NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Wilt TJ, Shaukat A, Shamliyan T, et al. Lactose Intolerance and Health. Rockville (MD): Agency for Healthcare Research and Quality (US); 2010 Feb. (Evidence Reports/Technology Assessments, No. 192.)
This publication is provided for historical reference only and the information may be out of date.
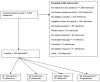
All work was conducted under the guidance of a Technical Expert Panel (TEP), whose members are identified in Appendix A. Figure 2 shows the inclusion/exclusion criteria and number and reasons for study inclusion and exclusion. The search strategies for the research questions are described in Appendix B. Excluded references are shown in Appendix C.
Key Question 1: What is the prevalence of lactose intolerance? How does this differ by race, ethnicity, and age?
Description of Study Characteristics
Our search strategy identified 2,450 articles from abstracts or full articles that were obtained to determine study eligibility. Each article was read by one extractor and was included for further review if the article either appeared to meet the inclusion criteria or if inclusion was uncertain. In cases where inclusion was not obvious, additional review by a senior investigator occurred.
A total of 54 articles met inclusion criteria (Figure 2). These articles include populations from the United States, as well as populations for Northern, Central, and Southern Europe, the Middle East, Central America, South America, Africa, Asia, and Australia. As described in our methods section, we over represented studies from the United States in order to make this review more relevant to U.S. populations. Although the majority of research has occurred outside the United States, our review includes 15 studies from the United States, with a total of 4,817 participants.
Only one randomly selected or population representative study of the United States was identified, and this study only included self reported LI on a questionnaire with no lactose challenge or objective confirmation.35 The vast majority of studies are convenience samples, which make extrapolation of results to the general public difficult to impossible.
Lactose Intolerance
Symptoms following blinded lactose challenge. We identified no studies in the United States or elsewhere that reported on the prevalence of LI based on our “gold-standard” definition of LI. Since abdominal symptoms can be caused by a large number of factors unrelated to lactose and biases in attributing abdominal symptoms following unblinded challenges of lactose, it is difficult to accurately identify the prevalence of symptoms truly attributable to lactose. This is made even more difficult since studies have rarely tried to obtain samples of participants that are representative of the overall U.S. population. Because of these limitations, we were unable to accurately define the true prevalence of LI or estimate the extent to which the true prevalence of LI differs depending on race/ethnicity or age.
The prevalence of symptoms estimated from studies not using blinded challenges is defined for the remainder of the report as “symptoms” so as to intentionally distinguish these results from the prior mentioned “gold standard” definition of prevalence of LI.
Symptoms following nonblinded lactose challenge. We identified 21 studies that reported LI related symptoms (abdominal pain, bloating, excess flatulence, and diarrhea) following a challenge of lactose.7–28 Detailed information about each of these studies, included in Table 3, is stratified into three different groups based on whether the participants self reported prior LI related symptoms prior to the challenge. Studies include results on a total of 8,174 people from various samples collected on every continent except Antarctica.
Table 3
Prevalence of lactose intolerance symptoms following challenge.
There is, however, little data available specifically from the United States to answer this question, and the data that are available offer little information about the overall prevalence of people who would report symptoms of LI if they were given a lactose challenge; moreover, these data are particularly limited for providing information on the impact of race/ethnicity and age on prevalence of symptoms. No U.S. studies from the past 30 years were identified. Four older, U.S. studies of convenience samples were identified.13,18,26,27 Newcomer reported results on a population of healthy Caucasian volunteers with no history of milk intolerance.18 This study reported no overall prevalence of symptoms following the lactose challenge, but it did report that all six of the participants with biopsy determined hypolactasia reported symptoms, while only 4 percent (2/57) of participants with normal lactase levels reported symptoms. U.S. studies of healthy volunteers from Texas reported results in adults26 and children27 for Hispanic and white non Hispanic participants. In adults, Hispanics were 43 percent more likely to report symptoms following a lactose challenge compared to white non Hispanics (Hispanics 67 percent versus non Hispanics 47 percent).26 Similarly, in children the rate of symptoms was much higher among Hispanic children (41 percent versus 20 percent in non Hispanic); however, even among Hispanic children, the majority did not experience symptoms and among Hispanic children less than 6 years old symptoms were rare (18 percent).27 The fourth U.S. study included black (n=69) and white (n=30) children between the ages of 4 and 9 years old.13 This study provided some information that is consistent with studies reported in other countries, showing the overall frequency of symptoms following a challenge is quite low in young children, but the rate increases with age and is significantly higher in black children compared to white children. Specific estimates of the prevalence in age or race strata are impossible, since confidence intervals were very wide.
Larger and more recent studies have been conducted outside of the United States, and these studies do provide more information, suggesting that there are substantial differences in the prevalence of reported symptoms depending on both the age and ethnicity of the population. These non U.S. populations included a total of nearly 7,260 participants from 16 different countries (Table 3).7–12,14–17,19–25,28 The results in Table 3 are separated according to whether the primary population does or does not have symptoms at baseline.
Many of the studies only reported symptoms in subgroups of their populations; for example, only in people who had positive breath hydrogen tests (LM) or only in people who reported previously having symptoms. Studies that reported results in people both with and without LM, reported significantly greater frequency of symptoms (typically around twice as high) in those with positive breath hydrogen tests compared to those with negative tests.11,15,22,25
Two studies reported doses of approximately 50 grams of lactose versus 12 grams of lactose and found much lower rates of symptoms with lower doses.15,16 While dose studies were uncommon, these dose results suggest that even among people positive for LM, symptoms might only occur in a minority of people when the dose is approximately one glass of milk. This might be particularly true for young children.15,16
Older age is a consistent predictor of increased symptoms following a lactose challenge.13,16,19,23,28,155 For almost all populations it appears as though very few children younger than 6 experience symptoms following lactose challenges. There was some evidence that children of African or Asian decent may experience increased frequencies of symptoms in childhood at younger ages compared to other populations, but even these studies still showed that the majority of young children did not experience symptoms.23,28,53
Symptoms without lactose challenge. Self reported history of LI related symptoms without empirical evidence of symptoms following a lactose challenge is very difficult to interpret. We identified seven studies reporting baseline self-reported symptoms representing 6,161 people.29–34,35 Study characteristics from the identified studies are provided in Table 4. The population based nationally representative sample of U.S. adults by Nicklas and colleagues provides some evidence regarding the prevalence self-reported LI.35 This study included 1,084 respondents 19 to 70 years of age, of which 486 were European American, 355 were African American, and 243 were Hispanic American. Data from this survey were combined with U.S. Census Bureau data to estimate an overall age adjusted prevalence of self reported LI of 12 percent. The specific racial/ethnic estimates were 8 percent for Caucasian adults, 20 percent for African American adults, and 10 percent for Hispanic Americans. This study did not attempt to validate the self reported results with either laboratory tests or clinician diagnoses.
Table 4
Prevalence of lactose intolerance by self report.
Among non U.S. studies, one additional population based random sample of 1,978 Iranian adults showed a population self reported prevalence of 28 percent with no variation by age.34 The generalizability of this one non U.S. study is difficult to put into a broader context without similar studies reporting different racial and ethnic populations and with greater variations in age.
Other than the one population based random sample in the United States, the rest of the self reported studies’ results provide little evidence to address our research questions about population prevalence and the impact of age and ethnicity. Overall, the prevalence of self reported symptoms was typically lower than the prevalence of symptoms following a lactose challenge.
Lactose Malabsorption
Determined by hydrogen breath test following lactose challenge. Prevalence of LM, as diagnosed via a hydrogen breath test following a lactose challenge, has been frequently assessed in a wide range of studies from around the world. We identified 31 studies, including a total of nearly 12,000 participants from a wide range of ages and ethnicities.7,8,10–12,14–17,20–25,28,30,32,36–42,44–48,156 The study characteristics from the identified studies are provided in Table 5. The studies in Table 5 are stratified into three different groups based on whether the participants self reported LI related symptoms prior to the challenge. Unfortunately, none of the U.S. studies were representative population based studies. In fact, all of the U.S. studies identified focused on reporting results in populations of patients with GI symptoms at baseline,36,42,47,48 with the exception of one three decade old study of American Indians30 and one convenience sample of adults from the Army, senior centers, nursing homes, and a university.44
Table 5
Prevalence of lactose malabsorption by challenge.
Within the U.S. studies, the prevalence of LM in Caucasian adult populations ranged from 6 percent to 24 percent.42,44,47 There were also some data suggesting high levels of LM among American Indians, but this effect was substantially attenuated among those with American Indian and Caucasian mixed ancestry.30 Few data were eligible for this review for other racial and ethnic groups within U.S. populations, but a prior review of smaller and older studies using blood glucose tests suggested that the prevalence of LM may be greater than 70 percent in African Americans, around 50 percent in Hispanic Americans, and even higher for Asian Americans.49 Data for various racial and ethnic groups within the United States can likely be best understood by looking at the LM rates in the ancestral homelands of each of these ethnic groups.
The high prevalence of LM in the majority of non Northern European countries has been well known for decades. Earlier reviews captured many of the smaller and earlier studies, particularly those that used blood glucose tests.49 The focus of this current review was more on LI as compared to LM. Similar to what has previously been reported, we found a wide range of LM rates that tended to be lowest among groups of Northern European ancestry, and relatively high in most other regions. Clearly, race and ethnicity have significant effects on the prevalence of LM; however, it is difficult to put precise estimates around the prevalence of LM for any group. In general, the majority of adults from populations with Northern European ancestry are able to digest lactose; whereas, the majority of adults who are Asian, African, American Indian, or from Sicily, Italy, (and actually much of the rest of the world) are unable to adequately digest 50 gram challenges of lactose. However, it is important to note that for many regions there is significant heterogeneity within the population in the ability of adults to digest lactose. This is particularly true within some regions in Africa,37,38 but it has also been seen in other areas, such as in Italy.11 However, much of the within country variation seen around the world is likely due to immigration that has occurred during the past couple of centuries.
Age is clearly an important contributor to the rate of LM, since nearly every population group identified, even those with high adult rates of LM, showed low rates of LM in the youngest age groups, particularly those less than 6 years of age.16,17,23,28,39,45,46 In populations with high adult rates of LM, rates often seemed to nearly peak between 10 and 16 years of age.
Not unexpectedly, the dose of the lactose challenge appears to be an important factor in the reported prevalence of lactose malabsorption. Studies that included a lower dose challenge appeared to identify significantly fewer case of malabsorption.12,16,23,41,46 Unfortunately, these lower dose studies were primarily only conducted in children, with the exception of a study of adults from Norway that found a 4 percent prevalence of LM following a 25 gram lactose challenge12 and a study from Spain that found, compared to the standard challenge, a single serving of milk and a single serving of yogurt were much less frequently malabsorbed (33 percent, 14 percent, and 4 percent, respectively).16
Lactase Nonpersisters (Adult-type Hypolactasia Biopsy)
Biopsy identification. Five studies were identified that reported on the prevalence of lactase persistence as diagnosed by biopsy assays.18,50–53 Generalizing results from these studies is more difficult since the studies were performed primarily in convenience samples of patients who had biopsy tissue available, often for clinical purposes, and these studies were all conducted decades ago (Table 6). The earliest study is the only study that provides estimates on lactase nonpersistence in a population of healthy U.S. Caucasians not thought to be intolerant to milk or to have GI symptoms.53 This study, among adults with a mean age of 39 years, found 6 percent (6/100) had lactase activity ≤0.5 units per gram, and from these data the authors estimated that a population prevalence of hypolactasia would be between 1.3 percent to 10.3 percent (95 percent confidence level) for asymptomatic Caucasian adults.
