Introduction and Timeline
Technology continues to transform the medical care system and to improve length and quality of life—but at substantial cost.
It is almost inconceivable to think about providing health care in today’s world without medical devices, machinery, tests, computers, prosthetics, or drugs. Medical technology can be defined as the application of science to develop solutions to health problems or issues such as the prevention or delay of onset of diseases or the promotion and monitoring of good health (1,2). Examples of medical technology include medical and surgical procedures (angioplasty, joint replacements, organ transplants), diagnostic tests (laboratory tests, biopsies, imaging), drugs (biologic agents, pharmaceuticals, vaccines), medical devices (implantable defibrillators, stents), prosthetics (artificial body parts), and new support systems (electronic medical records, e-prescribing, and telemedicine).
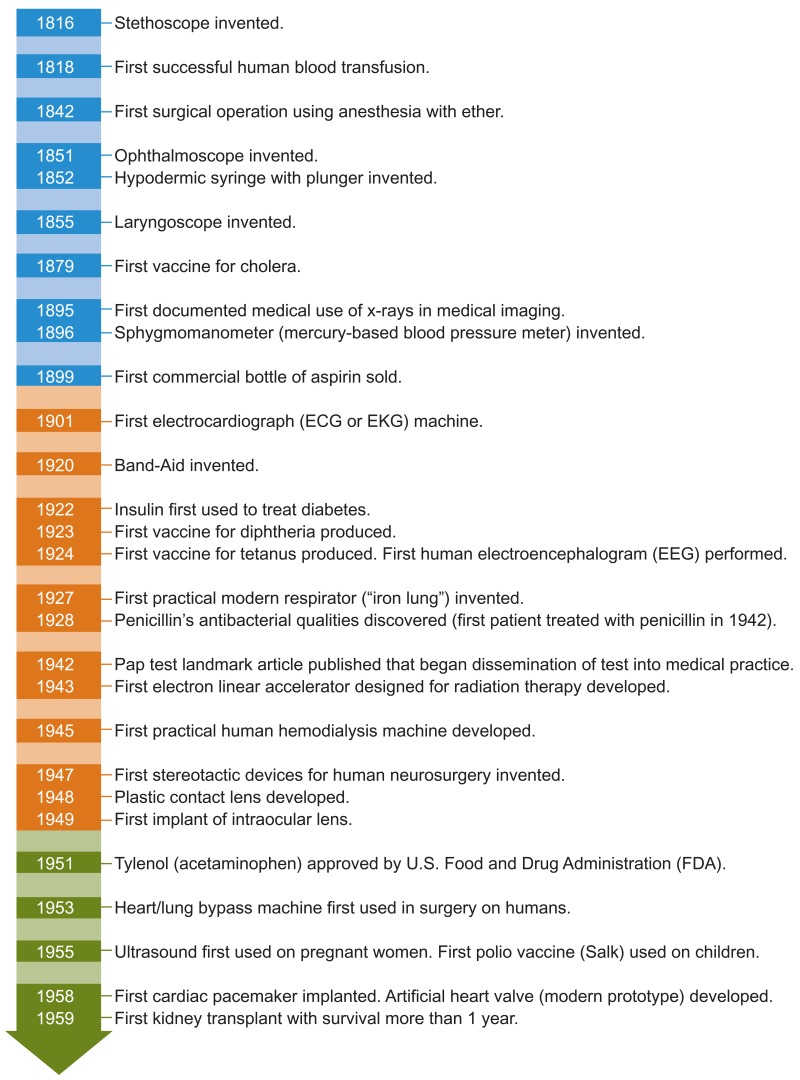
Figure 23 provides examples of selected key health care technologies developed in the past two centuries that have greatly influenced medical practice and health care outcomes. New vaccines may eliminate or greatly reduce the incidence and prevalence of many diseases, and antibiotics and other drugs can treat previously untreatable pathogens. Genetic typing offers the opportunity for early diagnosis and individualized therapies. New technologies can also improve on existing ones, such as new drugs that have fewer side effects and surgical advances such as laparoscopic techniques, which are less invasive and have a quicker recovery time than traditional surgery. New indications for existing therapies are common, such as fluoxetine, originally used for depression and now also used for premenstrual dysphoria, and atomoxetine, originally used for Parkinson disease and now also used for attention-deficit/hyperactivity disorder (3). Combinations of technologies can be more effective than individual ones, such as the combination “cocktail” now used to treat HIV/AIDS, combination chemotherapy for many types of cancers, and the recent creation of scanning machines that combine positron emission tomography and computed tomography (PET/CT) or PET and magnetic resonance imaging (PET/MRI).
As some technologies become easier to use and less expensive, as equipment becomes more transportable, and as recovery times for procedures are reduced, even complex technologies can diffuse out of hospitals and institutional settings and into ambulatory surgery centers, provider offices, outpatient facilities, imaging centers, and patients’ homes, making the technologies more accessible. Technologies have shifted out of institutional settings and into ambulatory surgery centers (Figure 29) and from hospitals into the home. Telemedicine, or the use of technology to remotely diagnose and treat conditions through electronic envisioning and data transfer, can provide services to remote or underserved areas (4).
New types of medical equipment, procedures, and devices have created the need for personnel with specialized training in their use, in some cases creating entirely new professions. Medical specialists such as radiation oncologists, medical geneticists, and surgical subspecialists, as well as allied and support professions such as medical sonographers, radiation technologists, and laboratory technicians, have all been created to use specific types of technology (Table 111).
The infrastructure necessary to support more complex technologies is also considered to be a part of medical technology. Use of electronic medical records and electronic prescribing are methods for coordinating the increasingly complex array of services provided, as well as allowing for electronic checks of quality to reduce medical errors (for example, drug interactions). The percentage of private office-based physicians who work in offices with fully functional electronic medical records remains low (4% in 2008) (5).
Because technologies have diffused into standard medical practice, there are concerns about whether they are consistently being used properly and about the quality of the information provided by tests, imaging, and other technological outputs (6,7). To address these concerns, several laws and regulations have been enacted. These include the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and the Mammography Quality Standards Act (MQSA, 1992). In July 2008 Congress passed the Medicare Improvements for Patients and Providers Act (MIPPA). Beginning January 1, 2012, MIPPA requires that “advanced diagnostic imaging services” (diagnostic MRI, CT, and nuclear medicine, including PET) be reimbursed by Medicare only if performed by accredited facilities (7) (also see Figure 26).
Technologies applied to new populations and conditions generally come at a cost to individuals and to society as a whole. Technologies can be very expensive (e.g., heart transplants, chemotherapy) or very inexpensive (e.g., the Band Aid). Total expenditures for a given technology, however, are determined by both use and cost; consequently, widely used inexpensive technologies can often have higher aggregate expenditures than rarely used expensive ones. Some new technologies can be cost-saving—for example annual influenza vaccinations in high-risk children (8). Many technologies, however, contribute to increases in overall health care expenditures because they increase utilization (e.g., more doctor visits may be needed to monitor new drug therapies); they may be used on a larger number of patients; they may be more expensive than technologies they replace; or they may increase life expectancy in populations and thus their lifetime health care costs (9). Therefore, although there is general agreement that new technologies and new uses for existing technologies are a major component of increases in health care expenditures, the cumulative contribution of all new technologies to rising medical expenditures, and how technology can be used in the most cost-effective manner, is a subject of much debate (10,11).
Medical technology expenditures are determined in large part by how technologies are used by practitioners and patients, and, for new technologies, how they diffuse into medical practice. In addition to the potential benefit of using technologies, use is also influenced by provider preferences, patient preferences, legal and regulatory constraints, and costs to both insurers and consumers (9,12). Use may be increased relative to what may be considered most cost effective because of overuse, errors in data interpretation, overestimation of the benefits of technology or underestimation of its risks, and defensive medicine. Patient demand may be influenced by advertising or information obtained from friends, the Internet, or other sources, and low tolerance of ambiguity by provider or patient (more information is always better) (13). Negative effects of technologies can include unnecessary expenditures, false positives that can spur additional testing or anxiety, and the inefficient use of resources. Some providers may be inclined to use the more profitable technologies, particularly when these technologies are less invasive or better accepted by patients than alternatives, such as counseling about lifestyle changes, that patients may not accept or implement and over which the provider has less control (9,14).
Once diffused into practice, it is often difficult to reduce the use of technologies, even in situations where they have been shown to be ineffective or not superior to less complex or less expensive alternatives. Widespread use of electronic fetal monitoring in low-risk deliveries continues, although there has been evidence for many years that it is unnecessary, perhaps even harmful (15). Diuretics have been shown to be more successful than newer, more expensive drugs in controlling hypertension for some patients (16).
In general, Americans—both providers and consumers—appear to be more willing and eager to adopt and use new technologies than people in other countries (17). More rapid acceptance of new technologies can be beneficial when they are effective, but in some cases harmful effects can be discovered only after widespread use. For example, use of nonsteroidal anti-inflammatory drugs (NSAIDs) increased substantially during the early 2000s, and it was not until reports of complications were reported to the FDA that studies showing adverse effects were publicized and use of these drugs decreased (18,19).
Technology diffusion can differ by population group (e.g., by income, race/ethnicity, gender, urbanization, or age), producing inequalities in treatment (overuse or underuse) (20). Women and black persons are significantly underrepresented among Medicare patients with ischemic cardiomyopathy who receive implantable cardioverter-defibrillators (21). Among patients who had an initial consultation for rectal cancer, black patients were 23% less likely to have chemotherapy and 12% less likely to have radiation than white patients, controlling for other factors (22). Higher spending is not necessarily associated with higher quality, so it is often difficult to determine whether some populations are overusing or underusing specific technologies relative to others (9,10,23).
The remainder of this Chartbook examines trends in, and use of, important medical technologies, including use of new types of imaging machinery; medical procedures that rely on devices, machines, or highly technical processes; and pharmaceuticals (Figures 25–30 and 32–35). Data are also presented on the association between regulation and growth in types of laboratories (Figure 24) and on differences in the use of intensive care services by geographic location (Figure 31). Figure 36 shows expenditures for selected hospital stays with highly technological procedures.
Technology provides an increasing ability to monitor, prevent, diagnose, control, and cure a growing number of health conditions and to improve quality and length of life. Questions remain, however, about how much innovation and improvement in new and existing technologies is possible when resources are constrained and health care expenditures are rising to unacceptable levels, about the opportunity costs of using one technology versus another (or neither), and whether target populations are appropriately and equitably served (11).
References
- 1.
- De Miranda MA, Doggett AM, Evans JT. Medical technology: Contexts and content in science and technology . Columbus, OH: The Ohio State University; 2005. Available from: http://teched
.vt.edu /CTTE/ImagesPDFs/MedicalTech2005.pdf. - 2.
- Snapshots: Health care costs How changes in medical technology affect health care costs [online]. The Kaiser Family Foundation; 2007. Available from: http://www
.kff.org/insurance /snapshot/chcm030807oth.cfm. - 3.
- Ashburn TT, Thor KB. Drug repositioning: Identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–83. [PubMed: 15286734]
- 4.
- Heinzelmann PJ, Lugn NE, Kvedar JC. Telemedicine in the future. J Telemed Telecare. 2005;11(8):384–90. [PubMed: 16356311]
- 5.
- Hsiao CJ, Burt CW, Rechtsteiner E, Hing E, Woodwell DA, Sisk JE. Preliminary estimates of electronic medical records use by office-based physicians: United States, 2008 [online] Health E-stats. NCHS. 2008. Available from: http://www
.cdc.gov/nchs /data/hestat/physicians08 /physicians08.htm. - 6.
- Howerton D, Anderson N, Bosse D, Granade S, Westbrook G. Good laboratory practices for waived testing sites: Survey findings from testing sites holding a Certificate of Waiver under the Clinical Laboratory Improvement Amendments of 1998 and recommendations for promoting quality testing. MMWR. 2005;54(RR–13):1–25. [PubMed: 16280973]
- 7.
- Mammography Quality Standards Act and program [online] U.S. Food and Drug Administration, Center for Devices and Radiological Health; 2009. Available from: http://www
.fda.gov/Radiation-EmittingProducts /MammographyQualityStandardsActandProgram/default.htm. - 8.
- Prosser LA, Bridges CB, Uyeki TM, Hinrichsen VL, Meltzer MI, Molinari N-AM, et al. Health benefits, risks, and cost-effectiveness of influenza vaccination of children. Emerg Infect Dis. 2006;12(10):1548–58. [PMC free article: PMC3290928] [PubMed: 17176570]
- 9.
