organisms associated with chronic otorrhea (e.g. Staphylococcus aureus and Pseudomonas aeruginosa).21
Conclusions
Overall, the evidence suggests that TT placed in children with persistent middle-ear effusion result in short term improvements in hearing compared to watchful waiting. However, there is no evidence of a sustained benefit.
Our network meta-analysis of hearing thresholds suggests the possibility of a more sustained improvement in hearing thresholds in at least some children who undergo adenoidectomy and TT placement. However, a nuanced understanding of which children may benefit from adenoidectomy is limited by the small evidence base and our use of aggregate data.
The evidence suggests that a period of watchful waiting does not worsen language, cognition, behavior, or quality of life. However, the current evidence base provides little guidance for the treatment of children who may be at increased risk for speech, language, or learning problems because of baseline sensory, physical, cognitive or behavioral factors.
Children with recurrent AOM may have fewer episodes after TT placement, but the evidence base is severely limited. It is unclear that quality of life outcomes are improved. The benefits of TT placement must be weighed against a variety of adverse events associated with TT placement.
In children in whom TT have been placed, there is no compelling evidence for the need to either avoid swimming or bathing or use ear plugs or bathing caps
Should otorrhea develop, the available evidence supports topical treatment of TT otorrhea.
Footnotes
- a
As seen in: van Dongen TM, van der Heijden GJ, Venekamp RP, et al. A trial of treatment for acute otorrhea in children with tympanostomy tubes. N Engl J Med. 2014 Feb 20;370(8):723–33. doi: 10.1056/NEJMoa1301630. PMID: 24552319 [PubMed: 24552319] [CrossRef]. (34 of 75 in watchful waiting arm)
- b
As seen in: Heslop A, Lildholdt T, Gammelgaard N, et al. Topical ciprofloxacin is superior to topical saline and systemic antibiotics in the treatment of tympanostomy tube otorrhea in children: the results of a randomized clinical trial. Laryngoscope. 2010 Dec;120(12):2516–20. doi: 10.1002/lary.21015. PMID: 20979100 [PubMed: 20979100] [CrossRef]. (12/26 cured in saline rinse (placebo) arm)
- c
As seen in: van Dongen TM, van der Heijden GJ, Venekamp RP, et al. A trial of treatment for acute otorrhea in children with tympanostomy tubes. N Engl J Med. 2014 Feb 20;370(8):723–33. doi: 10.1056/NEJMoa1301630. PMID: 24552319 [PubMed: 24552319] [CrossRef]. (43 of 77 cured in oral antibiotic arm)
- d
As seen in: Goldblatt EL, Dohar J, Nozza RJ, et al. Topical ofloxacin versus systemic amoxicillin/clavulanate in purulent otorrhea in children with tympanostomy tubes. Int J Pediatr Otorhinolaryngol. 1998 Nov 15;46(1–2):91–101. PMID: 10190709 [PubMed: 10190709]. (101 of 146 cured in oral antibiotic arm)
References
- 1.
- Rosenfeld RM, Schwartz SR, Pynnonen MA, et al. Clinical practice guideline: Tympanostomy tubes in children. Otolaryngol Head Neck Surg 2013 Jul;149(1 Suppl):S1–35. doi: 10.1177/0194599813487302. PMID: 23818543. [PubMed: 23818543] [CrossRef]
- 2.
- Schilder AG, Chonmaitree T, Cripps AW, et al. Otitis media. Nat Rev Dis Primers 2016;2:16063. doi: 10.1038/nrdp.2016.63. PMID: 27604644. [PMC free article: PMC7097351] [PubMed: 27604644] [CrossRef]
- 3.
- Boonacker CW, Rovers MM, Browning GG, et al. Adenoidectomy with or without grommets for children with otitis media: an individual patient data meta-analysis. Health Technol Assess 2014 Jan;18(5):1–118. doi: 10.3310/hta18050. PMID: 24438691. [PMC free article: PMC4780935] [PubMed: 24438691] [CrossRef]
- 4.
- Grindler DJ, Blank SJ, Schulz KA, et al. Impact of Otitis Media Severity on Children’s Quality of Life. Otolaryngol Head Neck Surg 2014 Mar 13;151(2):333–40. doi: 10.1177/0194599814525576. PMID: 24627408. [PMC free article: PMC4163281] [PubMed: 24627408] [CrossRef]
- 5.