Table 6
Prevalence of hypolactasia.
One additional study from the United Kingdom provides a comparison of the prevalence of hypolactasia in four different groups of British adults who had biopsy jejunal tissue available: white subjects with normal histopathic biopsy, nonwhite subjects with normal histopathic biopsy, subjects with diarrhea following gastric surgery, and subjects with irritable bowel syndrome.50 There were no statistically significant differences in the frequencies of hypolactasia for white subjects (7/150; 5 percent), subjects with diarrhea following gastric surgery (3/36, 8 percent), or subjects with IBS (16/200, 8 percent); however, the prevalence of hypolactasia was substantially higher in the nonwhite subjects (15/29, 75 percent). The three remaining studies offer little data on the population prevalence of hypolactasia, since the study samples were highly selected for patients with clinical GI symptoms.51–53 The first study found that both white children (ages 6 to 14) with recurrent abdominal pain and white children with chronic diarrhea had similar frequencies of hypolactasia—31 percent (8/26) and 36 percent (16/61), respectively.51 Similarly, another study found children with IBS had a similar frequency (p-value=0.16) of hypolactasia (40 percent, 45/112) compared to children with chronic abdominal pain (30 percent, 34/112).52 This study did report that within children with IBS, the nine black children had a significantly higher prevalence of hypolactasia compared to the 103 white children (78 percent versus 37 percent, respectively). The last study included a sample of 250 U.S. subjects with biopsy samples taken over a several year period with varied clinical reasons.53 This study did have a sample with both age (2–81) and racial (white=209 and black=39) diversity; however, the hypolactasia results were not stratified by race. The overall prevalence of hypolactasia in the sample was 34 percent, but without race or age stratification it is difficult to generalize these findings to create any meaningful population estimates.
Genetic test association. Adult-type hypolactasia is thought to be an inherited autosomal recessive trait leading to decreased lactase activity in the intestinal mucosa. The most commonly reported genetic mutation for adult-type hypolactasia is the single nucleotide polymorphism (SNP) located 13,910 base pairs upstream of the lactase (LCT) gene of which the C allele is the globally most prevalent allele, while the less common T allele is associated with lactase persistence.54
Nine studies were identified that reported genotype frequencies for adult-type hypolactasia‐linked LCT -13910C>T SNP mutation.29,45,55–57,59–61,91 These studies included a total of 8,581 participants; however, none of these studies were of U.S. populations, and the majority of the people included in these studies had Northern European ancestry (Table 7). Not unexpectedly, there were no obvious differences in genotype by age group.55,56 In North European studies, Caucasians had frequencies between 10–20 percent for the homozygous C/C genotype.29,55–57,59,61 The frequency of the C/C genotype was somewhat higher in the one study from Austria (C/C=27 percent). Two studies reported results for the Italian regions of Sardinia45,60 and Apulia60 where the prevalence of the C/C genotype was between 80 percent and 90 percent. One study from Finland reported results in a subgroup of 65 children from Africa in which the prevalence of the C/C genotype was 95 percent.
Table 7
Prevalence of adult-type hypolactasia genotype.
Results from genetic association tests consistently reported decreased consumption of milk (often on the order of twofold lower) in adults with the C/C genotype compared to those with at least one T allele.56,57,59,61,91 These differences were smaller in healthy children.59 The relative differences in calcium intake from all dairy and overall calcium intake were smaller than the differences in milk consumption.29,57,59,91 All of these studies were from populations in Finland with generally high dairy consumption, except for one study in Austrian men where milk consumption was low in all men.91
Summary
There are few data available from recent U.S. studies regarding any of the outcomes we reviewed. The data that were available tended to be highly selected and not likely representative of the overall U.S. population. Finally, the outcomes that have been reported do not directly assess LI, but instead assess either an inability to fully absorb lactose or somewhat subjective symptoms that are prone to biased reporting. This lack of data may in part be due to the fact that LI is a difficult condition to define. The lack of a clear, clinically meaningful, and commonly accepted definition of LI may partly explain the limited information available for characterizing the U.S. population prevalence.
While precise estimates of the U.S. prevalence of LI are not possible, there is evidence that the magnitude of LI will be very low in young children and likely remain low into adulthood for most populations of Northern European decent. For African American, Hispanic, Asian, and American Indian populations the rates of LI will likely be higher in late childhood and adulthood; however, smaller doses of lactose might be generally well tolerated in most populations.
Key Question 2. What are the health outcomes of dairy exclusion diets?
Association Between GI Symptoms and Dairy Exclusion Diets
We identified no studies that addressed the long-term impact (>1 month) of dairy exclusion diets on GI symptoms in the general population, vegans, or those diagnosed with LI or LM. Studies that reported symptoms in patients with milk allergies, IBS, or other diseases were beyond the scope of our review. In Key Questions 3 and 4 we report short-term GI outcomes from blinded RCTs among subjects with diagnosed LI or controls fed short-term diets containing varying doses of lactose or lactose free diets. We found low levels of indirect evidence that populations susceptible to LI avoid dairy consumption, presumably in an effort to reduce dairy induced GI symptoms. Postmenopausal Austrian women with TT genotype (lactase persistence) had lower odds of aversion to milk consumption than women with C/C genotype.68,69 Among children who avoided milk, those diagnosed with LI had much greater odds of milk related symptoms.76
Association Between Milk Intake With Genetic Polymorphism, Lactose Intolerance, or Malabsorption
As noted in Key Question 1, results from genetic association tests consistently reported decreased consumption of milk (often on the order of twofold lower) in adults with the C/C genotype compared to those with at least one T allele.56,57,59,61,91 These differences were smaller in healthy children.59 The relative differences in calcium intake from all dairy and overall calcium intake were smaller than the differences in milk consumption.29,57,59,91 All of these studies were from populations in Finland with generally high dairy consumption, except for one study in Austrian men where milk consumption was low in all men.91 The Finnish Cardiovascular Risk in Young Finns Study demonstrated that those with C/C genotype had lower than recommended calcium intake among young women (crude OR 1.91, 95 percent CI 1.12; 3.23) and men (crude OR 2.00, 95 percent CI 1.36; 2.95).70 Young women with C/C genotype had a 524 percent increase in odds of following a lactose free diet (OR 6.24, 95 percent CI 3.46; 11.24).70 Young men with C/C genotype had a 144 percent relative increase in odds of a lactose free diet when compared to those with T/T genotype (OR 2.44, 95 percent CI 1.22; 4.87).70
Children and adults with self reported symptoms of milk intolerance and diagnosed LM reported (or were assumed to be consuming) lactose free or low lactose diets.59,65–67 The association was more consistent for women.68,69 The association may diminish with aging.71,72 The American prospective “Project EAT: Eating Among Teens” study reported that adolescents with self-perceived lactose intolerance reported decreased dietary calcium intake during the transition to young adulthood.73
Association Between Dairy Exclusion Diets and Bone Health
We identified 55 publications of observational studies of 223,336 subjects (Appendix Table D1) that examined the association between lactose intake or factors associated with low lactose intake (i.e., diagnosis of LI/LM or biopsy or genetic test association for lactase nonpersistence in the absence of specific documentation of the amount of lactose intake) on bone health including clinical (fracture) and intermediate outcomes (osteoporosis, bone mineral density, and content). The absence of specific documentation of the amount of lactose consumed over long periods of time hampered synthesis so indirect associations between bone outcomes and proxy variables for lower lactose consumption were assessed. We identified seven RCTs of 1,207 children, and two RCTs of adult women62,63 that demonstrated causal effect of lactose intake on bone health. African American women were enrolled in one study.64
Sample sizes varied from a minimum of 19 to a maximum of 77,761 subjects, average = 4,06140,61±12,451 subjects. We identified 13 observational studies of 9,577 children or adolescents with an average sample size of 737±1,146 subjects.59,70,73,76,89,95–99,159–161
Adult men and women (N = 80,726) were examined in 11 publications with an average sample size of 7,339±14,826 subjects.5,65,67,83,88,90,92,94,100,162,163 Adult men (N=751) were examined in three publications with an average sample size of 250±24.57,66,91
The majority of the studies included women. We identified 28 publications of 132,282 women with an average sample size of 4,724±14,707.29,64,68,69,71,72,77–82,84–87,93,164–174
The majority of the studies (N=32) were cross-sectional evaluations that included on average 1,364 subjects. From 55 publications identified, 14 studies were prospective design, seven were case-control studies, one was a meta-analysis of the individual subject data, and one was a prospective observation of the placebo arm in an RCT. The majority of the studies were sponsored by grants from nonprofit resources, 29 studies enrolled an average of 5,929±15,418 subjects. Few (N=7) studies reported combined support from industry and grants, and one study was supported by industry alone. A large proportion of the studies (18/55) did not provide any information about funding sources.
U.S. studies represented 27 percent of all included studies (15/55) and enrolled an average of 7,324±19,795subjects. Studies from North European countries constituted 30 percent of the publications (seven from Austria, ten from Finland, and one from Sweden). Studies from the United Kingdom represented 6 percent of all eligible (3/55) but had larger sample sizes averaging around 25,475±20,363. Asian populations were examined in five studies; two were conducted in Taiwan, one in Hong Kong, one in China, and one in Japan. African American women were enrolled in one study.64 Other publications either did not report race or ethnical distribution of the subjects or enrolled predominately Caucasians.
Lactose metabolism was addressed in 29 publications.5,29,57,59,64–69,71,72,88,91,92,94,96,98‐100,159,162,164–170 The wide variety of definitions of milk intolerance and absence of the gold standard to diagnose LI hampered synthesis of evidence. Authors defined self reported symptoms as “perceived milk intolerance”99 or relied on clinical diagnosis that was made based on a positive hydrogen LI test and self reported symptoms after dairy consumption.66,91,92,100,168 Authors assessed symptoms during or after oral LI tests in few studies.5,64,166,167
Trained interviewers who were blinded to the results of oral LI tests assessed symptoms in one study.72 Two studies used blood glucose examination after oral lactose intake to diagnose malabsorption.162,170 Several studies obtained a hydrogen breath test after oral lactose intake without evaluating the symptoms of intolerance.71,98,164,165,169
One early study defined LI as positive oral lactose tolerance tests, positive glucose tolerance tests, and jejunal biopsy with impaired lactase activity.94 The remaining 23 publications evaluated the outcomes among populations with different dairy intake but unknown lactose metabolism.76–87,89,90,93,95,97,160,163,171–174 Randomized trials examined the effects of increased dairy administration in populations with baseline low lactose intake.