- Bodenheimer T. High and rising health care costs, Part 2, Technologic innovation. Ann Intern Med. 2005;142(11):932–7. [PubMed: 15941701]
- 10.
- Goldsmith J. The impact of new technology on health costs. Health Aff (Millwood). 1994;13(3):80–1. [PubMed: 7927163]
- 11.
- Garber AM, Fuchs V. Medical innovation: Promises and pitfalls [online] The Brookings Institution. Winter. 2003. Available from: http://www
.brookings .edu/articles/2003/winter _technology_fuchs.aspx?p=1. - 12.
- Wallner PE, Konski A. A changing paradigm in the study and adoption of emerging health care technologies: Coverage with evidence development. J Am Coll Radiol. 2008;5(11):1125–9. [PubMed: 18954812]
- 13.
- Deyo RA. Cascade effects of medical technology. Annu Rev Public Health. 2002;23:23–44. [PubMed: 11910053]
- 14.
- Lantos JD. Hooked on neonatology. Health Aff (Millwood). 2001;20(5):233–40. [PubMed: 11558709]
- 15.
- Thacker SB, Stroup DF. Revisiting the use of the electronic fetal monitor. Lancet. 2003;361(9356):445–6. [PubMed: 12583938]
- 16.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotension-converting enzyme inhibitor or calcium channel blocker vs diuretic. JAMA. 2002. pp. 2981–97. Available from: http://jama
.ama-assn .org/cgi/content/abstract/288/23/2981. [PubMed: 12479763] - 17.
- Kim M, Blendon RJ, Benson JM. How interested are Americans in new medical technologies? A multicountry comparison. Health Aff (Millwood). 2001;20(5):194–201. [PubMed: 11558703]
- 18.
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343(21):1520–8. [PubMed: 11087881]
- 19.
- Jacobs K. Do COX-2 inhibitors have a future? Harvard Science Review. 2005;19(1):26–9.
- 20.
- Stanley A, DeLia D, Cantor JC. Racial disparity and technology diffusion: The case of cardioverter defibrillator implants, 1996–2001. J Natl Med Assoc. 2007;99(3):201–7. [PMC free article: PMC2569614] [PubMed: 17393943]
- 21.
- Gauri AJ, Davis A, Hong T, Burke MC, Knight BP. Disparities in the use of primary prevention and defibrillator therapy among blacks and women. Am J Med. 2006;119(2):e17–e21. [PubMed: 16443424]
- 22.
- Morris AM, Billingsley KG, Hayanga AJ, Matthews B, Baldwin LM, Birkmeyer JD. Residual treatment disparities after oncology referral for rectal cancer. J Natl Cancer Inst. 2008 May 21;100(10):738–44. [PMC free article: PMC2766763] [PubMed: 18477800]
- 23.
- Skinner JS, Staiger DO, Fisher ES. Is technological change in medicine always worth it? The case of acute myocardial infarction. Health Aff (Millwood). 2006;25(2):w34–w47. [PMC free article: PMC2117353] [PubMed: 16464904]
History of medical technology: Selected milestones, 1816–2008
References
- Aquilina O. A brief history of cardiac pacing [online] Images Paediatr Cardiol. 2006. pp. 17–81. Available from: http://www
.impaedcard .com/issue/issue27/aquilinao2 /aquilinao.htm(pacemaker) [PMC free article: PMC3232561] [PubMed: 22368662] - BAND-AID brand celebrates 85 years of innovation with reinvention of the traditional bandage [online] Medical News Today . Apr 30, 2005. Available from: http://www
.medicalnewstoday .com/articles/23627.php(Band-Aid) - Blood pressure monitoring: The history of blood pressure measurement [online] . University College of London Department of Medical Physics and Bioengineering; 2003. Available from: http://www
.medphys.ucl .ac.uk/teaching/undergrad /projects/2003/group_03/history .html(blood pressure machine) - Drews J. Drug discovery: A historical perspective. Science. 2000;287(5460):1960–4. [PubMed: 10720314]
- Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol. 1929;10(31):226–36.
- Fusion imaging: A new type of technologist for a new type of technology [online]. Statements from the PET–CT Consensus Conference; 2002 Jul 31; New Orleans LA. Available from: www
.crcpd.org/PET-CT_Fusion_Imaging /PET-CT %20Consensus%20Paper.doc. - Gascoigne B. HistoryWorld medical timelines [online] Technology (Wellcome Trust). Available from: http://www
.historyworld .net/timelines/timeline .asp?back=existing .asp&from =existing&tid =yoby&title=Technology.
Surgery (Wellcome Trust). Available from: http://www.historyworld .net/timelines/timeline .asp?back=existing .asp&from =existing&tid =yobx&title=Surgery. - Gold RH, Bassett LW, Widoff BE. Highlights from the history of mammography. Radiographics. 1990;10(6):1111–31. [PubMed: 2259767]
- Hammerschmidt DE. The first commercial bottle of aspirin: March 6, 1899. J Lab Clin Med. 1998;132(6):556. [PubMed: 9851747]
- History of medicine [online] Wikipedia. 2009. Available from: http://en
.wikipedia.org /wiki/History_of_medicine#1000 .E2.80.94present. - Jenkins D, Gerred S. A (not so) brief history of electrocardiography [online] ECG Library. 2009. Available from: http://www
.ecglibrary.com/ecghist.html(electrocardiography) - Junod SW. Selections from the Food and Drug Law Institute update series on FDA history. U.S. Food and Drug Administration; 2007. Celebrating a milestone: FDA’s approval of first genetically-engineered product [online] Available from: http://www
.fda.gov/AboutFDA /WhatWeDo/History /ProductRegulation /SelectionsFromFDLIUpdate .SeriesonFDAHistory/ucm081964 .htm. - Lawrence G. The hypodermic syringe. Lancet. 2002. p. 1074. Available from: http://www
.sciencedirect .com/science?_ob =ArticleURL&_udi =B6T1B-45GK81X-1T&_user =856389&_rdoc =1&_fmt =&_orig =search&_sort =d&view =c&_version =1&_urlVersion =0&_userid =856389&md5 =e22aaa89e59d82bfb80bc1da970bb617. - Medical advances timeline [online] Infoplease. 2007. Available from: http://www
.infoplease .com/ipa/A0932661.html. - Milestones in medical diagnosis and diagnostic imaging [online] Imaginis. Available from: http://www
.imaginis.com/faq/milestones .asp. - Mishra RK. History of minimal access surgery [online] Laparoscopy Hospital. Available from: http://www
.laparoscopyhospital .com/history_of_laparoscopy .htm. - Okonek BAM, Morganstein L. Development of polio vaccines [online] Access Excellence Classic Collection. 2001. Available from: http://www
.accessexcellence .org/AE/AEC/CC/polio.php(polio vaccines) - Pearson HA, Anunziato D, Baker JP, Gartner LM, Howell DA, Strain JE, et al. American pediatrics: Milestones at the millennium Report of the Historical Archives Advisory Committee, American Academy of Pediatrics. Pediatrics. 2001. pp. 1482–91. Available from: http://pediatrics
.aappublications .org/cgi /content/full/107/6/1482(NICU) [PubMed: 11389283] - Sabbatini RME. The PET scan: A new window into the brain [online] Brain & Mind. 1997. Available from: http://www
.cerebromente .org.br/n01/pet/pet.htm(PET scan) - Samadi DB. History of robotic surgery [online] Robotic Oncology . http://www
.roboticoncology.com/history/(general laparoscopic surgery) - Science News: MRI/PET scanner combo made for first time [online] ScienceDaily . Mar 10 , 2008. Available from: http://www
.sciencedaily .com/releases/2008/03/080307095241 .htm. - Starr A. The artificial heart valve. Nat Med. 2007;13:1160–4. [PubMed: 17917665]
- Starzl TE. The mystique of organ transplantation. J Am Coll Surg. 2005;201(2):160–70. [PMC free article: PMC2987743] [PubMed: 16038811]
- Stephenson LW. Cohn LH, editor. Cardiac surgery in the adult. 3. New York: McGraw-Hill; 2008. History of cardiac surgery, Table 1–1, Twilight zone: Clinical status of open-heart surgery, 1951–1955. pp. 3–28. Available from: http:
//cardiacsurgery .ctsnetbooks.org/cgi /content/full/3/2008/3/T1?ck=nck(cardiac surgery) - Swartz BE, Goldensohn ES. Timeline of the history of EEG and associated fields. Electroencephalogr Clin Neurophysiol. 1998. pp. 173–6. Available from: http://www
.sciencedirect .com/science?_ob =MImg&_imagekey =B6SYX-4FV4S6H-1-1&_cdi =4846&_user =10&_orig =browse&_coverDate=02 %2F28 %2F1998&_sk =998939997&view =c&wchp =dGLbVzz-zSkWb&_valck =1&md5 =eb44bd7edfe0342bbfd71810e9810e94&ie=/sdarticle .pdf. [PubMed: 9741779] - The global HIV/AIDS timeline [online]. The Kaiser Family Foundation; 2007. Available from: http://www
.kff.org/hivaids /timeline/hivtimeline.cfm(HIV) - Valenti C, Schutta EJ, Kehaty T. Cytogenetic diagnosis of Down’s syndrome in utero. JAMA. 1969;207(8):1513–5. [PubMed: 4236853]
- Vilos GA. The history of the Papanicolaou smear and the odyssey of George and Andromache Papanicolaou. Obstet Gynecol. 1998. pp. 479–83. Available from: http://elib2
.cdc.gov:2071 /science?_ob=ArticleURL& _udi =B6TB2-3VMW382-C&_user =856389&_coverDate =03 %2F31%2F1998&_rdoc =30&_fmt =high&_orig =browse&_srch=doc-info( %23toc%235130 %231998%23999089996 %2351593%23FLP%23display %23Volume&_cdi =5130&_sort =d&_docanchor =&_ct =30&_acct =C000046148&_version =1&_urlVersion =0&_userid =856389&md5 =8a3c71bcfa4312b937094145c5f14bb2) [PubMed: 9491881]
Federally Regulated (CLIA) Laboratories
The number of federally regulated (CLIA) laboratories has grown substantially, fueled by an increase in laboratories or other sites that obtain Certificates of Waiver to perform only tests that are simple with low risk of an erroneous result.
Clinical laboratories perform testing on materials derived from the human body (including blood, urine, and tissues) (1). An estimated 7–10 billion medical tests are performed each year. Test results play a critical role in health assessment, influencing the majority of medical decisions (2).
Technological advances have increased the simplicity of some types of laboratory tests, while at the same time introducing sophisticated tests that may require complex equipment and highly trained staff (2).
Since 1992, the majority of facilities in the United States that perform laboratory testing on human specimens are regulated under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) (2). CLIA was enacted following reports of inaccurate Pap test results, which spurred an effort to regulate laboratory quality (3). The CLIA regulatory program is run cooperatively by the Centers for Medicare & Medicaid Services, CDC, and the Food and Drug Administration.
CLIA extended regulations to all nonexempt and nonexcepted laboratories that conduct testing on human specimens (3). The regulatory requirements are keyed to the type of testing a laboratory performs, with laboratories conducting more complicated tests subject to more stringent requirements. The three categories of testing under CLIA are as follows: waived (simple tests with little chance of erroneous result), moderate complexity, and high complexity. Laboratories performing only waived tests are not subject to routine CLIA oversight and must only acquire a certificate of waiver, pay fees, and follow manufacturer test instructions. Laboratories performing moderately or highly complex tests are subject to regulations setting minimum qualifications for all persons performing or supervising tests, must participate in approved proficiency testing programs, and must have systems and processes in place to ensure proper test performance and accurate results, and an overall plan to monitor the quality of all aspects of the laboratory’s operations (3). Laboratories in New York and Washington are exempt from CLIA regulations because those states have their own state-law-based laboratory oversight regulations that meet or exceed the CLIA requirements.
In 2008, approximately 209,000 laboratories were certified under CLIA (including in the two exempt states), an increase from 155,000 laboratories in 1993 (Figure 24). The number of waived laboratories in the 48 nonexempt states and the District of Columbia almost doubled between 1993 and 2008, increasing from 67,000 to 129,000 (Figure 24). In 2008, waived laboratories comprised 64% of all laboratories, up from 44% in 1993 (data table for Figure 24). The diffusion of testing to physician offices and other point-of-care sites increases the speed with which test results can be obtained and makes testing more convenient for providers and patients (2). The number of physician office laboratories (POLs) increased from about 91,000 in 1993 to about 109,000 in 2008, although the total number for all laboratories increased at a faster rate (data table for Figure 24). During this period, the percentage of CLIA laboratories located in physician offices decreased from 59% to 52% (data table for Figure 24).
