This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
Continuing Education Activity
Renal calculi are a common cause of blood in the urine (hematuria) and pain in the abdomen, flank, or groin. They occur in 1 in 11 people at some time in their lifetimes, with men affected twice as much as women. Development of the stones is related to decreased urine volume or increased excretion of stone-forming components such as calcium, oxalate, uric acid, cystine, xanthine, and phosphate. Calculi may also be caused by low urinary citrate levels or excessive urinary acidity. Dietary and medications can modify many of the risk factors leading to nephrolithiasis. However, depending on the size and location of the stone, lithotripsy or percutaneous intervention may be required, especially if pyonephrosis develops.
This activity explores the cause, pathophysiology, presentation, and treatment of renal calculi, providing learners with a comprehensive understanding of this condition. The various risk factors contributing to nephrolithiasis and the dietary and medical interventions aimed at prevention and management are explored. Moreover, the course emphasizes the importance of an interprofessional team approach in caring for patients with renal calculi, highlighting effective communication and collaboration among urologists, nephrologists, radiologists, nurses, and dietitians to optimize patient outcomes and provide holistic care.
Objectives:
- Compare the different types of renal calculi and frequency in different patient populations.
- Evaluate the risk factors for renal calculi based on diagnostic testing and detailed patient history.
- Develop a comprehensive treatment plan for patients depending on risk factors, metabolic abnormalities, and results of diagnostic testing.
- Strategize care coordination among interprofessional team members to provide education and improve outcomes for patients affected by renal calculi.
Introduction
Renal calculi are a common cause of blood in the urine (hematuria) and pain in the abdomen, flank, or groin. They occur in 1 of every 11 people in the United States at some time in their lifetimes, with men affected 2 to 1 over women.[1] Development of the stones is related to decreased urine volume or increased excretion of stone-forming components such as calcium, oxalate, uric acid, cystine, xanthine, and phosphate.
Calculi may also be caused by low urinary citrate levels (an inhibitor of stone formation) or excessive urinary acidity.[2][3][4] Renal calculi may present with excruciating pain, and most patients present to the emergency department in agony. A single event does not cause kidney failure, but recurrent renal calculi can damage the tubular epithelial cells, leading to functional loss of the renal parenchyma.
Etiology
Urolithiasis occurs when solutes crystallize out of urine to form stones. Urolithiasis may occur due to anatomic features leading to urinary stasis, low urine volume, dietary factors (eg, high oxalate or high sodium), urinary tract infections, systemic acidosis, medications, or, rarely, inheritable genetic factors such as cystinuria.[5][6]
Most patients with nephrolithiasis (75%-85%) form calcium stones, most composed primarily of calcium oxalate (monohydrate or dihydrate) or calcium phosphate. The other main types include uric acid (8%-10%), struvite (calcium magnesium ammonium phosphate, 7%-8%), and cystine stones (1%-2%).
The most common causes of urinary stone disease are inadequate hydration and low urine volume. The 4 most common chemical factors contributing to urinary stone formation are hypercalciuria, hyperoxaluria, hyperuricosuria, and hypocitraturia.[7][8]
The 4 major types and causes of renal calculi include:
- Struvite stones: caused by Gram-negative, urease-producing organisms that break down urea into ammonia [14]
- Cystine stones: due to an intrinsic metabolic defect causing the failure of the renal tubules to reabsorb cystine, lysine, ornithine, and arginine;[15] visually opaque and amber
Of these, uric acid and cystine are the most likely stone types that develop recurrences.[16]
Many drugs are known to cause renal stones, including the following:
- Atazanavir
- Guaifenesin
- Indinavir
- Silicate overuse
- Sulfonamide
There also appears to be a genetic association with the production of renal calculi.[16] In some families, mutations may cause a defect in the renal tubular handling of calcium and other substrates.[24]
Nephrolithiasis risk factors include the following:
- A positive family history of urolithiasis increases the incidence of new stone formation 2.5-fold.
- A prior personal history of urolithiasis increases the risk of new renal stones by 15% during the first year and an average of 50% over the following 10 years.[25]
- Diabetes, hypertension, gout, male sex, metabolic syndrome, and obesity appear to increase the risk of urolithiasis.[26]
- Increased oxalate absorption (from malabsorption syndromes, gastro bypass surgery, and similar) can greatly increase urinary oxalate levels and calcium oxalate stone production.[27]
- Low urinary volume due to inadequate fluid intake leads to relative dehydration with increased urinary concentrations and crystal formation.[28]
- Urinary tract infections increase urinary pH in the presence of bacteria that produce urease, predisposing the patient to struvite calculi formation.[14]
In general, higher total dietary fiber, fruit, and vegetable intake, reduced animal meat protein, and a low sodium diet tend to reduce nephrolithiasis. A moderate calcium (dairy) intake is also recommended. Dietary risk factors include the following:
- High salt intake increases urinary osmolarity and promotes hypercalciuria.
- High animal protein (eg, meat) intake increases uric acid production, and hyperuricosuria stimulates proximal renal tubular citrate reabsorption and causes low urinary pH, hypocitraturia, and hypercalciuria.
- High oxalate foods lead to hyperoxaluria.
- Very low dietary calcium intake increases oxalate absorption; excessive calcium intake should also be avoided, as it causes milk-alkali syndrome and hypercalciuria.
- Inadequate fluid intake is a risk factor. (Need sufficient fluids to maintain a minimum of 2000 mL urine output daily.)
- Higher sugar-sweetened beverages: 1 daily sugar-sweetened soda increased urinary stone production by 22% to 33% compared to drinking <1 per week.
- Lower coffee consumption appeared to be a dietary risk factor for nephrolithiasis. (Individuals with the highest caffeinated coffee consumption enjoyed a much lower incidence of nephrolithiasis).
Epidemiology
Overall, urinary stone prevalence in the United States (US) has increased from 3.8% in 1970 to 8.8% in 2010.[1] This rate has continued to increase and was last reported as 10.1% in 2016.[16][41] Men have a higher rate of stone disease than women, roughly now at 2:1, although the rate of increase among women is increasing faster than among men.[16]
For patients with a history of a previous urinary stone, recurrence rates approach 50% at 10 years. There has traditionally been a high incidence of urinary stones in the Southeastern and South Central US, termed the “stone belt,” which probably reflects the hot weather climate and relative dehydration in these areas.[16][42][43]
Before the development of modern urologic techniques for treatment, mortality from untreated staghorn (infection) calculi was 27% (see Image. Staghorn Renal Calculus). Currently, mortality from stone disease is rare, although there is still a significant rate (28%) of renal deterioration with certain stone types, particularly staghorn (struvite or infection) stones and pyonephrosis (obstructive pyelonephritis).[44][45][46]
Kidney stone risk increases with age. The highest incidence is in men aged older than 80 years.[41] The increase in incidence in children and adolescents is rising faster than the general population.[16][47][48] Unlike adults, in the pediatric age group, the highest incidence of nephrolithiasis is among female adolescents.[49] Black and Hispanic populations have the lowest incidence of nephrolithiasis.[50] This does not represent genetic differences but socioeconomic factors, healthcare access, and dietary preferences.[16]
A systematic review demonstrated that first-time stone formers had a 26% median recurrence rate over the following 5 years.[51] A recent comprehensive meta-analysis identified risk factors that increased the likelihood of a kidney stone recurrence.[52] These risk factors were the following:
- Diabetes
- Family history of nephrolithiasis
- Hypertension
- Obesity (higher body mass index)
- Prior personal history of urolithiasis
- Surgical intervention associated with the first or earlier stones
- Uric acid urolithiasis
- White race
Nephrolithiasis has been linked to various systemic disorders such as cardiovascular disease, hypertension, chronic renal failure, diabetes, metabolic syndrome, and obesity.[16][53] For example, patients with nephrolithiasis and diabetes have lower average urinary pH levels and greater urinary oxalate than stone formers without diabetes. Both of these conditions significantly increase the risk of calcium oxalate nephrolithiasis.[54]
Patients with nephrolithiasis are also more likely to develop hypertension, atherosclerosis, strokes, myocardial infarctions, and particularly renal failure than the non-stone-forming general population.[16][55][56][57][58][59][60][61][62][63][64][65][66][67] Chronic renal failure and permanent kidney damage are more likely in patients with multiple nephrolithiasis recurrences, primary hyperoxaluria, and renal tubular acidosis, as well as in those with uric acid, struvite (infection), staghorn, and cystine stones.[16][67][68][69][70]
Multiple extracorporeal shockwave lithotripsy treatments do not appear to significantly or permanently affect renal function.[71][72] Global warming and climate change are expected to further increase the incidence of nephrolithiasis in the US by 30% by the year 2050, especially in warmer regions.[16][73] Worldwide, the incidence of kidney stones has increased over the last 3 decades, placing a greater financial burden on global healthcare delivery systems.[1][16][74][75][76]
Pathophysiology
Most urinary stones start as Randall plaque at the junction of the nephron's collecting tubule and the renal pelvis in the papilla. These plaques start in the suburothelium and then gradually grow until they break through the urothelium into the renal pelvis. They form an anchored lithogenic nidus for stone formation.
Once in continuous contact with urine, calcium oxalate layers typically start forming on the calcium phosphate nidus (all Randall plaques are composed of calcium phosphate). Calcium oxalate stones tend to form when the urinary pH is lower than 7.2, while calcium phosphate will form in more alkaline urine.