We synthesized the evidence of the association between lactose diet and metabolism on clinical (fracture) and intermediate outcomes (osteoporosis, bone mineral density [BMD], and content) in children and adults. We provided the methodological characteristics of the studies when differences in results could be contributed to external or internal validity of the studies.
Association Between Lactose Intake and Metabolism and Bone Fractures
A low level of inconsistent evidence was available from observational studies that low milk consumers had fractures more often than higher milk consumers (Table 8). There are no data according to race. Observational studies with different quality provided low level evidence that childhood milk avoidance was associated with increased risk of bone fractures. Adults with C/C genotype, symptoms of milk intolerance, or diagnosed LM had reduced lactose intake and increased odds of bone fracture. One large cohort reported that vegans had an increased relative risk of fractures. The effects of lactose free or low lactose diet were more evident in women.
Table 8
Association between lactose intolerance and bone outcomes.
Diet
We found a low level of evidence that children who avoid milk intake had increased odds of bone fractures (Table 8).
The association between lactose intake and bone fracture was examined in 13 publications.76–88 The Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford) compared risk of fracture among vegans and dairy consumers (Table 9).90
Table 9
Association between low lactose diets and bone fractures.
Children. Low levels of evidence from two industry sponsored studies of prepubertal children from New Zealand found a significant association between lactose free diets and increased odds of bone fractures.76,89 Prepubertal children with a history of long-term milk avoidance had greater than a threefold increase in odds of the annual incidence of distal forearm fracture (age adjusted odds ratio 3.59, 95 percent CI 1.77; 7.29).76 Age adjusted odds of history of any fracture were four times higher (OR 4.13, 95 percent CI 1.61; 10.56) among children with lactose free diets when compared to the general population.89
Adults. We found a low level of inconsistent evidence in three studies of 44,552 adults that those with low lifetime or childhood milk intake had increased odds of any or osteoporotic fracture.80,83,88 The largest meta-analyses of individual data from 39,563 adults, participants in the European Vertebral Osteoporosis Study (EVOS/EPOS), the Canadian Multicentre Osteoporosis Study (CaMos), the Dubbo Osteoporosis Epidemiology Study (DOES), the Rotterdam Study, the Sheffield Study, and a cohort from Gothenburg, demonstrated a borderline nonsignificant 10 percent increase in relative risk of osteoporotic fracture in those who consume less than one glass of milk per day (multivariate adjusted RR 1.10, 95 percent CI 1.00; 1.21).88 The adjustment for body mineral density, however, attenuated the association to nonsignificant.
Women. Low level evidence from nine publications of 111,485 adult women suggested an inconsistent increase in risk of fracture in association with low dairy intake.77–79,81,82,84–87
Variability in definitions of lactose intake and types of fracture contributed to inconsistency in the results of the studies. All studies found increased odds of fracture in women with lower dairy intake; however, only five reported a significant association. For instance, an American study of 5,398 college alumnae, 2,622 former college athletes, and 2,776 non-athletes found a 92 percent increase in multivariate adjusted odds of the first fracture after 40 years of age in low milk consumers when compared to the rest of the population (OR 1.92, 95 percent CI 1.15;3.16).79 The third National Health and Nutritional Examination Survey demonstrated that older women with dairy intake of less versus more than two servings per day had greater crude odds of osteoporotic fracture.85 The European Mediterranean Osteoporosis Study showed that women with low lifetime intake of milk had 46 percent increased relative risk of hip fracture (RR 1.46, 95 percent CI 1.21; 1.76).82
In contrast, the Nurses’ Health Study of 77,761 women who had never used calcium supplements did not detect a significant association between milk or dairy calcium intake and risk of hip fracture at 12 years of followup.84 Moreover, the same study reported a 93 percent increase in relative risk of hip fracture among women with dairy calcium intake of >550 mg/day versus <175 mg/day (multivariate adjusted RR 1.93, 95 percent CI 1.09; 3.42). Elderly female participants in the Study of Osteoporotic Fractures, who rarely or never consumed dairy calcium during their adolescence, had a 77 percent increase in relative risk of fractured proximal humerus (multivariate adjusted RR 1.77, 95 percent CI 1.12; 2.80) with no differences in risk of fractured distal forearm.77 Three studies did not find a significant association between lifetime81,87 or adolescent milk intake78 and odds of bone fracture.
Men. One meta-analysis of individual data from 15,825 male participants in the EVOS/EPOS, CaMos, DOES, Rotterdam Study, and Sheffield Study, and a cohort from Gothenburg, did not detect a significant association between any osteoporotic or hip fracture in men.88
Type of fracture. Low lactose intake was associated with a history of any fracture in prepubertal children and elderly women (Figure 3).80,86,87,89 The association between low lactose intake and risk of hip fracture was significant in two studies of seven that examined this relationship (Figure 4).78,79,81–84,88

Figure 3
Association between milk intake and history of any fracture.
Osteoporotic fractures were not associated with lactose intake in the three studies that examined the relationship (Figure 5).85,86,88
Dairy calcium intake. Evidence from published studies did not suggest a significant association between dairy calcium intake and bone fractures. Low calcium intake was not associated with fracture in 50 prepubertal children (Appendix Table D3 and Figure 6),89 960 Italian women,81 or 4,342 adults from the National Health and Nutrition Examination Survey (NHANES) I Epidemiologic Follow-Up Study cohort.174

Figure 6
Association between dairy calcium intake (mg/day) and bone fractures.
Vegan diet. We found one study, the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford), that compared relative risk of bone fracture among vegan vegetarians (lactose free diet) with meat and dairy consumers (Table 10).90 Multivariate adjusted relative risk of incident fracture of bones other than the digits or ribs was 30 percent higher in vegan adults (RR 1.30, 95 percent 1.02; 1.66) but not significant in women or men separately.
Table 10
Association between vegan diet (lactose free) and incident fracture of bones other than the digits or ribs, results from the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford).
Genetic Polymorphism
A single nucleotide polymorphism of the LCT gene at chromosome 2q21–22 in association with fractures was examined in five publications.29,65,68,69,91
Children. We did not find studies that examined bone fractures in children with genetic polymorphism.
Women. Evidence of the association between bone fracture and genetic polymorphism from three studies of 895 postmenopausal women was inconsistent in direction and effect size (Table 11).29,68,69
Table 11
Association between genetic polymorphism and bone fractures.
A cross sectional Austrian study demonstrated that women with TT genotype had reduced crude odds of fracture (OR 0.26, 95 percent CI 0.13; 0.54).68 Another smaller prospective Austrian study, however, did not find a significant association between genetic polymorphism with interim vertebral or nonvertebral bone fractures.69 In contrast, a Finnish study reported greater crude odds of any and nonvertebral fractures in women with TT genotype when compared to C/C genotype.29 The authors did discuss why their results showed negative association between C/C genotype and bone fractures. They did not calculate odds ratios but compared fractures in three categories of genotype (TT, C/C, and TC). Authors reported a nonsignificant p value from χ2 tests, and concluded no differences in fractures in relation to genetic pattern.29
Adults. One population-based study “Vantaa 85+” of 601 Finnish elderly found that those with C/C genotype had a fourfold increase in crude odds of hip (OR 4.22, 95 percent CI 2.16; 8.26) and nearly threefold increase in crude odds of wrist fracture (OR 2.82, 95 percent CI 1.42; 5.59) when compared to TT genotype.65
Men. The Austrian Study Group on Normative Values on Bone Metabolism did not find a significant association between genetic polymorphism and bone fracture in elderly men.91
Lactose Intolerance
We synthesized the evidence with the exact definitions of lactose intolerance that were obtained by the primary investigators in the studies.
Children. Children who avoided drinking cow’s milk because of perceived milk intolerance did not have higher rates of fracture when compared to those milk avoiders who did not report symptoms of intolerance (Table 12).89
Table 12
Association between lactose intolerance or malabsorption and bone fractures.
Adults. Austrian men and women with self reported symptoms of lactose intolerance during the hydrogen breath test had twofold increased crude odds of any fracture (OR 1.96, 95 percent CI 1.11; 3.48).92 Estonian men and women with self reported milk intolerance had increased crude odds of osteoporotic fracture (OR 2.69, 95 percent CI 1.25; 5.78).67
Women. Finnish postmenopausal women with lactose intolerance did not have greater risk of any, vertebral, or nonvertebral fracture.29
Lactose Malabsorption
We synthesized the evidence of the association between LM that was diagnosed with objective breath hydrogen or blood glucose test and bone fractures (Table 12). As noted above, while we did not have information on dairy intake, we assumed that individuals with documented LM have lower dairy intake than absorbers.
Adults. Austrian adults with positive hydrogen breath test had an increase in crude odds of any fracture when compared to lactose absorbers (OR 2.63, 95 percent CI 1.52; 4.54).92 Adults with severe LI (ΔH2 >60ppm) had greater than threefold increase in crude odds of vertebral fractures when compared to lactose absorbers (OR 3.62, 95 percent CI 1.93; 6.79).92
Women. We found a low level of evidence that women with LM may have increased risk of bone fractures (Table 8).164,167,170
The Finnish Kuopio Osteoporosis Risk Factor and Prevention Study demonstrated that women with positive versus negative lactose tolerance test had 33 percent greater odds of any fracture (multivariate adjusted OR 1.33, 95 percent C 1.08; 1.64) after adjustment for age, body mass index (BMI), number of chronic health disorders, and menopausal and smoking status.167 Smaller case control studies of women failed to detect significant associations. One Finnish study of 18 elderly women with spinal fragility fractures, 28 elderly women with hip fractures, and 35 population controls did not find differences in crude odds of fracture when women with positive blood glucose tests were compared to those with negative tests.170 Elderly female malabsorbers from New Zealand did not have greater age adjusted odds of fracture when compared to those with negative breath hydrogen tests.164
Association Between Lactose Intake and Metabolism with Osteoporosis
Studies examined different populations, used different definitions of impaired lactose metabolism, and evaluated osteoporosis at different bone sites and with varying fracture definitions. Adults with lactose free or low lactose diets had osteopenia more often (Table 13).
Table 13
Association between low lactose diets, lactose intolerance or malabsorption, and osteoporosis.
Adults. Two studies addressed the odds of osteoporosis in association with lactose intake and reported different results, depending on ethnicity of the subjects and definitions of exposure. The study of Asian adults in Taiwan did not find a significant association between low milk intake and odds of osteoporosis.163 The U.S. study reported a significant increase in odds of osteoporosis in adults with LI or LM.94
Women. Postmenopausal Taiwanese women with lactose free diets had a fourfold increase in adjusted odds of femoral neck when compared to nonvegan vegetarians (multivariate adjusted OR 3.94, 95 percent CI 1.21; 12.82).93 Italian adults with symptoms of LI and positive hydrogen test an increase in crude odds of osteopenia.5 Women with different genetic polymorphism had the same odds of osteoporosis.29,69
Two small studies totaling 124 women examined crude odds of osteoporosis by LI and LM status.168,169 An Austrian study reported a large significant increase in crude odds of idiopathic osteoporosis among malabsorbers (OR 36.56, 95 percent CI 8.02; 166.69) and those with milk intolerance (OR 32.31, 95 percent CI 6.97; 149.75).168 In contrast, an Italian study of postmenopausal women did not find a significant association between osteoporosis and lactose intolerance or malabsorption.169
The magnitude and significance of the association varied, depending on definitions of exposure. Studies did not analyze all levels of exposure, including milk and dairy calcium intake, genetic polymorphism, perceived milk intolerance, and positive tests for lactose maldigestion. To address the issue of correlated definitions of exposure, we analyzed, when possible, the odds of lactose free diet in children and adults with genetic polymorphism or lactose malabsorption.