Although some studies indicate that waived laboratories generally take measures to perform tests according to manufacturers’ specifications, the lack of oversight has raised some concerns about the quality of the testing performed in POLs (2,4). Concerns also have been raised about the standards enforcement required by the CLIA regulations in nonwaiver laboratories that perform more complex testing, and whether this oversight is sufficient to ensure quality (5).
References
- 1.
- Clinical Laboratory Improvement Amendments (CLIA). How to obtain a CLIA Certificate of Waiver [online]. Centers for Medicare & MedicaidServices. ; 2006. Available from: http://www
.cms.hhs.gov /CLIA/downloads/HowObtainCertificateofWaiver.pdf. - 2.
- CDC. Good laboratory practices for waived testing sites: Survey findings from testing sites holding a Certificate of Waiver under the Clinical Laboratory Improvement Amendments of 1998 and recommendations for promoting quality testing. MMWR. 2005;54(RR–13):1–25. [PubMed: 16280973]
- 3.
- Centers for Medicare & Medicaid Services; 2006. CMS initiatives to improve quality of laboratory testing under the CLIA program [online] Available from: http://www
.cms.hhs.gov /CLIA/downloads/060630 .Backgrounder.rlEG.pdf. - 4.
- Overturf GD. CLIA waived testing in infectious diseases. Pediatr Infect Dis J. 2008;27(11):1009–12. [PubMed: 18955895]
- 5.
- U.S. Government, Accountability Office (GAO). GAO–06–416. Washington, DC: GAO; 2006. Clinical lab quality: CMS and survey organization oversight should be strengthened. Available from: http://www
.gao.gov/products/GAO-06-416.
Selected Imaging Technologies
The use of MRI/CT/PET scans in physician offices and hospital outpatient and emergency department settings has increased dramatically over the past decade.
Advanced imaging technologies offer the physician sophisticated tools for diagnosing and monitoring the status of a wide array of medical conditions (1). Advanced diagnostic medical imaging includes such technologies as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET). CT provides multidimensional and higher resolution information than x-ray. Hence, CT is preferable for imaging intracranial, head and neck, thoracic, and abdominal structures (2). The magnetic field aspect of MRI makes it better than CT for viewing soft tissue; therefore, it is often used to evaluate intracranial or spinal cord abnormalities, musculoskeletal tumors, or trauma (2). PET is often used for cancer, coronary, and neurologic evaluations (2). These technologies may be combined in hybrid machines to provide more diagnostic information.
Despite the significant costs of acquiring advanced imaging capability, the availability and use of imaging technologies in the United States has substantially increased since their introduction in the early 1980s (3). In 2006, there were more than 7,000 sites offering MRI, with an estimated 27 million MRI procedures performed (4) (also see Table 121). In 2007, more than 10,000 CT units were in operation at more than 7,600 hospital and nonhospital sites, and the availability of PET and other imaging modalities has been steadily increasing (5). The site of imaging services has diffused from hospital inpatient and outpatient settings to nonhospital settings such as physician offices or radiology centers (6). During the past decade, the number of freestanding diagnostic imaging centers owned by radiologists, other specialists, private investors, or for-profit companies has more than doubled (1).
Data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey were analyzed for 1996–2007 to examine trends in advanced imaging (CT, MRI, and PET scans), although the types of imaging procedures captured by the surveys varied during the period. Part of the increase in advanced imaging scanning may be due to improved survey questions and editing procedures (see Technical Notes). A visit with an advanced imaging scan is defined as a visit with a scan ordered or provided during the visit. Use of advanced imaging scans has increased substantially during physician office and hospital outpatient department (OPD) and emergency department (ED) visits since 1996 (Figure 25). Advanced imaging scan rates during visits to physician offices and OPDs more than tripled from 1996 to 2007 among persons under 65 years of age and among persons 65 years of age and over (Figure 25). In 2007, 3%–4% of physician office and OPD visits included advanced imaging scans ordered or provided during the visit (data table for Figure 25).
Between 1996 and 2007, the use of advanced imaging during ED visits increased fivefold among adults under 65 years of age and quadrupled among adults 65 years of age and over (Figure 25). In 2007, 12% of ED visits among persons under 65 years of age and 26% of ED visits among persons 65 years of age and over included advanced imaging scans ordered or provided during the visit (data table for Figure 25).
Although use of these technologies in ambulatory settings has increased, hospitals continue to perform them on an inpatient basis. The rate of use of at least one MRI during a given hospital stay among adults has remained relatively stable since 1990, but the rate of hospitalizations with at least one CT scan declined by 63% over this period (Table 103).
Most medical imaging is considered to be low risk; however, it is not without risk. The National Academy of Sciences’ Biologic Effects of Ionizing Radiation VII (BEIR VII) report on the effect of low-level ionizing radiation concludes that for any exposure to radiation, a linear relationship exists between the dose of radiation and an increased risk of cancer (7,8). Concerns have also been raised about standards for image quality and interpretation. A recent report by the Government Accountability Office concluded that the increase in imaging in physician offices, which have less oversight than more institutional settings, may be problematic (9). These and concerns about possible unnecessary use of imaging spurred provisions in the Medicare Improvements for Patients and Providers Act (MIPPA). Beginning January 1, 2012, MIPPA requires that advanced diagnostic imaging services (diagnostic MRI, CT, and nuclear medicine, including PET) be reimbursed by Medicare only if performed by accredited facilities (10).
Rapid growth in these relatively expensive imaging procedures has been the subject of several recent studies that attempt to examine the reasons for this growth and have raised concerns that some imaging may be unnecessary (11). Medicare Part B spending for imaging services under the physician fee schedule more than doubled between 2000 and 2006, from $6.9 billion to $14.1 billion (9). Between 2000 and 2006, the percentage of Medicare Part B spending for imaging performed in hospital settings decreased from 35% to 25%, while the share of imaging spending increased in physician offices from 58% to 64% and in independent diagnostic testing facilities from 7% to 11% (9).
References
- 1.
- Iglehart JK. The new era of medical imaging—Progress and pitfalls. N Engl J Med. 2006;354(26):2822–8. [PubMed: 16807422]
- 2.
- The Merck manual for healthcare professionals. 18th ed. Online Medical Library; Principles of radiologic imaging [online] Available from: http://www
.merck.com /mmpe/sec22/ch329/ch329a.html. - 3.
- Baker LC, Atlas SW, Afendulis CC. Expanded use of imaging technology and the challenge of measuring value. Health Aff (Millwood). 2008;27(6):1467–78. [PubMed: 18997202]
- 4.
- IMV Medical Information Division. Latest IMV market report shows continued demand for high field MRI systems. Des Plaines, IL: press release; Jan 16, 2007. Available from: http://www
.imvinfo.com /user/documents/content_documents /nws_rad /MS_MRI_PressRelease.pdf. - 5.
- IMV Medical Information Division. Latest IMV CT census shows slow-down in the purchase of CT technology [press release]. Des Plaines, IL: 20 Mar, 2008. Available from: http://www
.imvinfo.com /user/documents/content_documents /def_dis /2008_03_21_11_25_43_706.pdf. - 6.
- Levin DC, Rao VM, Parker L, Frangos AJ, Sunshine JH. Recent shifts in place of service for noninvasive diagnostic imaging: Have hospitals missed an opportunity? J Am Coll Radiol. 2009;6(2):96–9. [PubMed: 19179236]
- 7.
- Steenhuysen J. Overexposed: Imaging tests boost U.S. radiation dose. Reuters. 3 Mar, 2009. Available from: http://www
.reuters.com /article/scienceNews /idUSTRE5226IR20090304. - 8.
- National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. Washington, DC: National Academies Press; 2006. Available from: http://www
.nap.edu/openbook .php?isbn=030909156X. [PubMed: 25077203] - 9.
- U.S. Government Accountability Office (GAO). Medicare Part B imaging services: Rapid spending growth and shift to physician offices indicate need for CMS to consider additional management practices. GAO–08–452. Washington, DC: GAO; 2008. Available from: http://www
.gao.gov/products/GAO-08-452. - 10.
- Iglehart JK. Health insurers and medical imaging policy—A work in progress. N Engl J Med. 2009;360(10):1030–7. [PubMed: 19264694]
- 11.
- Gazelle GS, McMahon PM, Siebert U, Beinfeld MT. Cost-effectiveness analysis in the assessment of diagnostic imaging technologies. Radiology. 2005;235(2):361–70. [PubMed: 15858079]
Mammography
Between 1987 and 1999, recent mammography use among women 40 years of age and over more than doubled but decreased slightly between 1999 and 2008.
Mammography technology has advanced over the past 35 years, progressively becoming more accurate (1). Since the 1960s, technical developments in mammography have resulted in greater sensitivity and specificity in cancer detection and decreases in radiation exposure (1,2). Without mammography screening, many breast cancers would not be diagnosed until at least 1 year later (1). Newer forms of breast cancer screening are emerging, and some, like digital mammography, have been tested against current technologies and shown to have potential advantages for some groups, whereas others, like thermography, have not been proven equivalent to date (3–5). New technologies that complement traditional mammography include ductal lavage, MRI (magnetic resonance imaging technology), and ultrasound, and these are also evolving (6). New breast cancer treatments and drug regimens (including tamoxifen and herceptin), and earlier diagnoses—advanced by the invention of the modern-day mammogram machine, the development of quality standards for mammography machines and radiologists, and the promotion of regular mammography screening—have all contributed to declining mortality rates and reduced deaths due to breast cancer (1,2,6) (also see Tables 36 and 50).
Breast cancer ranks second as a cause of cancer death in women and is the most frequently diagnosed cancer in women after cancers of the skin (7,8) (also see Tables 36 and 49). In 2005, 186,000 women in the United States were diagnosed with breast cancer and 41,000 died from the disease (8).
The percentage of women 40 and over who had a mammogram in the past 2 years more than doubled, increasing from 29% in 1987 to 70% in 1999 (data table for Figure 26). Between 1999 and 2008, the percentage of women 40 years of age and over who had a mammogram within the past 2 years decreased slightly, from 70% to 68% (9) (data table for Figure 26; Table 86).
Over time, mammography screening rates have improved among women in all racial and ethnic groups, but disparities persist (Figure 26). Between 1987 and 1991, compared with other racial and ethnic groups, non-Hispanic white women had the highest recent mammography rates. Starting in 1993, mammography rates among non-Hispanic black and non-Hispanic white women have been similar. In 2008, the percentage of non-Hispanic black and non-Hispanic white women with recent mammograms was higher than for Hispanic women (Figure 26). Low-income and uninsured women also experience disparities in mammography screening, having consistently lower screening rates compared with insured and higher-income women (10). Recent increases in screening among uninsured, low-income women, and improvements in disparities for some racial and ethnic populations, may be attributable in part to programs promoting screening in underserved populations, such as the National Breast Cancer and Early Detection Program (NBCCEDP) (10). Not all women are using mammography technology equally, and adequate access, provider prescription, English proficiency, and health literacy, as well as knowledge, attitudes, and cultural beliefs, may serve as barriers to mammography access and use (11). Despite gains in the use of mammography across racial and ethnic subgroups, there are persistent mortality differences by race that remain unexplained because, although mammography use is equivalent, mortality rates are not (5).
References
- 1.
- Yaffe MJ, Mainprize JG, Jong RA. Technical developments in mammography. Health Phys. 2008;95(5):599–611. [PubMed: 18849694]
- 2.
- Linton OW, Schauer DA. Mammography: Better, safer, and more effective? Radiology. 2006;240(1):3–5. [PubMed: 16793970]
- 3.
- Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–83. [PubMed: 16169887]
- 4.
- Nishikawa RM, Acharyya S, Gatsonis C, Pisano ED, Cole EB, Marques HS, et al. Comparison of soft-copy and hard-copy reading for full-field digital mammography. Radiology. 2009;251(1):41–9. [PMC free article: PMC2663585] [PubMed: 19332845]
- 5.
- Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987–2005). Cancer Epidemiol Biomarkers Prev. 2009;18(1):121–31. [PubMed: 19124489]
- 6.