Hyperparathyroidism and similar metabolic disturbances like renal tubular acidosis typically form stones primarily or significantly composed of calcium phosphate. Overly acidic urine is the primary cause of uric acid stones (not hyperuricosuria).[77][78] Most renal calculi are made of radiopaque calcium compounds followed by radiolucent uric acid. Supersaturation of the urine is the common denominator in all cases of renal calculi.
In some cases, calcium oxalate stones may deposit in the renal papilla. Calcium phosphate stones usually precipitate in the basement membrane of the thin loop of Henle and may erode into the interstitium. The colicky pain is usually due to the dilatation and spasm of the ureter and stretching of the renal capsule.
Calcium oxalate stones are the most common type of renal calculi, comprising 70% to 75% of all urinary stones.[16][79] While chemically identical, they may present as 2 different crystalline forms: calcium oxalate monohydrate (whewellite, very hard) or a dihydrate (weddelite, brittle). These stones typically form in acidic urine but may be found with calcium phosphate, forming the central nidus.
- Calcium oxalate monohydrate calculi are extremely hard and usually present with a smooth, rounded surface. They are typically dark brown.
- Calcium oxalate dihydrate stones will be quite brittle with small, sharp, jagged edges. They are usually yellow to light brown.
Calcium phosphate calculi may be seen as the less soluble carbonate apatite (hydroxyapatite, apatite) and brushite (calcium hydrogen phosphate). They account for about 10% of all renal calculi.[16][79] Hydroxyapatite is more commonly found than brushite and is the calcium salt that forms bone. In general, calcium phosphate stones tend to grow faster and larger than calcium oxalate calculi. These stones are off-white, grayish-white, or yellowish in color. Calcium phosphate stones form in alkaline urine and are typically associated with abnormal metabolic factors, such as hyperparathyroidism and renal tubular acidosis.[10][80]
Uric acid calculi only form in acidic urine, usually with a pH less than 5.5. This acid is the most common composition of bladder stones and is typically radiolucent.[11] Uric acid accounts for 8% to 10% of urinary calculi, and the incidence is increasing worldwide.[16][79] This condition is most closely associated with diabetes, morbid obesity, metabolic syndrome, and older age at presentation.[16]
This is the only kidney stone that can be reasonably expected to dissolve if the urinary pH is sufficiently elevated and maintained.[11] This type of stone is also more likely to form from excessive urinary acidity rather than hyperuricosuria.[11][81] Uric acid stones may be yellow, orange, reddish, or brown, depending on the amount of blood-derived pigment they may have accumulated. Preventive treatment involves urinary alkalinization and possibly allopurinol if there is hyperuricosuria.[11]
Struvite or triple phosphate (calcium, ammonium, magnesium phosphate) stones are always associated with infection and increased pH levels.[14] They frequently form staghorn stones and comprise 7% to 8% of all urinary calculi worldwide.[16] Struvite stones are caused by the action of urease from bacteria, which increase the urinary pH and generate ammonia, leading to triple phosphate precipitation and stone formation.[14][82]
To treat the infection adequately, complete elimination of all stone material is necessary.[14] Struvite stones appear chalky, white, or grayish. Their surface is usually smooth and relatively brittle, as they can be broken relatively easily.
Cystine stones are caused by an uncommon familial genetic defect and account for only 1% to 2% of all urinary stones.[15] They tend to be amber, tan, or yellowish in color with a waxy appearance. Cystine stones may turn somewhat greenish after exposure to air. The stones are not calcified but resistant to shockwave therapy; therefore, laser lithotripsy is usually the preferred treatment.[15] Preventive treatment includes very high levels of hydration (>3 liters of urine/day), urinary alkalinization to a pH of 7.5 or more, and tiopronin, a reducing compound, if necessary.[15]
In addition to cystinuria, several other uncommon inherited disorders cause nephrolithiasis, including adenine phosphoribosyltransferase deficiency (causing dihyroxyadenine calculi), Bartter syndrome, Dent disease, distal tubular acidosis, familial hypomagnesemia with hypercalciuria and nephrocalcinosis, hereditary hypophosphatemic rickets, hereditary xanthinuria, Lesch-Nyhan syndrome, and primary hyperoxaluria.[80][83][84][85][86][87][88][89][90][91]
Natural urinary stone inhibitors include the following:
- Citrate
- Glycosaminoglycans
- Nephrocalcin
- Tamm-Horsfall protein
- Uropontin
- Water (increased urinary volume)
Histopathology
Urine microscopic crystal analysis can help identify the type of kidney stone present. Crystalluria alone does not necessarily indicate the presence of urinary calculi, but it does suggest the type of stone that may be produced. Crystalluria also indicates that conditions allowing a stone to form are present.
Common types of urinary crystals include the following:
- Calcium oxalate: envelope, spindles, ovals, octahedral, picket fences, or dumbbell-shaped. They often appear to have an "X" in their center. Ethylene glycol toxicity is associated with the picket fence type of urinary crystals.
- Calcium phosphate: colorless, they often appear as plates, stars, stellate, or as a collection of needles or rosettes. These are usually found in more alkaline urine.
- Cystine: hexagonal-shaped crystals. They appear as almost perfect benzene rings under the microscope.
- Struvite (magnesium, ammonium, calcium phosphate): This mineral is found only in alkaline urine and appears coffin-lid-shaped; this is always associated with urinary infections.
- Uric Acid: rhomboidal-shaped crystals, rosettes, or plates. Uric acid crystals are found in acidic urine with a pH of 5.5 or less.[92]
History and Physical
Patients with nephrolithiasis often present with hematuria, as 85% of patients demonstrate at least microscopic hematuria on urinalysis. Renal calculi may often be totally asymptomatic, with the stones being detected unexpectedly on imaging performed for a hematuria evaluation (or for unrelated reasons).[93] Symptoms are unlikely until or unless the calculi become infected or cause some degree of urinary obstruction, either within the kidney, at the ureteropelvic junction, or in the ureter.[94]
Patients with stone disease will also present with acute, severe flank pain (renal colic) that will often radiate to the abdomen and the groin, testicle, or labia.[94] The pain is often sharp, quite severe, and may also be colicky.[94] The pain is often associated with nausea and vomiting due to the embryological origins of the urogenital tract.[94]
Renal colic usually peaks within 90 to 120 minutes, and the pain radiation follows dermatomes T10 to S4.[94] The first phase may wake the patient up from sleep, is quite severe and somewhat intermittent, with excruciating pain coming in waves (renal colic). More constant pain characterizes the second phase, which may last 3 to 4 hours. The third phase is associated with mild pain relief, but intermittent waves of pain (colic) may persist. This phase may last 4 to 16 hours.[94]
The physical exam may reveal costovertebral tenderness and hypoactive bowel sounds. The testis and pubic area may also be tender to touch. Fever is rarely seen in renal colic without infection, but the presence of an elevated body temperature, pyuria, and leucocytosis may indicate infection, pyelonephritis, or pyonephrosis.
If infected, such patients will present with fever, chills, or other systemic signs of sepsis. This condition, called pyonephrosis or obstructive pyelonephritis, is potentially severe and life-threatening, requiring emergency decompression surgery to drain the renal pelvis.[95] The condition can rapidly progress to urosepsis, shock, and death if the renal pelvis is not quickly drained surgically, either percutaneously or cystoscopically, by double-J stenting or nephrostomy tubes.[95]
Patients who are more severely ill, hemodynamically unstable, or who have a large obstructing stone burden generally do better with percutaneous drainage, where manipulation of the infected renal unit and the obstructing stones is minimized.
High-risk factors for nephrolithiasis include the following:
- Bone disorders
- Chronic diarrhea, malabsorption
- Diabetes and obesity (especially in women)
- Excessive Vitamin C ingestion (>1,000 mg/day)
- Family history of kidney stones
- Gastrointestinal disease
- Gastro bypass surgery (especially Roux-en-Y)
- Gout
- Hyperparathyroidism
- Metabolic syndrome
- Obesity
- Prior urinary stones
- Renal failure
- Renal tubular acidosis
- Sarcoidosis (which can increase calcium and vitamin D levels)
Evaluation
Urinalysis should be obtained on every patient with a suspected kidney stone. Hematuria is usually present, but up to 15% of kidney stone patients will not demonstrate microscopic hematuria. The presence of urinary crystals may suggest urolithiasis. Positive nitrites, leukocytes, and bacteria suggest a possible urinary infection, which should be cultured and treated aggressively.[96] Other labs to obtain would include a WBC with differential if the patient is febrile or has a urinalysis suggestive of a possible infection.
KUB (flat plat abdomen including the kidneys, ureters, and bladder) can be obtained to screen for the presence of significant nephrolithiasis but may often miss small stones, calculi hidden by overlying bowel, or radiolucent stones if uncalcified.[97][98]
Ultrasound may be useful for assessing obstruction and resultant hydronephrosis, especially in pregnancy, where x-ray studies are discouraged. Ultrasound can also identify uric acid and other non-calcific renal stones if they are large enough (usually greater than 4 mm), but it can also miss the presence of stones less than 5 mm in size and cannot easily identify ureteral stones (see Image. Nephrolithiasis, Ultrasound).
The resistive index, measured using ultrasonography, can suggest ureteral obstruction. The formula is:
- Resistive index=(peak systolic velocity - end-diastolic velocity)/peak systolic velocity
- Values of 0.70 or less are considered normal, while higher values suggest obstructive uropathy.