Association Between Genetic Polymorphism, Milk Intake, or Self Reported Lactose Intolerance
Available evidence suggested that children and adults with self reported symptoms of milk intolerance and diagnosed LM reported lactose free or low lactose diets. Adults with C/C genotype reported reduced milk intake. The association was more consistent for women. The association may diminish with aging.
We identified five publications that examined genetic polymorphism in association with lactose intake.59,65–67,70 One study of children and adolescence, the Cardiovascular Risk in Young Finns Study, found that dietary intake of milk and milk products was significantly lower for girls with the C/C.59 The same study did not report significant difference in milk intake among boys (Appendix Table D4). During the transition to young adulthood, however, both genders with C/C genotype did not drink milk (OR 1.86, 95 percent CI 1.34; 2.59 among women and 2.00, 95 percent CI 1.36; 2.95 among men).70 The odds of following a low lactose or milk free diet at 24–39 years of age were also significantly higher in those with C/C genotype (OR 6.24, 95 percent CI 3.46; 11.24 in females and 2.44, 95 percent CI 1.22; 4.87 in males).70
Among adults, one study of Austrian men reported that milk tolerance and consumption were higher in those with TT genetic polymorphism compared to T/C or C/C types.66 Two studies of adults also reported that those with TT type had greater odds of using milk products (OR 2.06, 95 percent CI 1.38; 3.06)65 and greater daily milk intake.67
Two studies demonstrated smaller odds of positive tests for lactose malabsorption in adults with T/T when compared to C/C genotypes (Figure 7).66,69

Figure 7
Association between genetic polymorphism TT vs. C/C and positive tests for lactose malabsorption, crude odds ratios from two Austrian observational population based studies of genetic screening for osteoporosis.
The odds of self reported symptoms of lactose intolerance were higher in women with C/C genetic polymorphism (Appendix Table D5).68,69 Men with different genotypes, however, had the same frequency of milk related clinical symptoms.57,66,91
Studies demonstrated that children and adults diagnosed with LM had clinical symptoms more often than controls (Appendix Table D5). Adult malabsorbers reported symptoms of LI more often when compared to absorbers (OR 107.98, 95 percent CI 6.34; 1838.99).5 The association was dose response shaped with a greater than threefold increase in odds of symptomatic LI in adults with moderate (OR 3.58, 95 percent CI 1.43; 9.00) and with a six fold increase in those with severe LM (ΔH2 >60ppm) when compared to lactose absorbers (OR 6.22, 95 percent CI 2.87; 13.51).92 Postmenopausal lactose malabsorbers had milk-related clinical symptoms more often; however, the results did not achieve statistical significance.71,72
Summary. Observational studies with different quality provided low level evidence that childhood milk avoidance may be associated with increased risk of bone fractures. Selected adult populations with C/C genotype, symptoms of milk intolerance, or diagnosed LM and reduced lactose intake may have increased odds of bone fracture. One large cohort reported that vegan vegetarians had increased relative risk of fractures. The effects of lactose free or low lactose diet were more evident in women.
Association Between Lactose Intake and Metabolism and Bone Mineral Content or Density
We summarize here the results from seven RCTs in children,101–107 two RCTs of women,62,63 and 28 observational studies reporting bone mineral density or content.5,57,66–69,71,72,76,91–93,95–100,159–162,165–167,169,171,172
The studies suggest that children and adults with lactose free or low lactose diets may have reduced bone mineral content (BMC) and bone mineral density (BMD). The actual differences, however, varied across the studies, depending on the populations, definitions of exposure, time of followup, and measured bones (Table 8).
Diet
Children. We found a moderate level of evidence from RCTs that increased lactose intake resulted in improved BMC of lumbar spine and femoral neck in prepubertal children with low baseline milk intake (Table 14). Dairy intervention with 1,794 or 1,067 mg calcium per day for 12 months resulted in significant increases in total body BMC in boys and girls from Hong Kong (Figure 8).101 This open label trial included 344 boys and girls 10.0 ± 3 years of age with very low baseline milk intake of 35.6 percent of the recommended daily calcium consumption.101 One RCT that included prepubertal children with very low baseline milk intake of 30.8 percent from that recommended also reported a significant increase in total body BMC after dairy administration that provided 1,200 mg calcium per day.102 The effect, however, was not significant at 18 months of followup.102 The U.S.103 and British104 RCTs that included only girls consuming half the recommended daily calcium did not demonstrate significant improvement in total body BMC.
Table 14
Bone health outcomes in children and adolescents with low lactose diets (results from randomized controlled clinical trials of dairy products).

Figure 8
Bone mineral content from RCTs of dairy product use in children and adolescents with low lactose diets. Total body.
The same pattern was seen in BMC of femoral neck. Children with very low baseline calcium intake (36 percent from the recommended) experienced significant increase in BMC.101 Children that consumed half of the recommended calcium did not have a noticeable increase in BMC (Figure 9).106,107 The effects of dairy interventions on total hip BMC were significant in all three RCTs that examined the association (Figure 10).

Figure 9
Bone mineral content from RCTs of dairy product use in children and adolescents with low lactose diets. Femoral neck.

Figure 10
Bone mineral content from RCTs of dairy product use in children and adolescents with low lactose diets. Total hip.
Design, population gender, and baseline milk intake could explain study inconsistencies in increased lumbar spine BMC. Lumbar spine BMC was increased in three RCTs,101,102,105 while two trials did not report significant changes in this outcome106,107 (Figure 11). Children from Hong Kong with very low baseline calcium intake had the greatest increase in lumbar spine BMC.101 This evidence suggests that dairy intervention increased lumbar spine BMC in girls105 but not in boys106 because trials did not differ by country (both trials were conducted in Switzerland), baseline milk intake, and design (both trials were double blinded). Neither absolute levels of BMC nor changes from baseline in BMC or BMD differed in boys after dairy intervention (1,607 mg calcium/day) when compared to placebo (747 mg calcium/day) (Appendix Table D6).106

Figure 11
Bone mineral content from RCTs of dairy product use in children and adolescents with low lactose diets. Lumbar spine.
The improvement in BMD was less evident. Dairy interventions did not increase BMD in girls in two RCTs that reported absolute levels of the outcome.103,105 Dairy interventions increased BMD from baseline in one RCT of Finnish girls,107 while British girls104 and children from New Zealand102 or Hong Kong101 did not have significant changes in BMD (Table 15).
Table 15
Percent change in osteodensitometric values after administration of dairy products in children consuming low lactose diets (RCTs).
In contrast with RCTs, observational studies (Table 16) reported that children with very low milk intake had reduced BMD compared to the reference population.76,96,97 Long term milk avoiders had lower BMC.76,95–97 Studies did not address all confounding factors.
Table 16
Association between lactose intake and metabolism and BMC.
Adults. A low level of evidence in one study suggested that low milk consumers (<4dL/day) had decreased BMD when compared to high milk consumers (>4dL/day).67
Women. Inconsistent evidence of the association between low lactose diets and bone outcomes were limited to two RCTs62,63 and two observational studies.93,171 Dairy intervention resulted in a short term increase (6 months) in total spine BMD in young women with high adherence to their diet.62 Intention to treat analysis did not detect a significant improvement in BMD (Table 17). Dairy intervention reduced age related decline over a 3-year period in vertebral bone mineral density in pre-menopausal women.63 Asian women that followed a lactose free vegan diet had the same BMD as milk consumers (Appendix Table D7).93,171
Table 17
Effect of increased dairy intake on bone health in young and pre-menopausal women consuming low lactose diets (results from individual RCTs).
Lactose intolerance. We found low levels of evidence that children and adults with self reported milk intolerance (assumed low dairy intake) had reduced BMC or BMD (Table 8). American children98 and adolescent girls99 with LI had an inconsistent reduction in BMC (Table 16). Adults with self reported milk intolerance had a consistent reduction in BMD5,67,100 and BMC.5 A small observation of 58 postmenopausal Italian women, however, did not report a significant difference in BMD in those with symptoms of LI when compared to healthy asymptomatic milk consumers.169
Lactose malabsorption. We found low levels of evidence that, when compared to absorbers, children with diagnosed LM (and therefore assumed to be have low dairy intake) had lower BMC.99 LM in women was associated with inconsistent reduction in BMD72,166,167,169 with no differences in BMC.71 The studies of adults did not find a difference in either BMD5,92,162 or in BMC5 in malabsorbers compared to the general population.
Genetic polymorphism. We found low levels of evidence that women with C/C genotype had lower BMD when compared to TT genotype.68,69
Bone outcomes did not differ by genotype in adults67 or in men.57 Bone density did not differ by genotype in either gender (Appendix Table D8). However, one prospective Cardiovascular Risk in Young Finns study demonstrated that at 12 years of followup young men with C/C genotype tended to have greater bone loss when compared to those with T/T genotype (bone mineral density in lumbar spine p=0.081).161
Key Question 3: What amount of daily lactose intake is tolerable in subjects with diagnosed lactose intolerance?
Optimally, studies of this question would employ the following methodology.
- A large, randomly recruited group of subjects with a wide range of age and ethnicity would be tested for lactose malabsorption (the assumption being that subjects who do not malabsorb lactose cannot be lactose intolerant).
- The lactose malabsorbers would undergo double-blind testing with a maximal physiological dose of lactose (50 grams) or an identical placebo to identify which subjects had appreciably more symptoms with lactose than the placebo. This study would identify subjects for further study with lower, more physiological dosages of lactose.
- Subjects with lactose intolerance would then be tested in double-blind fashion with a range of doses of lactose or identical placebo in an attempt to determine at what dosage lactose symptoms convert from tolerable to intolerable. To simulate a true life situation, the lactose would be administered with meals throughout the day. The subjects would provide a global assessment of their symptoms as well as a daily severity assessment of various symptoms on a numerical scale. The dose of lactose that induced a global rating of unacceptable (“intolerable”), or a significant increase in symptom score relative to the rating of the placebo, would be determined. The numerical scoring system would be converted to biological relevance, i.e., what difference in symptom score differentiates “tolerable” versus “intolerable.”
- Lastly, data would be analyzed to determine if the tolerable dose of lactose in malabsorbers is influenced by age and ethnicity.