- Mammography screening and new technologies [online] Breast Cancer Action. Available from: http://bcaction
.org/index .php?page=mammography-and-new-tech. - 7.
- American Cancer Society (ACS). Cancer facts and figures 2008. Atlanta, GA: ACS; 2008. Available from: http://www
.cancer.org /downloads/STT/2008CAFFfinalsecured.pdf. - 8.
- United States cancer statistics: 1999–2005. Cancer incidence and mortality data [online] CDC. 2009. Available from: http://www
.cdc.gov/uscs. - 9.
- Breen N, Cronin KA, Meissner HI, Taplin SH, Tangka FK, Tiro JA, McNeel TS. Reported drop in mammography: Is this cause for concern? Cancer. 2007;109(12):2405–9. [PubMed: 17503429]
- 10.
- Sabatino SA, Coates RJ, Uhler RJ, Breen N, Tangka FK, Shaw KM. Disparities in mammography use among US women aged 40–64 years, by race, ethnicity, income, and health insurance status, 1993 and 2005. Med Care. 2008;46(7):692–700. [PubMed: 18580388]
- 11.
- Peek ME, Han JH. Disparities in screening mammography: Current status, interventions, and implications. J Gen Intern Med. 2004;19(2):184–94. [PMC free article: PMC1492136] [PubMed: 15009798]
Joint Replacement Procedures
The hospital discharge rate for total hip replacement increased by one-third, and the discharge rate for knee replacement increased by 70%, from 1996 to 2006.
Hip and knee joint replacements are among the most commonly performed and clinically successful surgical procedures in the United States (1–3). The most common reasons for knee and hip replacement procedures are pain and decreased quality of life from osteoarthritis (2,4). With one-third of Americans obese (Table 72) and an aging population (Figure 1A and Figure 1B), the prevalence of osteoarthritis is expected to increase, contributing to a growing demand for joint replacement procedures (2,3). According to one analysis, by 2030 the demand for total hip replacements is estimated to increase by about 175% and the demand for total knee replacements is projected to grow sixfold (5).
Modern hip and knee replacement techniques using prosthetic devices were developed in the 1960s (4). Since then, better prosthetic materials have increased the functioning and life span of joint replacements. Advances in surgical techniques, including minimally invasive methods and the use of computer-assisted surgical systems, aim to reduce post-operative pain and recovery time and improve surgical accuracy (6,7).
Although the majority of joint replacement procedures are among older patients, longer-lasting joints make these procedures a viable option for younger and more active patients (6,7).
Hospital discharges with at least one knee or hip replacement procedure among adults 45 years of age and over increased from 1996 to 2006 (Figure 27). Total hip replacement discharges increased by one-third, partial hip replacements increased by 60%, and total knee replacement discharges increased by 70% over that time period. In 2006, total hip replacement rates were similar among men (18.1 discharges per 10,000 population) and women (20.5) and increased with age (data table for Figure 27). Discharges for partial hip procedures were about twice as common among women (23.9 per 10,000 for age 45 years and over) as men (13.0 per 10,000). Partial hip procedures, which are often used to treat fractures, were also more common among older persons.
In 2006, knee replacement discharges were more common among women 45 years of age and over (54.0 per 10,000) than men (34.9). As with hip replacement procedures, knee replacement discharges were more than three times as high for those 65 years of age and over (84.1), compared with those 45–64 years of age (25.7). Although total hip and knee replacement discharges were more common among adults 65 years and over compared with adults 45–64 years of age, they increased at a faster rate among the younger group (data table for Figure 27).
References
- 1.
- Centers for Medicare & Medicaid Services (CMS). Data compendium. 2008. Baltimore, MD: CMS; 2009. Available from: http://www
.cms.hhs.gov /DataCompendium/16 _2008_Data_Compendium.asp#TopOfPage. - 2.
- Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487–97. [PubMed: 15995115]
- 3.
- Wilson NA, Schneller ES, Montgomery K, Bozic KJ. Hip and knee implants: Current trends and policy considerations. Health Aff (Millwood). 2008;27(6):1587–98. [PubMed: 18997215]
- 4.
- Montin L, Leino-Kilpi H, Suominen T, Lepistö J. A systematic review of empirical studies between 1966 and 2005 of patient outcomes of total hip arthroplasty and related factors. J Clin Nurs. 2008;17(1):40–5. [PubMed: 18088258]
- 5.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5. [PubMed: 17403800]
- 6.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: Total hip replacement. Lancet. 2007;370(9597):1508–19. [PubMed: 17964352]
- 7.
- Branson JJ, Goldstein WM. Primary total hip arthroplasty. AORN J. 2003;78(6):947–74. [PubMed: 14692668]
Angioplasty and Coronary Stenting Procedures
Since their introduction in 2003, drug-eluting stents have rapidly displaced non-drug-eluting stents and are used in three-quarters of angioplasty discharges.
Many technological advances have been directed at preventing, diagnosing, and treating heart disease, the leading cause of death in the United States (Table 28). Examples include drugs (statins), imaging (computed tomography, CT), procedures (angioplasty), and devices (stents). For many people with coronary artery disease (CAD), a common form of heart disease, coronary artery revascularization may be needed. One procedure to treat CAD is percutaneous transluminal coronary angioplasty (PTCA), more commonly called angioplasty. In PTCA, narrowed (or stenotic) arteries are treated to improve blood flow and reduce blockage (1). Compared with coronary artery bypass surgery, another widespread treatment for CAD, PTCA is relatively noninvasive and reduces length-of-stay in the hospital, recovery time, and expense (also see Figure 36 and Table 103). Therefore, PTCA is generally preferable in patients for whom both procedures are an option (1).
PTCA was first introduced about 30 years ago. Since then, additional modifications, including the introduction of stents, have improved the procedure. First introduced in the 1980s, stents are mesh-like devices that are inserted into the artery during PTCA to expand the artery and prevent restenosis (recurrent plaque development). One complication of early stents was clotting (thrombosis) at the site of the stent. To address this complication, drug-eluting stents were approved in 2003. Drug-eluting stents release short-term medication to reduce the risk of clotting and have been found to be better than bare stents at preventing restenosis and, consequently, the need for revascularization (2).
Data from the National Hospital Discharge Survey were used to examine changes that have occurred in PTCA procedures since the introduction of stents, and in particular, the introduction of bare (non-drug-eluting) stents. Discharges with PTCA procedures were separated into those including a drug-eluting stent (starting with 2003 data), those including a bare stent, and those with no stent (data table for Figure 28). Between 1996 and 2006, the rate of discharges with any PTCA procedure among persons 45 years of age and over was fairly steady, while the rate for PTCA discharges without a stent declined by 84% (data table for Figure 28). The diffusion of stent insertion was fairly rapid. In 1996, almost two-thirds of PTCA discharges among persons 45 years of age and over did not include stent insertion, but by 2006 less than one-tenth of discharges had no type of stent. Further, there was swift adoption of the drug-eluting stent, replacing the insertion of a bare stent. In 2002, the year before the first drug-eluting stent was approved, 82% of PTCA discharges among person 45 years of age and over had a bare stent inserted. In 2004, the year after drug-eluting stents were approved, 69% of PTCA discharges had a drug-eluting stent inserted, and by 2006, 77% of PTCA discharges included a drug-eluting stent (data table for Figure 28).
The rate of discharges with PTCA, and consequently the rate of PTCA with stent insertion, varied by age and sex. In 2006, the rate of PTCA discharges among those 65 years of age and over (86.2 per 10,000 persons) was double that for patients 45–64 years of age (39.7 per 10,000 persons; Figure 28). PTCA discharges were about twice as likely among men 65 years of age and over compared with women in that age group, and about two-and-a-half times as likely among men 45–64 years of age than women (data table for Figure 28). The likelihood of receiving a drug-eluting stent among PTCA discharges did not vary by age or sex.
The series of events accompanying the use of drug-eluting coronary artery stents—their introduction, adoption, rapid diffusion, and subsequent reconsideration—is an example of the complexities of technological advancement in medicine. The dilemma is how to best target new technologies, given that they are often more expensive than older options and their impact on broader and more diverse population subgroups is not fully known until they are more widely used and studied over longer periods. Initial studies of the use of drug-eluting stents indicated they were better than bare stents at preventing restenosis. On the basis of this evidence, drug-eluting stents were quickly adopted and used in place of bare stents, regardless of patient characteristics. More recent studies, after the diffusion of drug-eluting stents, suggest that patients receiving drug-eluting stents may be at risk for developing thrombosis, often up to a year after their PTCA (3,4). As more data are obtained, evidence suggests that drug-eluting stents may be best targeted at certain population subgroups with coronary artery disease, such as older patients and those with diabetes.
References
- 1.
- Baim DS. Percutaneous coronary intervention. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Harrison’s principles of internal medicine. 17th ed. New York, NY: McGraw-Hill; 2008. pp. 1544–8.
- 2.
- Windecker S, Jüni P. The drug-eluting stent saga. Circulation. 2009;119(5):653–6. [PubMed: 19204314]
- 3.
- Cook S, Windecker S. Early stent thrombosis: Past, present, and future. Circulation. 2009;119(5):657–9. [PubMed: 19204315]
- 4.
- Jeremias A, Kirtane A. Balancing efficacy and safety of drug-eluting stents in patients undergoing percutaneous coronary intervention. Ann Intern Med. 2008;148(3):234–8. [PubMed: 18087049]
Cholecystectomy Procedures
In 2006, laparoscopic procedures accounted for almost all ambulatory surgery visits for cholecystectomy and about three-quarters of hospital discharges for cholecystectomy.
Cholecystectomy—removal of the gallbladder—is one of the most commonly performed procedures in the United States (1). Cholecystectomy may be performed because of cancer of the gallbladder or, more commonly, because of symptoms from gallstones. Gallstones are more common among women, persons who are obese, and during pregnancy, and prevalence increases with age (2).
Laparoscopic cholecystectomy was introduced in the late 1980s and quickly became the standard of care for patients with symptomatic gallstones (2,3). In the laparoscopic procedure, the gallbladder is removed through small incisions in the abdomen, rather than the larger incision used in traditional, or open, cholecystectomy. This technological advance means a reduction in pain, in risk of postoperative infection, in recovery time, and in health care costs (1,2,4,5). The success of laparoscopic cholecystectomy is widely believed to account for the increased number of laparoscopic procedures performed by making the procedure more available to high-risk, reluctant, or mildly symptomatic patients (2). As the safety and effectiveness of laparoscopic cholecystectomy has been demonstrated, there has been a shift in cholecystectomy procedures from inpatient to outpatient settings (5,6). The improvement in patient outcomes measures and reduction in health care costs associated with laparoscopic cholecystectomy, compared with open cholecystectomy, have led to the use of laparoscopic techniques in other abdominal surgical procedures, including bariatric surgery, esophageal procedures (including those for gastroesophageal reflux disorder), and appendectomy (3,7).
Data on hospital discharges from the National Hospital Discharge Survey, and on ambulatory surgery visits from the National Survey of Ambulatory Surgery, were examined to identify cholecystectomy discharges and visits. Between 1996 and 2006, there was a shift in cholecystectomy procedures from the inpatient to outpatient settings. The hospital discharge rate among adults 18 years of age and over with cholecystectomy procedures in hospitals declined about 20% from 1996 (22.3 discharges per 10,000 population) to 2006 (18.1), while ambulatory surgery procedure visits increased more than 30%, from 16.1 visits per 10,000 population in 1996 to 21.2 in 2006 (Figure 29A and Figure 29B). In both 1996 and 2006, almost all cholecystectomy visits in ambulatory surgery centers were for laparoscopic procedures. The proportion of adult hospital discharges that were laparoscopic increased from about 70% of discharges in 1996 to 77% in 2006.
Consistent with the rates of underlying gallbladder disease, hospital cholecystectomy discharge and ambulatory surgery visit rates are higher among women than men and among older men compared with younger men. Focusing on ambulatory surgery visits in 2006, the rate of laparoscopic cholecystectomies among women 18 years of age and over (34.2 visits per 10,000 population) was more than four times higher than among men (7.3) (data table for Figure 29). The rate of laparoscopic cholecystectomy visits among men 45 years of age and over (9.8 visits per 10,000 population) was double that of younger men (5.1 visits). The visit rate was similar for younger (34.1 visits) and older (34.2 visits) women.
References
- 1.