- Bilateral high resistive indices suggest medical renal disease, while a unilateral high resistive index (0.75 or higher) suggests an obstruction, such as from a stone.
- Once a ureteral stone has been identified, the lower the resistive index, the more likely the stone will pass spontaneously.[99]
A non-contrast abdominal and pelvic computed tomography scan is considered the "gold standard" as it is the most sensitive and reliable test to diagnose urolithiasis and will also provide information regarding obstruction with resultant hydronephrosis.[100][101][102][103][104][105][106]
Even without intravenous contrast, the correct diagnosis can be easily made. If contrast is necessary, performing the non-contrast study first helps eliminate the diagnosis of urinary stones. Obscuring urinary stones with intravenous contrast can make it much more difficult to determine their size, number, or shape, complicating decisions on optimum treatment and possible surgery.[100][101][102][103][104] The initial use of intravenous contrast for computed tomography (CT) scans in patients with abdominal pain is not recommended. In many cases, an atypical abdominal pain will ultimately turn out to be a kidney stone that has moved or the presence of a urological anatomical variant such as a horseshoe kidney.
A KUB should be done immediately before the abdominal CT scan in all cases where urolithiasis is suspected or if the urinalysis shows gross or microscopic hematuria; this will provide vital information helpful in tracking or following the progress of the stone, its degree of calcification, and its shape, which cannot always be reliably determined from the CT scan alone.[97][98]
- Since contrast is used so often in abdominal CT scans, especially when ordered from the emergency department, it is recommended that the KUB be done first to facilitate tracking of any stones discovered. The cost and additional radiation exposure are minimal, and the information provided is extremely helpful to the follow-up clinician/urologist. Once intravenous contrast has been given, a clear x-ray without dye interference is impossible.
- A plain KUB done later in follow-up can easily determine whether a stone seen on the earlier KUB is still present. Otherwise, a repeat CT scan or a surgical retrograde pyelogram can be performed.
Combining a KUB with a renal ultrasound is an effective and reasonable alternative to CT scans as these can show hydronephrosis, measure resistive index, and demonstrate both calcified and non-calcified renal calculi with lower radiation exposure at a lower cost.
European Guidelines for Patients with Renal Calculi
- Check urine for hematuria, pH, and bacteria.
- Obtain a urine culture.
- Order a blood, urea, nitrogen (BUN) test and check serum creatinine.
- Order serum calcium, uric acid, sodium, and potassium levels.
- Order a complete blood count and C-reactive protein test.
- Obtain a coagulation profile in case surgical intervention is necessary.
- Obtain a non-contrast CT scan.[107]
Treatment / Management
Renal calculi can be extremely painful when they cause a ureteropelvic junction or ureteral obstruction or they become infected. Pain control may require opioids, but intravenous (IV) nonsteroidal anti-inflammatory drugs can also be quite effective while avoiding narcotic side effects. As patients with renal colic will often experience nausea and vomiting, IV hydration and antiemetics may be required acutely.
Many stones may be watched conservatively, with intervention planned as an outpatient. Smaller stones (<5 mm) have a greater chance (90%) of passing on their own with medical expulsion therapy (usually tamsulosin, alfuzosin, nifedipine, alfuzosin, silodosin, or mirabegron).[108][109][110][111][112][113][114] Any hint of a urinary tract infection should be treated aggressively with antibiotics, especially in patients with diabetes.[115][116][117]
Study results show that desmopressin can lower the pain of renal calculi. Anecdotal reports also indicate that calcium channel blockers or alpha-blockers can relieve pain due to the relaxation of the ureter and help the distal passage of the stone.[109][110][111] Mirabegron has shown similar efficacy in facilitating ureteral stone passage.[112][113] The urine should be strained for stones, and it should always be sent for chemical analysis.
There are several cases where urgent intervention is required:
- Obstructing stone in a patient with a urinary tract infection, fever, or sepsis—called pyonephrosis or obstructive pyelonephritis
- This condition requires urgent surgical decompression by urology or interventional radiology.
- Nausea or uncontrolled pain with outpatient care
- An obstructing stone in a solitary kidney
- Any degree of simultaneous bilateral obstruction (can easily lead to renal failure)
- Any degree of obstruction with a rising creatinine
In the case of a urinary tract infection or urosepsis with an obstructing stone, the obstruction should first be relieved with either a ureteral double J stent or nephrostomy tube placement. The decision of which treatment modality is most appropriate should be made by urology in consultation with the patient and family. The more severely ill the patient is, the greater the benefit will be from a nephrostomy tube (at least initially). Definitive stone management can then occur once the infection is no longer active. Patients who are morbidly obese and those who cannot be safely taken off blood thinners may require a double J stent, regardless.
Electively, renal calculi can be surgically managed in several ways:
(See the companion Statpearls reference article on "Renal Extracorporeal Therapy.")[118]
- This is a reliable treatment modality for stones with a high stone-free rate, but it depends on the surgeon's skill and experience and the kidney's internal anatomy.[121]
(See the companion StatPearls reference article on "Ureteroscopy.")[121]
- Combined treatments (ureteroscopy and percutaneous surgery) may be used in unusually difficult or complex cases.[125]
24-hour urine testing to identify the underlying chemical risk factors for future stone production so optimal prophylactic therapy can be administered should be carefully discussed with all patients with nephrolithiasis once the acute stone episode has been properly and adequately treated. This testing is strongly recommended by multiple published guidelines, including the American Urological Association.[128][129] Twenty-four-hour urine testing is particularly important if the patient has had multiple stones in the past, has a solitary kidney, has renal failure, or is a high surgical risk.[128][129][130][131]
Testing involves obtaining a basic metabolic panel, a serum uric acid, and a 24-hour urine collection for kidney stone prevention analysis.[130] Patients must understand that this represents their commitment to follow a long-term course of therapy for stone prevention and that no treatment plan is foolproof. An occasional stone may still be produced but is much less likely while on directed prophylaxis. Abnormal urinary chemistry contributing to recurrent stones will be found in over 93% of patients with nephrolithiasis tested by 24-hour urine and blood testing.[16][129][132] Physicians evaluating 24-hour kidney stone results should look at the normal ranges and what may be optimal.[130]
For example:
- Optimal 24-hour urinary calcium no more than 250 mg
- Oxalate less than 25 mg
- Citrate more than 600 mg
- Urinary volume of 2500 mL or more
While these levels may not be realistically obtainable in every patient, they are used as goals for treatment where the intention is to optimize as many urinary chemistry levels as possible (even if technically within normal limits).[130][134][135][136]
Analyses of 24-hour urine tests are often complicated but straightforward for most patients.[9][11][15][27][129][130][137][138][139][140][141]
- Patients with hypercalcemia should have parathyroid hormone levels checked for possible hyperparathyroidism, as these patients typically form calcium phosphate stones.
- Hydration should be optimized to produce at least two liters of urine daily. The optimal urinary volume is 2500 mL daily or more.
- Thiazides are used for hypercalciuria. They may also incidentally increase diuresis (but can also increase serum uric acid levels and decrease urinary citrate.)
- Potassium citrate and sodium bicarbonate are the most used urinary alkalinization agents for hypocitraturia and aciduria.
- Allopurinol will help lower high urinary and serum uric acid levels, but most patients with uric acid stones have aciduria and require urinary alkalinization, usually with potassium citrate.
- Patients with hyperoxaluria can use low-oxalate diets, dietary calcium supplements with higher-oxalate meals, and optimization of all other stone-related urinary chemistries.
- Cystine stone formers are treated with urinary alkalinization (to pH 7.5 or higher), vigorous hydration (sufficient to make 3 liters of urine a day or more), and sometimes with tiopronin, a reducing agent that makes cystine more soluble.
- Patients with struvite stones require infection control and complete removal of all infected stone pieces and fragments. Acetohydroxamic acid, a urease inhibitor, can be helpful in some cases but is associated with significant side effects.
Appropriate thiazide use has been shown to lower hypercalciuric calcium stone disease from 2.94 to 0.05 stones per year (P <0.001), while long-term use of potassium citrate can diminish calcium stone production by 80%.[142][143] Limiting sodium intake is important to allow thiazide to perform its hypocalciuric action.[144] A daily increase of 100 mEq of sodium will raise urinary calcium excretion by 50 mg.[9] These 3 drugs will be sufficient to treat the majority of patients.