Characteristics of Included Studies
Twenty-eight randomized (to treatment order), crossover, trials were included (Appendix Table D8).108–115,117–135 Nearly all trials reported utilizing a double blinded approach, but three studies were single blinded or did not attempt to mask the tastes of the test preparations.115,130,131 Trial populations ranged between six and 150 subjects. Women constituted 55 percent of the subjects (n=22 studies). The mean age of subjects was 37 years of age with a range between 10 and 77 (n=20 studies). Seven trials included children or adolescents, four exclusively.109,114,120,123,126,127,135 One trial enrolled elderly subjects (mean age 77 years).116 Within the 20 studies reporting race or ethnicity, 34 percent of subjects were white, 30 percent Hispanic, 20 percent black, 8 percent Asian, and 7 percent other.109–116,118,120,123,126–131,133–135 One study was exclusively American Indians.120 Fifteen studies were conducted in the United States,109–111,113,114,116,118–120,125–127,130,131,135 eight in Europe,108,112,121–124,132,134 three in Latin America,128,129,133 one in Asia,117 and one in Australia.115 Sixteen studies utilized commercial lactase products or hydrolyzed milk,108–111,113–115,121–125,128,130,133,135 two used milk products with lactose removed by ultrafiltration or chromatographically,112,134 and three assessed nonlactose solutions.116,126,127 An unclear or unreported method of lactose removal was noted in two trials.129,132 One trial involved probiotics,117 one was a colonic adaptation study,118 and three trials assessed varying levels of daily lactose (added to water or in sugar packets to be added to breakfast).119,120,131 In 11 studies, abdominal symptoms compatible with malabsorption of lactose prior to study entry were not required for study participation (based solely on biochemical diagnosis) or subjects were not reported to experience symptoms following ingestion of lactose. 115–117,120,122,126,127,129,130,133,135 Lactose malabsorption was diagnosed following lactose tolerance tests by the hydrogen breath test in 13 of the studies,108–120 and blood glucose test in 11 studies.121–131 Diagnosis based on urinary galactose concentration was reported in one study132 and biochemical method of diagnosis was not reported in three trials.133–135 Half of the trials included lactose digesting controls.110–113,116,120,122,125–129,133,135
Overview of Findings
Existing studies do not fulfill the ideal criteria described above. The vast majority of studies of LI have been small (<30 subjects). While age and ethnicity of the subjects is often provided, tolerance to lactose of these subgroups of subjects has not been studied. While subjects are routinely tested for LM, only a few studies have then tested the intolerant subjects in blinded fashion with increasing doses of lactose administered throughout the day to determine the daily tolerable dosage of lactose. Most studies have utilized a single dose of lactose and a lactose free control administered in water or milk without food, frequently in not totally blinded fashion (i.e., the taste of low lactose milk differs from milk). The statistical rating of symptoms is rarely related to biological significance. The probability that a given dose of lactose induces more symptoms than the control treatment has been assessed by standard statistical tests of the differences between group means. No attention has been paid to the possibility of outliers, i.e., selected subjects who consistently might be particularly sensitive to lactose induced symptoms. In contrast to the massive amount of data on LM and ethnicity, published data do not allow one to determine if the daily tolerable dose of lactose in lactose malabsorbers differs by age and ethnicity. Thus, it can be stated, a priori, that it is not possible to provide reliable answers to many of the questions raised in this section of the report. Results were heterogeneous in terms of patient populations, interventions, assessment methods, and outcome definitions, thus precluding pooling. We provide a description of the individual studies and their results stratified by key study design characteristics of interest.
Experimental Studies of the Tolerance of Individual Subjects to Lactose
A wide variety of methodologies have been employed to assess the ability of subjects to tolerate lactose. The vast majority of studies initially dosed a group of volunteers with a high (30 grams to 50 grams) dose of lactose, and the subjects were classified as malabsorbers or absorbers based on breath H2 measurements or blood glucose rise. In addition, the malabsorbing subjects were characterized as being lactose tolerant or intolerant based on the reporting of appreciable (variable from one study to the next) symptoms reported during this testing. A blinded control was virtually never employed during this portion of the study; thus, it is possible that some of the subjects categorized as lactose intolerant might have had similar symptoms following ingestion of a lactose free control solution.
In some studies only the lactose intolerant individuals were then tested in some sort of blinded fashion with a dosage or dosages of lactose, while in other studies both the lactose tolerant and the intolerant subjects were tested. The lactose free or lactose reduced milks that served as the controls usually were produced by prehydrolysis of milk with lactase, a process that produces a milk sweeter than that of conventional milk (glucose and galactose released from lactose is sweeter than lactose). Some studies did not blind for this taste difference, while other studies employed a variety of methods to disguise this taste difference, including the addition of an artificial sweetener to milk, chocolate, and commercial lactose free dietary supplements. A sizable variability of the response of malabsorbers to the placebo was observed in various studies, ranging from nil in some studies to very appreciable in others. In addition, there was large inter-study variability in the response of the absorbers/lactose tolerant to the lactose containing or lactose free treatments. A striking example of the potential for nonlactose induced symptoms in this testing was provided by the study of Haverberg, et al.,126 in which 32 percent of lactose absorbers reported symptoms after ingestion of 480 ml of lactose free milk. A further example of the potential importance for taste blinding was the study of Reasoner, et al.,125 in which the addition of 0.2 percent glucose to milk reduced the symptomatic response to milk. Presumably this low concentration of glucose induced its effect via an influence of the taste of the milk rather than lactose digestion/absorption. Some studies have administered lactose (or low lactose controls) with meals, while most studies have employed a single dose of milk or control ingested without food (usually in the morning after arising). The former is more physiological, while the latter eliminated the confounding effect of other food on symptom response.
Studies Using a Range of Dosages of Lactose
The study of Hertzler and Savaiano118 provided the literature’s most optimistic appraisal of the daily dosage of lactose that is tolerable by lactose intolerant subjects. Eighteen healthy young adult subjects with self diagnosed LI were demonstrated to be lactose malabsorbers. In a randomized, double blind crossover study, subjects received either sucrose or lactose for a 10-day period with a 2-day washout between feeding of the opposite sugar. The initial daily dosage of the sugar (lactose or sucrose) was 42 grams in evenly divided doses with meals, and this dose was incrementally increased to 70 grams/day over the 10-day period. Comparison of the daily symptom records showed no statistically significant difference between the sucrose and lactose feeding periods for any dosage of the sugars. Thus, subjects had negligible symptoms at the initiation of lactose feeding (42 grams per day) and by the end of the 10-day period, were tolerating 70 grams (almost 1.5 quarts of milk) per day. If the results of this study of 18 self diagnosed lactose intolerant subjects could be extrapolated to the universe of lactose intolerant individuals, LI would not represent an appreciable clinical problem, provided lactose was routinely ingested in divided doses with meals. The investigators attributed the apparent extraordinary tolerance to lactose at the end of the feeding period to adaptation of the colonic flora towards bacteria that ferment lactose via nongas producing pathways. Lactose ingestion was associated with a nonsignificantly greater flatus and diarrhea severity score on virtually each of the 10 days of the study, and a statistical analysis of the sum of the 10-day records, if provided, may have demonstrated a significant (but small) increase in symptoms with lactose.
Stephenson et al.,131 studied 14 healthy young adult subjects who were intolerant to a 50 gram dose of lactose. The subjects were then fed increasing dosages of lactose in water or in milk, with tolerance to lactose defined as two or less mild symptoms following lactose ingestion. All subjects tolerated the 15 gram dose, the vast majority tolerated 30 grams, while only 5/14 tolerated a 50 gram or greater dosage. Thus, 30 grams was the usual tolerable dose. Subjects were not blinded nor were the dosages of lactose randomly assigned.
Newcomer et. al.120 randomly fed 59 Native American lactose malabsorbers (three children and 56 adults) dosages of lactose ranging from 0–18 grams with a sweet roll and 8 ounces of Ensure® to disguise the difference in tastes of the test meals. Any symptom greater than slight was considered an appreciable problem. There was no significant correlation between the dosage of lactose and the frequency of appreciable symptoms up to a dosage of 18 grams of lactose. Jones, et al.130 fed variable doses of lactose in the form of milk or lactose reduced milk with breakfast to 16 lactose malabsorbers. Symptoms were comparable for 7.5 grams and 15 grams lactose dosages, but a significant increase in symptoms was observed with 30 grams. In a second study in this paper (15 subjects) symptoms were similar for placebo and milks containing 10 grams of lactose; however, symptoms increased significantly (p<0.05) when lactose dosage was increased to 25 grams. No effort was made to disguise the taste of the milk. This study shows that up to 15 grams of milk is tolerated by an unselected group of lactose malabsorbers, whereas 25 (or 30 grams) yields a statistically significant increase in symptoms.
In a study by Cavalli-Sforza, et al.122 40 adult lactose malabsorbers were randomly fed four different doses of lactose, each test period lasting 4 days. Dosages were 125, 250, 500, and 1,000 ml/day of milk or lactose hydrolyzed milk. A significant positive correlation between increasing dosage and symptoms was observed with milk. The percentage of subjects reporting symptoms with the 125 ml, 250 ml, 500 ml, 1,000 ml dosages were about 30 percent, 45 percent, 55 percent, and 65 percent, respectively. The symptomatic response to low lactase milk was about 10 percent less at each dosage. Symptoms seldom were severe. This study suggests that the frequency of mild symptoms increases with increasing dosage of lactose over the range of 125 ml of milk (6 grams of lactose) to 1,000 ml of milk (50 grams of lactose), with no clear-cut threshold for tolerance versus intolerance. Given the sizable percentage reporting symptoms with lactose hydrolyzed milk, lactose was only partially responsible for this symptom response.
Hertzler et al.119 fed 13 healthy adult lactose malabsorbers varying dosages of lactose (0 to 20 grams) in water without other food. Authors masked taste differences with aspartame. A statistically significant increase in symptoms was observed when the dose of lactose reached 20 grams, although mean symptom severity score was less than “slight.” Results suggest that the ability of lactose malabsorbers to ingest lactose without detectable symptoms occurs between a 12 gram and 20 gram dosage of lactose when the sugar is administered in water without other food.
Two studies of adolescents investigated the response to 240 and 480 ml of lactose-containing and lactose-free milk. Haverberg, et al.126 studied 43 lactose absorbers and 67 malabsorbers where the flavors were disguised with chocolate. There was no significant difference in symptomatic response of malabsorbers and absorbers to the 240 ml (12 grams lactose) dose nor was the response of malabsorbers to the two types significantly different. These comparisons showed greater differences for the 480 ml dosages. It was calculated for the lactose malabsorbers that the lactose content of 240 ml and 480 ml of milk might have induced symptoms in 5 percent and 24 percent of the subjects, respectively. The majority of the symptoms reported after milk ingestion by these subjects (particularly with the 240 ml milk dosage) were caused by factors other than LI. Kwon et al.,127 using similar methodology to that of Haverberg et al.,126 studied 45 malabsorbers and 42 absorbers. With the 240 ml dosage of milk, a higher percentage of absorbers (19 percent) had symptoms with the lactose containing milk than did malabsorbers (9 percent). However, with 480 ml of milk, a greater percentage of malabsorbers (27 percent) had symptoms versus absorbers (17 percent) and a greater percentage of the lactose malabsorbers had symptoms with the lactose containing (27 percent) than with the lactose free milk (16 percent). Statistical significance was not computed. This study showed that lactose malabsorbers tolerate the lactose content (11 grams) of 240 ml of milk, but a percentage of these subjects (about 16 percent) apparently experience lactose induced symptoms from a 22 gram dose of lactose (480 ml of milk).