- Robinson TN, Biffl WL, Moore EE, Heimbach JK, Calkins CM, Burch JM. Predicting failure of outpatient laparoscopic cholecystectomy. Am J Surg. 2002;184(6):515–9. [PubMed: 12488152]
- 2.
- Jacobson IM. Gallstones. In: Friedman SL, McQuaid KR, Grendell JH, editors. Current diagnosis and treatment in gastroenterology. 2nd ed. New York, NY: Lange Medical Books/McGraw-Hill; 2003. pp. 772–83.
- 3.
- Scott-Conner CEH. Laparoscopic gastrointestinal surgery. Med Clin North Am. 2002;86(6):1401–22. [PubMed: 12510458]
- 4.
- Ellison EC, Carey LC. Lessons learned from the evolution of the laparoscopic revolution. Surg Clin North Am. 2008;88(5):927–41. [PubMed: 18790146]
- 5.
- Jones K, DeCamp BS, Mangram AJ, Dunn EL. Laparoscopic converted to open cholecystectomy minimally prolongs hospitalization. Am J Surg. 2005;190(6):888–90. [PubMed: 16307938]
- 6.
- Fiorillo MA, Davidson PG, Fiorillo M, D’Anna JA Jr, Sithian N, Silich RJ. 149 ambulatory laparoscopic cholecystecomies. Surg Endosc. 1996;10:52–6. [PubMed: 8711607]
- 7.
- Melman L, Matthews BD. Current trends in laparoscopic solid organ surgery: Spleen, adrenal, pancreas, and liver. Surg Clin North Am. 2008;88(5):1033–46. [PubMed: 18790153]
Upper Endoscopy and Colonoscopy
Between 1996 and 2006, outpatient upper endoscopy and colonoscopy rates increased substantially, while inpatient rates remained unchanged.
Medical technology has affected the diagnosis and treatment of a wide variety of gastrointestinal (GI) diseases and conditions through the development of endoscopic procedures (1). Because endoscopic technology allows the direct visual inspection of the interior of organs, tissue sampling and minimally invasive diagnostic and therapeutic interventions are possible. Previously, these types of diagnostic and therapeutic interventions would have required major invasive surgery. As endoscopic technology has progressed, there have been improvements in the clarity of the images and in the types of scopes (thinner, more flexible, and more comfortable), in addition to the development of additional uses for the scopes. Examples of current endoscopic interventions include cauterization of gastric bleeding, application of clips to stop gastric bleeding, insertion of high-frequency ultrasound devices that produce highly detailed images, removal of stones (e.g., gallstones), and insertion of stents, often as a palliative cancer therapy (1). In addition to clinical uses, endoscopic technology has influenced medical training by providing higher quality static images for textbooks and journals and online collections of endoscopy video clips (2).
During an upper endoscopy (or esophagogastroduodenoscopy (EGD)) procedure, an image of the esophagus, stomach, and duodenum (the first part of the small intestine) is transmitted through a thin, flexible, lighted tube called an endoscope (3). The procedure can be used to diagnose upper gastrointestinal conditions such as gastroesophageal reflux disease (GERD) and Barrett’s esophagus (a rarely premalignant condition of the esophagus). Colonoscopy is a lower endoscopy procedure used to see inside the colon and rectum (4). Colonoscopy can be used to diagnose lower GI conditions and diseases, in addition to screening for colon cancer. The U.S. Preventive Services Task Force strongly recommends (for individuals without high-risk intestinal conditions) colorectal cancer screening for men and women 50–75 years of age, and colonoscopy is one of the recommended screening methods (5).
Data from the National Hospital Discharge Survey (NHDS) and the National Survey of Ambulatory Surgery (NSAS) were examined for EGD and colonoscopy procedures (see Technical Notes for codes used). Between 1996 and 2006, outpatient EGD visit rates per 10,000 population increased substantially among all age groups of adults 18–84 years of age and remained stable among adults 85 years of age and over (Figure 30). In 2006, outpatient EGD visit rates among adults increased with age until age 65–74 and declined sharply among those 85 and over. In contrast to the growth in outpatient visit rates for EGD from 1996 to 2006, inpatient EGD rates among adults in all age groups generally remained similar to 1996 levels (data table for Figure 30).
Between 1996 and 2006, outpatient colonoscopy visit rates tripled overall among adults 18 years of age and over and increased substantially in each age group (Figure 30). In 2006, outpatient colonoscopy procedure rates among adults increased with age until age 65–74 and then declined. As was the case with EGD, inpatient colonoscopy rates in all age groups remained basically unchanged from 1996 levels (data table for Figure 30).
Factors associated with the growth in EGD include the availability of new medications to treat GERD (proton pump inhibitors (“the purple pill”)); factors for colonoscopy include increased use for cancer screening, a change in Medicare reimbursement policy in 2001 for screening asymptomatic adults, and increases for cancer surveillance following the removal of polyps or cancers (6–10).
References
- 1.
- Mallery S, Van Dam J. Advances in diagnostic and therapeutic endoscopy. Med Clin North Am. 2000;84(5):1059–83. [PubMed: 11026918]
- 2.
- The DAVE (Digital Atlas of Video Education). Project—Gastro-enterology [online]. 2009. Available from: http://daveproject
.org/ - 3.
- National Digestive Diseases Information Clearinghouse. Upper GI endoscopy. NIH pub no 09-4333. Bethesda, MD: National Institutes of Health; 2009. Available from: http://digestive
.niddk .nih.gov/ddiseases /pubs/upperendoscopy/index.htm. - 4.
- National Digestive Diseases Information Clearinghouse. Colonoscopy NIH pub no 09-4331. Bethesda, MD: National Institutes of Health; 2008. Available from: http://digestive
.niddk .nih.gov/ddiseases /pubs/colonoscopy/index.htm. - 5.
- US Preventive Services Task Force, Agency for Healthcare Research and Quality; 2008. Screening for colorectal cancer: Recommendation statement [online] Available from: http://www
.ahrq.gov/CLINIC /uspstf/uspscolo.htm. - 6.
- Ofman JJ. The economic and quality-of-life impact of symptomatic gastroesophageal reflux disease. Am J Gastroenterol. 2003;98(3 suppl):S8–S14. [PubMed: 12644026]
- 7.
- DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100(1):190–200. [PubMed: 15654800]
- 8.
- Imperiale TF, Sox HC. Guidelines for surveillance intervals after polypectomy: Coping with the evidence. Ann Intern Med. 2008;148(6):477–9. [PubMed: 18347353]
- 9.
- Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141(4):264–71. [PubMed: 15313742]
- 10.
- Prajapati DN, Saeian K, Binion DG, Staff DM, Kim JP, Massey BT, Hogan WJ. Volume and yield of screening colonoscopy at a tertiary medical center after change in Medicare reimbursement. Am J Gastroenterol. 2003;98(1):194–9. [PubMed: 12526957]
Geographic Variation in Use of Intensive Care Units in the Last 6 Months of Life
In 2005, use of intensive care units in the last 6 months of life among Medicare decedents ranged from 23% of Medicare decedents in Vermont and North Dakota to 49% in New Jersey and Florida.
Intensive care units (ICUs), which include specialized units such as medical, surgical, or coronary care units, are defined by the American Hospital Association as separate units of a hospital that provide services of a more intensive nature than usual medical and surgical care, on the basis of physicians’ orders and approved nursing care plans. Units are staffed with specially trained personnel and contain monitoring and specialized support equipment for patients who require intensified comprehensive observation and care (1). The first dedicated ICU was established at Baltimore City Hospital in 1958 (2).
Because ICUs are technology- and resource-intensive, they are more costly than routine hospital care (3). In 2000, critical care medicine provided in ICUs and other types of critical care units made up an estimated 13% of all hospital costs and 4% of national health expenditures (4). Guidelines issued by the Society of Critical Care Medicine and the American Thoracic Society state that “Because of the utilization of expensive resources, ICUs should, in general, be reserved for those patients with reversible medical conditions who have a ‘reasonable prospect of substantial recovery’” (5).
Between 1994 and 2004, ICU use per 1,000 Medicare beneficiaries increased 16%, from 59 to 69 discharges per 1,000 beneficiaries (3). By 2004, one-third of all Medicare hospitalizations included ICU or coronary care unit (CCU) care at some time during the hospital stay. An estimated one in five Americans dies during hospitalizations that include ICU or CCU care (6).
The Dartmouth Atlas Group has created a database that allows examination of geographic variation in the use of ICU/CCU services in the last 6 months of life among Medicare decedents. This analysis was limited to those 65–99 years of age. ICU/CCU care includes care provided in medical, surgical, trauma, burn, or other types of critical care units. Nationwide, 39% of older Medicare decedents had an ICU/CCU stay in the last 6 months of life. The percentage of older Medicare decedents admitted to an ICU/CCU in their last 6 months of life varied widely, from 23% in Vermont and North Dakota to 49% in New Jersey and Florida (6) (Figure 31).
It is not evident what drives this variation in use of ICU/CCU care across the country. In general, states with higher ICU use also had higher Medicare state per capita expenditures and higher overall utilization (7). Most variation in health care spending cannot be explained by prices, health status of the population, demographics, or treatment preferences. However, the supply of physicians and other health care resources, including the number of ICU beds, appears to be correlated with spending (7,8).
Use of ICU/CCU care is determined by supply, provider practice patterns and preferences, patient preferences, and case mix or “need” (7,8). It is difficult to define the population in need of ICU/CCU care by using claims data and to determine how much of the geographic variation is based on patient needs or patient or provider preferences. Physicians use their judgment as to whether critically ill patients would benefit from ICU services, and patients and their families should also participate in this decision (8,9). Patients with ultimately or rapidly fatal preexisting chronic disease are often admitted to the ICU before death, and research indicates that many patients and their families do not have informed discussions with physicians about palliative or end-of-life care, which may include alternatives to ICU/CCU care (8–10). Some research has indicated that the majority of academic medical ICUs in the United States do not strictly employ ICU admission and restriction guidelines, as recommended by the Society of Critical Care Medicine and the American Thoracic Society (11). Debate continues about the ethical and economic tradeoffs in deciding who should be treated in ICUs/CCUs and how to reduce unnecessary use, both to improve quality of care and to reduce overall health care expenditures (5,7,9).
References
- 1.
- American Hospital Association. AHA Hospital Statistics 2009. Chicago, IL: Health Forum LLC; 2008.
- 2.
- Tisherman SA, Darby J, Peitzman AB. The intensive care unit as a trauma unit. Surg Clin North Am. 2000;80(3):783–90. [PubMed: 10897260]
- 3.
- Milbrandt EB, Kersten A, Rahim MT, Dremsizov TT, Clermont G, Cooper LM, et al. Growth of intensive unit resource use and its estimated cost in Medicare. Crit Care Med. 2008;36(9):2504–10. [PubMed: 18679127]
- 4.
- Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985–2000: An analysis of bed numbers, use, and costs. Crit Care Med. 2004;32(6):1254–9. [PubMed: 15187502]
- 5.
- Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Guidelines for intensive care unit admission, discharge, and triage. Crit Care Med. 1999;27(3):633–8. [PubMed: 10199547]
- 6.
- Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, Rubenfeld GD. Use of intensive care at the end of life in the United States: An epidemiologic study. Crit Care Med. 2004;32(3):638–43. [PubMed: 15090940]
- 7.
- Fisher E, Goodman D, Skinner J, Bronner K. Health care spending, quality, and outcomes: More isn’t always better. Dartmouth Atlas Project Topic Brief. Feb, 2009. Available from: http://www
.dartmouthatlas .org/atlases/Spending_Brief_022709 .pdf. [PubMed: 36454934] - 8.
- Barnato AE, Herndon MB, Anthony DL, Gallagher PM, Skinner JS, Bynum JPW, Fisher ES. Are regional variations in end-of-life care intensity explained by patient preferences? A study of the U.S. Medicare population. Med Care. 2007;45(5):386–93. [PMC free article: PMC2147061] [PubMed: 17446824]
- 9.
- Rady MY, Johnson DJ. Admission to intensive care unit at the end-of-life: Is it an informed decision? Palliat Med. 2004;18(8):705–11. [PubMed: 15623167]
- 10.
- Virnig BA. Toward a better understanding of the role of geography in intensity of end-of-life care: Must we first come to an understanding of end-of-life care? Med Care. 2007;45(5):374–6. [PubMed: 17446822]
- 11.