(See the companion StatPearls reference article, "24-Hour Urine Testing for Nephrolithiasis: Guide to Interpretation," for more details on testing, result analysis, and prophylactic therapy.)[130]
Hospital Admission is recommended in the following situations:
- Inadequate pain relief with oral analgesics
- Obstructed pyelonephritis (pyonephrosis)
- Patient with a transplanted kidney and renal calculi
- Presence of renal calculi and pyelonephritis or sepsis
- Intractable nausea and vomiting [141]
Dissolution therapy does not work for calcium stones but may be used to manage uric acid and cystine stones. Uric acid calculi can be dissolved by consistently making the urine alkaline with potassium citrate or sodium bicarbonate.[11][141][145] Potassium citrate is usually preferred due to its lower sodium load. In addition, allopurinol can be used to reduce renal uric acid excretion.[137][141] Cystine stones can be managed with tiopronin, aggressive fluid intake, increased urinary volume, and optimal alkalinization therapy (to a pH of 7.5 or higher).[15][141]
Struvite (triple phosphate) stones can sometimes be managed with acetohydroxamic acid—a urease inhibitor that irreversibly blocks urease.[128][141][146] Acetohydroxamic acid penetrates bacterial cells well, synergizes with many antibiotics, reduces urinary alkalinity levels, lowers ammonia production, and has a high renal clearance. Unfortunately, acetohydroxamic acid also has significant side effects (including hemolytic anemia), cannot be used in renal failure, and is generally considered adjunctive to surgical therapy. This therapy may also be used in patients who are not surgical candidates. Renacidin (hemiacidrin) irrigation may be used cautiously in highly selected cases to dissolve struvite stones, as improper use can be lethal.[14][141][147][148][149][150][151]
Differential Diagnosis
The following may present with similar symptoms to renal calculi:
- Appendicitis
- Benign familial hematuria
- Cholecystitis
- Costochondritis
- Diverticulitis
- Focal nephronia
- Glomerulonephritis
- Hernia
- Lobar pneumonia
- Pelvic inflammatory disease
- Pyelonephritis
Prognosis
Close to 80% to 90% of renal calculi pass spontaneously. About 3% of patients need admission because of pain, inability to pass the stone, sepsis, intractable nausea and vomiting, or dehydration. A few patients may develop urinary tract obstruction and an upper urinary tract infection, resulting in urosepsis, pyelonephritis, or pyonephrosis (obstructive pyelonephritis). Most of these patients require an urgent surgical procedure to bypass the stone or otherwise drain the renal pelvis until the infection is resolved; then, elective surgery can be safely performed to remove the calculus.
The recurrence rate of renal calculi has been reported to be about 50% within 10 years, but some patients form stones far more frequently. Twenty-four-hour urine testing and prophylactic therapy can significantly reduce the recurrence rate of nephrolithiasis, but the results must be properly interpreted, and patients need to be sufficiently motivated to follow treatment suggestions long-term, even if immediate relief is not evident.
Individuals with ongoing metabolic disorders or malignancies are also at greater risk for recurrences. The key for all patients with renal calculi is to stay hydrated as no medical therapy is likely to succeed without adequate hydration and sufficient urinary fluid output, generally considered at least 2000 mL daily (optimally 2500 mL or more).[133]
Complications
The following are possible complications of nephrolithiasis:
- Abscess formation
- Forniceal rupture
- Hydronephrosis
- Perinephric abscess
- Pyelonephritis
- Pyonephrosis (obstructive pyelonephritis)
- Renal colic
- Renal failure, atrophy, and end-stage kidney disease
- Sepsis and urosepsis
- Ureteral calculi with subsequent colic, obstruction, pain, and scarring
- Urinary extravasation
- Urinoma
Deterrence and Patient Education
Patents should generally avoid diets high in calcium while limiting excessive salt and animal protein intake. A low oxalate diet is also recommended, particularly for patients with hyperoxaluria or recurrent calcium oxalate stones. Overly restrictive dietary calcium is not recommended as it may lead to a lack of intestinal oxalate binding and hyperoxaluria.[27] Twenty-four-hour urine testing for kidney stone prophylaxis is available for any patient with urolithiasis who is interested, willing, and motivated to follow long-term preventive treatment.[128]
Pearls and Other Issues
Other important information to note includes:
- Ultimately, the success of any kidney stone preventive treatment program will depend on the patient's willingness to follow a long-term course of treatment that will involve some level of dietary modifications, medications, personal sacrifice, and lifestyle change, even if immediate benefits are not evident.
- Patients on optimal treatment may still make stones, albeit fewer than otherwise.
- Some patients may only partially follow their therapy regimens, and many will revert to their previous diets and behaviors; however, this can be minimized by warning patients of this natural tendency and seeing patients periodically to remind them of the benefits of maintaining preventive therapy.
- Patients may also develop an over-reliance on drug therapy, leading to failure to maintain the dietary changes and fluid intake goals requested.
- Patients at higher risk of developing chronic renal failure and end-stage renal disease include those with calcium phosphate, cystine, struvite, and uric acid stones, as well as those with primary hyperoxaluria, cystinuria, repeated stone recurrences, larger stones, and staghorn calculi.[16]
Enhancing Healthcare Team Outcomes
The presence of nephrolithiasis places a significant burden on the healthcare system and is a significant cause of patient morbidity. An interprofessional team of nurses, nurse practitioners, physician assistants, dieticians, pharmacists, urologists, nephrologists, and primary care physicians should educate and remind patients about the benefits of preventive therapy. Nutritionists and dieticians are especially important to help motivated individuals have long-term success.[136]
The health care team involved in the care of patients with nephrolithiasis has an absolute obligation to inform patients on preventative dietary changes and how to optimize urine chemistries, particularly in cases of multiple stone recurrences, solitary kidneys, high surgical risk factors, or those in the pediatric age group.[159][160]
Patients with recurrent renal calculi should be referred to a specialist urologist for workup and to rule out an anatomical or metabolic problem. Only through open and effective communication between the team members can the morbidity and recurrence rate of renal calculi be lowered.
References
- 1.
- Scales CD, Smith AC, Hanley JM, Saigal CS., Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012 Jul;62(1):160-5. [PMC free article: PMC3362665] [PubMed: 22498635]
- 2.
- Reesink DJ, Scheltema JMW, Barendrecht MM, Boeken Kruger AE, Jansonius A, Wiltink J, van der Windt F. Extracorporeal shock wave lithotripsy under intravenous sedation for treatment of urolithiasis. Scand J Urol. 2018 Oct-Dec;52(5-6):453-458. [PubMed: 30451054]
- 3.
- York NE, Zheng M, Elmansy HM, Rivera ME, Krambeck AE, Lingeman JE. Stone-free Outcomes of Flexible Ureteroscopy for Renal Calculi Utilizing Computed Tomography Imaging. Urology. 2019 Feb;124:52-56. [PubMed: 30391680]
- 4.
- Suliman A, Burki T, Garriboli M, Glass J, Taghizadeh A. Flexible ureterorenoscopy to treat upper urinary tract stones in children. Urolithiasis. 2020 Feb;48(1):57-61. [PubMed: 30370467]
- 5.
- Hoffman A, Braun MM, Khayat M. Kidney Disease: Kidney Stones. FP Essent. 2021 Oct;509:33-38. [PubMed: 34643363]
- 6.
- Kachkoul R, Touimi GB, El Mouhri G, El Habbani R, Mohim M, Lahrichi A. Urolithiasis: History, epidemiology, aetiologic factors and management. Malays J Pathol. 2023 Dec;45(3):333-352. [PubMed: 38155376]
- 7.
- Bowen DK, Tasian GE. Pediatric Stone Disease. Urol Clin North Am. 2018 Nov;45(4):539-550. [PubMed: 30316309]
- 8.
- Aune D, Mahamat-Saleh Y, Norat T, Riboli E. Body fatness, diabetes, physical activity and risk of kidney stones: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2018 Nov;33(11):1033-1047. [PMC free article: PMC6208979] [PubMed: 30066054]
- 9.
- Leslie SW, Sajjad H. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Feb 12, 2024. Hypercalciuria. [PubMed: 28846247]
- 10.
- Pokhrel B, Leslie SW, Levine SN. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Mar 1, 2024. Primary Hyperparathyroidism. [PubMed: 28722924]
- 11.
- KC M, Leslie SW. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Oct 15, 2023. Uric Acid Nephrolithiasis. [PubMed: 32809561]
- 12.
- George C, Leslie SW, Minter DA. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Oct 14, 2023. Hyperuricemia. [PubMed: 29083565]
- 13.
- Fenando A, Rednam M, Gujarathi R, Widrich J. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Feb 12, 2024. Gout. [PubMed: 31536213]
- 14.
- Karki N, Leslie SW. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 30, 2023. Struvite and Triple Phosphate Renal Calculi. [PubMed: 33760542]
- 15.
- Leslie SW, Sajjad H, Nazzal L. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 30, 2023. Cystinuria. [PubMed: 29262245]
- 16.
- Stamatelou K, Goldfarb DS. Epidemiology of Kidney Stones. Healthcare (Basel). 2023 Feb 02;11(3) [PMC free article: PMC9914194] [PubMed: 36766999]
- 17.
- Daudon M, Frochot V, Bazin D, Jungers P. Drug-Induced Kidney Stones and Crystalline Nephropathy: Pathophysiology, Prevention and Treatment. Drugs. 2018 Feb;78(2):163-201. [PubMed: 29264783]
- 18.
- Izzedine H, Lescure FX, Bonnet F. HIV medication-based urolithiasis. Clin Kidney J. 2014 Apr;7(2):121-6. [PMC free article: PMC4377784] [PubMed: 25852859]
- 19.
- Sighinolfi MC, Eissa A, Bevilacqua L, Zoeir A, Ciarlariello S, Morini E, Puliatti S, Durante V, Ceccarelli PL, Micali S, Bianchi G, Rocco B. Drug-Induced Urolithiasis in Pediatric Patients. Paediatr Drugs. 2019 Oct;21(5):323-344. [PubMed: 31541411]
- 20.
- Bennett S, Hoffman N, Monga M. Ephedrine- and guaifenesin-induced nephrolithiasis. J Altern Complement Med. 2004 Dec;10(6):967-9. [PubMed: 15673990]
- 21.
- Niyazov R, Sharman T. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 22, 2023. Triamterene. [PubMed: 32491582]
- 22.