Lybeck-Sorenson et al.134 tested 35 well nourished Latin American malabsorbers with 250 ml or 500 ml of lactose-containing and a low lactose milk from which 86 percent of the lactose had been removed. The products were said to be similar in taste and consistency. Doses of lactose fed (with a light breakfast) were 1.6 grams (250 ml, lactose-reduced milk), 3.2 grams (500 ml, lactose reduced milk), 11.3 grams (250 ml milk), 22.5 grams (500 ml milk), and 50 grams (lactose tolerance test). The respective median symptom scores for these lactose loads were 0.3, 0.2, 0.5, 1.1, and 6.1, with a maximal score of 12. No significant increase in symptoms was noted between conventional and low lactose milk at the 250 ml dosage, while a significant increase was noted with the 500 ml dosage, although symptoms tended to be slight (score 1.1 out of 12). When the lactose dosage was increased to 50 grams (1,000 ml of milk), symptoms became appreciable (score 6.1 out of 12), although there was no control for this phase of the study. This study demonstrates that 11.3 grams of lactose was tolerated, 22.5 grams yielded mild symptoms, and 50 grams was clearly intolerable.
Lisker et al.129 studied 97 lactose malabsorbing, healthy adult Mexican subjects. The subjects received 250 ml of milks containing 0 grams, 12.5 grams, and 37.5 grams of lactose, with taste difference disguised with chocolate. Compared to the lactose free preparation, the 12.5 gram dose induced a highly significant increase in symptoms (16 percent were severe) and the 37.5 gram dose resulted in very severe symptoms in 71 percent of subjects. This is the only study using multiple dosages of lactose in which appreciable symptoms were observed with 12 grams of lactose.
Vesa et al.112 tested 39 lactose malabsorbers with 250 ml of lactose free milk to which lactose was added in quantities of 0, 0.5, 1.5, and 7 grams. Symptoms were not significantly different for the various doses, showing that malabsorbers can tolerate small amounts of lactose (7 grams), such as might be used in coffee or cereal.
The above studies involving the feeding of incremental dosages of lactose to determine the amount of lactose tolerated by lactose intolerant subjects were all carried out with adult subjects, and no data were provided to correlate tolerance with age or ethnicity. All but one of the studies assessed tolerance to a single dose of lactose (frequently without food) and thus provided no data on the daily dosage of lactose that might be tolerated, assuming tolerance is improved if lactose intake is distributed throughout the day with meals. The one study that investigated symptoms when lactose was ingested for 1 week with each of the three meals showed that up to 70 grams of lactose/day could be tolerated without appreciable symptoms.118 The results of single feeding studies generally demonstrated that a 12 gram dose of lactose (one cup of milk) produces negligible symptoms with intolerance occurring at dosage ranging between 20 and 50 grams of lactose.
Studies Comparing Symptoms Resulting from the Ingestion of One Dosage of Lactose Versus that of a Lactose Reduced or Lactose Free Treatment
Adult and adolescent studies: Evaluating daily dosage of approximately 12 grams of lactose (250 ml of milk). Suarez et al.113 recruited 30 subjects who self reported extreme intolerance to milk. Nine of these subjects were demonstrated to be lactose absorbers via breath testing. This finding, which was observed in other studies, demonstrates the tendency of subjects to misdiagnose themselves as lactose intolerant. For 1-week periods, the lactose malabsorbers ingested 250 ml/day of conventional milk with their usual breakfast and during another week they receive 250/ml of lactose hydrolyzed milk, the taste difference masked with an artificial sweetener. There were no statistically significant differences in symptoms (gas, flatulence, abdominal discomfort, bloating) between the two testing periods. A surprising finding of this study was that symptoms were trivial during both testing periods, despite the pre-study perception of the subjects that lactose induced severe symptoms.
The finding of negligible symptoms with 12 grams of lactose was also observed by Rorick et al.116 in a study of 87 healthy elderly subjects (mean age 77). Either 240 ml of milk or 240 ml of lactose free milk (taste disguised with chocolate) was fed to 64 lactose absorbers and 23 lactose malabsorbers without food. The percentage of subjects with symptoms was similar (about 70 percent) for the absorbers and the malabsorbers. The percentage of subjects reporting symptoms to lactose-containing but not lactose-free milk (i.e., “lactose intolerance”) was actually higher for the lactose absorbers versus the malabsorbers.
Paige et al.135 studied 22 African American adolescent malabsorbers. Subjects received three 240 ml treatments: whole milk (12 grams of lactose), 50 percent lactose hydrolyzed milk (6 grams of lactose), and 90 percent lactose hydrolyzed milk (1.2 grams of lactose). Symptoms were reported by 3/22 subjects after ingestion of conventional milk, but two of these three subjects also had symptoms after ingestion of the 90 percent hydrolyzed milk. Thus, 1/22 subjects may have had symptoms attributable to LI.
In contrast, several groups have reported appreciable symptoms after ingestion of approximately 12 grams of lactose. Johnson, et al.114 fed 315 ml of milk (about 15 grams of lactose) or lactose free milk (taste difference disguised with artificial sweetener) to 45 lactose malabsorbing, young adult African Americans. Symptoms were reported by 100 percent of subjects with the lactose containing milk; however, 33 percent had symptoms with lactose free milk as well. Thus, 67 percent of this group of African American subjects appeared to have symptoms attributable to lactose, although the severity of symptoms was not studied. Brand et al.115 compared the symptomatic response of six lactose absorbers to conventional milk with that of lactose reduced milk with no blinding for taste differences. Five subjects had at least one symptom of flatulence, diarrhea, or cramps with conventional milks, whereas no subjects reported symptoms with 95 percent hydrolyzed milk. Symptom severity was recorded but not presented, other than that mild to moderate diarrhea was reported by three of the six subjects. Reasoner et al.125 studied nine milk intolerant individuals (defined as responding to a 50 gram lactose challenge with a positive breath test and appreciable symptoms). While multiple milks were tested, the three types pertinent to this study were conventional skim milk, conventional skim milk with added glucose (0.2 percent), and low lactose milk (approximately 80 percent lactose hydrolyzed). Taste differences were not disguised. The average scores for pain and gas were statistically significantly higher (“moderate”) for untreated skim milk versus the lactose hydrolyzed milk where symptoms were slight. No significant difference was observed for flatulence. Of interest, symptoms with the skim milk containing 0.2 percent glucose, added to simulate the taste of the hydrolyzed milk, induced less symptoms than did the skim milk. Although not analyzed statistically, differences between this milk and lactose hydrolyzed milk appeared to be insignificant.
Daily dosage of 18 to 25 grams of lactose (350 ml to 500 ml of milk). Suarez et al.111 studied the symptoms of 32 lactose malabsorbers when they ingested 240 ml of milk or lactose free milk with breakfast and dinner for 1-week periods (24 grams of lactose daily x 7 days with lactose-containing milk). Differences in milk flavors were disguised with artificial sweetener. While each of the symptoms was scored higher during the lactose ingestion period, none of the difference reached statistical significance. Mean symptom scores for gas, bloating, abdominal pain, and diarrhea were trivial with both types of milk. Of interest, symptoms during both the 24 gram lactose and the zero lactose test periods were significantly higher if, prior to testing, the subjects deemed themselves to be lactose intolerant.
Vesa et al.132 studied 30 Estonian malabsorbers. Subjects ingested 200 ml of conventional or lactose free milk twice daily (with breakfast and before lunch) for 2-day test periods (about 20 grams of lactose daily). No significant differences in symptoms were observed between the periods when subjects ingested lactose-containing versus lactose free milk.
Lin et al.117 fed 400 ml of milk (20 grams of lactose) to 20 healthy malabsorbers with a placebo or one of four different commercial beta-galactosidase preparations. The numerical ratings of symptoms of gas, “stomach” pain, and diarrhea were significantly less when each of the beta-galactosidase preparations was ingested with milk compared to milk ingested with placebo. However, the symptoms seemingly were relatively minor with a severity score, out of a maximum of 40, being 7.85 for gas and only 1.55 and 1.20 for “stomach pain” and diarrhea, respectively.
Montalto et al.108 studied 30 lactose malabsorbers who ingested 400 ml of milk (about 20 grams of lactose). The treatments consisted of conventional milk, milk pretreated with lactase, and milk taken 5 minutes after ingestion of a commercial beta-galactosidase preparation. The treatments were not blinded. Symptoms were significantly (p<0.001) higher for tests in which the milk was ingested without pretreatment with lactase. The mean overall symptom severity score when conventional milk was ingested without lactase was about 4, apparently out of a maximum of 12.
Rosado et al.133 studied 25 Mexican malabsorbers who ingested 360 ml of milk (18 grams of lactose) with and without pretreatment of the milk with a commercial beta-galactosidase. The study was not blinded. Symptoms were observed in 12 of 25 subjects with untreated milk and four of 12 with enzyme treated milk, and the median symptom grade in the 12 subjects ingesting untreated milk was “major.” A sizable reduction (p<0.01, paired t test) was observed in severity score with beta-galactosidase treated milk.
Rask Pedersen et al.124 studied 11 Danes with lactose malabsorption. Subjects received 500 ml of milk (25 grams of lactose) or lactose hydrolyzed milk without other food, apparently with no blinding for taste differences. Symptoms of diarrhea and flatulence were severe in 5/12 with milk and only 1/12 with lactose hydrolyzed milk, and statistical analysis showed the reduction in symptoms was significant (p<0.02).
Summary of results with 18–25 grams of lactose. The results of studies performed with 18 to 25 grams of lactose ranged from excellent tolerance by malabsorbers111,132 to a high frequency of appreciable intolerance symptoms.108,124,133 The two studies111,132 in which tolerance was observed supplied lactose in divided doses with meals, while studies that showed appreciable intolerance supplied lactose as a single dose without food. These few observations suggest that a daily dose of 18 to 25 grams of lactose may be tolerable to lactose malabsorbers if lactose intake is distributed throughout the day with meals.
Lactose dosage greater than 25 grams/day. Suarez et al110 enrolled 62 women, 31 lactose absorbers and 31 lactose malabsorbers, in a study to determine the tolerance to a diet that supplied 1,300 mg of calcium per day in the form of dairy products. To this end, for 1-week periods, each day the subjects ingested 480 ml of milk (240 ml at breakfast, 240 ml at dinner), 240 ml of yogurt at lunch, and 56 grams of hard cheese. One week the subjects ingested conventional products that had a total lactose content of 34 grams, and in another week the lactose in the milk and yogurt prehydrolyzed via treatment with beta-galactosidase (this diet contained 2 grams of lactose per day). Subjects rated symptoms twice daily on a zero to five scale. Perception of rectal gas, frequency of gas passages, bloating, and frequency of bowel movements all were significantly (p <0.05) greater when the lactose malabsorbers ingested the lactose rich diet. However, the mean symptom score seldom exceeded “slight.” No significant differences were observed between the two treatment weeks by the lactose absorbers. Despite the higher symptom severity scores recorded during the high lactose week, when queried as to the week they perceived their symptoms were greater, 15 identified the high lactose week, eight the low lactose week, and eight noted no difference (p = 0.21). Two-thirds of the malabsorbers felt that the symptoms during the high lactose week were less severe than they anticipated. A roughly equal percentage (about 50 percent) of lactose absorbers and malabsorbers indicated a willingness to obtain their calcium via the lactose rich diet, and conversely about 50 percent in each group indicated that they would prefer to obtain their daily calcium requirement via ingestion of calcium tablets. This study suggests that if dairy products are supplied as two cups of milk (distributed throughout the day), yogurt, and hard cheese, LI is not a major impediment to the daily ingestion of 34 grams of lactose.