- Rosenfeld RM, Jang DW, Tarashansky K. Tympanostomy tube outcomes in children at-risk and not at-risk for developmental delays. Int J Pediatr Otorhinolaryngol 2011 Feb;75(2):190–5. doi: 10.1016/j.ijporl.2010.10.032. PMID: 21106257. [PubMed: 21106257] [CrossRef]
- 6.
- Rettig E, Tunkel DE. Contemporary concepts in management of acute otitis media in children. Otolaryngol Clin North Am 2014 Oct;47(5):651–72. doi: 10.1016/j.otc.2014.06.006. PMID: 25213276. [PMC free article: PMC4393005] [PubMed: 25213276] [CrossRef]
- 7.
- Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report 2009 Jan 28(11):1–25. PMID: 19294964. [PubMed: 19294964]
- 8.
- Proceedings from the National Summit on Overuse. 2012. The Joint Commission. https://www
.jointcommission .org/assets/1 /6/National_Summit_Overuse.pdf. Accessed December 21, 2016. - 9.
- Hoffman HJ, Daly KA, Bainbridge KE, et al. Panel 1: Epidemiology, natural history, and risk factors. Otolaryngol Head Neck Surg 2013 Apr;148(4 Suppl):E1–E25. doi: 10.1177/0194599812460984. PMID: 23536527. [PubMed: 23536527] [CrossRef]
- 10.
- Casselbrant ML, Kaleida PH, Rockette HE, et al. In reference to What is the role of tympanostomy tubes in the treatment of recurrent acute otitis media? Laryngoscope. 2013 Dec;123(12):E127. doi: 10.1002/lary.24142. PMID: 24105683. [PubMed: 24105683] [CrossRef]
- 11.
- van Dongen TM, Schilder AG, Venekamp RP, et al. Cost-effectiveness of treatment of acute otorrhea in children with tympanostomy tubes. Pediatrics 2015 May;135(5):e1182–9. doi: 10.1542/peds.2014-3141. PMID: 25896832. [PubMed: 25896832] [CrossRef]
- 12.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Publication No. 10(12)-EHC063-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
- 13.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. doi: 10.1136/bmj.d5928. PMID: 22008217. [PMC free article: PMC3196245] [PubMed: 22008217] [CrossRef]
- 14.
- Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- 15.
- Berkman ND, Lohr KN, Ansari M, et al. AHRQ Methods for Effective Health Care: Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. [PubMed: 24404627]
- 16.
- Rovers MM, Black N, Browning GG, et al. Grommets in otitis media with effusion: an individual patient data meta-analysis. Arch Dis Child 2005 May;90(5):480–5. doi: 10.1136/adc.2004.059444. PMID: 15851429. [PMC free article: PMC1720375] [PubMed: 15851429] [CrossRef]
- 17.
- Gonzalez C, Arnold JE, Woody EA, et al. Prevention of recurrent acute otitis media: chemoprophylaxis versus tympanostomy tubes. Laryngoscope 1986 Dec;96(12):1330–4. PMID: 3537596. [PubMed: 3537596]
- 18.
- Goldstein NA, Mandel EM, Kurs-Lasky M, et al. Water precautions and tympanostomy tubes: a randomized, controlled trial. Laryngoscope 2005 Feb;115(2):324–30. doi: 10.1097/01.mlg.0000154742.33067.fb. PMID: 15689760. [PubMed: 15689760] [CrossRef]
- 19.
- Parker GS, Tami TA, Maddox MR, et al. The effect of water exposure after tympanostomy tube insertion. Am J Otolaryngol 1994 May–Jun;15(3):193–6. PMID: 8024107. [PubMed: 8024107]
- 20.
- Kay DJ, Nelson M, Rosenfeld RM. Meta-analysis of tympanostomy tube sequelae. Otolaryngol Head Neck Surg 2001 Apr;124(4):374–80. PMID: 11283489. [PubMed: 11283489]
- 21.
- Idicula WK, Jurcisek JA, Cass ND, et al. Identification of biofilms in post-tympanostomy tube otorrhea. Laryngoscope 2016 Aug;126(8):1946–51. doi: 10.1002/lary.25826. PMID: 27426942. [PMC free article: PMC4955598] [PubMed: 27426942] [CrossRef]
- Executive Summary - Tympanostomy Tubes in Children With Otitis MediaExecutive Summary - Tympanostomy Tubes in Children With Otitis Media
- LOC123991682 [Oncorhynchus gorbuscha]LOC123991682 [Oncorhynchus gorbuscha]Gene ID:123991682Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...