- Carr MC, Prien EL, Babayan RK. Triamterene nephrolithiasis: renewed attention is warranted. J Urol. 1990 Dec;144(6):1339-40. [PubMed: 2231920]
- 23.
- Roedel MM, Nakada SY, Penniston KL. Sulfamethoxazole-induced sulfamethoxazole urolithiasis: a case report. BMC Urol. 2021 Sep 17;21(1):133. [PMC free article: PMC8447800] [PubMed: 34535099]
- 24.
- Unno R, Taguchi K, Hosier G, Usawachintachit M, Sui W, Yang H, Hamouche F, Bayne D, Stoller M, Chi T. Maternal family history of urolithiasis is associated with earlier age of onset of stone disease. World J Urol. 2023 Jan;41(1):241-247. [PMC free article: PMC10044450] [PubMed: 36504337]
- 25.
- Kocvara R, Plasgura P, Petrík A, Louzenský G, Bartonícková K, Dvorácek J. A prospective study of nonmedical prophylaxis after a first kidney stone. BJU Int. 1999 Sep;84(4):393-8. [PubMed: 10468751]
- 26.
- Abate N, Chandalia M, Cabo-Chan AV, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004 Feb;65(2):386-92. [PubMed: 14717908]
- 27.
- Shah A, Leslie SW, Ramakrishnan S. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Mar 4, 2024. Hyperoxaluria. [PubMed: 32644413]
- 28.
- Lotan Y, Antonelli J, Jiménez IB, Gharbi H, Herring R, Beaver A, Dennis A, Von Merveldt D, Carter S, Cohen A, Poindexter J, Moe OW, Pearle MS. The kidney stone and increased water intake trial in steel workers: results from a pilot study. Urolithiasis. 2017 Apr;45(2):177-183. [PubMed: 27228999]
- 29.
- Wang JS, Chiang HY, Chen HL, Flores M, Navas-Acien A, Kuo CC. Association of water intake and hydration status with risk of kidney stone formation based on NHANES 2009-2012 cycles. Public Health Nutr. 2022 Sep;25(9):2403-2414. [PMC free article: PMC9991749] [PubMed: 35514256]
- 30.
- Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol. 2013 Aug;8(8):1389-95. [PMC free article: PMC3731916] [PubMed: 23676355]
- 31.
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Stampfer MJ. Prospective study of beverage use and the risk of kidney stones. Am J Epidemiol. 1996 Feb 01;143(3):240-7. [PubMed: 8561157]
- 32.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med. 1998 Apr 01;128(7):534-40. [PubMed: 9518397]
- 33.
- Littlejohns TJ, Neal NL, Bradbury KE, Heers H, Allen NE, Turney BW. Fluid Intake and Dietary Factors and the Risk of Incident Kidney Stones in UK Biobank: A Population-based Prospective Cohort Study. Eur Urol Focus. 2020 Jul 15;6(4):752-761. [PubMed: 31085062]
- 34.
- Yuan S, Larsson SC. Coffee and Caffeine Consumption and Risk of Kidney Stones: A Mendelian Randomization Study. Am J Kidney Dis. 2022 Jan;79(1):9-14.e1. [PubMed: 34690004]
- 35.
- Ferraro PM, Curhan GC. More Good News: Coffee Prevents Kidney Stones. Am J Kidney Dis. 2022 Jan;79(1):3-4. [PubMed: 34690003]
- 36.
- Gettman MT, Ogan K, Brinkley LJ, Adams-Huet B, Pak CY, Pearle MS. Effect of cranberry juice consumption on urinary stone risk factors. J Urol. 2005 Aug;174(2):590-4; quiz 801. [PubMed: 16006907]
- 37.
- Sorensen MD, Hsi RS, Chi T, Shara N, Wactawski-Wende J, Kahn AJ, Wang H, Hou L, Stoller ML., Women’s Health Initiative Writing Group. Dietary intake of fiber, fruit and vegetables decreases the risk of incident kidney stones in women: a Women's Health Initiative report. J Urol. 2014 Dec;192(6):1694-9. [PMC free article: PMC4241174] [PubMed: 24859445]
- 38.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993 Mar 25;328(12):833-8. [PubMed: 8441427]
- 39.
- Sorensen MD, Kahn AJ, Reiner AP, Tseng TY, Shikany JM, Wallace RB, Chi T, Wactawski-Wende J, Jackson RD, O'Sullivan MJ, Sadetsky N, Stoller ML., WHI Working Group. Impact of nutritional factors on incident kidney stone formation: a report from the WHI OS. J Urol. 2012 May;187(5):1645-9. [PMC free article: PMC4165387] [PubMed: 22425103]
- 40.
- Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol. 2009 Oct;20(10):2253-9. [PMC free article: PMC2754098] [PubMed: 19679672]
- 41.
- Chewcharat A, Curhan G. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis. 2021 Feb;49(1):27-39. [PubMed: 32870387]
- 42.
- Young BJ, Tejwani R, Wang HH, Wolf S, Purves JT, Wiener JS, Routh JC. Is the Economic Impact and Utilization of Imaging Studies for Pediatric Urolithiasis Across the United States Increasing? Urology. 2016 Aug;94:208-13. [PMC free article: PMC4969138] [PubMed: 27208819]
- 43.
- Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin H. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994 Sep;46(3):893-9. [PubMed: 7996811]
- 44.
- Jobs K, Rakowska M, Paturej A. Urolithiasis in the pediatric population - current opinion on epidemiology, patophysiology, diagnostic evaluation and treatment. Dev Period Med. 2018;22(2):201-208. [PMC free article: PMC8522892] [PubMed: 30056408]
- 45.
- Bauza JL, Pieras EC, Grases F, Tubau V, Guimerà J, Sabaté XA, Pizà P. Urinary tract infection's etiopathogenic role in nephrolithiasis formation. Med Hypotheses. 2018 Sep;118:34-35. [PubMed: 30037611]
- 46.
- Sabih A, Leslie SW. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Nov 12, 2023. Complicated Urinary Tract Infections. [PubMed: 28613784]
- 47.
- Moftakhar L, Jafari F, Ghoddusi Johari M, Rezaeianzadeh R, Hosseini SV, Rezaianzadeh A. Prevalence and risk factors of kidney stone disease in population aged 40-70 years old in Kharameh cohort study: a cross-sectional population-based study in southern Iran. BMC Urol. 2022 Dec 19;22(1):205. [PMC free article: PMC9764470] [PubMed: 36536352]
- 48.
- Shoag J, Tasian GE, Goldfarb DS, Eisner BH. The new epidemiology of nephrolithiasis. Adv Chronic Kidney Dis. 2015 Jul;22(4):273-8. [PubMed: 26088071]
- 49.
- Modi PK, Kwon YS, Davis RB, Elsamra SE, Dombrovskiy V, Olweny EO. Pediatric hospitalizations for upper urinary tract calculi: Epidemiological and treatment trends in the United States, 2001-2014. J Pediatr Urol. 2018 Feb;14(1):13.e1-13.e6. [PubMed: 28966022]
- 50.
- Abufaraj M, Xu T, Cao C, Waldhoer T, Seitz C, D'andrea D, Siyam A, Tarawneh R, Fajkovic H, Schernhammer E, Yang L, Shariat SF. Prevalence and Trends in Kidney Stone Among Adults in the USA: Analyses of National Health and Nutrition Examination Survey 2007-2018 Data. Eur Urol Focus. 2021 Nov;7(6):1468-1475. [PubMed: 32900675]
- 51.
- Ferraro PM, Curhan GC, D'Addessi A, Gambaro G. Risk of recurrence of idiopathic calcium kidney stones: analysis of data from the literature. J Nephrol. 2017 Apr;30(2):227-233. [PubMed: 26969574]
- 52.
- Wang K, Ge J, Han W, Wang D, Zhao Y, Shen Y, Chen J, Chen D, Wu J, Shen N, Zhu S, Xue B, Xu X. Risk factors for kidney stone disease recurrence: a comprehensive meta-analysis. BMC Urol. 2022 Apr 19;22(1):62. [PMC free article: PMC9017041] [PubMed: 35439979]
- 53.
- Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005 Sep;68(3):1230-5. [PubMed: 16105055]
- 54.
- Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. 2010 Jun;183(6):2244-8. [PubMed: 20400141]
- 55.
- Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. Am J Hypertens. 1998 Jan;11(1 Pt 1):46-53. [PubMed: 9504449]
- 56.
- Madore F, Stampfer MJ, Willett WC, Speizer FE, Curhan GC. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis. 1998 Nov;32(5):802-7. [PubMed: 9820450]
- 57.
- Reiner AP, Kahn A, Eisner BH, Pletcher MJ, Sadetsky N, Williams OD, Polak JF, Jacobs DR, Stoller ML. Kidney stones and subclinical atherosclerosis in young adults: the CARDIA study. J Urol. 2011 Mar;185(3):920-5. [PMC free article: PMC3827917] [PubMed: 21251678]
- 58.
- Rule AD, Roger VL, Melton LJ, Bergstralh EJ, Li X, Peyser PA, Krambeck AE, Lieske JC. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol. 2010 Oct;21(10):1641-4. [PMC free article: PMC3013539] [PubMed: 20616170]
- 59.
- Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Samuel S, Klarenbach SW, Curhan GC, Tonelli M., Alberta Kidney Disease Network. Kidney stones and cardiovascular events: a cohort study. Clin J Am Soc Nephrol. 2014 Mar;9(3):506-12. [PMC free article: PMC3944758] [PubMed: 24311706]
- 60.