Cheng et al.128 studied 15 Chilean penitentiary inmates who were lactose malabsorbers. For 30 days the subjects ingested a baseline diet which included 500 ml of low lactose milk taken twice daily at 8:30 am and 4:30 pm. On three occasions on weeks 2, 3, and 4 of this regimen, conventional milk sweetened with 5 percent sucrose was substituted for the low lactose milk. A marked increase in the frequency and severity of abdominal pain, diarrhea, distension, and flatulence (p <0.001 for each symptom) was observed on the days that conventional milk was substituted for the low lactose milk. No such increase in symptoms was observed in lactose absorbers. Although probably not perfectly blinded, this study indicated that in subjects ingesting a diet low in lactose, 50 grams of lactose in two divided doses during the day yields severe intolerance symptoms.
Xeno et al.121 dosed lactose malabsorbers with 100 grams of lactose in water, with a placebo or tablet, or a tablet containing beta-galactosidase. Symptoms were rated on a zero to four scale. While symptoms appeared to be more severe during the placebo phase of the study, no statistical analysis of the results was performed. Severe symptoms with the placebo were reported for abdominal cramping (3/8), bloating (1/8), flatulence (2/8), and diarrhea (2/8), and 2/8 reported vomiting. No severe symptoms were reported when beta-galctosidase was ingested. While the marked intolerance to 100 grams of lactose taken as a single dose was not unexpected, this study was unique in its use of such a large dose of lactose.
The studies testing the tolerance of lactose malabsorbing subjects to a single dose of lactose yielded discordant results. Multiple studies showed no appreciable increase in symptoms with the 12 gram dose, while others showed appreciable symptoms. The explanation for this discrepancy is not clear. When the dosage of lactose was increased to 18 to 25 grams, once again, the finding of intolerance varied between studies. However, the difference in tolerance observed in these studies could be explained by the better tolerance of lactose if the ingestion of this sugar was distributed throughout the day as opposed to ingestion of lactose as a single dose without food.
Studies in children. The tolerance of children to a given dose of lactose might differ from that of adults because of differing physiology in children and/or the greater dosage/kg of body weight.
Gremse et al.109 studied the effect of lactose on unexplained abdominal pain in 30 children (mean age 11.4 ± 2.5) lactose malabsorbers. The subjects were provided with 250 ml of regular or lactose hydrolyzed milk (taste disguised with artificial sweetener) for 2-week periods. Their abdominal pain scores increased from 4.1 on lactose free milk to 7.5 on lactose containing milk, a difference with a p value of 0. 021. No significant differences were observed for flatulence, diarrhea, or bloating. The mean pain score observed with milk (7.5) appeared to be trivial, given a maximal possible score of 54. Only 5/30 subjects appear to have had an appreciable increase in pain with introduction of lactose, and four of these subjects had the highest pain scores on the lactose free diet. Thus, the pain of these subjects was aggravated, but not solely caused, by lactose.
Nielsen et al,123 studied the tolerance of nine lactose malabsorbing children (mean age 10, range 9–16) via the feeding of 500 ml of conventional or lactose hydrolyzed milk, with no effort to disguise taste differences. Symptoms of abdominal pain, flatulence, and diarrhea were very significantly greater after ingestion of the nonhydrolyzed milk. Thus, clear-cut LI to a 25 gram dose of lactose was observed in these nine children. On a lactose dosage per kg body weight basis, the 25 gram dose to 10 year olds was roughly equivalent to a 50 gram dose for middle age adult subjects.
Summary
What amount of daily lactose intake is tolerable in subjects with diagnosed lactose intolerance? How does this differ by age and ethnicity? What are the diagnostic standards used? A number of problems arose when we attempted to answer these seemingly straightforward questions via a review of the existing literature.
- Patients enrolled in the studies did not have “diagnosed lactose intolerance.” The standard approach to the classification of patients enrolled in studies of intolerance was the demonstration via hydrogen breath testing or blood glucose measurements that the subject incompletely absorbed a sizable dosage of lactose (30 to 50 grams). While most studies recorded symptoms with this dosage of lactose, this information was seldom used in the selection of study subjects. Thus, the vast majority of the studies investigated subjects with proven LM, not proven LI.
- Although very seldom discussed in the literature, tests for LM are not 100 percent accurate. Most studies used H2 breath testing to identify lactose malabsorbers. It is known that this test has an appreciable, but not well defined, false negative rate, i.e., subjects with LM do not generate a diagnostic rise in breath H2. The incidence of false positives, i.e., production of the H2 in the small bowel with complete absorption of lactose, is not known. Thus, some patients were incorrectly classified as lactose malabsorbers or absorbers.
- The taste of conventional milk and lactose hydrolyzed milk differ. Many studies did not disguise this taste difference.
- Lactose was administered in a variety of ways in the intolerance tests. Most studies fed lactose in water or milk as a single does in the fasting state upon arising in the morning. The daily tolerable dose of lactose appears to be greater if lactose intake is distributed throughout the day and taken with meals.
- The response to lactose is primarily subjective symptoms – i.e., abdominal discomfort, gas, bloating – the severity of which the subjects rated on numerical scales. The finding of a statistically significant increase in symptoms with the lactose containing product versus the low lactase product was considered to provide evidence of intolerance. However, the biological significance of changes in numerical rating seldom was investigated. Only one study attempted to evaluate the association between the symptom score and the global assessment of symptom severity. In this study, the majority of a group of subjects who had a significant increase in symptom score when high and low lactose test periods were compared did not clearly identify the high lactose period as being particularly symptomatic.
- Some data supports the belief that the routine ingestion of lactose increases the quantity of lactose that is tolerable. Very few studies provided data on lactose ingestion by subjects prior to enrollment in controlled trials.
With the above problems in mind, the literature on this question can be summarized as follows: As shown in Figure 12, the majority of studies indicate that subjects with “lactose intolerance” can ingest 12 grams of lactose as a single dose (particularly if taken with food) with no or minor symptoms. In contrast, when lactose/milk is administered as a single test dose without other nutrients, dosages of 12 grams may be symptomatic (Figure 13). As the dose is increased above 12 grams, intolerance becomes more prominent, with single doses of 24 grams usually yielding appreciable symptoms. There is some evidence that if 24 grams of lactose are distributed throughout the day, many lactose malabsorbers will tolerate this dosage. Lactose in a dose of 50 grams induces symptoms in the vast majority of subjects. While the literature is laden with studies of the relationship of ethnicity to lactose malabsorption, no studies made it possible to determine if lactose malabsorbers of differing ethnicities have differing tolerance to lactose. Likewise, there was no data on the relationship of age or sex to the quantity of lactose that can be tolerated by lactose intolerant subjects.
Key Question 4. What strategies are effective in managing individuals with diagnosed lactose intolerance?
The details of our search strategy are presented in the methods section and in Figure 2. A total of 37 unique randomized studies (26 on lactase/lactose hydrolyzed milk supplements, lactose reduced milk, eight on probiotics, two on incremental lactose dose for colonic adaptation, and one on other agents) met inclusion criteria.108–147 The quality of the studies was low, with almost no study reporting adequate allocation concealment. Generally, studies had small sample sizes and reporting of symptoms was variable or not reported: composite scores of four to five symptoms or individual symptoms such as abdominal pain, diarrhea, bloating, and flatulence were reported, either as means or proportion. Many studies enrolled individuals who did not have a prior diagnosis of LI or did not have a prior history of LI like symptoms.
We focused our results on strategies grouped in the following categories, discussed below:
- Commercially available lactase/lactose hydrolyzed milk or nonlactose solutions and other dietary strategies
- Prebiotics and probiotics
- Incremental lactose for colonic adaptation
- Other strategies
Commercially Available Lactase/Lactose Hydrolyzed Milk, or Nonlactose Solutions
Characteristics of included studies. There was one study representing two trials that tested lactase supplements Lactodigest, DairyEase, and Lactaid,136 while the remaining 25 studies reported on lactose reduced or hydrolyzed milk by adding a lactase enzyme such as beta-galactosidase to the milk. Studies enrolled between six and 150 subjects. Women constituted 56 percent of the subjects (n=23 studies). The mean age of subjects was 37 years of age, with a range between 10 and 77 (n=19 studies). Six trials included children or adolescents.109,114,123,126,127,135 One trial enrolled elderly subjects (mean age 77 years).116 Within the 19 studies reporting race or ethnicity, 40 percent of subjects were white, 30 percent Hispanic, 20 percent black, and 9 percent Asian.109–116,123,126–130,133–135,137 Nineteen studies utilized commercial lactase products or hydrolyzed milk,108–111,113–115,121–125,128,133,135,136 two used milk products with lactose removed by ultrafiltration or chromatographically,112,134 and five assessed nonlactose solutions.116,126,127,137,138 Unclear or unreported methods of lactose removal were noted in two trials129,132 Subjects in 18 studies reported abdominal symptoms compatible with malabsorption of lactose prior to study entry.108–114,121,123–125,128,130,132,134,136–138 Abdominal symptoms were not required for study participation (based solely on biochemical diagnosis) or subjects were not reported to experience symptoms following ingestion of lactose in ten studies.115,116,122,126,127,129,130,133,135,136 LM was diagnosed following lactose tolerance tests by the hydrogen breath test in 11 of the studies108–116,136 and blood glucose test in 13 studies121–130,137,138 Diagnosis based on urinary galactose concentration was reported in one study132 and biochemical method of diagnosis was not reported in three trials.133–135 Over half of the trials included lactose digesting controls.110–113,116,122,125–129,133,135,137
Among the 18 studies that enrolled symptomatic subjects at baseline, 13 utilized lactose doses greater than 12 grams, comparable to one cup of milk.108,110,111,114,121,123–125,128,130,132,134,136,137 Hydrolyzed lactose doses typically ranged from zero to two grams per dose. In most of the studies, the lactose dose was consumed in a single serving. In six trials, the lactose dose was administered over multiple intervals per day for at least part of the study.110,111,122,125,128,132
Results. We found insufficient evidence that lactose reduced solution/milk, with lactose content of 0–2 grams, is effective in reducing symptoms among individuals with LI. Seven studies, representing nine comparisons that enrolled individuals who had symptoms compatible with LI reported inconsistent results that lactose reduced preparations reduced overall symptom scores compared to controls. None of the four studies reported a significant improvement in overall symptoms compared to control preparations of up to 12 grams of lactose. However, as noted in key question 3, doses of 12 grams of lactose or less are well tolerated and produce minimal to no symptoms. When compared to controls given greater than 12 grams of lactose, only two out of five trials reported statistically significant reductions in overall symptoms with lactose reduced/ hydrolyzed milk. Results for individual symptoms of abdominal pain, diarrhea, flatulence, and bloating were also inconsistent.