- Walter KL, Siegler M, Hall JB. How decisions are made to admit patients to medical intensive care units (MICUs): A survey of MICU directors at academic medical centers across the United States. Crit Care Med. 2008;36(2):414–20. [PubMed: 18091539]
Solid Organ Transplantation
Between 1997 and 2006, the number of new kidney and liver transplantations per 1 million population increased, while heart transplantations decreased.
Solid organ transplantation is the epitome of highly technological care: replacing failing organs with organs from living and deceased donors. Although many attempts were made to transplant tissues and organs prior to the 1980s (Figure 23), success was limited because in most cases the recipient’s immune system rejected incompatible donor organs and tissues. It was not until advances in the science of tissue typing and matching, and suppression of the host’s immune system to reduce transplant rejection, that transplantation became more common and successful (1).
In 1983, the U.S. Food and Drug Administration approved the first highly effective immunosuppressant, cyclosporine, a calcineurin inhibitor (1). Following the addition of cyclosporine to recipient’s drug regimens, 1-year graft survival rates for kidney transplantation exceeded 89%, and 1-year graft survival rates for heart and liver transplantations exceeded 70%. Prior to cyclosporine introduction, 1-year graft survival rates for all organ transplantations were significantly lower (1). For more than two decades, the core immunosuppression regimen for most organs has been based on the two-drug combination of a calcineurin inhibitor and a steroid, with the optional addition of an antiproliferative agent (traditionally azathioprine). In recent years, there has been a clear transition from cyclosporine to a newer calcineurin inhibitor, tacrolimus, for most organ recipients (with the exception of intestine and heart recipients). Similarly, azathioprine has been almost universally replaced by one of the newer antiproliferative versions of mycophenolate. The most common discharge regimen now is a triple-drug protocol of tacrolimus, mycophenolate mofetil, and steroid, providing even further improvement in graft survival rates. In addition, many programs have begun protocols aimed at reducing or eliminating steroids, in hopes of minimizing the well-recognized, debilitating long-term complications of this powerful drug (2).
Numerous technological advances have occurred in the field of organ transplantation. Advances in tissue and organ procurement include improved methods of obtaining multiple organs from a single donor and improved technologies allowing organs to be shared among previously incompatible recipients (3). Organ preservation and transportation have evolved to provide more high-quality organs that are less likely to be immediately rejected (3). Immunosuppressant drugs have become more effective and less toxic (3). Some types of organs can now be donated by both living and deceased donors (4). Technological innovations in surgical techniques have included new types of procedures and laparoscopic retrieval of organs or partial organs, which facilitates the donation process with a safer operation and more rapid recovery for the living donor. Despite these advances, the gap between the limited supply of donated organs and the burgeoning waiting list continues to widen every year, so more patients are dying while waiting for a transplant (4).
The U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients contain data regarding every solid organ donation and transplant event occurring in the United States since 1987. Solid organs include the heart, kidney, liver, pancreas, lung, and intestine. In 2006, there were approximately 28,000 solid organ transplantations in the United States, an increase from 20,000 in 1997 (data table for Figure 32). In 2006, 7% of transplant recipients were under 18 years of age, 39% were age 18–49 years, 42% were age 50–64 years, and 12% were 65 years of age and over (4).
Between 1997 and 2006, the rate of kidney transplantation increased 31% (Figure 32). In 2006, there were 16,600 new kidney transplantations, accounting for 59% of all solid organ transplantations (data table for Figure 32). Nearly 40% of kidney transplantations were from living donors in 2006 (4).
The rate of liver transplantation increased 42% during this same period (Figure 32). Liver transplantation was the second most common form of solid organ transplantation in 2006 (6,100), accounting for 22% of all solid organ transplantations (data table for Figure 32). In 2006, 5% of liver transplantations were from living donors (4).
Between 1997 and 2004, the rate of heart transplantation declined 20% and then increased slightly in the next 2 years (Figure 32). In 2006, heart transplantation was the third most common form of solid organ transplantation, accounting for 8% (2,100) of all solid organ transplantations (data table for Figure 32). The number of patients awaiting a heart transplantation has decreased steeply since 2000, likely reflecting improvements in medical therapy that have reduced the need for transplantation (4).
Organ transplantation and immunosuppressant drugs are extremely costly. Estimates from the Healthcare Cost & Utilization Project database show that the average cost of a hospital stay for a heart transplant in 2006 was about $114,000; for a kidney transplant about $44,000; and for a liver transplant about $92,000. These estimates do not include any pre- or postoperative visits or treatments (5). The average annual cost of immunosuppression drugs has been estimated at $11,000 and can reach over $20,000 (6).
Organ transplantation has also raised ethical, legal, and resource-allocation issues. It has raised questions about the clinical definition of death and when organs can ethically be removed from donors (1). Another issue is eligibility for transplanted organs; for example, whether people with comorbid conditions or a poor prognosis should receive scarce organs. Other ethical issues include prioritization of organ allocation, living donor transplantation, and quality of life for living donors (7–10).
References
- 1.
- Linden PK. History of solid organ transplantation and organ donation. Crit Care Clin. 2009;25(1):165–84. [PubMed: 19268801]
- 2.
- Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2006. 2005 Annual report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant data 1995–2004 [online] Available from: http://www
.ustransplant .org/annual_reports /archives/2005/default.htm. - 3.
- Starzl TE. History of clinical transplantation. World J Surg. 2000;24(7):759–82. [PMC free article: PMC3091383] [PubMed: 10833242]
- 4.
- Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2008. 2007 Annual report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant data 1997–2006 [online] Available from: http://www
.ustransplant .org/annual_reports/current/default .htm. - 5.
- HCUPnet [online] Healthcare Cost & Utilization Project online query system. Agency for Healthcare Research and Quality; Available from: http://hcupnet
.ahrq.gov/ - 6.
- Immunosuppressive drug fact sheet. Legislative fact sheet. American Society of Transplant Surgeons. 2007. Available from: http://www
.asts.org/Tools/Download .aspx?fid=494. - 7.
- Charpentier KP, Mavanur A. Removing patients from the liver transplant wait list: A survey of U.S. liver transplant programs. Liver Transpl. 2008;14(3):303–7. [PubMed: 18306339]
- 8.
- Brown RS Jr. Live donors in liver transplantation. Gastroenterology. 2008;134(6):1802–13. [PMC free article: PMC2654217] [PubMed: 18471556]
- 9.
- Hunt SA, Haddad F. The changing face of heart transplantation. J Am Coll Cardiol. 2008;52(8):587–98. [PubMed: 18702960]
- 10.
- Abbasoglu O. Liver transplantation: Yesterday, today and tomorrow. World J Gastroenterol. 2008;14(20):3117–22. [PMC free article: PMC2712841] [PubMed: 18506914]
Assisted Reproductive Technology (ART)
Between 1996 and 2006, the number of assisted reproductive technology (ART) cycles more than doubled and increased at the fastest rate among women over age 40.
Since 1978, assisted reproductive technology (ART) procedures have been used in the United States to overcome infertility. The first U.S. infant conceived using ART was born in 1981, and pregnancy rates using ART have shown continuous improvement with each year (1,2). In 2002, 12% of women of childbearing age (15–44 years) reported having an infertility-associated health care visit at some time in their lives, and according to birth certificate data, more than 1% of infants born in the United States were conceived using ART in 2006 (1,3,4). Although there is some controversy about whether the proportion of the population with self-reported infertility is increasing, stable, or decreasing, the utilization of ART has been increasing (5) (Figure 33).
The CDC definition of ART includes fertility treatments in which both eggs and sperm are handled in the laboratory for the purpose of establishing a pregnancy and excludes artificial (intrauterine) insemination or the use of fertility drugs without egg retrieval. ART involves surgically removing eggs from a woman’s ovaries, combining them with sperm in the laboratory, and returning them to the woman’s body or donating them to another woman. ART procedures are described in terms of cycles because ART services are performed in a series of several steps, over an interval of 2 weeks (4). A woman may have multiple cycles of treatment in 1 year. Types of ART treatment include in vitro fertilization, gamete intrafallopian transfer, and zygote intrafallopian transfer. In 2006, over 99% of all ART procedure cycles were in vitro fertilization treatments (4). ART procedures include fresh or frozen and nondonor or donor eggs or embryos.
The Fertility Clinic Success Rate and Certification Act of 1992 requires that fertility clinics publish their success rates and patient and treatment characteristics. Two of the ART success rates reported by CDC include the percentage of pregnancies per ART cycle and the percentage of live births (singleton only or singleton/multiple) per ART cycles initiated each year (4). In 2006, 30% of ART cycles resulted in a live-birth delivery (4).
The total number of ART cycles initiated doubled from 1996 to 2006 (data table for Figure 33). In 2006, 39% of ART cycles were initiated among women under 35 years of age, another 41% among women 35–40 years, and 19% among women 41 years of age and over (data table for Figure 33).
A woman’s age is an important factor associated with the chances of a live birth after ART (2,4). In 2006, 39% of ART cycles initiated in women under 35 years of age using fresh nondonor eggs or embryos resulted in a live birth, compared with 4% for women over 42 years (4). The growth in the number of ART cycles in women over age 40 has increased at a faster rate on average (11% per year) between 1996 and 2006 than the number of cycles in women 35–40 years of age (8% per year) and those under 35 years (7% per year) (Figure 33). This greater growth in the number of ART cycles among women over 40 may reflect in part a societal shift toward older motherhood (also see Table 4).
References
- 1.
- Wright VC, Chang J, Jeng G, Macaluso M. Assisted reproductive technology surveillance—United States, 2005. Surveillance Summaries. 2008 Jun 20; [PubMed: 18566567]
MMWR. 2008. pp. 1–23. Available from: http://www.cdc.gov/mmwr /preview/mmwrhtml/ss5705a1 .htm?s_cid=ss5705a1_e. - 2.
- Van Voorhis BJ. Outcomes from assisted reproductive technology. Obstet Gynecol. 2006;107(1):183–200. [PubMed: 16394060]
- 3.
- Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Vital Health Stat. Hyattsville, MD: NCHS; 2005. Fertility, family planning, and reproductive health of U.S. women: Data from the 2002 National Survey of Family Growth. Available from: http://www
.cdc.gov/nchs /data/series/sr_23/sr23_025.pdf. [PubMed: 16532609] - 4.
- CDC, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. Atlanta: CDC; 2006 . 2008. Assisted reproductive technology success rates: National summary and fertility clinic reports. Available from: http://www
.cdc.gov/ART /ART2006/508PDF/2006ART.pdf. - 5.
- Myers ER, McCrory DC, Mills AA, Price TM, Swamy GK, Tantibhedhyangkul J, et al. Evidence report/technology assessment no 167. AHRQ pub no 08–012. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Effectiveness of assisted reproductive technology. Available from: http://www
.ahrq.gov/downloads /pub/evidence /pdf/infertility/infertility.pdf.
Prescription Drugs
The use of statin drugs increased almost 10-fold from 1988–1994 to 2003–2006; during the same time period, the use of antidiabetic drugs increased by 50%.
Some of the most important medical advances have been the development and introduction of pharmacological treatments. These include the introduction of aspirin (1899), insulin (1922), penicillin (1942), and acetaminophen (1951) (Figure 23). Two important classes of drugs—antidiabetic and cholesterol-lowering statins—have continued this pattern of technological advancement.
Diabetes is a group of conditions in which insulin is not adequately secreted or utilized. Long-term complications of high glucose levels and diabetes include cardiovascular disease, renal failure, nerve damage, and retinal damage (1,2). The two most common forms of diabetes are Type 1 and Type 2. Type 1 diabetes, affecting an estimated 1 million Americans, is an autoimmune disorder in which insulin-producing cells in the pancreas are destroyed and, therefore, adequate insulin is not produced. Type 2 diabetes—which affects about 16 million Americans—is characterized by the body’s resistance to the effects of insulin (1,2). Diabetes may affect persons of all ages, although prevalence increases with age. Typically, Type 1 diabetes is diagnosed among children and young adults. In the past two decades, Type 2 diabetes has been reported among U.S. children and adolescents with increasing frequency. It is estimated that almost 200,000 persons 20 years of age and younger have been diagnosed with Type 1 or Type 2 diabetes (3). In 2003–2006, 2.5% of persons 20–39 years of age had diagnosed or undiagnosed diabetes, compared with 22.9% of adults 60 years and over (Table 51). Treatment guidelines for diabetes recommend dietary modifications, physical activity, weight loss (if overweight), and the use of needed medications (2,4).