- Chung SD, Liu SP, Keller JJ, Lin HC. Urinary calculi and an increased risk of stroke: a population-based follow-up study. BJU Int. 2012 Dec;110(11 Pt C):E1053-9. [PubMed: 22583934]
- 61.
- Liu Y, Li S, Zeng Z, Wang J, Xie L, Li T, He Y, Qin X, Zhao J. Kidney stones and cardiovascular risk: a meta-analysis of cohort studies. Am J Kidney Dis. 2014 Sep;64(3):402-10. [PubMed: 24797522]
- 62.
- Shoag J, Halpern J, Goldfarb DS, Eisner BH. Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J Urol. 2014 Nov;192(5):1440-5. [PubMed: 24929140]
- 63.
- Rule AD, Bergstralh EJ, Melton LJ, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009 Apr;4(4):804-11. [PMC free article: PMC2666438] [PubMed: 19339425]
- 64.
- El-Zoghby ZM, Lieske JC, Foley RN, Bergstralh EJ, Li X, Melton LJ, Krambeck AE, Rule AD. Urolithiasis and the risk of ESRD. Clin J Am Soc Nephrol. 2012 Sep;7(9):1409-15. [PMC free article: PMC3430957] [PubMed: 22745275]
- 65.
- Rule AD, Krambeck AE, Lieske JC. Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol. 2011 Aug;6(8):2069-75. [PMC free article: PMC3156433] [PubMed: 21784825]
- 66.
- Dhondup T, Kittanamongkolchai W, Vaughan LE, Mehta RA, Chhina JK, Enders FT, Hickson LJ, Lieske JC, Rule AD. Risk of ESRD and Mortality in Kidney and Bladder Stone Formers. Am J Kidney Dis. 2018 Dec;72(6):790-797. [PMC free article: PMC6252145] [PubMed: 30146423]
- 67.
- Keddis MT, Rule AD. Nephrolithiasis and loss of kidney function. Curr Opin Nephrol Hypertens. 2013 Jul;22(4):390-6. [PMC free article: PMC4074537] [PubMed: 23736840]
- 68.
- Evan A, Lingeman J, Coe FL, Worcester E. Randall's plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006 Apr;69(8):1313-8. [PubMed: 16614720]
- 69.
- Edvardsson VO, Goldfarb DS, Lieske JC, Beara-Lasic L, Anglani F, Milliner DS, Palsson R. Hereditary causes of kidney stones and chronic kidney disease. Pediatr Nephrol. 2013 Oct;28(10):1923-42. [PMC free article: PMC4138059] [PubMed: 23334384]
- 70.
- Prot-Bertoye C, Lebbah S, Daudon M, Tostivint I, Bataille P, Bridoux F, Brignon P, Choquenet C, Cochat P, Combe C, Conort P, Decramer S, Doré B, Dussol B, Essig M, Gaunez N, Joly D, Le Toquin-Bernard S, Méjean A, Meria P, Morin D, N'Guyen HV, Noël C, Normand M, Pietak M, Ronco P, Saussine C, Tsimaratos M, Friedlander G, Traxer O, Knebelmann B, Courbebaisse M., French Cystinuria Group. CKD and Its Risk Factors among Patients with Cystinuria. Clin J Am Soc Nephrol. 2015 May 07;10(5):842-51. [PMC free article: PMC4422236] [PubMed: 25717071]
- 71.
- Eassa WA, Sheir KZ, Gad HM, Dawaba ME, El-Kenawy MR, Elkappany HA. Prospective study of the long-term effects of shock wave lithotripsy on renal function and blood pressure. J Urol. 2008 Mar;179(3):964-8; discussion 968-9. [PubMed: 18207167]
- 72.
- Denburg MR, Jemielita TO, Tasian GE, Haynes K, Mucksavage P, Shults J, Copelovitch L. Assessing the risk of incident hypertension and chronic kidney disease after exposure to shock wave lithotripsy and ureteroscopy. Kidney Int. 2016 Jan;89(1):185-92. [PMC free article: PMC4911906] [PubMed: 26509587]
- 73.
- Brikowski TH, Lotan Y, Pearle MS. Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci U S A. 2008 Jul 15;105(28):9841-6. [PMC free article: PMC2474527] [PubMed: 18626008]
- 74.
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003 May;63(5):1817-23. [PubMed: 12675858]
- 75.
- Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010 Spring;12(2-3):e86-96. [PMC free article: PMC2931286] [PubMed: 20811557]
- 76.
- Hyams ES, Matlaga BR. Economic impact of urinary stones. Transl Androl Urol. 2014 Sep;3(3):278-83. [PMC free article: PMC4708578] [PubMed: 26816777]
- 77.
- Wiener SV, Ho SP, Stoller ML. Beginnings of nephrolithiasis: insights into the past, present and future of Randall's plaque formation research. Curr Opin Nephrol Hypertens. 2018 Jul;27(4):236-242. [PubMed: 29697409]
- 78.
- Cohen AJ, Borofsky MS, Anderson BB, Dauw CA, Gillen DL, Gerber GS, Worcester EM, Coe FL, Lingeman JE. Endoscopic Evidence That Randall's Plaque is Associated with Surface Erosion of the Renal Papilla. J Endourol. 2017 Jan;31(1):85-90. [PMC free article: PMC5220550] [PubMed: 27824271]
- 79.
- Siener R, Herwig H, Rüdy J, Schaefer RM, Lossin P, Hesse A. Urinary stone composition in Germany: results from 45,783 stone analyses. World J Urol. 2022 Jul;40(7):1813-1820. [PMC free article: PMC9236976] [PubMed: 35666268]
- 80.
- Mustaqeem R, Arif A. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 17, 2023. Renal Tubular Acidosis. [PubMed: 30085586]
- 81.
- Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002 Sep;62(3):971-9. [PubMed: 12164880]
- 82.
- Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, Traxer O, Tiselius HG. Kidney stones. Nat Rev Dis Primers. 2016 Feb 25;2:16008. [PMC free article: PMC5685519] [PubMed: 27188687]
- 83.
- Jaffer A, Joyce A, Koenig P, Biyani CS. Adenine Phosphoribosyltransferase Deficiency: A Rare Cause of Recurrent Urolithiasis. J Endourol Case Rep. 2017;3(1):49-51. [PMC free article: PMC5399732] [PubMed: 28466077]
- 84.
- Song Y, Zhao C, Li D. Research progress on renal calculus associate with inborn error of metabolism. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023 Apr 25;52(2):169-177. [PMC free article: PMC10293781] [PubMed: 37283101]
- 85.
- Bokhari SRA, Zulfiqar H, Mansur A. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Sep 4, 2023. Bartter Syndrome. [PubMed: 28723048]
- 86.
- Vall-Palomar M, Madariaga L, Ariceta G. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Pediatr Nephrol. 2021 Oct;36(10):3045-3055. [PubMed: 33595712]
- 87.
- Bergwitz C, Miyamoto KI. Hereditary hypophosphatemic rickets with hypercalciuria: pathophysiology, clinical presentation, diagnosis and therapy. Pflugers Arch. 2019 Jan;471(1):149-163. [PubMed: 30109410]
- 88.
- Kubihal S, Goyal A, Singla R, Khadgawat R. Urolithiasis due to Hereditary Xanthinuria Type II: A Long-term Follow-up report. Indian Pediatr. 2020 May 15;57(5):468-469. [PMC free article: PMC7240230] [PubMed: 32444521]
- 89.
- Nanagiri A, Shabbir N. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Apr 24, 2023. Lesch-Nyhan Syndrome. [PubMed: 32310539]
- 90.
- Milliner DS, Harris PC, Sas DJ, Lieske JC. Primary Hyperoxaluria Type 3. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. University of Washington, Seattle; Seattle (WA): Sep 24, 2015. [PubMed: 26401545]
- 91.
- Groothoff JW, Metry E, Deesker L, Garrelfs S, Acquaviva C, Almardini R, Beck BB, Boyer O, Cerkauskiene R, Ferraro PM, Groen LA, Gupta A, Knebelmann B, Mandrile G, Moochhala SS, Prytula A, Putnik J, Rumsby G, Soliman NA, Somani B, Bacchetta J. Clinical practice recommendations for primary hyperoxaluria: an expert consensus statement from ERKNet and OxalEurope. Nat Rev Nephrol. 2023 Mar;19(3):194-211. [PubMed: 36604599]
- 92.
- Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest. 2005 Oct;115(10):2598-608. [PMC free article: PMC1236703] [PubMed: 16200192]
- 93.
- Leslie SW, Hamawy K, Saleem MO. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Feb 29, 2024. Gross and Microscopic Hematuria. [PubMed: 30480952]
- 94.
- Patti L, Leslie SW. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jun 6, 2024. Acute Renal Colic. [PubMed: 28613743]
- 95.
- Rojas-Moreno C. Pyonephrosis and pyocystis. IDCases. 2016;6:104-105. [PMC free article: PMC5118591] [PubMed: 27882302]
- 96.
- Sandhu MS, Gulati A, Saritha J, Nayak B. Urolithiasis: Comparison of diagnostic performance of digital tomosynthesis and ultrasound. Which one to choose and when? Eur J Radiol. 2018 Aug;105:25-31. [PubMed: 30017289]
- 97.