For all included studies, regardless of symptom history, information from 16 (19 comparisons), mostly low quality, trials was insufficient to determine the effect of hydrolyzed milk, lactase, or non lactose preparations in reducing GI symptoms compared to lactose controls. Some studies did report substantial reductions (improvement from moderate and severe to mild or none, or an absolute reduction of at least 50 percent) in abdominal pain/cramping109,112,123,125,134 and diarrhea136 with use of lactose reduced solution/milk, with lactose content of 0–2 grams, compared to a lactose dose of 12 grams or more. However, even in studies where symptoms were reduced, statistically significant reductions were not consistently observed among all symptoms reported, or only a subset of symptoms was reported. For example, the overall symptom score was significantly reduced by 60 percent with 591 milliliters of lactose reduced milk containing 7.5 grams of lactose compared to a similar amount of milk with 30 grams of lactose,130 and by 13 percent with low lactose skim milk with 0.8–6.5 grams of lactose compared to skim milk with 6.1–49 grams of lactose,122 but the subjects in both studies were not symptomatic at enrollment, and improvement in individual symptoms was not provided. Mean and total symptom scores were also reduced, from 3.7 to 0.36 with 70 percent hydrolyzed milk compared to placebo with 20 grams of lactose,108 but subjects were also not symptomatic at enrollment, and improvement in individual symptoms was not provided. One study reported a score of 46 for skim milk with 11.3 grams of lactose which was reduced to a score of 17 with low lactose milk with 3.2 grams of lactose, but the difference was not statistically significant.134 Similar reductions were seen in summed scores for abdominal pain from 43 with milk containing 25 grams of lactose to one with lactose hydrolyzed milk containing 1.25 grams of lactose123 and a mean score for abdominal pain from 7.5 with milk containing 12 grams of lactose to 4.1 with milk containing lactase,109 both in children. Again, neither study required subjects to be symptomatic at baseline. One study showed a statistically significant reduction in abdominal pain from moderate to none or mild with low lactose milk containing 2.9 grams of lactose compared to skim milk containing 28.5 grams of lactose.125 One trial found a significantly greater percentage of subjects reporting abdominal pain and bloating compared to the 0.5 grams and 1.5 grams doses, respectively.112 Compared to placebo, use of lactase supplement Lactodigest, DairyEase, or Lactaid in doses of two to four capsules/tablets when taken with 400 ml of 2 percent milk containing 20 grams of lactose reduced overall symptom scores in subjects not symptomatic at enrollment. Of greater clinical relevance to management of patients with symptoms compatible with LI who wish to consume doses of lactose beyond the minimally tolerable dose, these products were found not to reduce symptoms when administered with a dose of 50 grams of lactose in subjects who had symptoms compatible with LI.136 Generally, studies had small sample sizes and reporting of symptoms was variable: composite scores of four to five symptoms or individual symptoms such as abdominal pain, diarrhea, bloating, and flatulence were reported, either as means or proportion, making pooling estimates difficult.

Figure 14
Percentage of subjects reporting abdominal pain.
Figure 15
Abdominal pain based on symptom scores (0 = none, 1 = mild, 3 = moderate, 5 = severe).
Prebiotics and Probiotics
Characteristics of included studies. Eight randomized trials were included; (Appendix Table D8) seven crossover117,139–141,143–145 and one parallel group design.142 The trials were generally small, enrolling between nine and 28 subjects (Table 18). Among the five studies reporting gender, women constituted 34 percent of the subjects.139–143 Two studies enrolled only male subjects.142,143 Subjects were typically young to middle-aged adults (between 18 and 45 years old), and only one study enrolled subjects older than 60 years of age.144 Half of the studies reported race or ethnicity. White subjects comprised two trials,140,141 one study evaluated black African immigrants to France,142 and one trial was conducted in Taiwan Chinese.117 Five of the studies were conducted in the United States,139,140,143–145 two in France,141,142 and one in Taiwan.117 Five trials assessed probiotic test products, prepared by adding strains of lactobacillus acidophilus, lactobacillus bulgaricus, or bifidobacterium longum to milk prior to consumption.117,139,140,144,145 Four studies evaluated yogurt products.141–143,145 LM was diagnosed by the hydrogen breath test in all studies.
Table 18
Summary of study characteristics for blinded LI treatment studies.
Results. We found insufficient evidence to determine the effectiveness of yogurt or probiotics to improve lactose intolerance symptoms (Table 19). The inclusion criteria and the studied type of yogurt and probiotics were variable—results either did not show a difference in symptom score, or reported clinically insignificant differences, mostly in the symptoms that are of low clinical relevance, such as flatulence. Only one study noted that the enrolled subjects reported symptoms compatible with malabsorption of lactose prior to study entry144 and reported no difference in symptom score in groups given milk or acidophilus milk (symptom score of 40 in both groups). In the remaining studies, study entry was based solely on breath hydrogen tests, and subjects were not reported to experience symptoms following ingestion of lactose. Lactose doses in the control tests were between 10 and 20 grams. Overall symptom score was reduced from 12.5 with 2 percent milk containing 20 grams of lactose to 2.8 with the same milk formulation but with added lactobacillus at 109 cfu/ml.117 Similar improvements were seen with the addition of lactobacillus at 108 cfu/ml (overall score 3.9) and lactoacidolphilus at 109 cfu/ml (overall score 6.5), but not with lactoacidolphilus at 108 cfu/ml. Overall symptom scores improved from fairly strong to mild with 400 ml of bulgofilus milk (Ofilus bacteria+L. bulgaircus) compared to control (lactulose 10 grams in 250 ml water), both with 18 grams of lactose.141 Reductions in other symptoms such as abdominal pain and diarrhea were either not reported, not significantly different, or of low clinical significance or relevance. The inclusion criteria were variable, the type, source, and concentration of yogurt and probiotics studied were variable, and no two studies studied the same agent. Based on these findings we found insufficient evidence for the use of yogurt or probiotics for lactose intolerance.
Table 19
Occurrence of GI symptoms in randomized trials.
Incremental Lactose for Colonic Adaptation
We found insufficient evidence to support the role of incremental doses of lactose for lactose intolerance symptoms (Table 19). Two studies met our inclusion criteria.118,146 In the first one, 20 healthy volunteers with LM on hydrogen breath testing were randomized to receive either dextrose or lactose in a blinded fashion for 10 days and crossed over for days 12 through 21. The dose of lactose and dextrose was 0.6 grams/kg body weight per day, increased by 0.2 grams/kg/day to a maximum of 1 gram/kg/day (approximately 42 to 70 grams of lactose per day for an average 70 kg adult). Subjects were also given lactose challenge doses of 0.35 grams/kg on days 11 and 22. The authors found that symptoms of flatulence after the lactose challenge decreased by 50 percent after lactose feeding compared to dextrose feeding, while symptoms of abdominal pain and diarrhea did not differ. These results suggest that colonic adaptation may occur, but there is no appreciable decrease in clinically relevant symptoms of abdominal pain and diarrhea. Though subjects were lactose malabsorbers at baseline, average symptom scores were 1 (scale 0–5) even with the highest doses of lactose (70 grams), and very similar to scores were seen with sucrose. The second study evaluated colonic adaptation to lactose compared to sucrose in a double blinded fashion. The study enrolled 46 healthy volunteers in France, 21 males, 25 females, all of Asian origin, with a mean age of 33 (range 20–47 years) that were lactose malabsorbing by hydrogen breath testing. Subjects were fed their regular diet and underwent hydrogen breath testing and symptom evaluation on days 1 and 14. For the 13 days in between, subjects were fed either 34 grams of lactose or sucrose in a double blind fashion. The overall clinical score improved from 42 to 20 in the group randomized to lactose, as did the individual mean scores for pain, flatulence, bloating and borborygmi, but similar improvements were seen with sucrose (overall score improvement from 42 to 24), suggesting a placebo response.
Other Strategies
We found insufficient evidence regarding rifaximin for treatment of lactose intolerance. A single small study met inclusion criteria147 and showed reduction in symptom score after rifaximin treatment compared to placebo and similar to a lactose free control. The study enrolled 40 patients with lactose malabsorption on hydrogen breath test, 16 were randomized to 10-day treatment with rifaximin 800 mg/day, 16 to a 40-day lactose free diet, while eight were given 10 days of placebo. On a scale of 0–4, compared to baseline, there was reduced abdominal pain (2.0 versus 1.0), diarrhea (1.3 versus 0.2), bloating (2.5 versus 1.6), and distention (2.4 versus 1.5) at day 40 for the rifaximin group. Similar decreases were seen for the lactose free group. The clinical significance of the change in score is not clear.
Studies on Management Strategies in Subjects with IBS and LM/LI
We found insufficient evidence that low lactose diet or probiotics were effective in reducing symptoms of lactose intolerance among subjects with IBS and LM/LI. Four small, double blinded, trials assessed management strategies in subjects with IBS and LI/LM with conflicting results.144,175–177 A British study of 23 IBS subjects identified with lactose malabsorption based on the hydrogen breath test found patients with LI were not distinguishable from other IBS subjects based on GI symptoms, and treatment with a low lactose diet led to disappointing results.175 They concluded there was no real advantage to segregating IBS subjects with LI from other IBS subjects. In contrast, a Dutch study investigating the prevalence of lactose malabsorption in 70 IBS patients found statistically significant improvement in GI symptoms in 17 IBS subjects identified to have LM following 6 weeks of treatment with a low lactose diet.176 They concluded that LM should be excluded prior to a diagnosis of IBS. A Mexican study of 12 IBS subjects, eight of whom were noted to be lactase nonpersistent, found that IBS symptoms appeared to be independent of LM following 3 months of treatment with hydrolyzed milk or placebo.177 An American study by Newcomer assessed whether unfermented acidophilus milk was beneficial in relieving symptoms in subjects with lactase deficiency or IBS.144 Sixty one subjects with IBS who were lactase sufficient and 18 lactase deficient (based on the hydrogen breath test) subjects each received lactobacillus acidophilus milk or regular milk for 2 week intervals each. Within the lactase deficient group, symptoms were not significantly reduced during the acidophilus milk period compared to the regular milk period. In subjects with IBS, acidophilus milk did not relieve their symptoms. These studies are summarized in Table 19, section F.
Footnotes
Appendixes and evidence tables cited in this report are available at http://www
.ahrq.gov/downloads /pub/evidence /pdf/lactoseint/lactint.pdf.
- Key Question 1: What is the prevalence of lactose intolerance? How does this differ by race, ethnicity, and age?
- Key Question 2. What are the health outcomes of dairy exclusion diets?
- Key Question 3: What amount of daily lactose intake is tolerable in subjects with diagnosed lactose intolerance?
- Key Question 4. What strategies are effective in managing individuals with diagnosed lactose intolerance?
- Other Strategies
- Results - Lactose Intolerance and HealthResults - Lactose Intolerance and Health
Your browsing activity is empty.
Activity recording is turned off.
See more...