New and emerging technologies have made it easier for people with diabetes to manage their disease. For years, people could only check their glucose levels by testing urine—a method that recognized high, but not dangerously low, glucose levels and reflected past, not current, glucose levels (5). In the 1960s, the first meter to measure glucose in the blood was invented (6). By the 1980s, blood glucose meters were widely used and, with further improvements, remain so today. Improved technology came in the form of a continuous glucose monitor, which was first approved by the FDA in 1999 (7). The new technology enables people with diabetes to monitor their blood glucose levels continuously, rather than just a few times per day.
The key drug treatment for Type 1 diabetes is the use of insulin (2,4). In the 1970s, the invention of the insulin pump gave people another way to administer insulin besides self-injection. Insulin pumps are small, pager-sized machines that can deliver insulin to patients continuously in a small basal amount and provide larger boluses when needed, such as at mealtime. In addition, for many decades people had to use insulin derived from animals. The biotechnology revolution led to the production of biosynthetic human insulin (8). Since that time, other improved forms of insulin have been developed, such as long-lasting insulin for treating both forms of diabetes (9). Persons with Type 2 diabetes are often treated with oral antidiabetic medications and, in some cases, with insulin (4). The first oral antidiabetic medication was introduced in 1956, providing Type 2 diabetics with an alternative to insulin (10).
The increase in the use of antidiabetic drugs over time mirrors the increase in diagnosed diabetes. In 1988–1994, 10% of adults 45 years of age and over had been diagnosed by their physician with diabetes. By 2003–2006, this had grown to 13% (11) (also see Table 51). The use of antidiabetic drugs by adults 45 years and over increased about 50%, from 7% in 1988–1994 to 11% in 2003–2006 (data table for Figure 34). In 2003–2006, adults 65 and over were significantly more likely to take antidiabetic drugs than adults 45–64 years, reflecting differences in diabetes rates by age (also see Table 51). Consistent with the prevalence of diagnosed diabetes, there were no differences in the use of antidiabetic drugs by sex (11) (Figure 34).
High cholesterol—particularly elevated levels of low density lipoprotein (LDL) cholesterol—is a risk factor for heart disease. Cholesterol levels may be reduced by dietary modifications, increased physical activity, and the use of medications (12). Studies in the 1980s demonstrated that some drugs were effective at lowering cholesterol (13,14), but there was no widespread acceptance of the value of drug therapy to lower cholesterol, and questions lingered about whether lowering cholesterol reduced mortality from heart disease (14,15). In 1987, the first statin drug (also known as HMG–CoA reductase inhibitor) to lower cholesterol was marketed in the United States (16) (Figure 23). Other statin drugs soon followed. Statin drugs lowered cholesterol levels significantly, and studies demonstrated that statin therapy reduced the incidence of coronary artery disease and deaths from heart disease (13,14). These findings helped gain acceptance for the use of cholesterol-lowering drugs. Although there are four classes of cholesterol-lowering drugs (14,15), statins have become the drug class of choice to lower cholesterol levels because of their demonstrated efficacy and safety (14,17).
From 1988–1994 to 2003–2006, the use of statin drugs by adults 45 years of age and over increased almost 10-fold, from 2% to 22% (data table for Figure 34; see Technical Notes for the specific drugs included in this analysis). There was a concurrent decline in the percentage of Americans with high cholesterol over this time period, largely attributable to increased use of cholesterol-lowering medications, especially statins (11,18) (also see Table 69). Regardless of age category, both men and women 45 years and over saw increases in statin drug use and declines in high cholesterol. However, women 65 years and over were more likely to have high cholesterol in 2003–2006 (22%) than older men (10%) (11) but had lower use of statin drugs (33% of women compared with 39% of men; Figure 34). The higher cholesterol levels among older women may be due to hormonal changes after menopause and because women often have higher levels of high-density lipoprotein (HDL), a component of total cholesterol (18,19).
References
- 1.
- Beers MH, editor. The Merck manual of medical information. 2nd ed. Whitehouse Station, NJ: Merck Research Laboratories; 2003. home ed. Available from: http://www
.merck.com /mmhe/sec13/ch165/ch165a .html?qt=diabetes&alt=sh. - 2.
- Masharani U. Diabetes mellitus and hypoglycemia. In: McPhee SJ, Papadakis MA, editors. Current medical diagnosis and treatment. 48th ed. New York, NY: McGraw-Hill; 2009. pp. 1052–94.
- 3.
- CDC. Atlanta, GA: CDC; 2008. National diabetes fact sheet: General information and national estimates on diabetes in the United States, 2007. Available from: http://www
.cdc.gov/diabetes /pubs/pdf/ndfs_2007.pdf. - 4.
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(1 suppl):S12–S51.
- 5.
- Free AH, Adams EC, Kercher ML, Free HM, Cook MH. Simple specific test for urine glucose. Clin Chem. 1957;3(3):163–8. [PubMed: 13437464]
- 6.
- Clemens AH, inventor. Miles Laboratories, Inc. Reflectance meter. 3,604,815. U.S. patent. 1971 September 14; assignee.
- 7.
- June 1999 PMA approvals [online] U.S. Food and Drug Administration. 1999. Available from: http://www
.fda.gov/MedicalDevices /ProductsandMedicalProcedures /DeviceApprovalsandClearances /PMAApprovals/ucm114805.htm. - 8.
- Goeddel DV, Kleid DG, Bolivar F, Heyneker HL, Yansura DG, Crea R, et al. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci USA. 1979;76(1):106–10. [PMC free article: PMC382885] [PubMed: 85300]
- 9.
- Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23(5):639–43. [PubMed: 10834423]
- 10.
- Teuscher A. Insulin: A voice for choice . New York, NY: Karger; 2007.
- 11.
- CDC/NCHS. National Health and Nutrition Examination Survey, unpublished analysis.
- 12.
- NIH pub no 01–3670. Bethesda, MD: National Heart, Lung, and Blood Institute, National Institutes of Health; 2001. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Executive summary. Available from: http://www
.nhlbi.nih .gov/guidelines/cholesterol/atp3xsum.pdf. - 13.
- Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999;341(7):498–511. [PubMed: 10441607]
- 14.
- Steinberg D, Gotto AM Jr. Preventing coronary artery disease by lowering cholesterol levels: Fifty years from bench to bedside. JAMA. 1999;282(21):2043–50. [PubMed: 10591387]
- 15.
- LaRosa JC. Unresolved issues in early trials of cholesterol lowering. Am J Cardiol. 1995;76(9):5C–9C. [PubMed: 7572687]
- 16.
- Junod SW. Statins: A success story involving FDA, academia and industry [online] U.S. Food and Drug Administration. 2007. Available from: http://www
.fda.gov/AboutFDA /WhatWeDo/History /ProductRegulation /SelectionsFromFDLIUpdateSeriesonFDAHistory/ucm082054.htm. - 17.
- Evans M, Roberts A, Davies S, Rees A. Medical lipid-regulating therapy: Current evidence, ongoing trials and future developments. Drugs. 2004;64(11):1181–96. [PubMed: 15161326]
- 18.
- Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294(14):1773–81. [PubMed: 16219880]
- 19.
- Schober SE, Carroll MD, Lacher DA, Hirsch R. NCHS data brief. 2. Hyattsville, MD: NCHS; 2007. High serum total cholesterol—An indicator for monitoring cholesterol lowering efforts: U.S. adults, 2005–2006. [PubMed: 19389314]
Highly Active Antiretroviral Therapy (HAART)
The introduction of highly active antiretroviral therapy (HAART) led to substantial declines in mortality from HIV disease, including a 65% decline in HIV disease mortality among males from 1995 to 1997.
During the late 1980s, human immunodeficiency virus (HIV) disease, as well as the associated acquired immunodeficiency syndrome (AIDS), emerged as a leading cause of death among adults 25–44 years of age in the United States (1). Although rates for most other leading causes of death for adults 25–44 years of age declined or remained stable during the 1980s and early 1990s, the death rate for HIV disease among this age group steadily increased (2). During the early years of HIV, there were few treatment options for those living with HIV other than palliative care and the management of opportunistic infections, and mortality was high (3,4). Soon the virus was identified, and a blood test to detect the virus was developed (3,5). The first medication to treat HIV disease— zidovudine (AZT)—was approved in 1987 (4) (Figure 23). AZT was followed by the introduction of other antiretroviral drugs. About 20 drugs, in four classes, have been developed to control HIV disease (4–7).
Initially, researchers and clinicians thought that HIV disease could be controlled with the use of one or two antiretroviral drugs (4,5,8), but mortality and morbidity rates remained high with this treatment approach. The health of individuals living with HIV improved dramatically when clinicians began to treat individuals with a combination of three or more antiretroviral drugs that act at different stages of the HIV life cycle (4,5,8,9). These regimens of proven combinations of drugs are known as highly active antiretroviral therapy (HAART). HAART allows clinicians the flexibility to change the regimen for each patient as the course of their disease and the complex nature of HIV warrant (9,10). After HAART became the standard of care in 1996 (5–7,11), there were marked reductions in morbidity and mortality associated with HIV disease (3,5,10,12). HAART has significantly improved the prognosis of those with HIV disease (Table 48) by reducing the severity and range of opportunistic infections, thereby reducing hospital admissions (9) (Table 101). For many with access to HAART, HIV is now regarded as a chronic manageable disease (6,9).
The success of HAART is demonstrated by the sharp decline in death rates from HIV disease after HAART’s adoption as the standard of care in 1996. From 1987 to 1995 (pre-HAART), HIV mortality increased sharply (Figure 35). From 1995 (pre-HAART) to 1997 (widespread HAART use), the death rate from HIV disease among males declined by two-thirds, from 27.3 deaths per 100,000 population in 1995 to 9.6 in 1997 (data table for Figure 35). Declines in HIV death rates were also observed for females and all racial and ethnic groups. The rate of decline from 1995 to 1997 ranged from 44% for black females to 73% for non-Hispanic white males (data table for Figure 35). These differences in mortality declines by racial and ethnic group and sex reflect differences in access to and use of HAART (10,12). After 1997, the rate of decline for HIV mortality slowed across all groups (Figure 35).
In 2006, gender and racial and ethnic differences in HIV mortality persisted (Figure 35). Some research focusing on access to HAART therapy suggests that Hispanic and black persons are less likely to have access to and utilize HAART treatment than non-Hispanic white persons, and women are less likely to have access to and utilize HAART treatment compared with men (10,13,14).
References
- 1.
- CDC. Current trends mortality attributable to HIV infection/AIDS—United States, 1981–1990. MMWR . 1991. pp. 41–4. Available from: http://www
.cdc.gov/mmwr /preview/mmwrhtml/00001880.htm. [PubMed: 1898757] - 2.
- CDC. Mortality attributable to HIV infection/AIDS among persons aged 25–44 years—United States, 1990, 1991. MMWR. 1993. pp. 481–6. Available from: http://www
.cdc.gov/mmwr /preview/mmwrhtml/00021017.htm. [PubMed: 8510635] - 3.
- Fauci AS. Twenty-five years of HIV/AIDS [editorial] Science. 2006;313(5786):409. [PubMed: 16873613]
- 4.
- Sepkowitz KA. AIDS—The first 20 years. N Engl J Med. 2001;344(23):1764–72. [PubMed: 11396444]
- 5.
- Weiss RA. Special anniversary review: Twenty-five years of human immunodeficiency virus research: Successes and challenges. Clin Exp Immunol. 2008;152(2):201–10. [PMC free article: PMC2384092] [PubMed: 18373700]
- 6.
- Wynn GH, Zapor MJ, Smith BH, Wortmann G, Oesterheld JR, Armstrong SC, Cozza KL. Antiretrovirals, part I: Overview, history, and focus on protease inhibitors. Psychosomatics. 2004;45(3):262–70. [PubMed: 15123854]
- 7.
- Zapor MJ, Cozza KL, Wynn GH, Wortmann GW, Armstrong SC. Antiretrovirals, part II: Focus on non-protease inhibitor antiretrovirals (NRTIs, NNRTIs, and fusion inhibitors). Psychosomatics. 2004;45(6):524–35. [PubMed: 15546830]
- 8.
- Weston R, Portsmouth S, Benzie A. An update on HAART: Part 1. Pharm J. 2006;276:631–4.
- 9.
- Weston R, Portsmouth S, Benzie A. An update on HAART: Part 2. Pharm J. 2006;276:693–6.
- 10.