- Kaul I, Moore S, Barry E, Pareek G. Renal Imaging in Stone Disease: Which Modality to Choose? R I Med J (2013). 2023 Dec 01;106(11):31-35. [PubMed: 38015782]
- 98.
- Brisbane W, Bailey MR, Sorensen MD. An overview of kidney stone imaging techniques. Nat Rev Urol. 2016 Nov;13(11):654-662. [PMC free article: PMC5443345] [PubMed: 27578040]
- 99.
- Erbay G, Yalcın A, Gultekin MH. Predictor Role of Pretreatment Resistive and Pulsatile Indexes in the Success of Medical Expulsive Therapy of Ureteral Stones. Urology. 2018 Aug;118:47-51. [PubMed: 29729361]
- 100.
- Batura D, Hashemzehi T, Gayed W. Should contrast CT urography replace non-contrast CT as an investigation for ureteric colic in the emergency department in those aged 65 and over? Emerg Radiol. 2018 Dec;25(6):621-626. [PubMed: 29946802]
- 101.
- Rodger F, Roditi G, Aboumarzouk OM. Diagnostic Accuracy of Low and Ultra-Low Dose CT for Identification of Urinary Tract Stones: A Systematic Review. Urol Int. 2018;100(4):375-385. [PubMed: 29649823]
- 102.
- Kennish SJ, Wah TM, Irving HC. Unenhanced CT for the evaluation of acute ureteric colic: the essential pictorial guide. Postgrad Med J. 2010 Jul;86(1017):428-36. [PubMed: 20634253]
- 103.
- Dalla Palma L, Pozzi-Mucelli R, Stacul F. Present-day imaging of patients with renal colic. Eur Radiol. 2001;11(1):4-17. [PubMed: 11194915]
- 104.
- Pfister SA, Deckart A, Laschke S, Dellas S, Otto U, Buitrago C, Roth J, Wiesner W, Bongartz G, Gasser TC. Unenhanced helical computed tomography vs intravenous urography in patients with acute flank pain: accuracy and economic impact in a randomized prospective trial. Eur Radiol. 2003 Nov;13(11):2513-20. [PubMed: 12898174]
- 105.
- Patel VA, Popat NP. Essentials of Computed Tomography Imaging of Hematuria. Saudi J Kidney Dis Transpl. 2023 Jan 01;34(1):61-79. [PubMed: 38092717]
- 106.
- Khandelwal S, Dhande R, Sood A, Parihar P, Mishra GV. Role of Multidetector Computed Tomography Urography in the Evaluation of Obstructive Uropathy: A Review. Cureus. 2023 Oct;15(10):e48038. [PMC free article: PMC10688237] [PubMed: 38034148]
- 107.
- Güven S, Sönmez MG, Somani BK, Gözen AS, Sarica K, Rivas JG, Nagele U, Tokas T. Current management of renal colic across Europe and its compliance to the European Association of Urology Guidelines on Urolithiasis: a survey from the European Section of Uro-technology, European Section of Urolithiasis, Young Academic Urologists study groups. Cent European J Urol. 2022;75(2):182-190. [PMC free article: PMC9326703] [PubMed: 35937652]
- 108.
- Gnyawali D, Pradhan MM, Sigdel PR, Parajuli P, Chudal S, Poudyal S, Chapagain S, Luitel BR, Chalise PR, Sharma U, Gyawali PR. Efficacy of Tamsulosin plus Tadalafil versus Tamsulosin as Medical Expulsive Therapy for Lower Ureteric Stones: A Randomized Controlled Trial. Adv Urol. 2020;2020:4347598. [PMC free article: PMC7204220] [PubMed: 32411212]
- 109.
- Talebi Doluee M, Shams A, Keshvari Shirvan M, DadgarMoghadam M, Sadrzadeh SM, VafadarMoradi E. Renal Colic Pain Management by Tamsulosin with Morphine Versus Morphine in Adults: A Randomized Clinical Trial. Anesth Pain Med. 2023 Apr;13(2):e134627. [PMC free article: PMC10439690] [PubMed: 37601962]
- 110.
- Ye Z, Zeng G, Yang H, Tang K, Zhang X, Li H, Li W, Wu Z, Chen L, Chen X, Liu X, Deng Y, Pan T, Xing J, Wang S, Cheng Y, Gu X, Gao W, Yang J, Zhang Y, Mi Q, Qi L, Li J, Hu W, Liang P, Sun Z, Xu C, Long Y, Liao Y, Liu S, Liu G, Xu X, He W, Chen Z, Xu H. Efficacy and Safety of Tamsulosin in Medical Expulsive Therapy for Distal Ureteral Stones with Renal Colic: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. Eur Urol. 2018 Mar;73(3):385-391. [PubMed: 29137830]
- 111.
- Cui Y, Chen J, Zeng F, Liu P, Hu J, Li H, Li C, Cheng X, Chen M, Li Y, Li Y, Yang Z, Chen Z, Chand H, Chen H, Zu X. Tamsulosin as a Medical Expulsive Therapy for Ureteral Stones: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Urol. 2019 May;201(5):950-955. [PubMed: 30694932]
- 112.
- Morsy S, Nasser I, Aboulela W, Abdelazim MS, Ali H. Efficacy of Mirabegron as Medical Expulsive Therapy for Distal Ureteral Stones: A Prospective, Randomized, Double-Blinded, Controlled Study. Urol Int. 2022;106(12):1265-1271. [PubMed: 35100594]
- 113.
- Tang QL, Wang DJ, Zhou S, Tao RZ. Mirabegron in medical expulsive therapy for distal ureteral stones: a prospective, randomized, controlled study. World J Urol. 2021 Dec;39(12):4465-4470. [PubMed: 34241685]
- 114.
- Abdel-Kader MS, Sayed AM, Sayed SM, AbdelRazek M. Evaluation of the efficacy and safety of either or both mirabegron and silodosin, as a medical expulsive therapy for distal ureteric stones. Int Urol Nephrol. 2024 May;56(5):1605-1610. [PMC free article: PMC11001674] [PubMed: 38041752]
- 115.
- Yildirim K, Olcucu MT, Colak ME. Trends in the treatment of urinary stone disease in Turkey. PeerJ. 2018;6:e5390. [PMC free article: PMC6074772] [PubMed: 30083475]
- 116.
- Fedrigon DC, Jain R, Sivalingam S. Current use of medical expulsive therapy among endourologists. Can Urol Assoc J. 2018 Sep;12(9):E384-E390. [PMC free article: PMC6143505] [PubMed: 29787372]
- 117.
- Diri A, Diri B. Management of staghorn renal stones. Ren Fail. 2018 Nov;40(1):357-362. [PMC free article: PMC6014528] [PubMed: 29658394]
- 118.
- Manzoor H, Leslie SW, Saikali SW. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 6, 2024. Extracorporeal Shockwave Lithotripsy. [PubMed: 32809722]
- 119.
- Almeras C, Abid N, Meria P., lithiasis committee of the French Association of Urology (CLAFU). 2022 Recommendations of the AFU Lithiasis Committee: Extracorporeal shock wave lithotripsy (ESWL). Prog Urol. 2023 Nov;33(14):812-824. [PubMed: 37918981]
- 120.
- Manukian MV. [Probability of development of "steinstrasse" depending on the number of extracorporeal shock wave lithotripsy in ureteral stones]. Georgian Med News. 2010 Apr;(181):13-7. [PubMed: 20495220]
- 121.
- Wason SE, Monfared S, Ionson A, Klett DE, Leslie SW. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Apr 20, 2024. Ureteroscopy. [PubMed: 32809391]
- 122.
- Raynal G, Malval B, Panthier F, Roustan FR, Traxer O, Meria P, Almeras C., lithiasis committee of the French Association of Urology (CLAFU). 2022 Recommendations of the AFU Lithiasis Committee: Ureteroscopy and ureterorenoscopy. Prog Urol. 2023 Nov;33(14):843-853. [PubMed: 37918983]
- 123.
- Ibrahim A, Wollin D, Preminger G, Andonian S. Technique of Percutaneous Nephrolithotomy. J Endourol. 2018 May;32(S1):S17-S27. [PubMed: 29774824]
- 124.
- Abid N, Conort P, Franquet Q, Roustan FR, Meria P, Almeras C., Lithiasis Committee of the French Association of Urology (CLAFU). 2022 Recommendations of the AFU Lithiasis Committee: Percutaneous nephrolithotomy. Prog Urol. 2023 Nov;33(14):854-863. [PubMed: 37918984]
- 125.
- Estrade V, Meria P, Almeras C., Lithiasis Committee of the French Association of Urology (CLAFU). 2022 Recommendations of the AFU Lithiasis Committee: Combined approach for the management of kidney and ureteral stones (Endoscopic Combined IntraRenal Surgery, ECIRS). Prog Urol. 2023 Nov;33(14):871-874. [PubMed: 37918986]
- 126.
- Sellers J, George A, Camacho J, Cammack JT, Medway A. Robotic-assisted laparoscopic pyelolithotomy with intracorporeal pyeloscopy in a horseshoe kidney. Urol Case Rep. 2023 Jan;46:102305. [PMC free article: PMC9794879] [PubMed: 36590647]
- 127.
- Yeh YJ, Weng SC, Lin YH, Chen CL, Tsao SH, Tsai HY, Juang HH, Chang PL, Hou CP. Comparative Analysis of Surgical Outcomes of Flexible Ureteroscopy and Da Vinci Robotic Surgery in Community Patients with Renal Pelvic Stones Larger than 2 cm. Medicina (Kaunas). 2023 Jul 29;59(8) [PMC free article: PMC10456386] [PubMed: 37629685]
- 128.
- Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR., American Urological Assocation. Medical management of kidney stones: AUA guideline. J Urol. 2014 Aug;192(2):316-24. [PubMed: 24857648]
- 129.
- Amaro CR, Goldberg J, Damasio PC, Leitão VA, Turney B, Padovani CR, Amaro JL. An update on metabolic assessment in patients with urinary lithiasis. World J Urol. 2015 Jan;33(1):125-9. [PubMed: 24623379]
- 130.
- Leslie SW, Sajjad H, Bashir K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Apr 30, 2024. 24-Hour Urine Testing for Nephrolithiasis: Interpretation and Treatment Guidelines. [PubMed: 29494055]
- 131.
- Skolarikos A, Straub M, Knoll T, Sarica K, Seitz C, Petřík A, Türk C. Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol. 2015 Apr;67(4):750-63. [PubMed: 25454613]
- 132.
- Vale L, Ribeiro AM, Costa D, Morgado A, Antunes-Lopes T, Dinis P, Silva C, Pacheco-Figueiredo L. Metabolic evaluation in urolithiasis - study of the prevalence of metabolic abnormalities in a tertiary centre. Cent European J Urol. 2020;73(1):55-61. [PMC free article: PMC7203776] [PubMed: 32395325]
- 133.
- Courbebaisse M, Travers S, Bouderlique E, Michon-Colin A, Daudon M, De Mul A, Poli L, Baron S, Prot-Bertoye C. Hydration for Adult Patients with Nephrolithiasis: Specificities and Current Recommendations. Nutrients. 2023 Nov 22;15(23) [PMC free article: PMC10708476] [PubMed: 38068743]
- 134.
- Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, Pace KT, Pais VM, Pearle MS, Preminger GM, Razvi H, Shah O, Matlaga BR. Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART I. J Urol. 2016 Oct;196(4):1153-60. [PubMed: 27238616]
- 135.
- Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, Pace KT, Pais VM, Pearle MS, Preminger GM, Razvi H, Shah O, Matlaga BR. Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART II. J Urol. 2016 Oct;196(4):1161-9. [PubMed: 27238615]
- 136.
- Morgan MS, Pearle MS. Medical management of renal stones. BMJ. 2016 Mar 14;352:i52. [PubMed: 26977089]
- 137.
- Kaur P, Bhatt H. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 15, 2023. Hyperuricosuria. [PubMed: 32965872]
- 138.
- Leslie SW, Bashir K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 30, 2023. Hypocitraturia and Renal Calculi. [PubMed: 33232062]
- 139.
- Akbari P, Khorasani-Zadeh A. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jan 23, 2023. Thiazide Diuretics. [PubMed: 30422513]
- 140.
- Qurie A, Preuss CV, Musa R. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jun 26, 2023. Allopurinol. [PubMed: 29763117]
- 141.
- Segall M, Mousavi A, Eisner BH, Scotland K. Pharmacologic treatment of kidney stones: Current medication and pH monitoring. Actas Urol Esp (Engl Ed). 2024 Jan-Feb;48(1):11-18. [PubMed: 38043680]
- 142.
- Pak CY, Heller HJ, Pearle MS, Odvina CV, Poindexter JR, Peterson RD. Prevention of stone formation and bone loss in absorptive hypercalciuria by combined dietary and pharmacological interventions. J Urol. 2003 Feb;169(2):465-9. [PubMed: 12544288]
- 143.
- Pak CY, Fuller C, Sakhaee K, Preminger GM, Britton F. Long-term treatment of calcium nephrolithiasis with potassium citrate. J Urol. 1985 Jul;134(1):11-9. [PubMed: 3892044]
- 144.
- Park SM, Jee J, Joung JY, Cho YY, Sohn SY, Jin SM, Hur KY, Kim JH, Kim SW, Chung JH, Lee MK, Min YK. High Dietary Sodium Intake Assessed by 24-hour Urine Specimen Increase Urinary Calcium Excretion and Bone Resorption Marker. J Bone Metab. 2014 Aug;21(3):189-94. [PMC free article: PMC4170081] [PubMed: 25247156]
- 145.
- Ong A, Brown G, Tokas T, Hameed BMZ, Philip J, Somani BK. Selection and Outcomes for Dissolution Therapy in Uric Acid Stones: A Systematic Review of Literature. Curr Urol Rep. 2023 Aug;24(8):355-363. [PubMed: 37079196]
- 146.
- Williams JJ, Rodman JS, Peterson CM. A randomized double-blind study of acetohydroxamic acid in struvite nephrolithiasis. N Engl J Med. 1984 Sep 20;311(12):760-4. [PubMed: 6472365]
- 147.
- Gonzalez RD, Whiting BM, Canales BK. The history of kidney stone dissolution therapy: 50 years of optimism and frustration with renacidin. J Endourol. 2012 Feb;26(2):110-8. [PMC free article: PMC3311908] [PubMed: 21999455]
- 148.
- Schillebeeckx C, Vander Eeckt K, Ost D, Van den Branden M, Deconinck S. Kidney Stone Dissolution Therapy in Phosphate Stones: A Case Report. J Endourol Case Rep. 2020;6(1):45-48. [PMC free article: PMC7383401] [PubMed: 32775674]
- 149.
- Øbro LF, Sloth Osther S, Osther PJS, Jung H. Case of the month from Lillebaelt hospital, University Hospital of South Denmark, Denmark: Renacidin® - still a useful adjunct to endoscopic surgery for complex renal struvite stone disease. BJU Int. 2022 Oct;130(4):437-440. [PMC free article: PMC9543700] [PubMed: 36102583]
- 150.
- Wall I, Tiselius HG, Larsson L. Hemiacidrin: a useful component in the treatment of infectious renal stones. Eur Urol. 1988;15(1-2):26-30. [PubMed: 3215233]
- 151.
- Kachrilas S, Papatsoris A, Bach C, Bourdoumis A, Zaman F, Masood J, Buchholz N. The current role of percutaneous chemolysis in the management of urolithiasis: review and results. Urolithiasis. 2013 Aug;41(4):323-6. [PubMed: 23743991]
- 152.
- Taylor EN, Curhan GC. Dietary calcium from dairy and nondairy sources, and risk of symptomatic kidney stones. J Urol. 2013 Oct;190(4):1255-9. [PMC free article: PMC4393945] [PubMed: 23535174]
- 153.
- Chewcharat A, Thongprayoon C, Vaughan LE, Mehta RA, Schulte PJ, O'Connor HM, Lieske JC, Taylor EN, Rule AD. Dietary Risk Factors for Incident and Recurrent Symptomatic Kidney Stones. Mayo Clin Proc. 2022 Aug;97(8):1437-1448. [PubMed: 35933132]
- 154.
- Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004 Dec;15(12):3225-32. [PubMed: 15579526]
- 155.
- Knight J, Madduma-Liyanage K, Mobley JA, Assimos DG, Holmes RP. Ascorbic acid intake and oxalate synthesis. Urolithiasis. 2016 Aug;44(4):289-97. [PMC free article: PMC4946963] [PubMed: 27002809]
- 156.
- Traxer O, Huet B, Poindexter J, Pak CY, Pearle MS. Effect of ascorbic acid consumption on urinary stone risk factors. J Urol. 2003 Aug;170(2 Pt 1):397-401. [PubMed: 12853784]
- 157.
- Thomas LD, Elinder CG, Tiselius HG, Wolk A, Akesson A. Ascorbic acid supplements and kidney stone incidence among men: a prospective study. JAMA Intern Med. 2013 Mar 11;173(5):386-8. [PubMed: 23381591]
- 158.
- Abdullah M, Jamil RT, Attia FN. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 1, 2023. Vitamin C (Ascorbic Acid) [PubMed: 29763052]
- 159.
- Reynolds LF, Kroczak T, Pace KT. Indications and contraindications for shock wave lithotripsy and how to improve outcomes. Asian J Urol. 2018 Oct;5(4):256-263. [PMC free article: PMC6197584] [PubMed: 30364729]
- 160.
- Li X, Zhu W, Lam W, Yue Y, Duan H, Zeng G. Outcomes of long-term follow-up of asymptomatic renal stones and prediction of stone-related events. BJU Int. 2019 Mar;123(3):485-492. [PubMed: 30253029]
Disclosure: Stephen Leslie declares no relevant financial relationships with ineligible companies.
Disclosure: Hussain Sajjad declares no relevant financial relationships with ineligible companies.
Disclosure: Patrick Murphy declares no relevant financial relationships with ineligible companies.
Figures
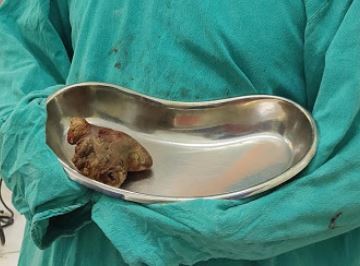
Staghorn Renal Calculus. These are complex renal stones that occupy a majority of the renal collecting system and are associated with high morbidity. Contributed by S Munakomi, MD
Nephrolithiasis, Ultrasound. The image shows a normal kidney with grades of hydronephrosis, twinkle artifact, and ureterovesical junction stone. Contributed by MK Herbst, MD