- Ghani AC, Donnelly CA, Anderson RM. Patterns of antiretroviral use in the United States of America: Analysis of three observational databases. HIV Med. 2003;4(1):24–32. [PubMed: 12534956]
- 11.
- CDC. Epidemiology of HIV/AIDS—United States, 1981–2005. MMWR . 2006. pp. 589–92. Available from: http://www
.cdc.gov/mmwr /preview/mmwrhtml/mm5521a2.htm. [PubMed: 16741494] - 12.
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer JF, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–60. [PubMed: 9516219]
- 13.
- Lemly DC, Shepherd BE, Hulgan T, Rebeiro P, Stinnette S, Blackwell RB, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;199(7):991–8. [PMC free article: PMC7822728] [PubMed: 19220139]
- 14.
- Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38(1):96–103. [PubMed: 15608532]
Costs for Hospitalizations With Procedures
Aggregate national costs adjusted for inflation for hospitalizations with five out of the six most costly hospital procedures have increased substantially since 1999.
Advances in technology contribute to overall health care costs and expenditures. In 2007, $697 billion was spent for care in hospitals—where the most complex procedures are performed and the most complex technologies used— representing 37% of personal health care expenditures in that year (data table for Figure 21). In 2006, almost two-thirds of hospital discharges among adults had at least one procedure performed during the stay (Table 103), and almost all procedures require some type of medical technology.
Using data from the Healthcare Cost & Utilization Project, Nationwide Inpatient Sample, Figure 36 shows the costs for hospital discharges with the six principal procedures that contributed the most to aggregate national hospital costs in 2006. The costs shown were for the entire hospital stay, not just the cost of performing the principal procedure (see Technical Notes for information on how costs are derived). Principal procedures were identified using Clinical Classification Software, which combines relevant International Classification of Diseases, ninth revision, Clinical Modification (ICD–9–CM) procedure codes into meaningful groups (1) (see Technical Notes).
The principal procedure contributing the most to national hospital costs in 2006 was respiratory intubation and mechanical ventilation (Figure 36). This technology provides machinery that breathes for patients when they cannot breathe on their own for a variety of medical reasons, or for administering anesthesia during surgery (2). Virtually all (98%) hospital discharges in 2006 with a principal diagnosis of respiratory intubation and mechanical ventilation were for medical reasons and were not associated with an operating room procedure (3). The number of hospital discharges in which respiratory intubation and mechanical ventilation was the principal procedure increased from 548,000 in 1999 to 712,000 in 2006 (data table for Figure 36). Hospital discharges with this principal procedure were estimated to have hospital costs of approximately $15.7 billion dollars in 2006, an increase of almost 50% since 1999 (in 2006 dollars) (Figure 36). Respiratory intubation and mechanical ventilation have been estimated to contribute an extra $1,500 per day (in 2002) to the cost for an intensive care unit stay (4).
In 2006, the patient died during 26% of the stays with a principal procedure of respiratory intubation and mechanical ventilation (5).
Heart disease is the leading cause of death in the United States (Table 28), and the next three most expensive principal procedures are all cardiac-related. During percutaneous transluminal coronary angioplasty (PTCA) procedures, narrowed (or stenotic) arteries are treated by means of a catheter with a balloon tip, to reduce blockages and improve blood flow. Stents are inserted during most PTCA procedures (6) (also see Figure 28). Cardiac pacemakers, cardioverters, and defibrillators are medical devices inserted to regulate heart rate or rhythm (7). Coronary artery bypass graft (CABG) procedures are used when less invasive PTCA cannot be performed or is not medically indicated and involve bypassing a blocked artery or arteries with a blood vessel taken from another part of the body (6).
The number of hospital discharges with PTCA as the principal procedure increased steadily since 1999, from 502,000 to 828,000 in 2006 (a 65% increase), and inflation-adjusted national hospital costs associated with PTCA discharges increased 108% (Figure 36). Hospitalizations with cardiac pacemaker, cardioverter, or defibrillator as the principal procedure increased 64% during the period, while inflation-adjusted aggregate costs increased 147%. In contrast to PTCA hospitalizations, which increased substantially during the period, hospitalizations with CABG as the principal procedure decreased by 24%. However, aggregate costs for CABG hospitalizations declined only by 3%, and hospitalizations with these procedures ranked as the fourth most expensive in terms of aggregate hospital costs in 2006.
The next two principal procedures with the highest contribution to national hospital costs in 2006 were orthopedic in nature: knee arthroplasty (or knee replacement) (also see Figure 27) and spinal fusion. During knee arthroplasty procedures, part of or the entire knee joint is replaced by a prosthesis (8). Technologies associated with knee replacement procedures have evolved over time through improved prosthetic materials and surgical techniques (8). The number of hospital discharges with knee arthroplasty procedures increased 76% from 311,000 in 1999 to 547,000 in 2006, and inflation-adjusted national hospital costs associated with knee replacement discharges have increased 122% since 1999 (Figure 36).
Spinal fusion surgery uses bone grafts, with or without screws, plates, cages, or other devices, to stabilize the back by joining together vertebrae or spinal bones (9). This surgery is commonly performed in conjunction with removal of a herniated disk. The efficacy of spinal fusion for the most common indication (degenerative disk disease) remains unclear, and there is concern that rising procedure rates are being driven by technological advances (improved anesthesia, imaging, types of prosthetics and devices) and financial incentives (10). Hospital discharges with spinal fusion as the principal procedure increased 82% during the period, while aggregate costs increased 189% in 2006 dollars (Figure 36).
References
- 1.
- Clinical Classifications Software (CCS) for ICD–9–CM [online]. Agency for Healthcare Research and Quality, Healthcare Cost & Utilization Project (HCUP); Available from: http://www
.hcup-us.ahrq .gov/toolssoftware/ccs/ccs.jsp. - 2.
- Gehlbach BK, Hall J. Overview of mechanical ventilation [online]. Merck Manuals Online Medical Library; 2007. Available from: http://www
.merck.com /mmpe/print/sec06/ch065/ch065b.html. - 3.
- Agency for Healthcare Research and Quality (AHRQ). Healthcare Cost & Utilization Project (HCUP), unpublished analysis. [PubMed: 21413206]
- 4.
- Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–71. [PubMed: 15942342]
- 5.
- HCUPnet [online] Healthcare Cost & Utilization Project online query system. Agency for Healthcare Research and Quality; Available from: http://hcupnet
.ahrq.gov/ - 6.
- Michaels AD, Chatterjee K. Angioplasty versus bypass surgery for coronary artery disease. Circulation. 2002;106(23):e187–e190. [PubMed: 12460885]
- 7.
- Medline Plus . National Institutes of Health, National Library of Medicine; 2009. Pacemakers and implantable defibrillators [online] Available from: http://www
.nlm.nih.gov /medlineplus/pacemakersandimplantabledefibrillators.html. - 8.
- Medline Plus . National Institutes of Health, National Library of Medicine; 2009. Knee joint replacement [online] Available from: http://www
.nlm.nih.gov /medlineplus/ency/article/002974.htm. - 9.
- Medline Plus . National Institutes of Health, National Library of Medicine; 2009. Spinal fusion [online] Available from: http://www
.nlm.nih.gov /medlineplus/ency/article/002968.htm. - 10.
- Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery—The case for restraint. N Engl J Med. 2004;350(7):722–6. [PubMed: 14960750]
Figures
Figure 29ACholecystectomy procedures among adults 18 years of age and over, by location of care: United States, 1996 and 2006
Click here for spreadsheet version Click here for Powerpoint slide 29A
* Nearly all ambulatory surgery visits that did not result in hospital admission were laparoscopic procedures.
NOTES: Cholecystectomy is gallbladder removal. See data table for Figure 29.
SOURCES: CDC/NCHS, National Hospital Discharge Survey and National Survey of Ambulatory Surgery.
Figure 29BType of cholecystectomy procedure among adults 18 years of age and over, by location of care: United States, 2006
Click here for spreadsheet version Click here for Powerpoint slide 29B
* Nearly all ambulatory surgery visits that did not result in hospital admission were laparoscopic procedures.
NOTES: Cholecystectomy is gallbladder removal. See data table for Figure 29.
SOURCES: CDC/NCHS, National Hospital Discharge Survey and National Survey of Ambulatory Surgery.
Figure 26Use of mammography within the past 2 years among women 40 years of age and over, by race and Hispanic origin: United States, 1987–2008
Click here for spreadsheet version Click here for Powerpoint
NOTE: See data table for Figure 26.
SOURCE: CDC/NCHS, National Health Interview Survey.
Figure 24Federally regulated (CLIA) laboratories: United States, 1993–2008
Click here for spreadsheet version Click here for Powerpoint
NOTES: CLIA is Clinical Laboratory Improvement Amendments of 1988. New York state and Washington state are exempt from CLIA because they have their own regulatory requirements. Waived laboratories perform only tests that have been classified as waived, which are very simple tests with low risk of erroneous results. See data table for Figure 24.
SOURCE: Centers for Medicare & Medicaid Services, CLIA Database.
Figure 25Ambulatory care visit with MRI/CT/PET scans ordered or provided during the visit, by age and location of care: United States, 1996–2007
Click here for spreadsheet version Click here for Powerpoint
NOTES: OPD is hospital outpatient department. ED is hospital emergency department. See data table for Figure 25.
SOURCES: CDC/NCHS, National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey.
Figure 27Discharges with at least one knee or hip replacement procedure in nonfederal short-stay hospitals among adults 45 years of age and over, by type of procedure: United States, 1996–2006
Click here for spreadsheet version Click here for Powerpoint
NOTE: See data table for Figure 27.
SOURCE: CDC/NCHS, National Hospital Discharge Survey.
Figure 28Hospital discharges with a PTCA procedure among persons 45 years of age and over, by type of procedure and age: United States, 1996–2006
Click here for spreadsheet version Click here for Powerpoint
NOTES: PTCA is percutaneous transluminal coronary angioplasty. See data table for Figure 28.
SOURCE: CDC/NCHS, National Hospital Discharge Survey.
Figure 30Ambulatory surgery visits for upper endoscopy or colonoscopy procedures among adults 18 years of age and over, by age: United States, 1996 and 2006
Click here for spreadsheet version Click here for Powerpoint
NOTES: EGD is esophagogastroduodenoscopy. See data table for Figure 30.
SOURCE: CDC/NCHS, National Survey of Ambulatory Surgery.
Figure 31Medicare decedents 65 years of age and over with an ICU/CCU stay in the last 6 months of life, by state: United States, 2005
Click here for spreadsheet version Click here for Powerpoint
NOTE: See data table for Figure 31.
SOURCE: Dartmouth Atlas of Health Care.
Figure 32Selected solid organ transplantation, by type of organ: United States, 1997–2006
Click here for spreadsheet version Click here for Powerpoint
NOTES: Transplantation rates are shown for the three most common types of organ transplantations. See data table for Figure 32.
SOURCE: Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients (OPTN/SRTR). Data as of May 1, 2007.
Figure 33Assisted reproductive technology (ART) cycles initiated among women, by age: United States, 1996–2006
Click here for spreadsheet version Click here for Powerpoint
NOTE: See data table for Figure 33.
SOURCE: CDC/National Center for Chronic Disease Prevention and Health Promotion, National ART Surveillance System.
Figure 34Adults 45 years of age and over reporting prescription drug use in the past month for selected drug categories, by age and sex: United States, 1988–1994 and 2003–2006
Click here for spreadsheet version Click here for Powerpoint
* Estimates are considered unreliable. Data preceded by an asterisk have a relative standard error of 20%–30%.
NOTES: See data table for Figure 34.
SOURCE: CDC/NCHS, National Health and Nutrition Examination Survey.
Figure 35Death rates for human immunodeficiency virus (HIV) disease for all ages, by sex and race and Hispanic origin: United States, 1987–2006
Click here for spreadsheet version Click here for Powerpoint
NOTES: Data are age-adjusted. HAART is highly active antiretroviral therapy. See data table for Figure 35.
SOURCE: CDC/NCHS, National Vital Statistics System.
Figure 36Costs for hospital stays with the six most expensive principal procedures: United States, 1999–2006
Click here for spreadsheet version Click here for Powerpoint
NOTES: The six most expensive principal procedures were selected based on aggregate national hospital costs in 2006. Costs were for the entire hospital stay, not just the cost of performing the principal procedure. See data table for Figure 36.
SOURCE: Agency for Healthcare Research and Quality, Healthcare Cost & Utilization Project.